Abstract
Mechanosensitive ion channels, transmembrane proteins that directly couple mechanical stimuli to ion flux, serve to sense and respond to changes in membrane tension in all branches of life. In plants, mechanosensitive channels have been implicated in the perception of important mechanical stimuli such as osmotic pressure, touch, gravity, and pathogenic invasion. Indeed, three established families of plant mechanosensitive ion channels play roles in cell and organelle osmoregulation and root mechanosensing—and it is likely that many other channels and functions await discovery. Inspired by recent discoveries in bacterial and animal systems, we are beginning to establish the conserved and the unique ways in which mechanosensitive channels function in plants.
Introduction
The ability to sense intrinsic or extrinsic mechanical cues is as basal to the tree of life as the ownership of a cell membrane [1]. Several aspects of growth and development in land plants involve mechanical signals, including touch, osmotic stress, vibration, and gravity responses, the perception of pathogen invasion, and proprioception. One well-established component of the mechanosensory apparatus of cells in every kingdom of life is the mechanosensitive (also called stretch-activated) (MS) ion channel [2–4]). These multimeric pore-forming proteins convert mechanical force into ion flux. In some cases, the flow of ions through an open MS ion channel is sufficient for the desired response to mechanical stimulation. For example, the canonical bacterial MS ion channel MscS acts as an osmotic safety valve to protect the cell from hypo-osmotic stress; passage of ions out of the cell through channel directly accomplishes the primary function of the channel [5]. In other cases, mechanosensitive ion flux generates bioelectric signals that in turn trigger organismal sensory perception. For example, the MS ion channel NOMPC mediates touch perception in Drosophila larvae [6]. The line between the two examples above may not be so clear, as a recent report demonstrated entry of the second messenger Ca2+ into the bacterial cell through MscS during hypoosmotic shock [7]. In this article, we summarize recent exciting developments in the field of plant MS channels, speculate on their evolution, describe a few areas of limited knowledge, and propose potential solutions to technical challenges.
The Tip of the Iceberg: Known Families of Plant Mechanosensitive Channels
The first MS channel activities in plant membranes were characterized by patch clamp electrophysiology [8,9] shortly after they were discovered in animal cells (see [10] for a historical perspective). Dozens of MS channel activities in the plasma and vacuolar membranes of a wide variety of cell types and species have been described over the past 30 years (summarized in [11]), suggesting that they are used broadly in plants to respond to diverse signals. Despite this apparent ubiquity, the underlying genes/proteins and physiological function of only a handful of MS ion channel activities have been elucidated. So far, three MS channel families have so far been characterized as membrane stretch-activated in plant systems; as described in further detail below, these channels exhibit diverse, yet overlapping localization, structure, channel properties and proposed function. As a result, the activity of channels with different ionic affinities in the same or in different compartments is likely to result in crosstalk and have complex effects on ion flux into and out of the cytoplasm and apoplast (Figure 1). These three families are unlikely to provide all observed MS channel activities in plants, and a major challenge for the field will be the development of functional (rather that homology-based) screens capable of identifying additional MS channels. Intriguing candidates have been identified [12–14] but have not yet been shown to respond directly to membrane tension.
Figure 1. Subcellular Localization and Ionic Preference for Known Plant Mechanosensitive Ion Channels.
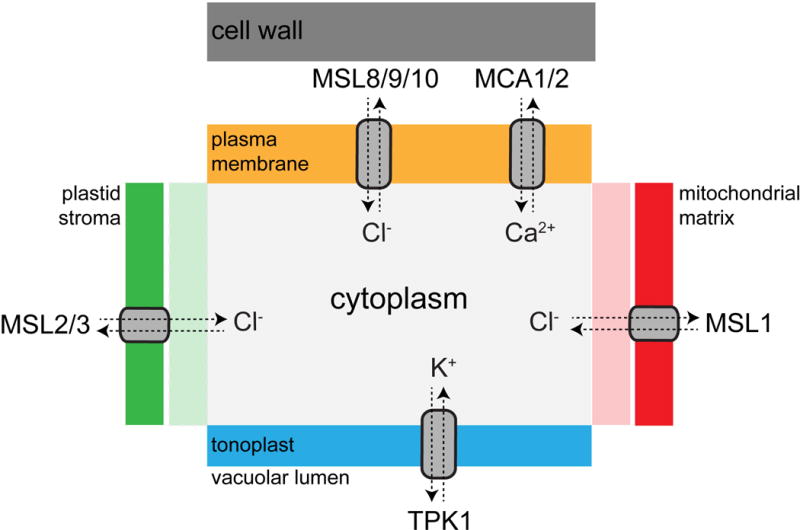
The subcellular localization of MS ion channel proteins so far identified in land plants is indicated [20–23,32,58]. The outer membrane of the chloroplast is permeable to ions [59], and Voltage-dependent Anion Channels (VDACs) are thought to mediate flux across the outer mitochondrial membrane [60]. MSL, MscS-Like; TPK, Two-pore K+; MCA, Mid1-Complementing Activity. Note that only general ion permeability preferences are indicated; these channels are likely to be permeable to additional species.
MscS-Like (MSL) Channels
Escherichia coli MscS is one of the best-understood MS ion channels in any system. It is an essentially non-selective ion channel, gated directly by membrane tension, with a large conductance of 1.2 nS. The classic function of EcMscS is to serve as an osmotic safety valve, protecting cells from rupture during extreme hypo-osmotic downshock. MscS-Like channels, or MSLs, are found throughout bacteria, archaea, some fungi, algae, and plants [15]. MSL gene families have been described and characterized to various degrees in Arabidopsis, papaya, rice, and common bean [16–19]. There are 10 MSL proteins in Arabidopsis, most of which are predicted to localize to the plasma membrane. Unexpectedly, MSL1, MSL2, and MSL3 were found to localize to the inner membrane of plastids and mitochondria (Figure 1, [20–23]).
Electrophysiological analyses of MSL9 and MSL10 in plant cells [22], MSL10 and MSL8 expressed heterologously in Xenopus oocytes [23,24], and MSL1 expressed heterologously in giant E. coli spheroplasts [21] all revealed channel characteristics that are similar (though not identical) to EcMscS. MSLs are anion-preferring (e.g. 2 to 6 anions pass for every cation) MS ion channels with conductances ranging from ~0.1 to 1 nS, depending on buffer conditions. Several lines of evidence support the model that, like EcMscS, AtMSLs function to relieve osmotic stress. This was first demonstrated with MSL2 and MSL3, two plastid-localized channels that directly maintain plastid osmoregulation. Plastids in msl2 msl3 mutants exhibit altered size, shape and fission [20,25,26]. The loss of MSL2/3 also leads to stress responses associated with drought and the development of callus tissue at the apex of the plant [27,28]. While the pleiotropic phenotypes associated with this mutant have illustrated the importance of plastid osmoregulation during normal plant growth and development, any mechanistic insights await the electrophysiological analysis of MSL2 and MSL3—a challenging prospect for plastid-localized proteins. Adding to the complexity is a recent report demonstrating that mitochondria-localized MSL1 is required to ameliorate the oxidative burden imposed upon mitochondria during abiotic stress [21]. The potential role of membrane tension, redox state, and transmembrane voltage in regulating MSL1 channel activity in vivo remains to be determined. For plasma membrane-localized MSLs, recent reports both support their role as osmotic safety valves and suggest more complex function, as discussed below.
Two-Pore Domain K+ (TPK) Channels
TREK1, TREK2, and TRAAK are MS channels from the TPK family that are expressed in the mammalian nervous system and are proposed to modulate mechanical, heat and cold-associated pain perception [29]. AtTPK1 is a voltage-independent K+ channel required for normal guard cell closure kinetics [30], and, along with homologs from rice and barley, has been demonstrated to be mechanosensitive [31]. Whether the mechanosensitive activity of AtTPK1 is important for its function in guard cells, and how it is integrated with other regulatory signals such as low pH, Ca2+ and binding to 14-3-3 proteins is not yet understood [30].
Mid1-Complementing Activity (MCA) Channels
The Mid1-Complementing Activity (MCA) proteins were identified based on their ability to rescue the mating-induced lethality of the yeast mid1 mutant [32]. MCA proteins are plant-specific and show no homology to the yeast Mid1 channel. In fact, MCA proteins have only 1 transmembrane (TM) domain [33], placing them outside the norm for ion channel subunits. Cryo-EM imaging followed by single particle reconstruction of a MCA2 tetramer did not reveal a pore [34]. However, heterologously expressed MCA1 and 2 produce increased current in response to osmotic swelling in whole cells and to membrane stretch in excised patches [35], providing evidence that they directly form a MS ion channel. MCA expression is correlated with enhanced Ca2+ influx in response to hypoosmotic shock and mechanical stimulus in several plant species [32,36,37]. Arabidopsis MCAs are required for normal rates of root penetration into hard agar and for proper response to cellulose biosynthesis inhibition, implying a role in the maintenance/response to extracellular mechanical stress [32,38]. MCAs may be involved in the perception of developmentally imposed mechanical signals, as a maize MCA homolog was recently identified in a screen for leaf patterning mutants [39].
Getting our Sea Legs: Recent Advances in Understanding Plasma Membrane Localized MSL Channels
MSL8 Fully Meets the Criteria for a Mechanoreceptor
A recent analysis of MSL8, a MS ion channel expressed exclusively in mature pollen grains and tubes, advanced our understanding of the function of plasma membrane-localized MSL channels and underscores the essential role of osmoregulation during fertilization. The correct level of MSL8 activity appears critical for pollen to survive hydration and germination and for full male fertility. Disruption of MSL8 results in high rates of bursting during pollen hydration and germination, but the overall rate of in vitro germination is higher than the wild type. On the other hand, overexpressing MSL8 inhibits pollen germination and no bursting is observed [23]. These opposing effects can be attributed to the inability to relieve excess turgor during hydration (in msl8 mutants) or to maintain necessary turgor during germination, and tube growth (in lines that overexpress MSL8) (Figure 2). Lesions that disrupt the ion conducting properties of MSL8 also disrupt its ability to accomplish these functions in pollen [40], providing further evidence that it serves directly as an osmotic mechanosensor in pollen membranes. MSL8 is thus the first plant protein to fill the stated criteria for a mechanoreceptor [2].
Figure 2. Proposed Role of MSL8 in Controlling Turgor During Pollen Hydration, Germination, and Tube Growth.
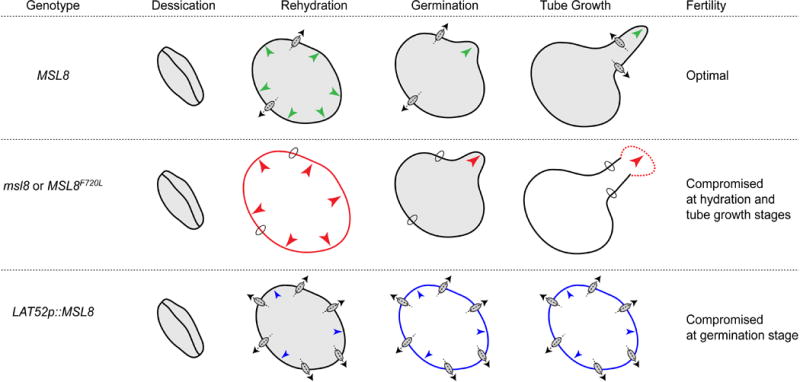
Wild-type pollen grains successfully survive hydration in distilled water, germinate effectively in germination media, produce intact pollen tubes, and are optimally fertile. Pollen grains from msl8-4 null mutants, or null mutants expressing the MSL8F720L allele, display reduced viability upon hydration in distilled water, and we propose that this is due to an inability to relieve turgor pressure by releasing ions upon hypoosmotic shock. Excess turgor after hydration leads both to germination at a rate higher than the wild type, but also to frequent bursting, and an overall loss of fertility. When MSL8 is overexpressed from the pollen-specific, strong LAT52 promoter, pollen grains survive hydration but are unable to maintain the threshold turgor pressure required for pollen germination or tube elongation. Green arrows, optimal turgor; red arrows, excessive turgor; blue arrows, insufficient turgor.
Links Between MSLs and Stress Responses
The role or roles of MSLs at the plasma membrane in cells other than pollen grains has remained stubbornly opaque. Both MSL and MCA gene expression responds to vibration [41] and nodulation [42], but the physiological relevance of these observations have yet to be demonstrated. While a mutant harboring lesions in 5 MSL genes (msl4 msl5 msl6 msl9 msl10) ablated the primary MS channel activity in Arabidopsis root protoplasts, the quintuple mutant does not produce an observable mutant phenotype in response to a wide range of mechanical, touch or osmotic stimuli [22]. However, overexpression of MSL10 results in dwarfing, ROS accumulation, and ectopic lesions, and all of these effects are negatively regulated by phosphorylation of the N-terminus [43]. Dwarfing and ectopic lesions are also observed in response to a single EMS-induced point mutation in the C-terminus of MSL10 [44], suggesting that these overexpression phenotypes reflect some aspect of the normal gene function. In addition, a recent study implicated MSL4 in pathogen-triggered immunity [45], and MSL6 phosphorylation was observed in response to oligogalacturonide treatment [46]. We propose that plasma membrane-localized MSLs serve as sensors of cellular mechanical homeostasis, or “mechanostasis”. This idea is supported by a recent meta-analysis of Arabidopsis microarray datasets wherein MSL10 expression levels were altered in a wide range of mutant backgrounds [47].
An intriguing aspect to the MSL10 study was the discovery that the soluble N-terminus of MSL10 is on its own able to trigger cell death in an overexpression system, indicating that the protein harbors at least one function independent of the production of a channel pore [43]. Determining if this non-conducting function is regulated by membrane tension is an important next step. If so, MSLs (and possibly other MS channels or MS channel homologs [39]) may have evolved to couple changes in membrane tension to a wide range of signaling outputs beyond ion flux.
Beyond the Horizon: Innovations in MS Channel Studies
Plant MS Channel Structure and Gating Dynamics
Structural information about bacterial and animal MS channels derived from a multiplicity of approaches has led to a rapid uptick in our understanding of the structural and biophysical basis of mechanosensitivity. A number of recent reports utilizing crystallography, EPR spectroscopy, PELDOR, and/or molecular dynamics add exciting and provocative new detail to the force-from-lipid concept/principle [1], see Box 1, and suggest that lipid acyl chains filling voids or pockets in the channel surface could “drag” MS channels open under increased membrane tension [48,49] or even block the permeation pathway [50](but see [51]). While these new ideas are sparking a great deal of discussion in the field, MS channels from plants have yet to contribute to the conversation. The cryo-EM structure of MCA2 provides only low resolution information (26 Å) [34], and nothing is yet known about the structure or even oligomeric state of any MSL channel.
Box 1: The force-from-lipid principle.
According to the force-from-lipid principle, anisotropic forces inherent to the lipid bilayer impinge on the conformation of membrane-embedded proteins. Ion channels classified as mechanosensitive allow the passage of ions when forces directly transmitted from the lipid bilayer are transduced into conformational rearrangements of the protein. This concept is proposed to underlie the mechanosensitivity of channels from multiple kingdoms and evolutionarily unrelated families. It follows from this principle that all channels are to some degree mechanosensitive; enhanced sensitivity, dynamic range, and spatio-temporal control are accomplished through structural arrangement and/or by tethering to cytoskeletal elements or extracellular matrix.
Solving the structure of plant MSLs would do more than contribute to our view of MS channel gating dynamics. Arabidopsis MSL family members differ substantively from EcMscS (and from each other) not only in terms of the number of TM helices, but in the presence of soluble domains at the N- and C-termini and in inter-TM loops [11,52]. We have previously proposed that this diversity in structure within the MscS family implies that MSL channels in plants may have functions and regulatory mechanisms that are specific to multicellular eukaryotes [53]. A three-dimensional structure of these channels would reveal the spatial relationship between the regions thought to serve as tension sensors, the channel pore, and soluble domains. This would help us determine how membrane tension is transmitted from the channel-membrane interface to the channel pore—and potentially to other domains within the protein (see non-conducting functions, above).
Closing the Gap between Channel Behavior in the Patch Pipette and in the Intact Plant Cell
While patch clamp electrophysiology has proven a powerful way to identify and characterize MS ion channels, in plants takes place in the absence of a cell wall, sometimes in an isolated membrane patch, in tightly regulated and non-physiological ionic conditions, and in the case of heterologous expression, not in the native lipid environment. Thus, the next great challenge for the field will be developing approaches that allow the analysis of MS ion channel action in their native context. Controlled activation of MS channels from inside a plant cell might be possible through the application of focused ultrasound, as was recently demonstrated for animal TPKs expressed in oocytes [54]. Integration of localized extracellular ion flux measurements with genetically encoded ion or voltage biosensors may allow the study of MS channel function in some cellular contexts, such as pollen tubes [55]. To date, the genetically encoded sensors for transmembrane voltage used extensively in animal systems to monitor ion channel activity in vivo [56] do not yet function well in plants [57].
Conclusion
Membrane tension is a force intrinsic to all cells, and every branch of life expresses ion channels that serve specifically to sense and respond to it. In plants, MS ion channels are widely distributed across multiple species, cell types, and intracellular compartments. In Arabidopsis, MS ion channels are required for roots to penetrate hard agar and mediate osmoregulation of pollen and plastids during normal growth and development. Future work should reveal the physiological function of channels we know, add more channel genes and proteins to our short list, and develop the methodologies that will allow in vivo analysis of ion channel function, regulation, and mechanism.
Highlights.
Mechanosensitive ion channels are capable of transducing force into ion flux
Three families of plant MS channels are described to date; many more likely exist
MS channels were repurposed during evolution to perform novel functions in plants
They play roles in root mechanosensing and osmoregulation
Mechanistic insight will require novel structural studies and in vivo analyses
Acknowledgments
We apologize to those whose work we were unable to include due to space constraints. We are grateful for the experimental and intellectual contributions of past and present members of the Haswell lab. Our work is currently supported by National Science Foundation MCB-1253103, National Institutes of Health R01GM084211, the National Science Foundation Science and Technology Center for Engineering Mechanobiology Award #1548571, and a Faculty Scholar grant from the Howard Hughes Medical Institute and the Simons Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anishkin A, Loukin SH, Teng J, Kung C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc Natl Acad Sci USA. 2014;111:7898–7905. doi: 10.1073/pnas.1313364111. • The authors of this Perspective argue that the force-from-lipid principle can and should be applied to membrane-embedded proteins in all organisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranade SS, Syeda R, Patapoutian A. Mechanically Activated Ion Channels. Neuron. 2015;87:1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyronnet R, Tran D, Girault T, Frachisse J-M. Mechanosensitive channels: feeling tension in a world under pressure. Front Plant Sci. 2014;5:558. doi: 10.3389/fpls.2014.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinac B. Mechanosensitive ion channels: an evolutionary and scientific tour de force in mechanobiology. Channels (Austin) 2012;6:211–213. doi: 10.4161/chan.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levina N, Tötemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox CD, Nomura T, Ziegler CS, Campbell AK, Wann KT, Martinac B. Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nature Communications. 2013;4:2137. doi: 10.1038/ncomms3137. [DOI] [PubMed] [Google Scholar]
- 8.Falke LC, Edwards KL, Pickard BG, Misler S. A stretch-activated anion channel in tobacco protoplasts. FEBS Lett. 1988;237:141–144. doi: 10.1016/0014-5793(88)80188-1. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder JI, Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends in Biochemical Sciences. 1989;14:187–192. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]
- 10.Morris CE. Mechanosensitive ion channels. J Membr Biol. 1990;113:93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton ES, Schlegel AM, Haswell ES. United in diversity: mechanosensitive ion channels in plants. Annu Rev Plant Biol. 2015;66:113–137. doi: 10.1146/annurev-arplant-043014-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2014;514:367–371. doi: 10.1038/nature13593. [DOI] [PubMed] [Google Scholar]
- 13.Hou C, Tian W, Kleist T, He K, Garcia V, Bai F, Hao Y, Luan S, Li L. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 2014;24:632–635. doi: 10.1038/cr.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pivetti CD, Yen M-R, Miller S, Busch W, Tseng Y-H, Booth IR, Saier MH. Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. – table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silvia GHUL de S, Adriana PDS, Tania MI, Eduardo GDS. Genome-wide analysis of mechanosensitive channel of small conductance (MscS)-like gene family in common bean. Afr J Biotechnol. 2016;15:580–592. [Google Scholar]
- 17.Porter BW, Zhu YJ, Webb DT, Christopher DA. Novel thigmomorphogenetic responses in Carica papaya: touch decreases anthocyanin levels and stimulates petiole cork outgrowths. Ann Bot. 2009;103:847–858. doi: 10.1093/aob/mcp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saddhe AA, Kumar K. In silico identification and expression analysis of MscS like gene family in rice. Plant Gene. 2015;1:8–17. doi: 10.1016/j.compbiolchem.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Haswell ES. Mechanosensitive Ion Channels, Part A. Elsevier; 2007. MscS-Like Proteins in Plants; pp. 329–359. [Google Scholar]
- 20.Haswell ES, Meyerowitz EM. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Lee CP, Maksaev G, Jensen GS, Murcha MW, Wilson ME, Fricker M, Hell R, Haswell ES, Millar AH, Sweetlove L. MSL1 is a mechanosensitive ion channel that dissipates mitochondrial membrane potential and maintains redox homeostasis in mitochondria during abiotic stress. Plant J. 2016 doi: 10.1111/tpj.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse J-M. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol. 2008;18:730–734. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton ES, Jensen GS, Maksaev G, Katims A, Sherp AM, Haswell ES. Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science. 2015;350:438–441. doi: 10.1126/science.aac6014. •• MSL8 is shown to be a pollen-specific, membrane tension-gated, ion channel required for pollen to survive the hypoosmotic shock of rehydration and for full male fertility. MSL8 negatively regulates pollen germination, but is required for cellular integrity during germination and tube growth. These data suggest that homologs of bacterial MscS have been repurposed in eukaryotes to sense and respond to mechanical stimuli in a developmental context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maksaev G, Haswell ES. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci USA. 2012;109:19015–19020. doi: 10.1073/pnas.1213931109. • This study established that MSL10 is a bona fide mechanosensitive ion channel using single-channel patch clamp electrophysiology and a heterologous expression system, and characterized its behavior. Thus, plant genomes do indeed encode mechanosensitive ion channels evolutionarily related to those from bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson ME, Jensen GS, Haswell ES. Two mechanosensitive channel homologs influence division ring placement in Arabidopsis chloroplasts. Plant Cell. 2011;23:2939–2949. doi: 10.1105/tpc.111.088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veley KM, Marshburn S, Clure CE, Haswell ES. Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol. 2012;22:408–413. doi: 10.1016/j.cub.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson ME, Basu MR, Bhaskara GB, Verslues PE, Haswell ES. Plastid osmotic stress activates cellular stress responses in Arabidopsis. Plant Physiol. 2014;165:119–128. doi: 10.1104/pp.114.236620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson ME, Mixdorf M, Berg RH, Haswell ES. Plastid osmotic stress influences cell differentiation at the plant shoot apex. Development. 2016;143:3382–3393. doi: 10.1242/dev.136234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brohawn SG. How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann N Y Acad Sci. 2015;1352:20–32. doi: 10.1111/nyas.12874. [DOI] [PubMed] [Google Scholar]
- 30.Voelker C, Gomez-Porras JL, Becker D, Hamamoto S, Uozumi N, Gambale F, Mueller-Roeber B, Czempinski K, Dreyer I. Roles of tandem-pore K+ channels in plants - a puzzle still to be solved*. Plant Biology. 2010;12:56–63. doi: 10.1111/j.1438-8677.2010.00353.x. [DOI] [PubMed] [Google Scholar]
- 31.Maathuis FJM. Vacuolar two-pore K+ channels act as vacuolar osmosensors. New Phytol. 2011;191:84–91. doi: 10.1111/j.1469-8137.2011.03664.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. •• The authors employed a novel functional screen in yeast to identify MCA1, a plant-specific membrane protein that facilitates Ca2+ influx in response to mechanical and osmotic stress. Seedlings lacking functional MCA1 are unable to efficiently penetrate hard agar. MCA1 and close homolog MCA2 are leading candidates for the long-sought plant stretch-activated Ca2+ channel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamano S, Kume S, Iida K, Lei K-J, Nakano M, Nakayama Y, Iida H. Transmembrane Topologies of Ca2+-permeable Mechanosensitive Channels MCA1 and MCA2 in Arabidopsis thaliana. J Biol Chem. 2015;290:30901–30909. doi: 10.1074/jbc.M115.692574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shigematsu H, Iida K, Nakano M, Chaudhuri P, Iida H, Nagayama K. Structural characterization of the mechanosensitive channel candidate MCA2 from Arabidopsis thaliana. PLoS ONE. 2014;9:e87724. doi: 10.1371/journal.pone.0087724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuichi T, Iida H, Sokabe M, Tatsumi H. Expression of Arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant Signal Behav. 2012;7:1022–1026. doi: 10.4161/psb.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurusu T, Yamanaka T, Nakano M, Takiguchi A, Ogasawara Y, Hayashi T, Iida K, Hanamata S, Shinozaki K, Iida H, et al. Involvement of the putative Ca2+-permeable mechanosensitive channels, NtMCA1 and NtMCA2, in Ca2+ uptake, Ca2+-dependent cell proliferation and mechanical stress-induced gene expression in tobacco (Nicotiana tabacum) BY-2 cells. J Plant Res. 2012;125:555–568. doi: 10.1007/s10265-011-0462-6. [DOI] [PubMed] [Google Scholar]
- 37.Kurusu T, Nishikawa D, Yamazaki Y, Gotoh M, Nakano M, Hamada H, Yamanaka T, Iida K, Nakagawa Y, Saji H, et al. Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol. 2012;12:11. doi: 10.1186/1471-2229-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H, Terashima A, Iida K, Kojima I, Katagiri T, et al. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 2010;152:1284–1296. doi: 10.1104/pp.109.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosa M, Abraham-Juárez MJ, Lewis MW, Fonseca JP, Tian W, Ramirez V, Luan S, Pauly M, Hake S. The Maize MID-COMPLEMENTING ACTIVITY Homolog CELL NUMBER REGULATOR13/NARROW ODD DWARF Coordinates Organ Growth and Tissue Patterning. THE PLANT CELL ONLINE. 2017;29:474–490. doi: 10.1105/tpc.16.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton ES, Haswell ES. The Tension-sensitive Ion Transport Activity of MSL8 is Critical for its Function in Pollen Hydration and Germination. Plant and Cell Physiology. 2017 doi: 10.1093/pcp/pcw230. •• Point mutations were introduced into the presumptive channel pore of MSL8 and resulting variants assessed for channel behavior and function in pollen hydration, germination, and tube growth. Lesions in the pore that change channel behavior also change physiological function, and it is concluded that MSL8 serves as a mechanoreceptor in pollen. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh R, Gururani MA, Ponpandian LN, Mishra RC, Park S-C, Jeong M-J, Bae H. Expression Analysis of Sound Vibration-Regulated Genes by Touch Treatment in Arabidopsis. Front Plant Sci. 2017;8:100. doi: 10.3389/fpls.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damiani I, Drain A, Guichard M, Balzergue S, Boscari A, Boyer J-C, Brunaud V, Cottaz S, Rancurel C, Da Rocha M, et al. Nod Factor Effects on Root Hair-Specific Transcriptome of Medicago truncatula: Focus on Plasma Membrane Transport Systems and Reactive Oxygen Species Networks. Front Plant Sci. 2016;7:3389–22. doi: 10.3389/fpls.2016.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veley KM, Maksaev G, Frick EM, January E, Kloepper SC, Haswell ES. Arabidopsis MSL10 has a regulated cell death signaling activity that is separable from its mechanosensitive ion channel activity. Plant Cell. 2014;26:3115–3131. doi: 10.1105/tpc.114.128082. •• This study used molecular genetics, a transient expression assay, and electrophysiology to show that MSL10 has two genetically separable functions, each attributable to a different domain of the protein. These results implicate MSL10 in ROS-mediated cell death, and provide evidence that some MscS homologs have non-conducting functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou Y, Chintamanani S, He P, Fukushige H, Yu L, Shao M, Zhu L, Hildebrand DF, Tang X, Zhou J-M. A gain-of-function mutation in Msl10 triggers cell death and wound-induced hyperaccumulation of jasmonic acid in Arabidopsis. J Integr Plant Biol. 2016;58:600–609. doi: 10.1111/jipb.12427. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Tateda C, Jiang S-C, Shrestha J, Jelenska J, Speed DJ, Greenberg JT. A Suite Of Receptor-Like Kinases and a Putative Mechano-Sensitive Channel Are Involved in Autoimmunity and Plasma Membrane–Based Defenses in Arabidopsis. Mol Plant Microbe Interact. 2017 doi: 10.1094/MPMI-09-16-0184-R. • The authors identify MSL4 as one of several proteins that interact with ACD6, a plasma membrane-localized defense protein. msl4 mutants show multiple defense-related defects, including reduced resistance to Pseudomonas syringae pv. maculicola ES4326 hrcC–. [DOI] [PubMed] [Google Scholar]
- 46.Kohorn BD, Hoon D, Minkoff BB, Sussman MR, Kohorn SL. Rapid Oligo-Galacturonide Induced Changes in Protein Phosphorylation in Arabidopsis. Mol Cell Proteomics. 2016;15:1351–1359. doi: 10.1074/mcp.M115.055368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Salle P, Incerti G, Colantuono C, Chiusano ML. Gene co-expression analyses: an overview from microarray collections in Arabidopsis thaliana. Brief Bioinform. 2016 doi: 10.1093/bib/bbw002. [DOI] [PubMed] [Google Scholar]
- 48.Bavi N, Cox CD, Perozo E, Martinac B. Towards a structural blueprint for bilayer-mediated channel mechanosensitivity. Channels (Austin) 2016 doi: 10.1080/19336950.2016.1224624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pliotas C, Naismith JH. Spectator no more, the role of the membrane in regulating ion channel function. Curr Opin Struct Biol. 2016;45:59–66. doi: 10.1016/j.sbi.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Brohawn SG, Campbell EB, MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature. 2014;516:126–130. doi: 10.1038/nature14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aryal P, Jarerattanachat V, Clausen MV, Schewe M, McClenaghan C, Argent L, Conrad LJ, Dong YY, Pike ACW, Carpenter EP, et al. Bilayer-Mediated Structural Transitions Control Mechanosensitivity of the TREK-2 K2P Channel. Structure. 2017 doi: 10.1016/j.str.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cox CD, Nakayama Y, Nomura T, Martinac B. The evolutionary “tinkering” of MscS-like channels: generation of structural and functional diversity. Pflugers Arch. 2015;467:3–13. doi: 10.1007/s00424-014-1522-2. [DOI] [PubMed] [Google Scholar]
- 53.Haswell ES, Phillips R, Rees DC. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–1369. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kubanek J, Shi J, Marsh J, Chen D, Deng C, Cui J. Ultrasound modulates ion channel currents. Nature Publishing Group. 2016;6:24170. doi: 10.1038/srep24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damineli DSC, Portes M-T, Feijó JA. Oscillatory signatures underlie growth regimes in Arabidopsis pollen tubes: computational methods to estimate tip location, periodicity, and synchronization in growing cells. Journal of Experimental Botany. 2017 doi: 10.1093/jxb/erx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.St-Pierre F, Chavarha M, Lin MZ. Designs and sensing mechanisms of genetically encoded fluorescent voltage indicators. Curr Opin Chem Biol. 2015;27:31–38. doi: 10.1016/j.cbpa.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matzke AJM, Matzke M. Expression and testing in plants of ArcLight, a genetically-encoded voltage indicator used in neuroscience research. BMC Plant Biol. 2015;15:245. doi: 10.1186/s12870-015-0633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Czempinski K, Frachisse J-M, Maurel C, Barbier-Brygoo H, Mueller-Roeber B. Vacuolar membrane localization of the Arabidopsis “two-pore” K+ channel KCO1. Plant J. 2002;29:809–820. doi: 10.1046/j.1365-313x.2002.01260.x. [DOI] [PubMed] [Google Scholar]
- 59.Heldt HW, Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971;234:83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- 60.Homblé F, Krammer E-M, Prévost M. Plant VDAC: facts and speculations. Biochim Biophys Acta. 2012;1818:1486–1501. doi: 10.1016/j.bbamem.2011.11.028. [DOI] [PubMed] [Google Scholar]


