Abstract
Fetal alcohol spectrum disorders (FASD) are caused by ethanol exposure during the pregnancy and is the leading cause of mental retardation. Ethanol exposure during the development results in the loss of neurons in the developing brain, which may underlie many neurobehavioral deficits associated with FASD. It is important to understand the mechanisms underlying ethanol-induced neuronal loss and develop appropriate therapeutic strategies. One of the potential mechanisms involves neuroimmune activation. Using a third trimester equivalent mouse model of ethanol exposure, we demonstrated that ethanol induced a wide-spread neuroapoptosis, microglial activation, and neuroinflammation in C57BL/6 mice. Minocycline is an antibiotic that inhibits microglial activation and alleviates neuroinflammation. We tested the hypothesis that minocycline may protect neurons ethanol-induced neuron death by inhibiting microglial activation and neuroinflammation. We showed that minocycline significantly inhibited ethanol-induced caspase-3 activation, microglial activation, and the expression of pro-inflammatory cytokines. In contrast, minocycline reversed ethanol inhibition of anti-inflammatory cytokines. Minocycline blocked ethanol-induced activation of GSK3β, a key mediator of neuroinflammation and microglial activation in the developing brain. Consistent with the in vivo observations, minocycline inhibited ethanol-induced the expression of pro-inflammatory cytokines and activation of GSK3β in a microglia cell line (SIM-9). GSK3β inhibitor eliminated ethanol activation of pro-inflammatory cytokines in SIM-9 cells. Co-cultures of cortical neurons and SIM-9 microglia cells sensitized neurons to alcohol-induced neuronal death. Minocycline protected neurons against ethanol-induced neuronal death in neurons/microglia co-cultures. Together, these results suggest that minocycline may ameliorate ethanol neurotoxicity in the developing by alleviating GSK3β-mediated neuroinflammation.
Keywords: Apoptosis, development, fetal alcohol syndrome, inflammation, microglia, neurodegeneration
Introduction
Ethanol exposure during pregnancy may cause fetal alcohol spectrum disorders (FASD) which are characterized by a spectrum of structural anomalies and neurocognitive and behavioral disabilities (Riley et al., 2011). Fetal Alcohol Syndrome (FAS) is the most severe form of FASD and displays the complete phenotype of characteristic intrauterine growth restriction, central nervous system (CNS) malformations, mental retardation, and craniofacial and skeletal defects. The incidence of FASD in the United States is between 2 and 5% (May et al., 2009). FASD represent the leading cause of mental retardation in North America (K. et al., 2008; May and Gossage, 2001). Ethanol affects divers developmental events at all stages ranging from neurogenesis to myelination (Saito et al., 2016; Yang and Luo, 2015; Young et al., 2008). Among them, permanent depletion of neurons is the most devastating effect. Despite attempts to increase public awareness of the risks involved, the number of women drinking during pregnancy has not declined in the USA (Ebrahim et al., 1999; Surveys, 2013). It is therefore important to understand the mechanisms underlying ethanol-induced neuron death and develop strategies prevent or ameliorate alcohol-induced CNS damages.
It is well established that neuroinflammation plays an important role in alcohol use disorder (AUD) (Crews et al., 2017). Recently findings indicate that ethanol induces neuroimmune activation in the developing brain as well. Microglia, the resident macrophages, are the mediator of neuroinflammation in the CNS, and it has been proposed that ethanol could activate microglial and induce proinflammatory molecules which impair neuronal survival and function, leading to long-term neuropathological and cognitive defects observed in FASD (Chastain and Sarkar, 2014; Drew and Kane, 2014; Kane and Drew, 2016).
Minocycline, a second generation broad-spectrum antibiotic, is a tetracycline derivative that is capable of crossing the blood brain barrier (BBB). It is frequently postulated to be a “microglia inhibitor” (Moller et al., 2016) and has a potent anti-microglial activation and anti-inflammatory property. Minocycline has been shown to protect neurons in various neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), cerebral ischemia, spinal cord injury (Budni et al., 2016; Chen et al., 2012; Cox et al., 2015; Dheen et al., 2007; Seidl and Potashkin, 2011). In this study, we tested the hypothesis that minocycline may protect neurons ethanol-induced neuron death by inhibiting microglial activation and neuroinflammation in the developing brain. We used a well-established third trimester equivalent mouse model in which ethanol produces a wide-spread neurodegeneration in the developing brain. We showed that minocycline effectively inhibited ethanol-induced microglial activation/neuroinflammation and ameliorated neurodegeneration. It appears that minocycline may target AKT/GSK3β-mediated neuroinflammation in microglia.
Material and Methods
Reagents
Minocycline was purchased from Sigma Chemical Co. (St. Louis, MO, USA). TRIzol was purchased from Life Technologies (Gaithersburg, MD, USA). Anti-MCP-1 and anti-CD68 antibodies were purchased from Bio-Rad AbD Serotec, Inc. (Raleigh, NC, USA). Anti-CCR-2 antibody was purchased from BioVision, Inc. (Milpitas, CA, USA). Anti-Iba-1 antibody was purchased from Wako Chemicals USA, Inc. (Richmond, VA, USA). Anti-OX-42 antibody was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All other antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Other reagents used in this project were obtained from Sigma-Aldrich, Inc (St. Louis, MO, USA) unless stated otherwise.
Animals and ethanol exposure
C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). All animals were housed in a specific pathogen-free room within the animal facilities at the University of Kentucky, and were handled according to the Institutional Animal Care and Use Committee, University of Kentucky. Minocycline and ethanol were administrated on postnatal day 5 (PD5). Pups were weighed, toe-clipped, and randomly assigned to 4 groups: control, minocycline, ethanol, and ethanol plus minocycline. Minocycline was dissolved in PBS and administered by two subcutaneous (SC) injections at a concentration of 15 mg/kg or 30 mg/kg each at 12 and 2 hours prior to ethanol exposure. The concentrations of minocycline were selected based on previous studies in rodents investigating the neuroprotection of minocycline (Beheshti Nasr et al., 2013; Kobayashi et al., 2013). We injected minocycline before ethanol exposure to ensure the minocycline inhibition of microglial activation. Pups received a total of 5 g/kg ethanol in two SC injections; each consisted of 2.5 g/kg and was 2 hours apart (Alimov et al., 2013; Ikonomidou et al., 2000; Olney et al., 2002). Pups were returned to the dam immediately following each injection. Control animals received SC injections of the same volume of sterile saline. At 8 hours after the first ethanol injection, pups were sacrificed for and processed for neurochemical and molecular analyses
Culture and treatment of microglial cells and neurons
Immortalized mouse microglia cells (SIM-A9) were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). SIM-A9 cells are a spontaneously immortalized microglial cell line cloned from mouse cerebral tissues of C57BL6 mice. They have phenotypic and functional properties characteristic of primary microglia and are capable of switching their profiles to pro- or anti-inflammatory phenotypes in response to exogenous stimuli (Nagamoto-Combs et al., 2014). SIM-A9 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium supplemented with 5% heat-inactivated horse serum, 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2 in a humidified atmosphere. At 80–90% confluence, the culture medium was replaced with DMEM/F12 medium containing 0.1% FBS overnight before indicated treatment. The cultures were exposed to ethanol (0.2%, 0.4%, or 0.8%) in sealed containers. The containers were placed in a humidified environment and maintained at 37°C with 5 % CO2. With this method, the ethanol concentration in the culture medium can be accurately maintained (Luo and Miller, 1997). For minocycline treatment, cells were exposed to minocycline (50 μM) 2 hours prior to ethanol treatment as previously described (Song et al., 2008).
Primary cortical neurons were generated from the brain of C57BL6 mice on postnatal day 1. The method for the isolation and culture of primary cortical neuronal has been previously described in detail (Chen et al., 2016; Wang et al., 2007). Briefly, the pups were decapitated and the brain immediately transferred into dissection medium (97.5% Hank’s balanced salt solution, 0.11 mg/ml sodium pyruvate, 0.1% glucose, 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin). The meninges were removed and the cerebral cortices dissected. Cerebral cortical tissues were dissociated with 0.25% trypsin for 15 min at 37 °C followed by 0.1% DNase treatment for 5 min. Then, tissues were carefully triturated and the cell suspension was mixed with 4% bovine serum albumin and centrifuged. The cell pellet was resuspended in Neuronbasal/B27 medium containing B27 (2%), glutamine (1 mM/L), penicillin (100 U/ml) and streptomycin (100 μg/ml). Cells were plated onto poly-D-lysine (50 μg/ml)-coated cell culture wells or dishes and maintained at 37°C in a humidified environment containing 5% CO2 for 7 days before the initiation of experiment.
The co-cultures of microglia cells and primary cortical neurons were established in 24-well cell culture plates with inserts as described previously (Ock et al., 2010; Wang et al., 2015a). The cortical neurons were maintained in the cell culture plate containing 500 μl of medium at a density of 2.5 ×107 cells/well. SIM-A9 cells were grown in the culture insert (0.2 μm pore size) in a separate plate at a density of 6 × 106 cells/per insert in 200 μl of medium. SIM-A9 cells were cultured overnight at 37°C and 5% CO2, then treated with minocycline at 50 μM for 30 min. After that, the inserts containing SIM-A9 cells were placed into the wells containing the cortical neurons. The co-cultures were then treated with ethanol with or without minocycline for 48 hours. The viability of neurons was determined by the 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as previously described (Wang et al., 2007).
Immunoblotting
After treatment, mice were anesthetized by intraperitoneal injection of ketamine/xylazine and the cerebral cortices, cerebella, and the rest of brain were immediately dissected from four groups of mice. The tissues were frozen in liquid nitrogen and stored at −80°C. The proteins in brain tissues or SIM-A9 cells were extracted using previously described method with some modification (Wang et al., 2007). Briefly, tissues or cells were homogenized and lysed in an ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EGTA, 1 mM PMSF, 0.5% NP-40, 0.25% SDS, 5 μg/ml leupeptin, and 5 μg/ml aprotinin. Homogenates were centrifuged at 20,800 x g for 30 min at 4°C and the supernatant fractions were collected. The protein concentration in the supernatant was determined
The procedure for immunoblotting has been previously described (Wang et al., 2007). Briefly, aliquots of proteins (50 μg) were loaded into the lanes of a sodium dodecyl sulfate-polyacrylamide gel. The proteins were separated by electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 5% BSA in 0.01 M Tris-buffered saline (TBS) (pH 7.4) and 0.05% Tween-20 (TBST) at room temperature for 1 hour, then incubated with primary antibodies directed against target proteins overnight at 4°C. The final dilutions for primary antibodies were: anti-cleaved caspase-3 antibody, 1:1,000; anti-phospho-GSK3β (Ser9) antibody, 1:1,000; phospho-GSK3β (Tyr216) antibody, 1:1,000; anti-MCP-1 antibody, 1:500; anti-CCR-2 antibody, 1:1,000; anti-CD-68 antibody, 1:1,000; anti-OX-42 antibody, 1:1,000; anti-phospho-Akt (Ser473) antibody, 1:1000; anti-phospho-Akt (Thr308) antibody, 1:1,000; anti-active β-catenin antibody, 1:1000; anti-phospho-CREB antibody, 1:1,000; and anti-phospho-c-Jun antibody, 1:500. After two quick washes in TBST, the membranes were incubated with secondary antibodies conjugated to horseradish peroxidase (Amersham, Arlington, Heights, IL) diluted at 1:5, 000 in TBST containing 5% BSA for 1 hour. The immunocomplexes were detected by the enhanced chemiluminescence method (Amersham). The density of immunoblotting was quantified with the software of ImageJ (version 1.48; National Institutes of Health, Bethesda, MD).
Quantitative real-time PCR
Total RNA was extracted from brain tissues samples which were stored in liquid nitrogen or SIM-A9 cells using TRIzol reagent as previously described (Yang et al., 2012). RNA concentration was determined by spectrophotometric optical density measurement at 260 and 280 nm. The ratio 260 nm and 280 nm (OD260/280) was used estimate the purity of the nucleic acid. OD260/280 in all samples ranged between 1.7 and 2.0. The cDNA synthesis was performed using High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was performed using ABI 7500 Real-Time PCR system (Applied Biosystems). 18S RNA was used as an internal control. Results were expressed as mean fold changes of gene expression relative to control group using 2−ΔΔCT method.
Immunohistochemistry
The procedure for immunohistochemistry (IHC) has been previously described (Wang et al., 2015a). Briefly, mice were deeply anesthetized by intraperitoneal injection of ketamine/xylazine and then intracardially perfused with PBS followed by 4% paraformaldehyde in PBS (pH 7.4). The brain tissues were removed, post fixed in 4% paraformaldehyde for additional 24 hours and then transferred to 30% sucrose in PBS until the brain sunk to the bottom. Sagittal brain sections (20–40 μm) were cut on a freezing microtome. Floating sections were permeabilized with 0.1% Triton X-100 in PBS, incubated in 0.3% H2O2/50% methanol in PBS for 10 min. After washing with PBS, sections were mounted on slides and dried. The slides were blocked with 5% normal goat serum containing 0.5% Triton X-100 in PBS for 1 hour at room temperature, and then incubated with primary antibodies (diluted in PBS with 1% BSA) overnight at 4°C. The dilution for the primary antibodies was: anti-cleaved caspase-3 antibody, 1:6,000; and anti-Iba1 antibody, 1:1,000. After washing with PBS, slides were incubated with a biotin-conjugated goat anti-rabbit secondary antibody (1:1,000) for 1 hour at room temperature and followed by washing with PBS. Avidin-biotin-peroxidase complex was prepared according to the manufacturer’s instructions. The slides were incubated in the complex for 1 hour at room temperature. After rinsing, the slides were developed in 0.05% 3, 3′-diaminobenzidine (DAB) containing 0.003% H2O2 in PBS.
Statistical analysis
The data were expressed as mean ± SEM. All analyses were performed using the SPSS software (SPSS, Chicago, IL, USA). Differences in indicated protein expression, mRNA expression, or cell viability in experimental groups were determined by the one-way Analysis of Variance (ANOVA). Differences in which the p value was less than 0.05 were considered statistically significant. In cases where significances were detected, specific post hoc comparisons between treatment groups were examined with Student-Newman-Keuls tests or Dunnett’s T3 tests.
Results
Minocycline blocks ethanol-induced caspase-3 activation in the developing brain
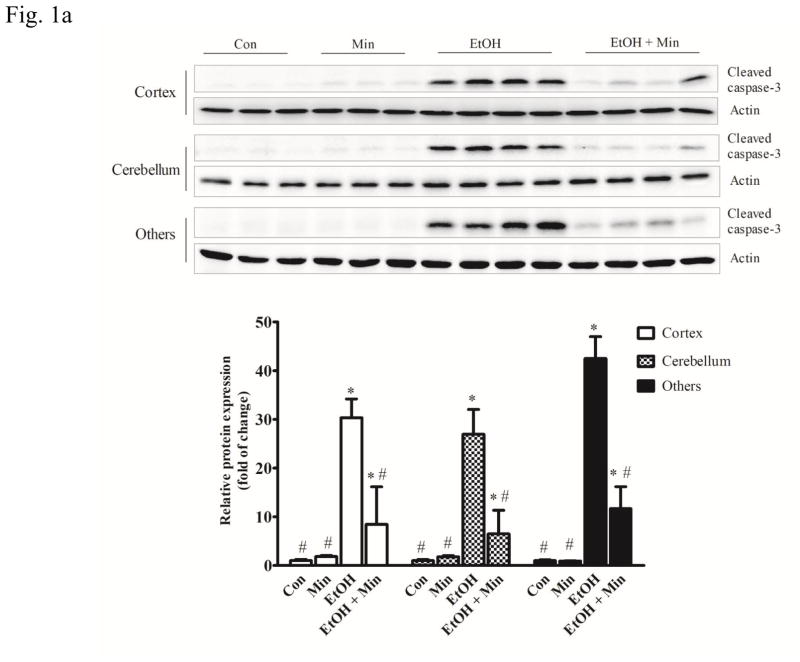
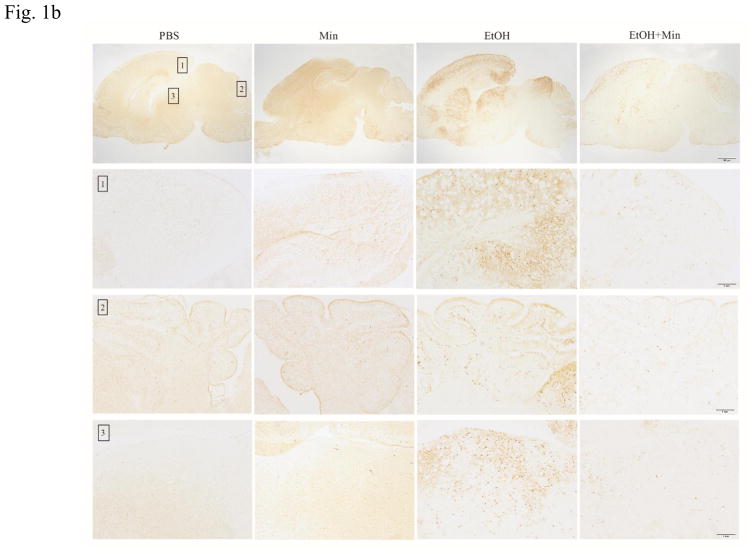
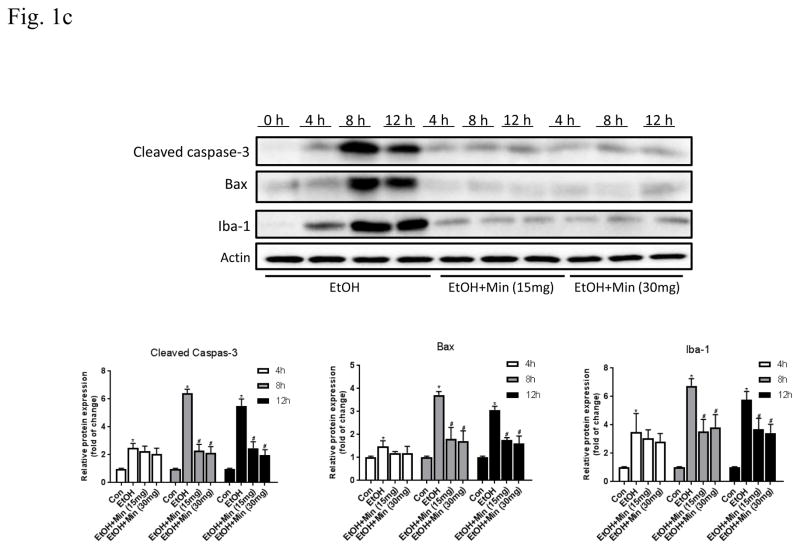
First, we sought to determine whether minocycline offer protection against ethanol-induced neuronal death in the developing brain. We used a well-established mouse model of postnatal ethanol exposure (Carloni et al., 2004; Ikonomidou et al., 2000; 2001; Olney et al., 2002; Young et al., 2005; 2008). As shown in Fig. 1A, the alterations in cleaved caspase-3 were analyzed by one-way ANOVA with the treatments as a variable. A significant alteration in cleaved caspase-3 was observed in the cortex (F(3,36)=21.12; p <0.05), cerebellum (F(3,36)=16.21; p <0.05), and other parts of brain (F(3,36)=32.54; p <0.05). Ethanol exposure caused a drastic increase in cleaved caspase-3 in the cerebral cortex, cerebellum, and other parts of brain (p <0.05). However, minocycline treatment significantly diminished ethanol-induced caspase-3 activation (p <0.05). For the saline-injected control and minocycline treatment alone, there was little caspase-3 activation. The results from the immunoblotting analysis were confirmed by the IHC study (Fig. 1B). Ethanol induced a wide-spread expression of cleaved caspase-3 in the cerebral cortex, midbrain, thalamus, anterior olfactory nucleus, and cerebellum. Minocycline treatment drastically reduced the immunoreactivity of cleaved caspase-3. We examined the time course of the effect of minocycline on cleaved caspase-3, Bax and Iba-1 (Fig. 1C). At each time points we examined minocycline reduced ethanol-induced expression of cleaved caspase-3, Bax and Iba-1; minocycline at 15 mg/kg and 30 mg/kg offered similar protection.
Figure 1.
Effect of minocycline on ethanol-induced apoptotic neuronal death in the developing mouse brain. Pups of postnatal day 5 (PD5) were randomly assigned to 4 groups: control, minocycline, ethanol, and ethanol with minocycline. Minocycline was administered by two subcutaneous injections at a dose of 30 mg/kg each on 12 hour and 2 h before the ethanol exposure. The Pups were exposed to ethanol by subcutaneous injections as described in the Materials and Methods. Eight hours after ethanol injection, the pups were sacrificed. A: The expression of cleaved caspase-3 in cortex, cerebellum, and other parts of the brain were determined with immunoblotting. The expression of actin served as an internal control. The relative amounts of cleaved caspaser-3 were quantified microdensitometrically and normalized to the expression of actin. Each data point was the mean ± SEM of three independent experiments. N = 10 for each group. *p < 0.05, statistically significant difference from control group, #p < 0.05, statistically significant difference from ethanol group. B: The expression of cleaved caspaser-3 was examined by immunohistochemistry (IHC); bar = 100 μm. An image of higher magnification showed layers in the indicated brain areas: 1. cortical retrosplenial; 2, cerebellum; 3. Periaqueductal gray. Bar= 1 mm. C: Mouse pups were treated with minocycline (0, 15 mg/kg and 30 mg/kg) and ethanol as described above. Mice were sacrificed at 4, 8, 12 hours after ethanol injection. The expression of active-caspase, Bax and Iba-1 was determined and quantified. N = 6 for each group. *p < 0.05, statistically significant difference from control group, #p < 0.05, statistically significant difference from ethanol group.
Minocycline alleviates ethanol-induced inflammation in the developing brain
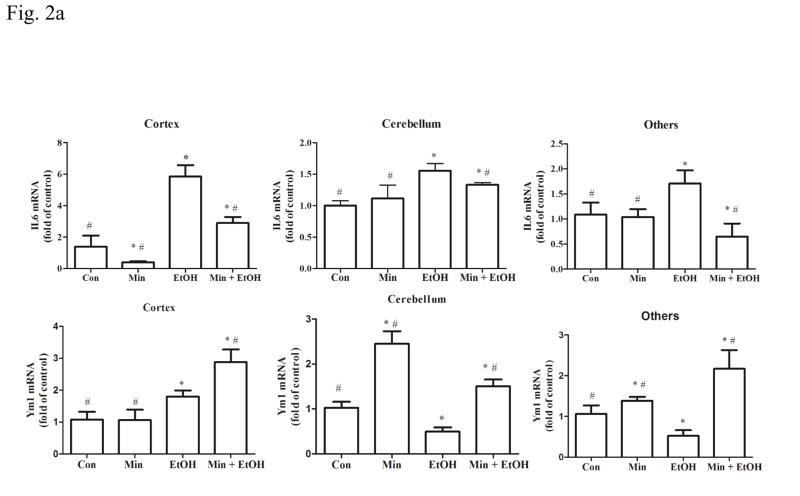
To confirm that minocycline reduced ethanol-induced neuroinflammation, we examined the expression of a number of pro-inflammatory as well as anti-inflammatory cytokines/chemokines in the developing brain. Inerleukin-6 (IL-6) is a pro-inflammatory cytokine that plays multiple roles in central nervous system (CNS) during infections and injuries (Vallieres and Rivest, 1999; Wang et al., 2015b). As shown in Fig. 2A, a significant alteration in IL-6 mRNA was observed in the cortex (F(3,28)=15.08; p <0.05), the cerebellum (F(3,28)=3.89, p <0.05), and other part of brains (F(3,28)=3.44, p <0.05). Ethanol significantly increased the mRNA level of IL-6 in the cortex, cerebellum, and other parts of brain, compared with the control group (p <0.05) (Fig. 2A). While minocycline alone had little effect on the basal level of IL-6 mRNA (p >0.05), it inhibited ethanol-induced increase of IL-6 mRNA compared with ethanol group (p <0.05) (Fig. 2A). In contrast to its effect on IL-6, ethanol’s effect on Ym1, an anti-inflammatory marker (Roszer, 2015), was complex. One-way ANOVA revealed statistically significant alterations in the cortex (F(3,28)=6.15, p <0.05), the cerebellum (F(3,28)=5.16, p <0.05), and other brain parts (F(3,28)=4.29, p <0.05). Ethanol significantly reduced the mRNA level of Ym1 by 30% and 48% in the cerebellum and other parts of the brain (p <0.05), respectively, but significantly increased it by 80% in the cortex (p <0.05), compared control group. Minocycline in combination with ethanol increased the expression of Ym1 (p <0.05), compared with the ethanol-treated group.
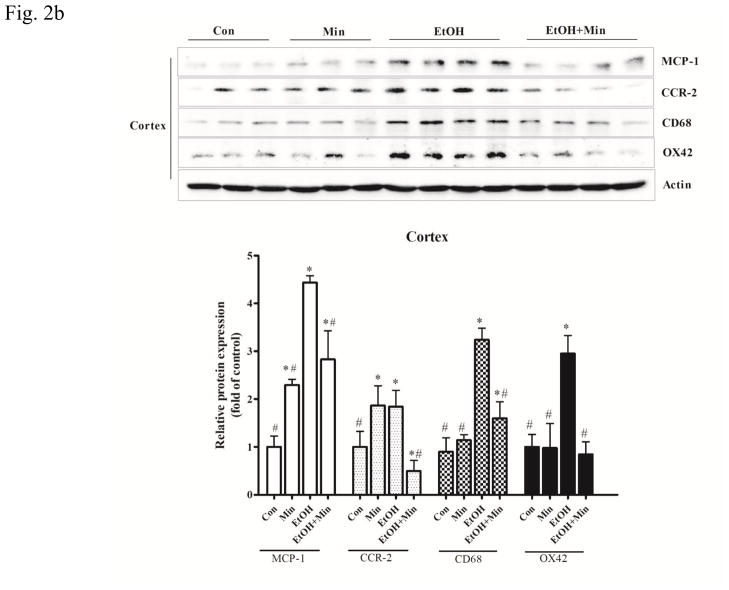
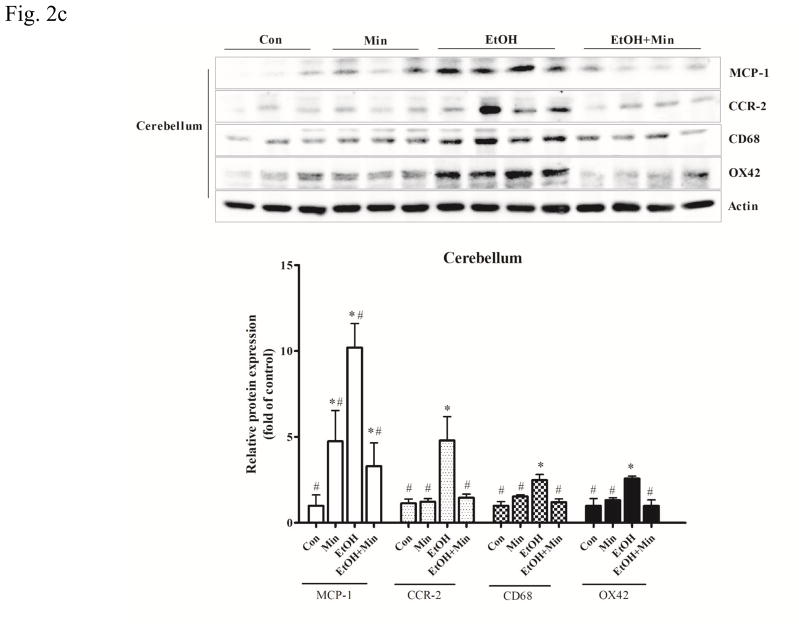
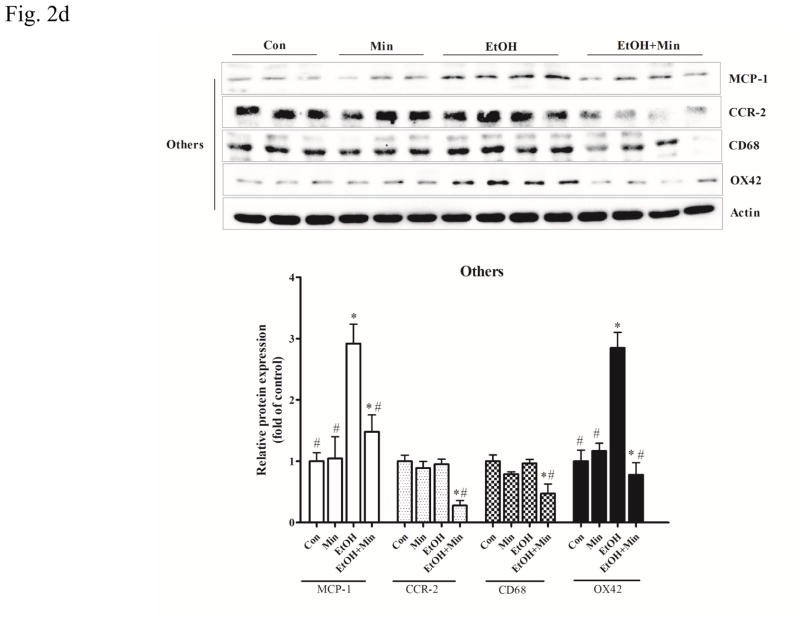
Figure 2.
Effect of minocycline on ethanol-induced inflammation. The treatment of minocycline and ethanol was same as described in Fig. 1. A: The levels of mRNA for IL-6, a pro-inflammatory cytokine, and Ym-1, an anti-inflammatory cytokine in the cortex, cerebellum and other part of brain were determined by qRT-PCR using 18s as an internal control. Minocycline- or ethanol-induced changes were expressed as fold difference over the control. Data are the mean ± SEM of three independent experiments. N = 8 for each group. *p < 0.05, statistically significant difference from control group, #p < 0.05, statistically significant difference from ethanol group. B–D: The expression of MCP-1, CCR-2, CD-68 and OX42 in cortex, cerebellum, and other parts of the brain were determined with immunoblotting. The expression of actin served as an internal control. The relative amounts of indicated proteins were quantified microdensitometrically and normalized to the expression of actin. Each data point was the mean ± SEM of three independent experiments. n = 10 for each group. *p < 0.05, statistically significant difference from control group, #p < 0.05, statistically significant difference from ethanol-treated group.
Monocyte chemoattractant protein-1 (MCP1, also known as CCL2) and its receptor (CCR-2) are pro-inflammatory mediators and up-regulated in many CNS disorders (Dimitrijevic et al., 2007; Hinojosa et al., 2011; Yao and Tsirka, 2014). In the cortex, both minocycline or ethanol treatment alone increased the expression of MCP-1 and CCR-2 protein; however, when they were applied together, minocycline significantly inhibited ethanol-induced increase in MCP-1 and CCR-2 protein levels (Fig. 2B): For MCP-1, F(3,36)=43.24, p <0.05; for CCR-2, F(3,28)=8.23, p <0.05; for CD68, F(3,28)=23.05, p <0.05; for OX42, F(3,28)=18.78, p <0.05. In the cerebellum, ethanol significantly increased the expression of both MCP-1 and CCR-2 (p <0.05), while minocycline only significantly increased MCP-1 (p <0.05), but not CCR-2 expression, compared with the control group (Fig. 2C). As in the cortex, minocycline significantly inhibited ethanol-induced increase in MCP-1 and CCR-2 protein levels (p <0.05), compared with the ethanol group. In the other part of brain, ethanol only significantly increased the expression of MCP1 (p <0.05), but not CCR-2, compared with the control group; minocycline alone did not affect their expression (Fig. 2D). Minocycline significantly inhibited ethanol-induced expression of MCP-1 and significantly reduced the CCR-2 expression below basal levels (p <0.05), compared with ethanol group (Fig. 2D). Therefore, in general, minocycline inhibited ethanol-induced expression of pro-inflammatory cytokines/chemokines, but increased anti-inflammatory markers.
Minocycline inhibits ethanol-induced microglial activation in the developing brain
Microglial activation plays a key role in the neuroinflammation associated with many CNS disorders (Dheen et al., 2007). Since minocycline is a “microglia inhibitor”, we sought to determine whether minocycline was able to inhibit ethanol-induced microglial activation. Both CD68, a cell surface marker of M1-poalrized microglia, and OX42 (CD11b), a major adhesion receptor of neutrophils, are inflammatory markers in the brain and indicative of microglial activation (Jeong et al., 2013; Kobayashi et al., 2013). In the cortex, ethanol significantly increased the expression of CD68 and OX42 (p <0.05), compared with control group; while pretreatment of minocycline blocked ethanol-induced increase of these proteins (p <0.05), compared with ethanol group (Fig. 2B). A similar observation was obtained in the cerebellum (Fig. 2C). In the other part of brain, minocycline blocked ethanol-induced increase in OX42 (p <0.05), compared with ethanol group (Fig. 2D). Although minocycline and ethanol alone did not alter the expression of CD68, they acted together to significantly reduced its expression below the basal levels (p <0.05), compared with control group.
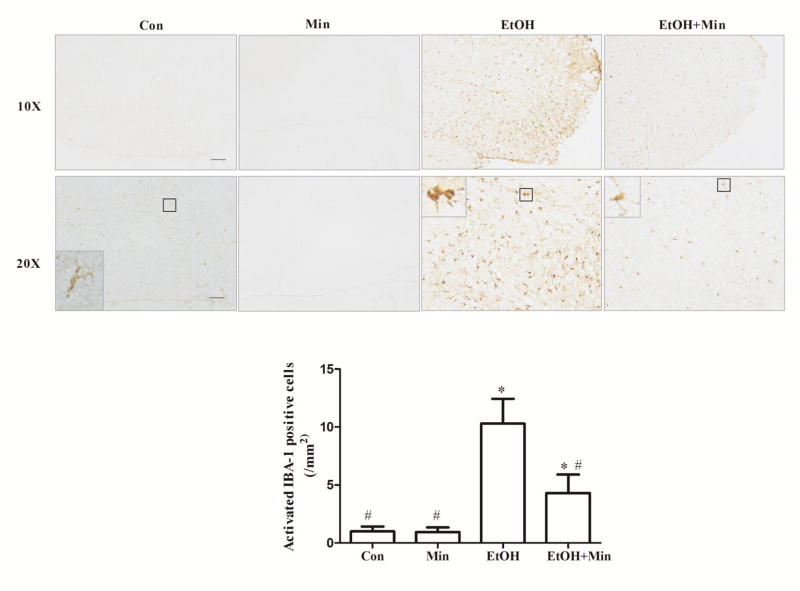
Microglial activation was further assessed morphologically by IHC of Iba-1. Iba-1 is expressed in both active and resting microglia and used as a microglial marker. The resting microglia cell is characterized by a small cell body and much elaborated thin processes, which send multiple branches and extend in all directions, while the activated microglia have fewer and thicker processes with larger cell body (Chen et al., 2012). Ethanol significantly increased the number of active microglia (p <0.05), compared with the control group; minocycline significantly inhibited ethanol-induced activation of microglia, (p <0.05), compared with ethanol group (Fig. 3).
Figure 3.
Effect of minocycline on ethanol-induced microglia activation. The treatment of minocycline and ethanol was same as described in Fig. 1. Microglia were identified by IHC of IBA-1 in thalamus of brain area; bar = 200 μm. Images of higher magnification are shown in the lower panel; bar = 100 μm). Activated microglia were determined morphologically and the number of activated IBA-1 positive cells per mm2 was determined. Data are the mean ± SEM of three independent experiments. *p < 0.05, statistically significant difference from control group, #p < 0.05, statistically significant difference from ethanol group.
Minocycline inhibits ethanol-induced GSK3β activation in the developing brain
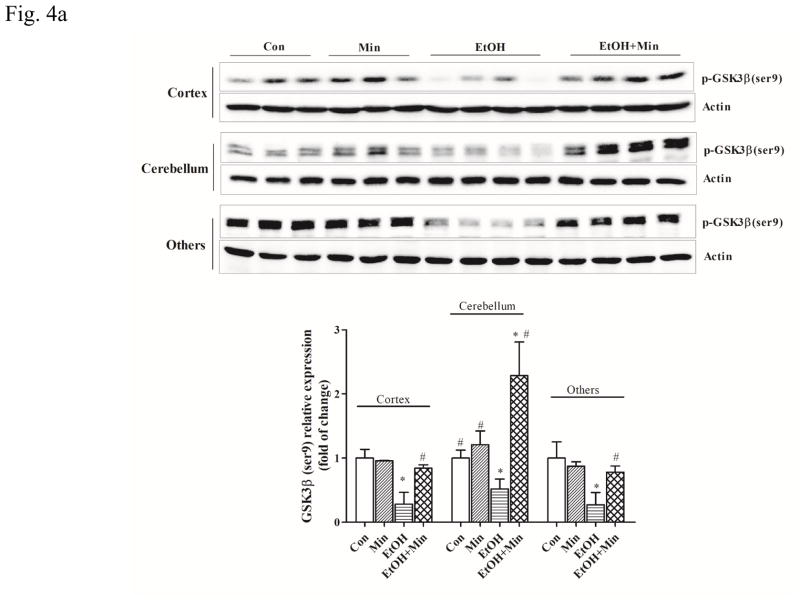
Glycogen synthase kinase 3 beta (GSK3β) plays an important role in neuroinflammation and microglial activation (Maixner and Weng, 2013). The dysregulation of GSK3β contributes to the development and progression of many neurological diseases through regulating the neuroinflammation processes (Golpich et al., 2015; Maixner and Weng, 2013; Sandberg et al., 2014; Verdile et al., 2015). Inhibitors of GSK3β have been shown to be beneficial in many various models of neuroinflammatory diseases including Alzheimer’s disease, multiple sclerosis, and AIDS dementia complex (Maixner and Weng, 2013). The activity of GSK3β is mainly regulated by the phosphorylation at serine 9 which results in the inhibition of GSK3β (Golpich et al., 2015; Luo, 2009; Maixner and Weng, 2013). As shown in Fig. 4A, co-treatment with minocycline significantly increased the expression of GSK3β in the cortex (F(3,36) = 30.45; p <0.05), the cerebellum (F(3,36) = 50.97; p <0.05), and other parts of brain (F(3,36) = 26.86; p <0.05). Ethanol significantly decreased the expression of phosphorylated GSK3β [p-GSK3β (Ser9)] by 72%, 49%, and 73% in the cortex, cerebellum, and other parts of the brain, respectively, compared with the control group (p <0.05) (Fig. 4A), indicating an activation of GSK3β. Minocycline blocked ethanol’s inhibitory effect on p-GSK3β (Ser9) by increasing the levels of p-GSK3β (p <0.05) in the cortex, cerebellum, and other parts of brain (Fig. 4A). The activity of GSK3β can also be positively regulated by the phosphorylation at tyrosine 216 (Medina and Wandosell, 2011). Our results showed that neither ethanol nor minocycline affected expression of total GSK3β and p-GSK3β (Tyr216) in the developing brain (data not shown).
Figure 4.
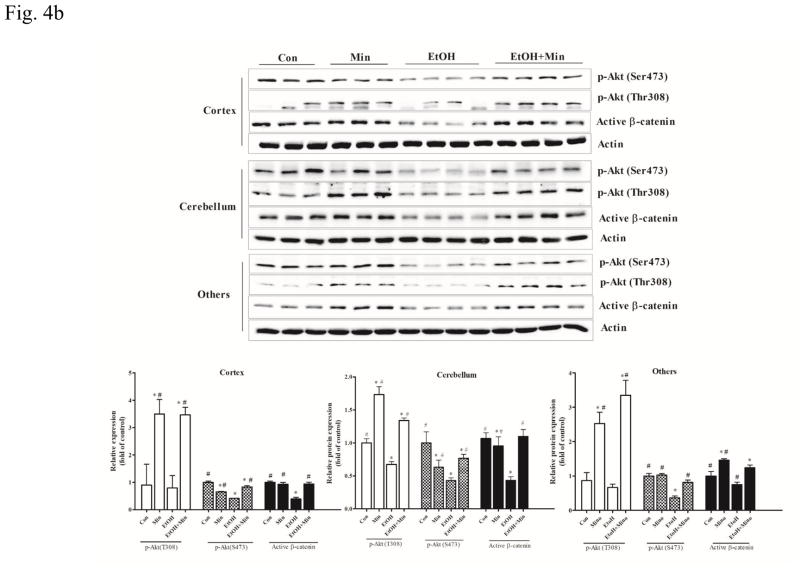
Effect of minocycline on ethanol-induced GSK3β activation. The treatment of minocycline and ethanol was same as described in Fig. 1. A: the expression of phospho-GSK3βSer9 (A) was determined by immunoblotting. B: The expression of GSK3β upstream regulator AKT (phospho-AKTSer473 and phospho-AKTThr308), and down-stream target, active β-catenin in the cortex, cerebellum, and other parts of the brain was determined with immunoblotting. The expression of actin served as an internal control. The relative amounts phospho-GSK-3βSer9, phospho-AKTSer473, phospho-AKTThr308, and active β-catenin were quantified microdensitometrically and normalized to the expression of actin. Each data point was the mean ± SEM of three independent experiments. *p < 0.05, statistically significant difference from control group, #p < 0.05, statistically significant difference from ethanol group.
We then examined the effect of ethanol on the upstream and downstream components of GSK3β. AKT is upstream of GSK3β and the activation of AKT results in the inhibition of GSK3β through inducing its phosphorylation at serine 9 (Zhang et al., 2003). β-catenin is a substrate of GSK3β which induces its degradation through destabilizing it; activation of GSK3β destabilizes β-catenin while inactivation of GSK3β increases the stabilized (non-phosphorylated) form of β-catenin, resulting in higher levels of β-catenin (Zhu et al., 2009). AKT/GSK3β/β-catenin signaling plays a critical role in neuroinflammation and neurodegeneration (Kitagishi et al., 2012; Orellana et al., 2015; Zhao et al., 2012). AKT activation involves the phosphorylation of two residues: threonine 308 (Thr308) in the activation loop and serine 473 (Ser473) in the C-terminal hydrophobic motif. In general, ethanol inhibited AKT phosphorylation (p <0.05), compared with the control group, and minocycline abolished ethanol inhibition of AKT phosphorylation (p <0.05), compared with the ethanol group (Fig. 4B). Ethanol and minocycline did not affect the levels of total AKT (data not shown). Similarly, ethanol decreased the expression of the active form of β-catenin (p <0.05), compared with the control group, and minocycline restored the levels of β-catenin in the cortex and cerebellum to the controls. In other parts of brain, minocycline significantly increased the levels of p-AKT(T308) and active form of β-catenin over the control group (p <0.05) (Fig. 4B).
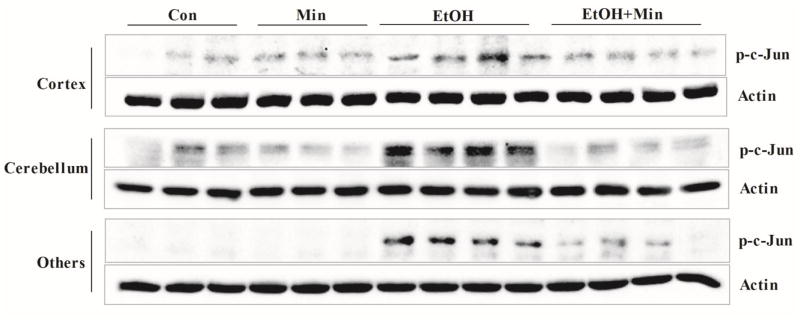
Since p38 MAPK and JNKs are other important mediators of microglial activation and neuroinflammation in the brain (Badshah et al., 2016; Dheen et al., 2007; Zhao et al., 2016b), we investigated the effect of ethanol on these signaling pathways. Ethanol had little effect on p38 MAPK (data not shown) but increased the phosphorylation of c-Jun (p-c-Jun), the substrate of JNKs in the cortex, cerebellum, and other parts of brain (Fig. 5). Phosphorylated c-Jun contributes to the formation of the activator protein 1 (AP-1) transcription factor complex which is involved in the expression of many pro-inflammatory genes and cytokines, such as IL-6 and TNF-α (Zhao et al., 2016a). p-c-Jun is also one of the GSK3β target proteins (Luo, 2009). Minocycline blocked ethanol-induced increase in p-c-Jun (Fig. 5).
Figure 5.
Effect of minocycline on ethanol-induced activation of JNKs. The treatment of minocycline and ethanol was same as described in Fig. 1. The expression of phospho-c-Jun in the cortex, cerebellum, and other parts of the brain were determined with immunoblotting. The expression of actin served as an internal control.
Minocycline inhibits ethanol-stimulated pro-inflammatory cytokines in cultured microglial cells
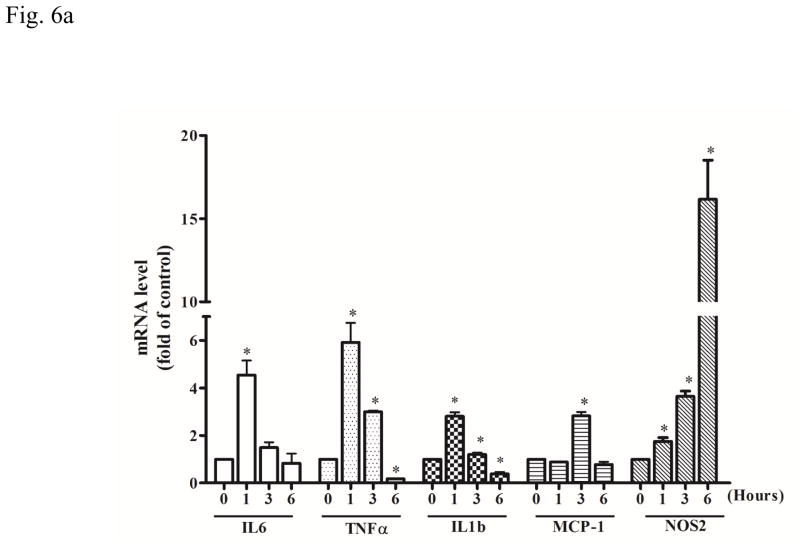
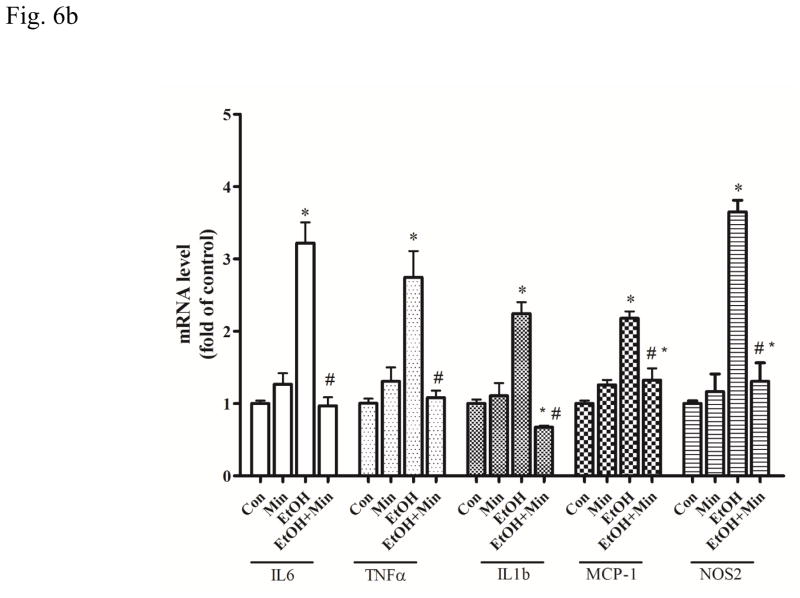
We used a mouse microglia cell line, SIM-A9 to assess the direct effect of ethanol and minocycline on microglia in the context of minocycline neuroprotection. We first investigated the effect of ethanol on SIM-A9 cells. Ethanol rapidly increased the mRNA levels of IL-6 (F(3, 16)=35.17, p <0.05), TNF-α (F(3, 16)=42.31, p <0.05), IL1b (F(3, 16)=7.51, p <0.05), MCP-1 (F(3, 16)=4.87, p <0.05), and NOS2 (F(3, 16)=67.85, p <0.05) in SIM-A9 cells (Fig. 6A). The increase in IL-6, TNF-α, and IL1b mRNA peaked at 1 hour after ethanol exposure (p <0.05), compared with the control group. The increase in MCP-1 and NOS2 mRNA peaked at 3 and 6 hours after ethanol exposure (p <0.05), respectively, compared with the control group (Fig. 6A). Minocycline blocked ethanol-induced increase in the expression of IL-6, TNF-α, IL1b, MCP-1, and NOS2 at 1.5 hours after ethanol exposure (p <0.05), compared with the ethanol group (Fig. 6B).
Figure 6.
Effect of ethanol on the expression of cytokines and chemokines in microglia cells. A: SIM-A9 microglial cells were exposed to ethanol (0.4 %) for 1, 3, and 6 hours. The mRNA levels for IL-6, TNF-α, IL-1b, MCP-1, and NOS-2 were determined by quantitative PCR. B: SIM-A9 cells were pretreated with minocycline (50 μM) for 2 hours, and then exposed to ethanol (0.4%) for 1.5 hours. The mRNA levels of IL-6, TNF-α, IL-1b, MCP-1, and NOS-2 were determined by quantitative PCR. Data are the mean ± SEM of three independent experiments. *p < 0.05, statistically significant difference from control group, #p < 0.05, statistically significant difference from ethanol-treated group.
Minocycline inhibits ethanol-induced GSK3β activation in cultured microglial cells
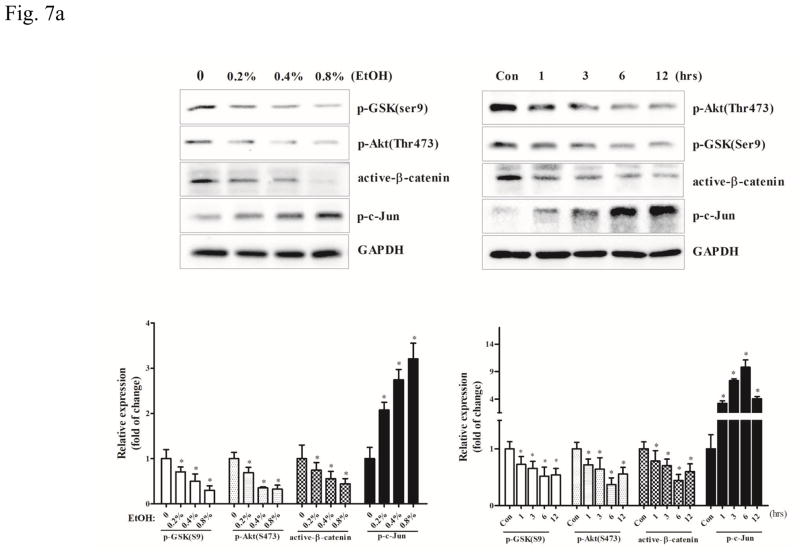
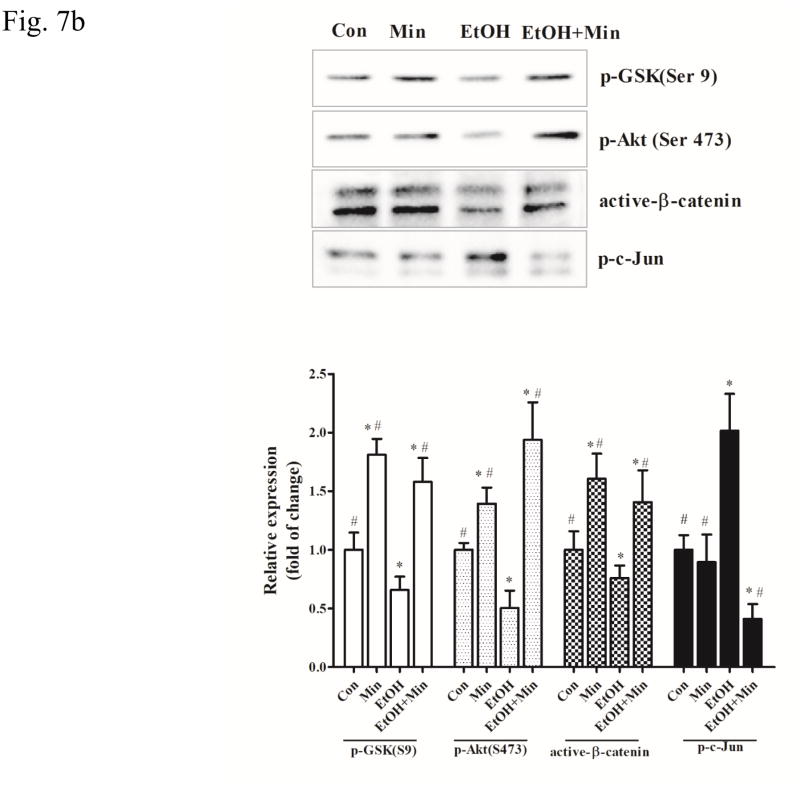
Then, we examined the role of GSK3β in ethanol-induced inflammation and the effect of minocycline on microglial cells. Ethanol activated GSK3β by decreasing p-GSK3β (Ser9) in a concentration- and duration-dependent manner in SIM-A9 cells (p <0.05), compared with the control group (Fig. 7A). As a result, ethanol caused a concentration- and duration-dependent decrease of active β-catenin (p <0.05), compared with the control group. As observed in vivo, ethanol also decreased the level of p-AKT (Ser473), but increased p-c-Jun in SIM-A9 cells (Fig. 7A) (p <0.05), compared with the control group. Minocycline inhibited ethanol-induced GSK3β activation and decrease in active β-catenin levels (p <0.05), compared with the ethanol group; it blocked ethanol-mediated alterations in p-AKT (Ser473) and p-c-Jun (p <0.05), compared with the ethanol group (Fig. 7B).
Figure 7.
Effect of minocycline on ethanol-induced GSK3β activation in the microglia cells. A: SIM-A9 were exposed to ethanol (0, 0.2%, 0.4, or 0.8%) for 6 hours. The expression of phospho-GSK-3βSer9, phospho-AKTSer473, phospho-c-Jun, and active β-catenin was determined by immunoblotting. B: SIM-A9 cells were exposed to ethanol (0.4%) for 0, 1, 3, 6, or 12 hours. The expression of phospho-GSK3βSer9, phospho-AKTSer473, phospho-c-Jun, and active β-catenin was determined by immunoblotting. C: SIM-A9 cells were pretreated with minocycline (50 μM) for 2 hours, and then exposed to ethanol (0.4%) for 6 hour. The expression of phospho-GSK3βSer9, phospho-AKTSer473, phospho-c-Jun, and active β-catenin was determined with immunoblotting. The expression of GAPDH served as an internal control. The relative amounts phospho-GSK-3βSer9, phospho-AKTSer473, phospho-AKTThr308, phospho-c-Jun, and active β-catenin were quantified microdensitometrically and normalized to the expression of GAPDH. Each data point was the mean ± SEM of three independent experiments. *p < 0.05, statistically significant difference from control group, #p < 0.05, statistically significant difference from ethanol group.
Blocking GSK3β inhibits ethanol-stimulated pro-inflammatory in microglial cells
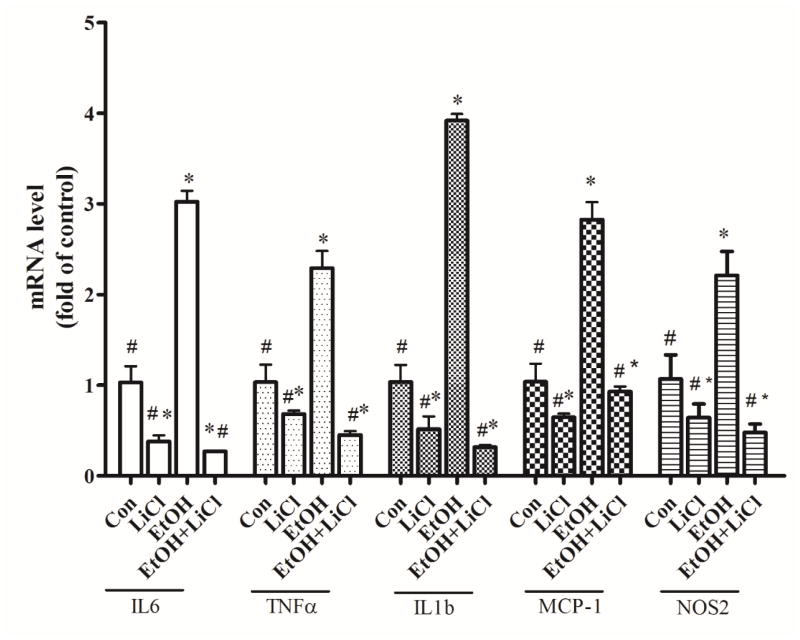
To determine the role of GSK3β in ethanol-induced inflammation, we used a GSK3β inhibitor, lithium to inhibit the activity of GSK3β in SIM-A9 cells. Previous studies has used lithium to evaluate the role of ethanol-induced neuronal death in vitro and in vivo (Luo, 2010; Young et al., 2008; Zhong et al., 2006). As shown in Fig. 8, lithium alone significantly decreased the intrinsic mRNA levels of IL-6, TNF-α, IL1b, MCP-1, and NOS2 in comparison with the no treatment controls (p <0.05). More importantly, lithium eliminated ethanol-induced increase in mRNA levels of IL-6, TNF-α, IL1b, MCP-1, and NOS2 (p <0.05), compared with the ethanol group (Fig. 8). These data suggested that the GSK3β played an important a role in microglia-mediated inflammation.
Figure 8.
Effect of GSK3β inhibitor on ethanol-induced inflammation in microglia cells. SIM-A9 cells were pretreated with GSK3β inhibitor LiCl (10 mM) for 2 hours and exposed to ethanol (0.4%) for 1.5 hour. The levels of mRNA for IL-6, TNF-α, IL-1b, MCP-1, and NOS-2 were determined by qRT-PCR using 18s as an internal control. Ethanol- or LiCl-induced changes were expressed as fold difference over the control. Data are the mean ± SEM of three independent experiments. *p < 0.05, statistically significant difference from control group, #p < 0.05, statistically significant difference from ethanol-treated group.
Minocycline protects neurons against ethanol-induced neuronal death in the co-culture of mouse cortical neurons and microglia cells
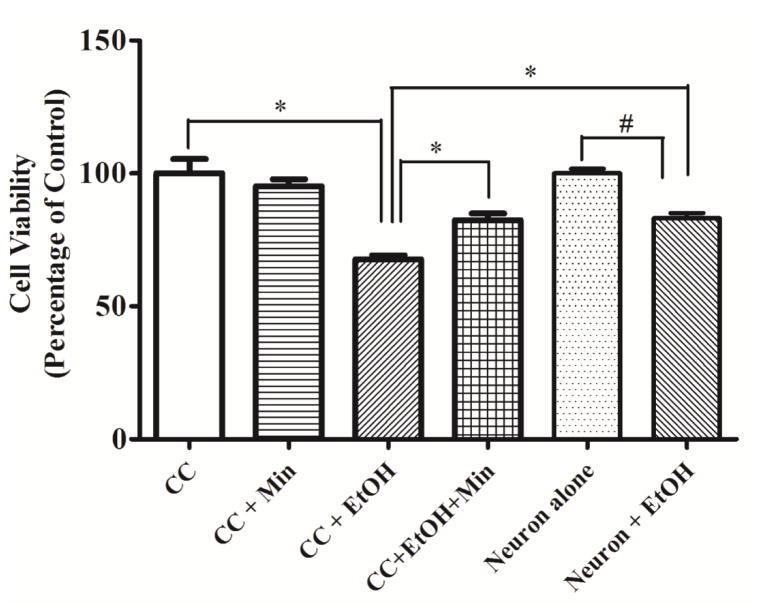
To investigate the role of microglial activation on ethanol-induced neuronal death we used a co-culture system in which mouse cortical neurons and SIM-A9 cells were cultured together. In primary cortical neuron cultures alone, ethanol caused significant but relatively modest decrease in cell viability 17.0% (F(3,16)=4.63, p <0.05) (Fig. 9). In the co-culture system, ethanol caused more decrease in neuronal viability around 32.4% (F(3,16)=12.47, p <0.05). Minocycline significantly ameliorated ethanol-induced neuronal death in the co-cultures; ethanol plus minocycline only reduced neuronal viability by 17.6% (p <0.05), compared with the control group. In primary cortical neuron cultures alone, minocycline offered little protection (data not shown). These results suggested that microglia contributed to ethanol-induced neuronal death and minocycline protection was, at least in part, mediated through the inhibition of microglial activation.
Figure 9.
Effect of minocycline on ethanol-induced neuronal death in co-cultures of mouse cortical neurons and microglia. Co-cultures of SIM-A9 cells and primary cortical neurons were established as described in the Materials and Methods. Co-cultures were exposed to ethanol (0 or 0.4%) exposure with or without minocycline (50 μM) for 48 hours. The viability of neurons was determined by MTT as described in the Materials and Methods. Data were the mean ± SEM of three independent experiments. &p < 0.05, statistically significant difference from cortical neuron alone; $p < 0.05, statistically significant difference from cortical neuron with ethanol exposure; *p < 0.05, statistically significant difference from co-culture group without ethanol; #p < 0.05, statistically significant difference from ethanol group in co-culture. CN: Cortical neuron; CC: Co-culture.
Discussion
This study used a well-established third trimester equivalent mouse model of ethanol exposure to demonstrate the neuroprotective role of minocycline. Minocycline inhibits ethanol-induced caspase-3 activation, microglial activation, and the expression of pro-inflammatory factors. Minocycline reverses ethanol inhibition of anti-inflammatory cytokines and blocks ethanol-induced activation of GSK3β, a key mediator of neuroinflammation/microglial activation in the developing brain. Using an in vitro microglial model, we demonstrate that GSK3β mediates ethanol-stimulated pro-inflammatory cytokines in cultured microglial cells and minocycline blocks ethanol-induced activation of GSK3β as well as the increase in pro-inflammatory factors. Furthermore, minocycline protects primary cortical neurons against ethanol-induced neuronal death in neurons/microglia co-cultures. This is the first to show that minocycline can ameliorate ethanol-induced apoptotic neurodegeneration in the developing brain, implying a potential therapeutic role of minocycline.
The mechanisms underlying ethanol-induced neuronal death in the developing brain are complex; it may be mediated by the interference with signaling by neurotrophic factors, disruption of microRNAs (miRNAs), oxidative stress, endoplasmic reticulum (ER) stress (Boyadjieva and Sarkar, 2013a; Luo, 2012; Miranda, 2012; Yang and Luo, 2015). Neuroinflammation plays important role in both alcohol use disorders (AUD) and FASD (Chastain and Sarkar, 2014; Kane and Drew, 2016; Crews et al., 2017). There is extensive evidence showing that ethanol activate microglia in experimental models of AUD and FASD (Chastain and Sarkar, 2014; Kane and Drew, 2016; Saito et al., 2016; Wilhelm and Guizzetti, 2015). Microglial activation is often categorized into pro-inflammatory (M1), anti-inflammatory (M2), and intermediate phenotypes (Kane and Drew, 2016; Saito et al., 2016; Zhao et al., 2016b). M1 microglia may contribute to dysfunction of the neurotrophic system by producing pro-inflammatory cytokines, such as tumor necrosis factor-α (TNFα), IL-1β, IL-6, iNOS, and CCL2, usually leading to neurotoxicity. The M2 phenotype, sometimes called the neuroprotective microglial phenotype, enhances clearance of debris and produces anti-inflammatory mediators including Ym1, arignase1 (Arg1), IL-4, IL-10, and TGFβ to antagonize inflammation-induced damage in the CNS (Zhao et al., 2016b). In our model system, ethanol-induced microglial activation appears to be associated with an increase in pro-inflammatory cytokines and chemokines, suggesting that ethanol promotes M1-like phenotype of microglia. The ethanol-induced microglial activation appears transient. In SIM-A9 cells, ethanol induces a rapid increase in pro-inflammatory factors within hours and then declines (Fig. 6A).
Minocycline, a second-generation tetracycline with anti-inflammatory and anti-apoptotic property, has high lipid solubility (Good and Hussey, 2003) and readily crosses blood-brain barrier (Saivin and Houin, 1988; Yong et al., 2004). It has been tested for therapeutic benefits in animal models of various CNS diseases (Garrido-Mesa et al., 2013). Minocycline was shown to be beneficial in several neurological disorders, such as multiple sclerosis and spinal cord injury, stroke and traumatic injury, and some several neurodegenerative conditions including Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and Alzheimer’s disease (Budni et al., 2016; Chen et al., 2000; Choi et al., 2007; Du et al., 2001; Garrido-Mesa et al., 2013). The main biological effects of tetracycline are inhibition of microglial activation, attenuation of apoptosis, and suppression of reactive oxygen species production (Orsucci et al., 2009). In humans, long-term treatment with minocycline is generally safe and well tolerated (Garrido-Mesa et al., 2013). However, minocycline may have some toxic effects especially for developing brain depending on animal models, route/dosage, and timing of administration (Zhu et al., 2014; Arnoux et al., 2014; Hanlon et al., 2017; Majidi et al., 2016; Strahan et al., 2017). In our paradigm, minocycline dis not have apparent toxic effects on the CNS. Minocycline inhibits ethanol-induced microglial activation in the developing brain (Fig. 3). Generally, minocycline inhibits ethanol-stimulated pro-inflammatory cytokines but increases the expression of anti-inflammatory factors (Fig. 2). These findings are verified by in vitro studies which demonstrate that minocycline inhibits ethanol-induced pro-inflammatory factors in cultured microglial cells (Fig. 6), suggesting that minocycline attenuates microglia-mediated neuroinflammation. More importantly, minocycline provides a dramatic protection against ethanol-induced apoptotic neurodegeneration in wide-spread brain regions of PD5 mice (Figs. 1–2). The positive correlation between its neuroprotective role and inhibition of microglial activation/neuroinflammation suggests that minocycline may alleviate ethanol neurotoxicity by blocking microglia-mediated neuroinflammation. Furthermore, our study using a neurons/microglia co-culture system indicates that ethanol is more toxic for primary cortical neurons in the presence of microglial cells and minocycline offers a significant neuroprotection in this co-culture system (Fig. 9). Together, these results suggest that ethanol-induced microglial activation/neuroinflammation may be harmful for the developing neurons in our model system and minocycline protects neurons by attenuating microglia-mediated neuroinflammation. The view that microglia-mediated neuroinflammation contributes neurotoxicity in the developing brain is supported by many both in vitro and in vivo studies (Chastain and Sarkar, 2014). Particularly, Boyadjieva and Sarkar (2013) showed that the conditioned media collected from ethanol-activated microglial enhanced apoptotic death of cultured fetal hypothalamic neurons probably through the ROS and pro-inflammatory cytokines presented in the conditioned media.
However, microglial activation may also have a regenerative and neuroprotective role ethanol exposure (Chastain and Sarkar, 2014; Saito et al., 2016). It is believed that transient activation of microglia may be a response to neuronal damage and results in phagocytosis of degenerating neurons; while phagocytosis of pathogens requires initiation of an inflammatory response, removal of apoptotic neurons by microglia may prevent excessive inflammation and offer protection (Saito et al., 2016). For example, systemic administration of lipopolysaccharide (LPS), an agonist of TLR4 prior to ethanol exposure in PD7 mice, activates microglia and causes neuroinflammation in the developing brain, and attenuates ethanol-induced neurodegeneration (Saito et al., 2016). The evidence suggests that activation of microglia may offer neuroprotection in the neonatal brain. We show that ethanol increases the expression of CD68, a marker for phagocytes (Fig. 2), suggesting that these microglia are phagocytes engulfing degenerating neurons. However, minocycline inhibits the expression of CD68, which does not support that phagocytosis of degenerating neurons is sufficient to provide neuroprotection.
Ethanol-induced microglial activation may be either a secondary response to neuronal damage and/or results from a direct effect on microglia. It is difficult to distinguish these effects in animal studies. We therefore used an in vitro microglia model (SIM-A9) to evaluate the direct effect of ethanol and minocycline on microglial cells. We show that ethanol can directly affect microglial cells and induce the expression of pro-inflammatory factors in these cells (Fig. 7). This finding is supported by previous studies showing that ethanol can direct activate microglia in culture (Boyadjieva and Sarkar, 2013b; Liu et al., 2012; Liu et al., 2009; Yang et al., 2014). GSK3β and p38 MAPK are two key intracellular signaling components mediating microglia activation and neuroinflammation (Wang et al., 2010; Badshah et al., 2016). In our model system, ethanol has little effect on p38 MAPK (data not shown) and therefore we focused on GSK3β. GSK3β is critical mediator of ethanol neurotoxicity and ethanol activates GSK3β in neurons in the developing brain (Luo, 2009, 2012). Ethanol activates GSK3β by inhibiting p-GSK3β (Ser9) in the developing brain; over-expression of GSK3β makes neurons more sensitive to ethanol while inhibition of GSK3β protects neurons against ethanol-induced neurodegeneration in vitro and in vivo (Liu et al., 2009). Lithium, a GSK3β inhibitor, protects neurons against ethanol-induced neurodegeneration and inhibition of neurite outgrowth (Chen et al., 2012; Liu et al., 2009). The current study confirms the previous observation and expands it to microglial cells (Fig. 7). The ethanol-induced activation of GSK3β in microglial cells may be mediated by its inhibition of AKT, an upstream signaling component (Fig. 7). The role of GSK3β in ethanol-induced neuroinflammation is further demonstrated by that lithium blocks ethanol-induced expression of pro-inflammatory factors (Fig. 8). Minocycline inhibits ethanol-induced activation of GSK3β in the developing brain (Fig. 4) and cultured microglial cells (Fig. 7); the effect may result from the activation of upstream AKT. It remains to be determined whether minocycline also inhibits ethanol-induced GSK3β activation in neurons. Since GSK3β activation is involved in pro-apoptotic pathways in neuron and microglia-mediated neuroinflammation, it is conceivable that lithium-mediated protection may result from its inhibition of GSK3β in both neurons and microglia. Although lithium is a GSK3β inhibitor, it may protect developing brain against ethanol-induced neuroapoptosis through modulating other signaling pathways, such as the activation of ERK pathway (Young et al., 2008).
Effects of ethanol on pro-inflammatory signaling appear not limited to the AKT/GSK3β pathway. c-Jun N-terminal kinases (JNK) are also important mediator of microglia-mediated neuroinflammation (Badshah et al., 2016). We show that ethanol activates JNKs which is evident by the increases of their substrate, phosphorylated c-Jun, and more importantly minocycline inhibits ethanol-induced p-c-Jun (Fig. 5). Other investigators also show that ethanol activates the JNK system. However, JNK activation does not occur in the same neuronal populations that are killed by ethanol (Young et al., 2008). The exact role of JNK system in ethanol-induced microglial activation and neuroapoptosis remains to be investigated. In addition to intracellular signaling components, microglia can be activated through transmembrane receptors, such as Toll-like receptor 4 (TLR4) by ethanol directly or indirectly (Saito et al., 2016). Future study will need to determine the effect of ethanol and minocycline on TLR4 activation.
In this paradigm, ethanol-induced neuroapoptosis is accompanied by the activation of intrinsic apoptotic pathway, such as upregulation/release of Bax, Bad, and cytochrome C (Ikonomidou et al., 2000; Olney et al., 2002). We confirm that ethanol activates the intrinsic apoptotic pathway (Fig, 1C). Although the current findings indicate that the inhibition microglial activation is involved in minocycline’s protective effect, we do not conclude that the inhibition microglial activation is the sole pathway for minocycline’s protective effect. Minocycline may directly act on neurons and offer protection. It would be interesting to determine whether minocycline could protect adult brain against ethanol’s neurotoxicity. Since some of ethanol’s effects are mediated by NMDA and GABA receptors, it would be also interesting to examine whether minocycline could offset the toxicity of other NMDA receptor antagonists and GABA receptor agonists.
In summary, this is the first study to show that minocycline offers protection against ethanol-induced neurotoxicity in the developing brain. Although our understanding of minocycline-induced neuroprotection is incomplete, the inhibition of microglia-mediated neuroinflammation is a potential mechanism.
Minocycline inhibited ethanol-induced apoptosis and microglia activation in the CNS
Ethanol activated GSK3β in the developing brain and cultured microglial cells
GSK3β mediated microglial activation and was inhibited by minocycline
Minocycline protected neurons by inhibiting GSK3β-dependent neuroinflammation
Abbreviation
- AD
Alzheimer’s disease
- CNS
central nervous system
- CCR2
- FASD
fetal alcohol spectrum disorder
- GSK3β
Glycogen synthase kinase 3 beta
- Iba-1
ionized calcium binding adaptor molecule 1
- IFN-γ
interferon γ
- IHC
immunohistochemistry
- IL
interleukin
- MCP-1
monocyte chemoattractant protein-1
- PD
Parkinson’s disease
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alimov A, Wang H, Liu M, Frank JA, Xu M, Ou X, Luo J. Expression of autophagy and UPR genes in the developing brain during ethanol-sensitive and resistant periods. Metab Brain Dis. 2013;28:667–676. doi: 10.1007/s11011-013-9430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoux I, Hoshiko M, Sanz Diez A, Audinat E. Paradoxical effects of minocycline in the developing mouse somatosensory cortex. Glia. 2014;62:399–410. doi: 10.1002/glia.22612. [DOI] [PubMed] [Google Scholar]
- Badshah H, Ali T, Shafiq-ur R, Faiz-ul A, Ullah F, Kim TH, Kim MO. Protective Effect of Lupeol Against Lipopolysaccharide-Induced Neuroinflammation via the p38/c-Jun N-Terminal Kinase Pathway in the Adult Mouse Brain. J Neuroimmune Pharmacol. 2016;11:48–60. doi: 10.1007/s11481-015-9623-z. [DOI] [PubMed] [Google Scholar]
- Beheshti Nasr SM, Moghimi A, Mohammad-Zadeh M, Shamsizadeh A, Noorbakhsh SM. The effect of minocycline on seizures induced by amygdala kindling in rats. Seizure. 2013;22:670–674. doi: 10.1016/j.seizure.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Sarkar DK. Cyclic adenosine monophosphate and brain-derived neurotrophic factor decreased oxidative stress and apoptosis in developing hypothalamic neuronal cells: role of microglia. Alcohol Clin Exp Res. 2013a;37:1370–1379. doi: 10.1111/acer.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjieva NI, Sarkar DK. Microglia play a role in ethanol-induced oxidative stress and apoptosis in developing hypothalamic neurons. Alcohol Clin Exp Res. 2013b;37:252–262. doi: 10.1111/j.1530-0277.2012.01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budni J, Garcez ML, de Medeiros J, Cassaro E, Bellettini-Santos T, Mina F, Quevedo J. The Anti-Inflammatory Role of Minocycline in Alzheimer s Disease. Curr Alzheimer Res. 2016;13:1319–1329. doi: 10.2174/1567205013666160819124206. [DOI] [PubMed] [Google Scholar]
- Carloni S, Mazzoni E, Balduini W. Caspase-3 and calpain activities after acute and repeated ethanol administration during the rat brain growth spurt. J Neurochem. 2004;89:197–203. doi: 10.1111/j.1471-4159.2004.02341.x. [DOI] [PubMed] [Google Scholar]
- Chastain LG, Sarkar DK. Role of microglia in regulation of ethanol neurotoxic action. Int Rev Neurobiol. 2014;118:81–103. doi: 10.1016/B978-0-12-801284-0.00004-X. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- Chen X, Rivard L, Naqvi S, Nakada S, Padbury JF, Sanchez-Esteban J, Stopa EG, Lim YP, Stonestreet BS. Expression and localization of Inter-alpha Inhibitors in rodent brain. Neuroscience. 2016;324:69–81. doi: 10.1016/j.neuroscience.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012;32:11706–11715. doi: 10.1523/JNEUROSCI.0730-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Kim HS, Park CH, Jeong YH, Yoo J, Lee JP, Chang KA, Kim S, Suh YH. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology. 2007;32:2393–2404. doi: 10.1038/sj.npp.1301377. [DOI] [PubMed] [Google Scholar]
- Cox A, Varma A, Banik N. Recent advances in the pharmacologic treatment of spinal cord injury. Metab Brain Dis. 2015;30:473–482. doi: 10.1007/s11011-014-9547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG., Jr The role of neuroimmune signaling in alcoholism. Neuropharmacology. 2017;122:56–73. doi: 10.1016/j.neuropharm.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- Drew PD, Kane CJ. Fetal alcohol spectrum disorders and neuroimmune changes. Int Rev Neurobiol. 2014;118:41–80. doi: 10.1016/B978-0-12-801284-0.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim SH, Diekman ST, Floyd RL, Decoufle P. Comparison of binge drinking among pregnant and nonpregnant women, United States, 1991–1995. Am J Obstet Gynecol. 1999;180:1–7. doi: 10.1016/s0002-9378(99)70139-0. [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpich M, Amini E, Hemmati F, Ibrahim NM, Rahmani B, Mohamed Z, Raymond AA, Dargahi L, Ghasemi R, Ahmadiani A. Glycogen synthase kinase-3 beta (GSK-3beta) signaling: Implications for Parkinson’s disease. Pharmacol Res. 2015;97:16–26. doi: 10.1016/j.phrs.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Good ML, Hussey DL. Minocycline: stain devil? Br J Dermatol. 2003;149:237–239. doi: 10.1046/j.1365-2133.2003.05497.x. [DOI] [PubMed] [Google Scholar]
- Hanlon LA, Raghupathi R, Huh JW. Differential effects of minocycline on microglial activation and neurodegeneration following closed head injury in the neonate rat. Exp Neurol. 2017;290:1–14. doi: 10.1016/j.expneurol.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW. Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol. 2001;62:401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Jeong HK, Ji K, Min K, Joe EH. Brain inflammation and microglia: facts and misconceptions. Exp Neurobiol. 2013;22:59–67. doi: 10.5607/en.2013.22.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard K, Rovet E, Koren JG. Understanding fetal alcohol spectrum disorders (FASDs): toward identification of a behavioral phenotype. ScientificWorldJournal. 2008;8:873–882. doi: 10.1100/tsw.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Drew PD. Inflammatory responses to alcohol in the CNS: nuclear receptors as potential therapeutics for alcohol-induced neuropathologies. J Leukoc Biol. 2016;100:951–959. doi: 10.1189/jlb.3MR0416-171R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagishi Y, Kobayashi M, Kikuta K, Matsuda S. Roles of PI3K/AKT/GSK3/mTOR Pathway in Cell Signaling of Mental Illnesses. Depress Res Treat. 2012;2012:752563. doi: 10.1155/2012/752563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4:e525. doi: 10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Barber DS, Stevens SM., Jr Stable isotope labeling with amino acids in cell culture-based proteomic analysis of ethanol-induced protein expression profiles in microglia. Methods Mol Biol. 2012;829:551–565. doi: 10.1007/978-1-61779-458-2_35. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen G, Ma C, Bower KA, Xu M, Fan Z, Shi X, Ke ZJ, Luo J. Overexpression of glycogen synthase kinase 3beta sensitizes neuronal cells to ethanol toxicity. J Neurosci Res. 2009;87:2793–2802. doi: 10.1002/jnr.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. GSK3beta in ethanol neurotoxicity. Mol Neurobiol. 2009;40:108–121. doi: 10.1007/s12035-009-8075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. Lithium-mediated protection against ethanol neurotoxicity. Front Neurosci. 2010;4:41. doi: 10.3389/fnins.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. The role of GSK3beta in the development of the central nervous system. Front Biol (Beijing) 2012;7:212–220. doi: 10.1007/s11515-012-1222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Miller MW. Differential sensitivity of human neuroblastoma cell lines to ethanol: correlations with their proliferative responses to mitogenic growth factors and expression of growth factor receptors. Alcohol Clin Exp Res. 1997;21:1186–1194. [PubMed] [Google Scholar]
- Maixner DW, Weng HR. The Role of Glycogen Synthase Kinase 3 Beta in Neuroinflammation and Pain. J Pharm Pharmacol (Los Angel) 2013;1:001. doi: 10.13188/2327-204X.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi J, Kosari-Nasab M, Salari AA. Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety- and depression-like behaviors, hippocampal inflammation, and HPA axis activity in adult mice. Brain Res Bull. 2016;120:1–13. doi: 10.1016/j.brainresbull.2015.10.009. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Medina M, Wandosell F. Deconstructing GSK-3: The Fine Regulation of Its Activity. Int J Alzheimers Dis. 2011;2011:479249. doi: 10.4061/2011/479249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda RC. MicroRNAs and Fetal Brain Development: Implications for Ethanol Teratology during the Second Trimester Period of Neurogenesis. Front Genet. 2012;3:77. doi: 10.3389/fgene.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller T, Bard F, Bhattacharya A, Biber K, Campbell B, Dale E, Eder C, Gan L, Garden GA, Hughes ZA, Pearse DD, Staal RG, Sayed FA, Wes PD, Boddeke HW. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia. 2016;64:1788–1794. doi: 10.1002/glia.23007. [DOI] [PubMed] [Google Scholar]
- Ock J, Han HS, Hong SH, Lee SY, Han YM, Kwon BM, Suk K. Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Br J Pharmacol. 2010;159:1646–1662. doi: 10.1111/j.1476-5381.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res. 2002;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Orellana AM, Vasconcelos AR, Leite JA, de Sa Lima L, Andreotti DZ, Munhoz CD, Kawamoto EM, Scavone C. Age-related neuroinflammation and changes in AKT-GSK-3beta and WNT/beta-CATENIN signaling in rat hippocampus. Aging (Albany NY) 2015;7:1094–1111. doi: 10.18632/aging.100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsucci D, Calsolaro V, Mancuso M, Siciliano G. Neuroprotective effects of tetracyclines: molecular targets, animal models and human disease. CNS Neurol Disord Drug Targets. 2009;8:222–231. doi: 10.2174/187152709788680689. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Chakraborty G, Hui M, Masiello K, Saito M. Ethanol-Induced Neurodegeneration and Glial Activation in the Developing Brain. Brain Sci. 2016:6. doi: 10.3390/brainsci6030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15:355–366. doi: 10.2165/00003088-198815060-00001. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Patil J, D’Angelo B, Weber SG, Mallard C. NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl SE, Potashkin JA. The promise of neuroprotective agents in Parkinson’s disease. Front Neurol. 2011;2:68. doi: 10.3389/fneur.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xu A, Pan W, Wallin B, Kivlin R, Lu S, Cao C, Bi Z, Wan Y. Minocycline protects melanocytes against H2O2-induced cell death via JNK and p38 MAPK pathways. Int J Mol Med. 2008;22:9–16. [PubMed] [Google Scholar]
- Strahan JA, Walker WH, 2nd, Montgomery TR, Forger NG. Minocycline causes widespread cell death and increases microglial labeling in the neonatal mouse brain. Dev Neurobiol. 2017;77:753–766. doi: 10.1002/dneu.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveys N. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings 2013 [Google Scholar]
- Vallieres L, Rivest S. Interleukin-6 is a needed proinflammatory cytokine in the prolonged neural activity and transcriptional activation of corticotropin-releasing factor during endotoxemia. Endocrinology. 1999;140:3890–3903. doi: 10.1210/endo.140.9.6983. [DOI] [PubMed] [Google Scholar]
- Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE, Newsholme P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediators Inflamm. 2015;2015:105828. doi: 10.1155/2015/105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang X, Ke ZJ, Comer AL, Xu M, Frank JA, Zhang Z, Shi X, Luo J. Tunicamycin-induced unfolded protein response in the developing mouse brain. Toxicol Appl Pharmacol. 2015a;283:157–167. doi: 10.1016/j.taap.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015b;3:136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fan Z, Wang B, Luo J, Ke ZJ. Activation of double-stranded RNA-activated protein kinase by mild impairment of oxidative metabolism in neurons. J Neurochem. 2007;103:2380–2390. doi: 10.1111/j.1471-4159.2007.04978.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Guizzetti M. Fetal Alcohol Spectrum Disorders: An Overview from the Glia Perspective. Front Integr Neurosci. 2015;9:65. doi: 10.3389/fnint.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Peng LX, Huang TJ, Yang GD, Chu QQ, Liang YY, Cao X, Xie P, Zheng LS, Huang HB, Cai MD, Huang JL, Liu RY, Zhu ZY, Qian CN, Huang BJ. Cancer stem-like cell characteristics induced by EB virus-encoded LMP1 contribute to radioresistance in nasopharyngeal carcinoma by suppressing the p53-mediated apoptosis pathway. Cancer Lett. 2014;344:260–271. doi: 10.1016/j.canlet.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Yang F, Dong A, Mueller P, Caicedo J, Sutton AM, Odetunde J, Barrick CJ, Klyachkin YM, Abdel-Latif A, Smyth SS. Coronary artery remodeling in a model of left ventricular pressure overload is influenced by platelets and inflammatory cells. PLoS One. 2012;7:e40196. doi: 10.1371/journal.pone.0040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Luo J. Endoplasmic Reticulum Stress and Ethanol Neurotoxicity. Biomolecules. 2015;5:2538–2553. doi: 10.3390/biom5042538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci. 2014;71:683–697. doi: 10.1007/s00018-013-1459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744–751. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]
- Young C, Roth KA, Klocke BJ, West T, Holtzman DM, Labruyere J, Qin YQ, Dikranian K, Olney JW. Role of caspase-3 in ethanol-induced developmental neurodegeneration. Neurobiol Dis. 2005;20:608–614. doi: 10.1016/j.nbd.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Young C, Straiko MM, Johnson SA, Creeley C, Olney JW. Ethanol causes and lithium prevents neuroapoptosis and suppression of pERK in the infant mouse brain. Neurobiol Dis. 2008;31:355–360. doi: 10.1016/j.nbd.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang L, Dong X, Hu X, Zhou L, Liu Q, Song B, Wu Q, Li L. The c-Jun N-terminal kinase (JNK) pathway is activated in human interstitial cystitis (IC) and rat protamine sulfate induced cystitis. Sci Rep. 2016a;6:19670. doi: 10.1038/srep19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wu X, Yan S, Xie X, Fan Y, Zhang J, Peng C, You Z. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARgamma-mediated alteration of microglial activation phenotypes. J Neuroinflammation. 2016b;13:259. doi: 10.1186/s12974-016-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Fu J, Liu X, Wang T, Zhang J, Zhao Y. Activation of Akt/GSK-3beta/beta-catenin signaling pathway is involved in survival of neurons after traumatic brain injury in rats. Neurol Res. 2012;34:400–407. doi: 10.1179/1743132812Y.0000000025. [DOI] [PubMed] [Google Scholar]
- Zhong J, Yang X, Yao W, Lee W. Lithium protects ethanol-induced neuronal apoptosis. Biochem Biophys Res Commun. 2006;350:905–910. doi: 10.1016/j.bbrc.2006.09.138. [DOI] [PubMed] [Google Scholar]
- Zhu D, Kang Q, Huang PY, He TC, Xie P. Neurogenesis-related genes expression profiling of mouse fibroblastic stem cells induced by Wnt signaling. Neurol Res. 2009;31:200–203. doi: 10.1179/174313209X393915. [DOI] [PubMed] [Google Scholar]
- Zhu F, Zheng Y, Liu Y, Zhang X, Zhao J. Minocycline alleviates behavioral deficits and inhibits microglial activation in the offspring of pregnant mice after administration of polyriboinosinic-polyribocytidilic acid. Psychiatry Res. 2014;219:680–686. doi: 10.1016/j.psychres.2014.06.046. [DOI] [PubMed] [Google Scholar]