Abstract
Purpose
Among HIV-infected persons, antiretroviral therapy (ART) and depression are strongly associated with mortality. We estimated reductions in 5-year mortality in Women’s Interagency HIV Study (WIHS) participants under plausible hypothetical increases in ART initiation and reductions in depression (CES-D score≥16).
Methods
We followed 885 ART-naïve WIHS participants for 5 years from their first study visit after April 1998 to death or censoring. We used the parametric extended g-formula to estimate cumulative mortality under the natural course (NC) and alternative exposure distributions.
Results
Baseline prevalence of depression was 52% and 62% initiated ART by 5 years. Compared to mortality under NC (13.2%), immediate ART and elimination of 36% or 67% of depressive episodes were associated with risk differences (RD) of −5.2% (95% CI: −7.7%, −2.6%) and −5.7 (95% CI: −8.7, −2.7). Compared to immediate ART and NC for depression, additionally eliminating 67% of depressive episodes was associated with RD = −1.6 (95% CI: −3.9, 0.8). Compared to 5-year mortality under NC for ART and elimination of 67% of depression, also initiating ART immediately was associated with RD = −2.6 (95% CI: −5.0, −0.3).
Conclusions
Increasing ART initiation and reducing depression were associated with moderate reductions in 5-year mortality among HIV-infected women.
Keywords: Antiretroviral Therapy, Cohort Studies, Depression, HIV, Survival Analysis, Mortality
INTRODUCTION
Antiretroviral therapy (ART) has reduced immunodeficiency-associated morbidity and mortality in persons infected with HIV (1, 2), exposing the importance of managing comorbid conditions. Depression is two to four times more common in HIV-infected persons than in the general population (3). Untreated depression is associated with lower probabilities of ART initiation and ART adherence (4, 5). Furthermore, depression is associated with faster progression of HIV disease (6) and AIDS-related and non-AIDS-related mortality (7), even after adjusting for ART use and adherence. ART and depression may interact biologically or psychosocially (3). It is necessary to consider ART and depression changes together to explore this interaction (8, 9).
A recent analysis by Todd et al. in the Women’s Interagency HIV Study (WIHS) was one of the first to explicitly consider joint effects (10) of ART and depressive symptoms (8). In that study, the relative hazard (HR) of mortality associated with depressive symptoms was similar to the HR associated with no ART (8). Todd et al. contrasted mortality hazards under four extreme counterfactual exposure distributions: 1) everyone initiates ART immediately and never has depressive symptoms; 2) everyone initiates ART immediately and always has depressive symptoms; 3) everyone delays ART initiation indefinitely (i.e., never initiates ART) and never has depressive symptoms; and 4) everyone delays ART initiation indefinitely and always has depressive symptoms.
Our goal in the present analysis was to extend this prior work by estimating 5-year, all-cause mortality under several less extreme, hypothetical interventions to jointly increase ART uptake and reduce depressive symptoms, compared to the natural course (i.e., the 5-year mortality under the observed exposure distributions) (11). This type of contrast has been called a “generalized intervention contrast” (12).
Hypothetical interventions
We estimated effects of two sets of hypothetical interventions on 5-year mortality. First, we imposed no direct intervention on ART initiation (ART initiation was allowed to be affected indirectly through interventions on depression) and simultaneously either: 1) eliminated depressive symptoms with probability 36% at a given visit when a woman would have reported being depressed if all interventions were discontinued right before that visit (the natural value of depression) (13); 2) eliminated depressive symptoms with probability 67% at a given visit when a woman would have reported her natural value of depression as depressed; or 3) eliminated 100% of depressive symptoms for all women at all visits. Next, set ART initiation to be immediate for all women and simultaneous reduction of either 0% (no direct intervention on depression), 36%, 67% or 100% in the probability of depressive symptoms at a given, for woman-visits when the natural value of depression would have been depressed. All interventions assumed no loss to follow-up.
Reductions of 36% and 67% in depressive symptoms generally correspond to the reductions in depression seen among outpatients with non-psychotic major depressive disorder in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial (see Discussion). Thirty-six percent of participants experienced depressive symptom elimination following first-line depression treatment and 67% experienced symptom elimination after switching or augmenting treatment if their current regimen was not working (14).
METHODS
Study population
A full description of the WIHS cohort, recruitment, study enrollment, and study procedures is provided elsewhere (15, 16). Briefly, between 1994 and 2015, the WIHS enrolled 4982 women in ten sites across the US, of whom approximately 3 in 4 were HIV seropositive at enrollment. Women attended semiannual follow-up visits, at which time detailed measurements on HIV disease history and progression, medications, laboratory results and behavioral characteristics, were collected. Institutional review boards at each site approved the WIHS, and this analysis was approved by the institutional review board of the University of North Carolina.
We took 1 April 1998 to be the start of the modern ART era and eligibility for this analysis. HIV-seropositive, ART-naïve WIHS participants with detectable viral load at their earliest WIHS visit on or after 1 April 1998 (defined as baseline for this analysis) were included. Thus study sample was a mix of women enrolled in the first wave of WIHS who remained ART-naïve through April 1998 and women who were ART-naïve when they enrolled in the second (2001/2002) or third (2011/2012) wave of WIHS recruitment. Of 3,567 seropositive WIHS participants, 928 were ART-naïve at their first visit during the analytic period. We excluded 43 (5%) who did not have at least one depression measure, one CD4 cell count and one HIV RNA viral load determination within one year of baseline. The final study population included 885 participants.
Mortality Ascertainment
Mortality for women enrolled in the WIHS is obtained from clinic sources, follow-up with family members for participants lost to follow-up, and regular matches against the National Death Index.
Statistical analysis
We followed women from their first eligible WIHS visit on or after 1 April 1998 until first of death, loss to follow-up (defined as 12 months since the most recent WIHS visit), or administrative censoring at five years follow-up or site-specific administrative end of follow-up (most recent check for deaths in the National Death Index; 31 December 2010 for 12% of women and 31 December 2012 for 88%). Time-fixed baseline covariates included: race (black or other); age in years; current smoking; and at-risk drinking (>7 drinks/week) (17). Time-varying covariates included CD4 cell count (cells/μL), HIV viral load (log10 copies/mL) and illicit drug use (any use of crack, cocaine, heroin, amphetamines, or other drugs, excluding marijuana, since the last visit). Women reported whether they had initiated ART since their last visit, where ART was defined as ≥3 antiretroviral medications, one of which was a protease inhibitor, a non-nucleoside reverse transcriptase inhibitor, abacavir, tenofovir, an integrase inhibitor (e.g., raltegravir) or an entry inhibitor (e.g., Maraviroc or enfuvirtide) (18). Presence of depressive symptoms was defined as a score of ≥16 on the Center for Epidemiological Studies Depression scale [CES-D], measured at each visit (19). The CES-D has been shown to have reasonably high sensitivity (95%) and specificity (70%) among low income women (20); positive predictive value of the CES-D should be high in the WIHS where prevalence of depression is high.
We used the parametric g-formula and the parametric extended g-formula to estimate 5-year cumulative mortality under several scenarios (21, 22). In instances where confounders also serve as mediators, in contrast to standard methods, g-methods remain unbiased (23, 24); for the current research question, CD4 cell count and viral load are confounders and mediators of the effect of ART, and illicit drug use may be a confounder and mediator of the effect of depressive symptoms. We employed the parametric extended g-formula (11, 23) because it allows estimation of the effects of hypothetical exposure distributions that may depend on the natural value of exposure. The trade-off for increased flexibility of the parametric g-formula is increased assumptions about correct joint-model fit. As an informal check on the plausibility of the modeling assumptions required in the parametric g-formula, we compared results from the g-formula with results from a marginal structural model, for always/never exposure distributions for ART and depression (static deterministic regimes). Because the parametric g-formula and marginal structural models rely on different modeling assumptions, agreement between the two results increases confidence in model specification (25). The marginal structural model we fit for comparison modeled joint effects on 5-year mortality of always versus never having depressive symptoms and immediately versus never initiating ART. The denominator of the weights for ART were estimated using logistic models for the probability of ART initiation conditional on time since baseline, black race, age, illicit drug use reported at baseline, baseline smoking, baseline heavy drinking, baseline CD4 cell count, baseline viral load, and 6-months’ lagged depressive symptoms, CD4 cell count, and detectable viral load. The denominator for the weights for depression were estimated using logistic models for the probability of depressive symptoms, stratified by 6-months’ lagged history of depressive symptoms, conditional on time since baseline, black race, age, illicit drug use reported at baseline, baseline smoking, baseline heavy drinking, baseline CD4 cell count, baseline viral load, ART initiation, and 6-months’ lagged CD4 cell count, and detectable viral load. Both sets of weights were stabilized by time since baseline; inverse probability of depression weights were also stabilized by ART initiation. Censoring weights were conditional on the same set of confounders as both treatment weights, as well as prior ART initiation, prior depression, and a product term for the two exposures. All continuous variables were modeled with restricted cubic splines with knots at the 5th, 35th, 65th and 95th percentile of their distribution (26). The final weights were the product of the ART, depression, and censoring weights.
A formal description of methods for the parametric extended g-formula appears in the appendix. Descriptions of the parametric g-formula and parametric extended g-formula have been published elsewhere (11, 21, 22, 27–29). Briefly, we first fit parametric (pooled logistic or linear) models in the following order for: 1) reporting recent illicit drug use at visit j = 1 … J conditional all time-fixed covariates and time-varying covariates at visit j − 1 (see table 1) including an interaction term between depression and ART (note: this and subsequent models were also implicitly conditional on survival and retention in the cohort until visit j); 2) undetectable HIV RNA viral load (<200 copies/mL) at visit j, conditional on time-fixed covariates, time-varying covariates other than illicit drug use at visit j − 1, and illicit drug use at visit j; 3) CD4 cell count at visit j, conditional on time-fixed covariates, time-varying covariates other than illicit drug use and viral load at visit j − 1, and illicit drug use and detectable viral load at visit j; 4) depressive symptoms at visit j (exposure), conditional on time-fixed covariates, ART initiation prior to j, and time-varying covariates at visit j; 5) ART initiation in the interval from j to j +1 (exposure), conditional on time-fixed covariates, time-varying covariates at visit j, and not yet having initiated ART; 6) remaining uncensored until visit j + 1, conditional on time-fixed covariates, time-varying covariates at visit j, and ART initiation in the interval starting with j; and 7) death before visit j + 1 (outcome), conditional on time-fixed covariates, time-varying covariates at visit j, and ART initiation in the interval starting with j. We saved parameter estimates from each model for use in the Monte Carlo simulation. We explored alternative orderings of modeling time-varying covariates (27) and saw little change in final estimates.
TABLE 1.
Characteristics of 885 Women’s Interagency HIV Study (WIHS) participants who were ART-naïve at baseline and followed for up to 5 years
| WIHS participants at baseline N=885 |
Person-years of follow-upa N=3377 |
|
|---|---|---|
| Black raceb | 571 (65) | 2193 (64) |
| Age, yearsc | 38 (32, 45) | 40 (34, 46) |
| Baseline past or current smokingb | 658 (74) | 2591 (75) |
| Baseline heavy drinking (>7 drinks/week)b | 121 (14) | 420 (12) |
| Illicit drug use in past 6 monthsb,d | 199 (22) | 614 (18) |
| Most recent CD4 cell countc | 441 (273, 649) | 433 (277, 623) |
| Most recent log10 viral loadc | 3.5 (2.7, 4.3) | 3.3 (2.3, 4.2) |
| Current depressive symptomsb,e | 457 (52) | 1639 (48) |
| CES-D scorec | 16 (7, 27) | 15 (6, 25) |
Abbreviations: ART, antiretroviral treatment; CES-D, Center for Epidemiological Studies Depression scale; HIV, human immunodeficiency virus
For time-updated covariates, N (%) and Median (IQR) are summaries of the covariate across all person-years of follow-up
N (%)
Median (IQR)
Participant reported use of crack, cocaine, heroin, methadone, methamphetamine, or other drug use (excluding marijuana) since the previous visit
Defined as CES-D≥16
We used Monte Carlo simulation to estimate cumulative mortality curves approximated by the joint distribution of the models above. We sampled WIHS participants at baseline with replacement 50,000 times, discarding follow-up data. We simulated follow-up data for each of the 50,000 resamples based on the parameters estimated from the models above to estimate the natural course. Inability to recapture the observed distributions of time-varying covariates or cumulative incidence of the outcome imply model misspecification. Thus, we compared observed and natural course cumulative incidence curves for ART initiation, drop-out and mortality, and mean variable values over time for CD4 cell count, viral suppression, illicit drug use and depression. We did a further check of model specification by setting covariate values for ART initiation and depression (in addition to removing all drop-out) to estimate cumulative incidence of mortality under exposure distributions corresponding to immediately/never initiating ART and always/never having depressive symptoms for comparison with marginal structural model effect estimates (8). Finally, we estimated cumulative mortality curves under interventions by first predicting the natural value of depression at visit j (that is, the value of depression that would have been observed if the intervention were discontinued right before j) and then modifying the probability of depression at visit j + 1 if the woman was depressed at visit j (13).
We estimated the standard error of estimates from the standard deviation from 200 bootstrap resamples (30). Unrestricted random samples were drawn with replacement from the study sample. Models described above were fit and Monte Carlo simulation was run within each bootstrap resample. All analyses were carried out using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Of 885 women in the WIHS who met inclusion criteria and had complete data, 65% were black, 74% reported ever smoking, 14% reported at-risk drinking and 22% reported recent illicit drug use. Median age was 38 years (interquartile range [IQR]: 32, 45). Median CD4 cell count and viral load were 441 cells/μL (IQR: 273, 649) and 3.5 log10 copies/mL (IQR: 2.7, 4.3), respectively. Over half of participants had prevalent depressive symptoms at the first visit (52%). Median CES-D score was 16 (IQR: 7, 27) (Table 1).
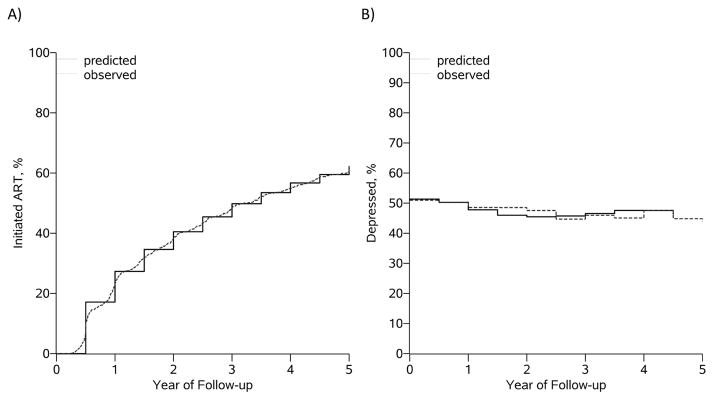
During 3,377 person-years of follow-up, 191 patients were lost to follow-up, and 92 patients died. Overall, 5-year mortality risk was 12.6% (95% CI: 10.1%, 15.1%). Cumulative incidence of ART initiation by 5 years was 62% (Figure 1). Prevalence of depressive symptoms was steady across the study period, around 45% (Figure 1). We were able to recapture the observed data with the parametric g-formula for all modeled covariates, exposures and outcome.
Figure 1.
Natural history (observed) and natural course (modeled/predicted) for (A) ART initiation and (B) prevalence of depression
Using the parametric g-formula, we estimated that, compared to never initiating ART and always being depressed, never initiating ART and never being depressed was associated with a 5-year mortality risk difference of −12.3 (95% CI: −19.7, 4.9), immediately initiating ART and always being depressed was associated with a 5-year mortality risk difference of −10.8 (95% CI: −18.7, −2.9), and immediately initiating ART and never being depressed was associated with a 5-year mortality risk difference of −16.0 (95% CI: − 23.2, −8.9) (Table 2).
TABLE 2.
Exposure effects of ART and depression on 5-year risk of mortality estimated among 885 ART-naïve women enrolled in the Women’s Interagency HIV Study (WIHS)
| Parametric g-formula | ||||
|---|---|---|---|---|
|
| ||||
| 5-year Mortality Risk | Risk Difference | Risk Ratio | Hazard Ratio | |
| No ART, always depressive symptoms | 23.2 (16.9, 29.4) | 0. | 1. | 1. |
| No ART, never depressive symptoms | 10.9 (6.5, 15.2) | −12.3 (−19.7, −4.9) | 0.47 (0.37, 0.59) | 0.50 (0.28, 0.89) |
| Immediate ART, always depressive symptoms | 12.4 (6.7, 18.0) | −10.8 (−18.7, −2.9) | 0.53 (0.40, 0.71) | 0.44 (0.24, 0.75) |
| Immediate ART, never depressive symptoms | 7.1 (3.8, 10.5) | −16.0 (−23.2, −8.9) | 0.31 (0.26, 0.37) | 0.28 (0.15, 0.52) |
| No ART | 18.6 (14.0, 23.1) | 0. | 1. | 1. |
| Immediate ART | 9.0 (6.3, 11.8) | −9.6 (−14.9, −4.3) | 0.49 (0.33, 0.72) | 0.46 (0.30, 0.71) |
| Always depressive symptoms | 16.4 (12.5, 20.3) | 0. | 1. | 1. |
| Never depressive symptoms | 9.4 (6.3, 12.6) | −7.0 (−12.0, −2.0) | 0.57 (0.38, 0.87) | 0.55 (0.35, 0.86) |
|
| ||||
| Inverse probability weighted marginal structural model | ||||
|
| ||||
| No ART, always depressive symptoms | 25.0 (9.6, 40.4) | 0. | 1. | 1. |
| No ART, never depressive symptoms | 12.1 (5.1, 19.2) | −12.8 (−29.8, 4.2) | 0.49 (0.21, 1.13) | 0.45 (0.19, 1.05) |
| Immediate ART, always depressive symptoms | 11.1 (5.8, 16.3) | −13.9 (−29.8, 2.0) | 0.44 (0.21, 0.92) | 0.39 (0.17, 0.89) |
| Immediate ART, never depressive symptoms | 4.7 (2.0, 7.5) | −20.2 (−36.0, −4.4) | 0.19 (0.08, 0.45) | 0.17 (0.07, 0.42) |
| No ART | 17.5 (11.7, 23.3) | 0. | 1. | 1. |
| Immediate ART | 8.2 (5.4, 11.1) | −9.3 (−15.6, −2.9) | 0.47 (0.29, 0.76) | 0.45 (027, 0.74) |
| Always depressive symptoms | 17.7 (12.7, 22.6) | 0. | 1. | 1. |
| Never depressive symptoms | 8.4 (5.1, 11.7) | −9.3 (−15.5, −3.0) | 0.48 (0.28, 0.80) | 0.46 (0.27, 0.78) |
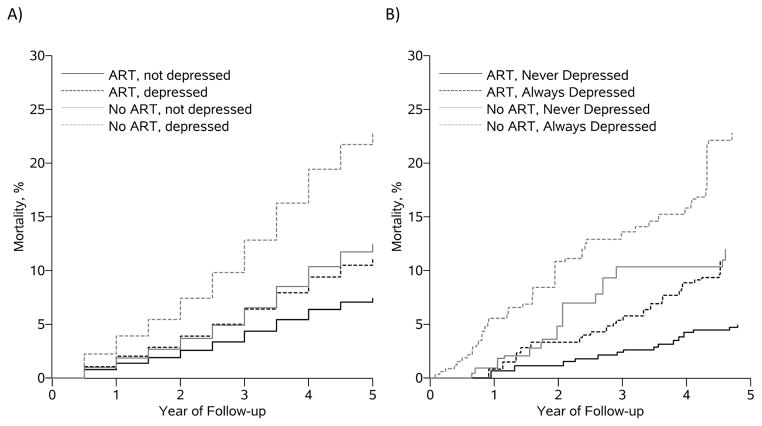
When we estimated the same exposure contrasts using a joint marginal structural model all estimates were slightly larger (Table 2). Cumulative mortality curves for each of the four always/never exposure effects from the parametric g-formula and marginal structural models coincide at 5 years, but there are some differences in predicted mortality associated with the treatment policy never initiate ART/never be depressed (Figure 2).
Figure 2.
Cumulative mortality curves from (A) the parametric g-formula and (B) inverse probability weighted marginal structural model under intervention regimens: 1) immediately initiate ART and never be depressed; 2) immediately initiate ART and always be depressed; 3) never initiate ART and never be depressed; and 4) always initiate ART and always be depressed
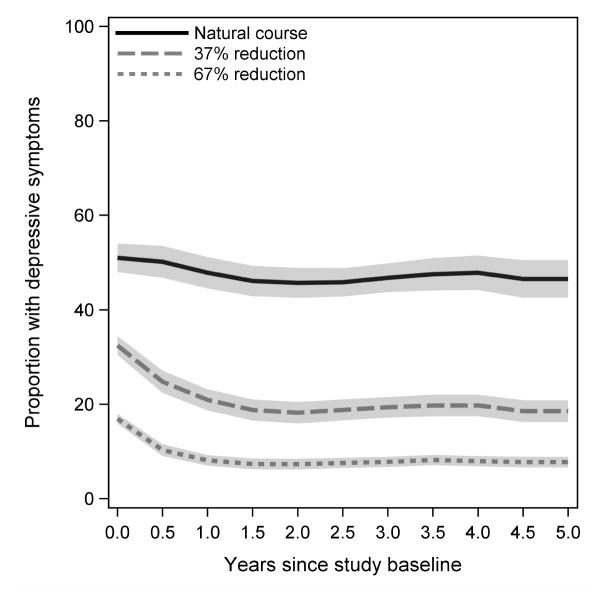
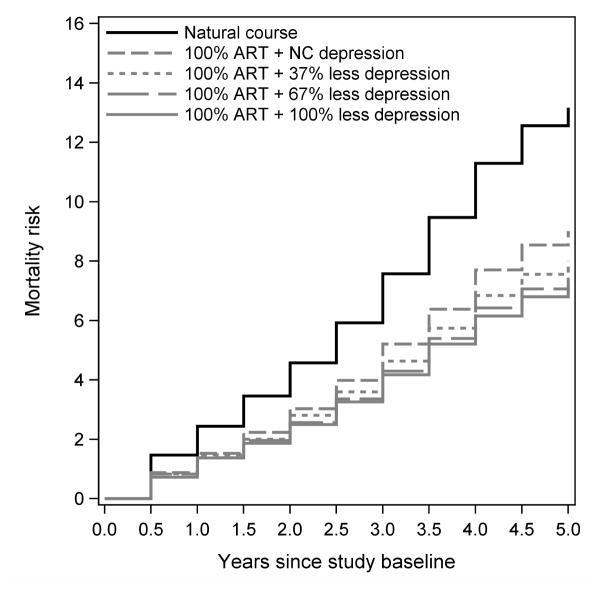
Generalized intervention contrasts were attenuated compared to exposure effects (Table 3). If depressive symptoms were treated with 36%, 67%, or 100% probability of elimination at the next visit (Figure 3) and if ART initiation was immediate, associated 5-year mortality risk differences were −4.2 (95% CI: −6.3, −2.0), −5.2 (95% CI: −7.7, −2.6), and −6.0 (95% CI: −9.4, −2.6), respectively. Compared to only intervening to reduce depression by 67% at the next visit, also intervening to initiate everyone on ART immediately was associated with risk difference of −2.6 (95% CI: −5.0, −0.3). Compared to only intervening to initiate everyone on ART, also reducing depressive symptoms by 67% at the next visit would further reduce 5-year mortality by −1.6 (95% CI: −3.9, 0.8). For completeness, we present cumulative incidence functions for modeled interventions in Figure 4 and risk differences, risk ratios and hazard ratios in Table 3.
TABLE 3.
Generalized effect of several plausible interventions on antiretroviral treatment and depression (CES-D score≥16) on 5-year risk of mortality among 885 ART-naïve women enrolled in the Women’s Interagency HIV Study (WIHS)
| Parametric g-formula | ||||
|---|---|---|---|---|
|
| ||||
| 5-year Mortality Risk | Risk Difference | Risk Ratio | Hazard Ratio | |
| Natural course | 13.2 (10.6, 15.9) | 0. | 1. | 1. |
| Natural course for ART and… | ||||
| …reduce depression 36% at next visit | 11.4 (8.9, 14.0) | −1.8 (−3.4, −0.2) | 0.87 (0.76, 0.99) | 0.86 (0.74, 0.99) |
| …reduce depression 67% at next visit | 10.1 (7.2, 12.9) | −3.1 (−5.4, −0.9) | 0.76 (0.62, 0.94) | 0.75 (0.60, 0.94) |
| …eliminate all depression | 9.4 (6.3, 12.6) | −3.7 (−6.5, −1.0) | 0.72 (0.54, 0.95) | 0.70 (0.52, 0.94) |
| Immediate ART and… | ||||
| …natural course for depression | 9.0 (6.3, 11.8) | −4.2 (−6.3, −2.0) | 0.68 (0.55, 0.86) | 0.67 (0.53, 0.85) |
| …reduce depression 36% at next visit | 8.0 (5.3, 10.7) | −5.2 (−7.7, −2.6) | 0.61 (0.45, 0.81) | 0.59 (0.44, 0.80) |
| …reduce depression 67% at next visit | 7.4 (4.4, 10.5) | −5.7 (−8.7, −2.7) | 0.57 (0.39, 0.82) | 0.55 (0.37, 0.81) |
| …eliminate all depression | 7.1 (3.7, 10.5) | −6.0 (−9.4, −2.6) | 0.54 (0.34, 0.86) | 0.53 (0.33, 0.85) |
|
| ||||
| Immediate ART and reduce depression 67% at next visit versus… | ||||
| …natural course for ART and reduce depression 67% at next visit (ref) | −2.6 (−5.0, −0.3) | 0.74 (0.54, 1.01) | 0.73 (0.53, 1.01) | |
| …immediate ART and natural course for depression (ref) | −1.6 (−3.9, 0.8) | 0.83 (0.60, 1.1) | 0.82 (0.59, 1.14) | |
Abbreviations: ART, antiretroviral treatment; CES-D, Center for Epidemiological Studies Depression scale; HIV, human immunodeficiency virus
FIGURE 3.
Prevalence of depressive symptoms (CES-D score ≥16) under the natural course and various interventions to reduce the probability of depressive symptoms at the next visit given depressive symptoms at a given visit. Shaded areas indicate pointwise 95% confidence intervals.
Figure 4.
Cumulative mortality curves from the parametric g-formula for the natural course (no intervention) and interventions that would treat everyone with ART immediately and treat depressive symptoms with success rates of 34%, 67%, and 100%
DISCUSSION
We used Robins’ parametric g-formula (21, 22) to substantiate the strong association between constant exposure to depressive symptoms and long-term delay of ART initiation and higher mortality estimated with a marginal structural model (8). However, contrasts of mortality under various reductions in depression symptoms and increases in ART compared with mortality under the natural course provide more realistic, generalizable (12) estimates of the effect that interventions might produce. Mortality reductions attainable with immediate ART initiation in contemporary HIV-infected cohorts may be even less because ART use is more common (and fewer people have not yet started ART under the natural course). Mortality reductions associated with depression treatment, assuming ART initiation was immediate for all women, were modest; in particular, there was arguably not a meaningful difference between a reduction in episodes of depression of 37% or 67%. This may be due to sub-additive causal interactions between the two exposures or it may be due to the relatively minor differences in depression prevalence under the two hypothetical interventions (figure 3). However, our results indicated that improving ART coverage and reducing depressive symptoms were both associated with clinically meaningful mortality reductions that were similar in magnitude, and reducing depressive symptoms reduced mortality (marginally) even in the presence of 100% ART coverage.
ART use has an undisputed direct effect (not through depression) on mortality (2, 31), and it is possible that it also has a weak indirect effect on mortality by reducing depressive symptoms (32). In addition the indirect effects of depression on mortality through HIV progression and treatment, depression may have a direct effect (not through HIV) on mortality. Depression in HIV-infected adults has also been linked with higher non-AIDS-related mortality, including mortality due to liver disease, drug overdose, violence (homicide/suicide/accident), and non-AIDS-related malignancy (7). Because of these interactions between depression and ART use, a major strength of our analysis was our consideration of them jointly.
A set of assumptions sufficient for a causal interpretation of our results includes conditional exchangeability (13, 24), treatment version irrelevance (33), positivity (22, 34), correct model specification (24), no measurement error (35), and no interference (36). We frame the limitations of this analysis in terms of potential violations of these assumptions. Perhaps most significant is that depression treatment was not collected during part of the study period, and the format in which it was collected was inconsistent across time; thus we focused our analysis on depressive symptoms rather than depression treatment. This decision could result in violations of several assumptions. First, women who were never depressed may be meaningfully different from formerly depressed women whose depressive symptoms are eliminated, violating the exchangeability assumption. However, HIV-infected women receiving treatment for depression (specifically serotonin reuptake inhibitors) had ART adherence and laboratory value trajectories similar to non-depressed women (5). Furthermore, while we are fairly confident that key confounders of the effect of ART on mortality are measured, it is less plausible that we accounted for all possible confounders of the effect of depression on mortality; time-varying smoking, risky sexual behaviors, and engagement in medical care are just some possible confounders whose influence should be explored in future analyses. Second, because we did not model a specific intervention on depression, assuming treatment version irrelevance is more problematic. Acknowledging this limitation, we chose to model reductions in depression that coincided with effects seen in the STAR*D trial (14). If women with depressive symptoms were already receiving depression treatment and were resistant to treatment, first-line depression treatment may be less effective for an unidentifiable subgroup of the cohort. However, less than 20% of HIV-infected women with prevalent depression are estimated to be receiving any depressive treatment (37), and thus depressive symptoms are likely indicative of untreated depression. Our modeled intervention did not exactly correspond to the STAR*D trial result in that the probability of remission found per individual in the STAR*D trial was applied to each depressive episode in our analysis, and thus the overall individual probability of remission of depressive symptoms over a 5-year period in our cohort was higher than was found in the trial (figure 3). As a consequence, our results may be overly optimistic about the reduction in mortality attainable by treating depression in this population. Alternatively, we could have assigned each woman a latent probability of having depression that was responsive to treatment, but such an approach has not been implemented nor formalized before. Third, because we modeled the effect of a change in depressive symptoms rather than modeling the effect of a specific intervention on clinical depression, if CES-D were a poor indicator of the presence of clinical depression, we may have misclassified women as eligible for intervention when they were not. However, to the first point, the CES-D has been shown to have high sensitivity (95%) and specificity (70%) for clinical depression among low income women (20). Finally, we believe that in this analysis, there is little potential for violations of the assumptions of positivity and no interference.
One strength of our analysis was our decision to fit both a marginal structural model and the parametric g-formula for comparisons of always/never having depressive symptoms and no/immediate ART initiation (static deterministic treatment regimes). We did not check our findings from the parametric extended g-formula (in which the intervention depends on the natural value of exposure) against a comparable marginal structural model (22), which would be computationally more complicated. Fitting either a marginal structural model or using g-computation should arguably be standard operating procedure when employing the parametric g-formula to triangulate toward the truth (22, 25, 27). The parametric g-formula assumes correct model specification for the outcome and all time-varying covariates; in contrast, marginal structural models rely on correct specification of the model(s) for exposure and (possibly) censoring. If results from alternate estimators disagree, this is an indication that at least one assumption of at least one of the estimators is violated. Using double robust methods or an alternate study design [e.g., instrumental variable methods (38, 39) or structural nested models (40)] may give clues as to which estimator is biased.
We chose to estimate joint effects of ART and depression given their high potential to interact in determining mortality. We report a generalized impact contrast, which is arguably more useful to policy makers than exposure effects. While intervention effects associated with realistic interventions on ART and depressive symptoms were attenuated compared to exposure effects, they were still clinically significant. Our results provide further evidence supporting immediate ART initiation for persons with HIV, and some evidence that depression screening and treatment may provide marginal additional benefit for prevention of all-cause mortality.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI- 035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young and Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
The authors also wish to thank Jessica Young for her incredibly helpful comments on an earlier draft of this manuscript.
FUNDING: This analysis was supported by National Institutes of Health grants U01 AI103390, R01 AI100654 and U01 HL121812.
LIST OF ABBREVIATIONS
- AIDS
Acquired Immunodeficiency Syndrome
- ART
Antiretroviral therapy
- CES-D
Center for Epidemiological Studies Depression scale
- HIV
Human Immunodeficiency Virus
- HR
Hazard ratio
- STAR*D
Sequenced Treatment Alternatives to Relieve Depression (trial)
- WIHS
Women’s Interagency HIV Study
APPENDIX
We follow the convention of denoting random variables with uppercase letters and possible realizations of those random variables with lowercase letters. Let i = 1, …, N index WIHS participants, and let j = 1, …, J denote completed months of follow-up. For participant i, let Yij be an indicator of death in month j; let Cij be an indicator of censoring (having gone 12 months since the last study visit) in month j; let Aij be an indicator of antiretroviral therapy (ART) in month j; let Dij be an indicator of depression in month j; and finally, let Zij be a vector of time-fixed and time-varying covariates that confound the effect of A on Y or that confound the effect of D on Y, in month j. By design, Ai(−1) = Yi0 = Ci0 = 0, since we restricted to persons who were ART naïve and alive and at risk for death at baseline. By definition, if Yik = 1 then Yi(k+1) = 1, and if Cik = 1 then Ci(k+1) = 1. Also, because we analyzed the data using an intent-to-treat approach for initiation of ART, if Aik = 1 then Ai(k+1) = 1 by definition.
The cumulative incidence of death observed in the WIHS by month j + 1 (the natural course) (25) can be written:
where P(·|·) is the conditional probability evaluated at the observed covariates values for a given participant; f(·|·) is the conditional density function; and F(j)is the cumulative incidence function of death evaluated at time j. We assumed that censoring was not informative of subsequent outcome, exposure or covariates, given measured covariate and exposure history.
Many typical epidemiologic analyses estimate exposure effects. That is, they ask the question, “what would be the difference in the incidence of death in the study sample had everyone initiated ART immediately and no one been depressed ever, versus had no one initiated ART and everyone always been depressed?” To answer that question, we would estimate the cumulative incidence of death under a deterministic intervention g of the form:
We use g to indicate interventions on a and d, g, = (ga, gd). Note that here we compare mortality under exposure regimens and (always treated and never depressed) versus and (never treated and always depressed), for example. Ensuring no loss to follow-up, that is, intervening to set c̄j = (0,…,0), is a component of both regimens.
In this paper, we aimed to estimate an intervention effect (21). That is, we asked the question, “what would be the difference in the incidence of death in the study sample had we intervened to get all women initiated on ART immediately, treated depression with effectiveness T% for all women with depressive symptoms, and had no one been lost to follow-up, versus the incidence of death in the absence of any intervention?” This is a contrast of mortality under the natural course F(j) compared to a contrast of mortality under an intervention g of the form:
The intervention depends on the natural value of depression at time k, which is defined as the value of depression that would have been observed if the intervention were discontinued right before k (13). We denote the natural value of depression at time k as . is set according to exposure regimens and , and is set according to exposure regimens:
If , then
And if , then
By definition . The intervention specified above is equivalent to: “Initiate everyone on ART immediately, and among those who are depressed (assuming any intervention on depression were discontinued right before depression was measured), treat them all with T% probability of remitting depressive symptoms.” Individuals who are treated for depression are not prevented from relapsing and experiencing depressive symptoms at a future visit.
Footnotes
CONFLICTS OF INTEREST: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.HIV-CAUSAL Collaboration. Ray M, Logan R, Sterne JA, Hernández-Díaz S, Robins JM, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24(1):123–37. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337(11):725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 3.Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep. 2015;17(1):530. doi: 10.1007/s11920-014-0530-4. [DOI] [PubMed] [Google Scholar]
- 4.Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDS. 2008;22(3):233–43. doi: 10.1089/apc.2007.0092. [DOI] [PubMed] [Google Scholar]
- 5.Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):384–90. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- 6.Bouhnik AD, Preau M, Vincent E, Carrieri MP, Gallais H, Lepeu G, et al. Depression and clinical progression in HIV-infected drug users treated with highly active antiretroviral therapy. Antivir Ther. 2005;10(1):53–61. [PubMed] [Google Scholar]
- 7.Cohen MH, French AL, Benning L, Kovacs A, Anastos K, Young M, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. The American journal of medicine. 2002;113(2):91–8. doi: 10.1016/s0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todd JV, Cole SR, Pence BW, Lesko CR, Bacchetti P, Cohen MH, et al. Effects of antiretroviral therapy and depressive symptoms on all-cause mortality among HIV infected women. Am J Epidemiol. 2016 doi: 10.1093/aje/kww192. [DOI] [PMC free article] [PubMed]
- 9.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21(9):1175–83. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 10.VanderWeele TJ. On the distinction between interaction and effect modification. Epidemiology (Cambridge, Mass) 2009;20(6):863–71. doi: 10.1097/EDE.0b013e3181ba333c. [DOI] [PubMed] [Google Scholar]
- 11.Westreich D, Cole SR, Young JG, Palella F, Tien PC, Kingsley L, et al. The parametric g-formula to estimate the effect of highly active antiretroviral therapy on incident AIDS or death. Stat Med. 2012;31(18):2000–9. doi: 10.1002/sim.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westreich D. From exposures to population interventions: pregnancy and response to HIV therapy. Am J Epidemiol. 2014;179(7):797–806. doi: 10.1093/aje/kwt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson TS, Robins J. Series UoW, editor. Single World Intervention Graphs (SWIGs): a unification of the counterfactual and graphical approaches to causality. Center for Statistics and the Social Sciences; 2013. [Google Scholar]
- 14.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 15.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–25. [PubMed] [Google Scholar]
- 16.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.What’s “at risk” or “heavy” drinking? [Available from: http://rethinkingdrinking.niaaa.nih.gov/How-much-is-too-much/Is-your-drinking-pattern-risky/Whats-At-Risk-Or-Heavy-Drinking.aspx.
- 18.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2013. [Google Scholar]
- 19.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appliead Psych Meas. 1977;1(3):385–401. [Google Scholar]
- 20.Thomas JL, Jones GN, Scarinci IC, Mehan DJ, Brantley PJ. The utility of the CES-D as a depression screening measure among low-income women attending primary care clinics. The Center for Epidemiologic Studies-Depression. Int J Psychiatry Med. 2001;31(1):25–40. doi: 10.2190/FUFR-PK9F-6U10-JXRK. [DOI] [PubMed] [Google Scholar]
- 21.Robins J, Hernán MA, Siebert U. Effects of multiple interventions. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks. World Health Organization; 2004. pp. 2191–230. [Google Scholar]
- 22.Young JG, Hernán MA, Robins JM. Identification, estimation and approximation of risk under interventions that depend on the natural value of treatment using observational data. Epidemiol Method. 2014;3(1):1–19. doi: 10.1515/em-2012-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robins J. A new approach to causal inference in mortality studies with sustained exposure periods - application to control of the healthy worker survivor effect. Mathe Model. 1986;7:1393–512. [Google Scholar]
- 24.Hernan MA, Robins JM. Estimating causal effects from epidemiological data. Journal of epidemiology and community health. 2006;60(7):578–86. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young JG, Cain LE, Robins JM, O’Reilly EJ, Hernán MA. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat Biosci. 2011;3(1):119–43. doi: 10.1007/s12561-011-9040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–5. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taubman SL, Robins JM, Mittleman MA, Hernan MA. Intervening on risk factors for coronary heart disease: an application of the parametric g-formula. Int J Epidemiol. 2009;38(6):1599–611. doi: 10.1093/ije/dyp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keil AP, Edwards JK, Richardson DB, Naimi AI, Cole SR. The parametric g-formula for time-to-event data: intuition and a worked example. Epidemiology. 2014;25(6):889–97. doi: 10.1097/EDE.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins J. A graphical approach to the identification and estimation of causal parameters in mortality studies with sustained exposure periods. Journal of chronic diseases. 1987;40(Suppl 2):139S–61S. doi: 10.1016/s0021-9681(87)80018-8. [DOI] [PubMed] [Google Scholar]
- 30.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. p. xvi.p. 436. [Google Scholar]
- 31.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Low-Beer S, Chan K, Yip B, Wood E, Montaner JS, O’Shaughnessy MV, et al. Depressive symptoms decline among persons on HIV protease inhibitors. J Acquir Immune Defic Syndr. 2000;23(4):295–301. doi: 10.1097/00126334-200004010-00003. [DOI] [PubMed] [Google Scholar]
- 33.VanderWeele TJ. Concerning the consistency assumption in causal inference. Epidemiology. 2009;20(6):880–3. doi: 10.1097/EDE.0b013e3181bd5638. [DOI] [PubMed] [Google Scholar]
- 34.Westreich D, Cole SR. Invited commentary: positivity in practice. Am J Epidemiol. 2010;171(6):674–7. doi: 10.1093/aje/kwp436. discussion 8–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernán MA, Cole SR. Invited Commentary: Causal diagrams and measurement bias. Am J Epidemiol. 2009;170(8):959–62. doi: 10.1093/aje/kwp293. discussion 63–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudgens MG, Halloran ME. Toward Causal Inference With Interference. J Am Stat Assoc. 2008;103(482):832–42. doi: 10.1198/016214508000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pence BW, O’Donnell JK, Gaynes BN. Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS. 2012;26(5):656–8. doi: 10.1097/QAD.0b013e3283519aae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brookhart MA, Wang PS, Solomon DH, Schneeweiss S. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology. 2006;17(3):268–75. doi: 10.1097/01.ede.0000193606.58671.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernan MA, Robins JM. Instruments for causal inference: an epidemiologist’s dream? Epidemiology. 2006;17(4):360–72. doi: 10.1097/01.ede.0000222409.00878.37. [DOI] [PubMed] [Google Scholar]
- 40.Robins J, Hernán MA. Estimation of the causal effects of time-varying exposures. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, editors. Longitudinal Data Analysis. Boca Raton, FL: Chapman & Hall/CRC; 2009. pp. 553–99. [Google Scholar]