SUMMARY
Both behavioral and neural responses to sounds are generally modified by the acoustic context in which they are encountered. As an example, in the auditory cortex, preceding sounds can powerfully suppress responses to later, spectrally similar sounds—a phenomenon called forward suppression (FWS). Whether cortical inhibitory networks shape such suppression or whether it is wholly regulated by common mechanisms such as synaptic depression or spike frequency adaptation is controversial. Here, we show that optogenetically suppressing somatostatin-positive (Sst+) interneurons weakens forward suppression, often revealing facilitation in neurons that are normally forward-suppressed. In contrast, inactivating parvalbumin-positive (Pvalb+) interneurons strengthens forward suppression and alters its frequency dependence. In a simple network model, we show that these effects can be accounted for by differences in short-term synaptic dynamics of inputs onto Pvalb+ and Sst+ interneurons. These results demonstrate separate roles for somatostatin and parvalbumin interneurons in regulating the context dependence of auditory processing.
In Brief
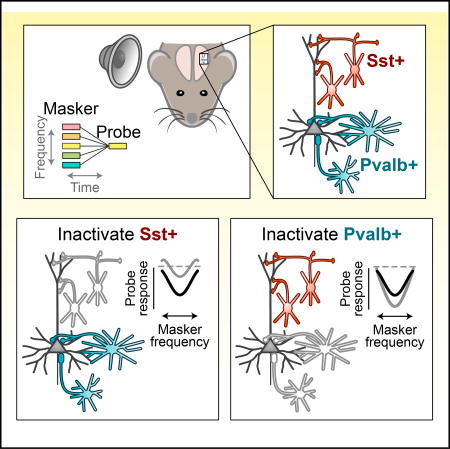
Auditory cortical responses to sounds are profoundly altered by preceding sounds. Using optogenetic inactivation and computational modeling, Phillips et al. find that specific aspects of these changes are mediated by somatostatin-positive interneurons and parvalbumin-positive interneurons, which differentially alter the strength and frequency dependence of this forward suppression.
INTRODUCTION
In multiple sensory areas, including visual (Nelson, 1991), auditory (Ulanovsky et al., 2004), somatosensory (Simons, 1985), and olfactory (Wilson, 1998) cortices, neural responses to stimuli are suppressed by similar preceding stimuli. This history-dependent suppression is considered a mechanism for ignoring redundancies, detecting changes, and matching neural responses to the statistics of the sensory scene (Barlow, 1961); however, the underlying circuitry is not well understood. Throughout the auditory system, history-dependent suppression is especially prominent. Neural responses to a sound are often completely abolished when preceded by a spectrally similar sound and more weakly suppressed when preceded by a spectrally dissimilar sound—a phenomenon called forward suppression (FWS) (Brosch and Schreiner, 1997; Calford and Semple, 1995). Although forward suppression is also present subcortically (Malone and Semple, 2001; Schreiner, 1981; Watanabe and Simada, 1971), responses in the auditory cortex (AC) tend to recover more slowly from forward suppression (Fitzpatrick et al., 1999) and are unable to follow fast repetition rates (Creutzfeldt et al., 1980; Miller et al., 2002; Yao et al., 2015), suggesting that forward suppression is enhanced within the AC. How does this enhancement occur?
Diverse populations of specialized gamma-aminobutryic acid (GABA)ergic interneurons interact to dynamically regulate neural activity. In the AC, their influence on spectral processing has been well established through direct intracellular recordings (Tan et al., 2004; Volkov and Galazjuk, 1991; Wehr and Zador, 2003) and application of GABA receptor agonists and antagonists (Kaur et al., 2004; Wang et al., 2002), but direct evidence of their contributions to temporal processing is lacking. Moreover, other neural mechanisms, such as spike frequency adaptation (Abolafia et al., 2011) and short-term synaptic depression (Bayazitov et al., 2013; Wehr and Zador, 2005) are pronounced in AC and have been hypothesized to dominate such history-dependent interactions. Assessing the role of inhibition is further complicated by the diversity of cortical interneurons, whose distinct contributions cannot be resolved by standard extracellular recording techniques or pharmacological methods and whose activity is profoundly affected by both behavioral state (Fu et al., 2014; Steriade et al., 2001) and anesthesia (Adesnik et al., 2012; Haider et al., 2013). Thus, whether intracortical inhibition plays a role in forward suppression in awake animals and how the various inhibitory cell types are involved remains unresolved.
Here, in the AC of awake mice, we use optogenetics to test whether synaptic inhibition contributes to forward suppression and whether different interneuron types support history-dependent interactions in distinct ways.
RESULTS
Recent Auditory Stimulation Affects Tone Responses in Diverse Ways
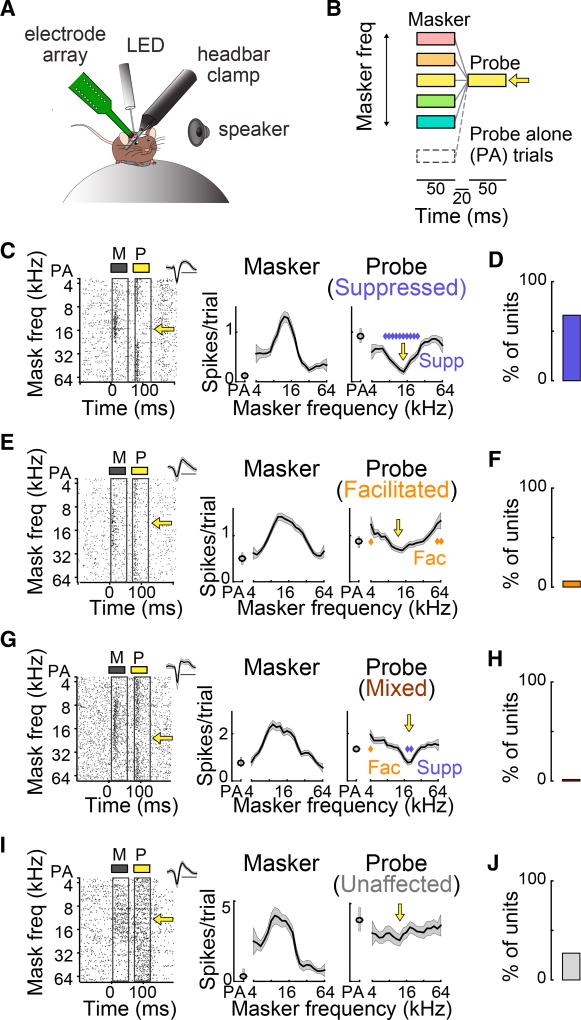
We placed mice on a free-floating spherical ball, fixed their heads in place, and used 16-channel linear probes to record single units (SUs) while the mice passively listened to sounds (Figure 1A). To measure the effects of stimulus history on auditory responses, we presented 10–50 trials of a forward suppression stimulus: a 50-ms “masker” tone (whose frequency varied) followed by a 50-ms “probe” tone (whose frequency was fixed at the unit’s preferred frequency) separated by a 20-ms gap (Figure 1B). The masker tone was omitted on random trials (probe-alone [PA] trials).
Figure 1. Prior Tones Affect Responses to Later Tones in Diverse Ways.
(A) Recordings are made in the AC of awake head-fixed mice.
(B) Forward suppression stimulus: a masker tone (varied frequency) precedes a probe tone (fixed frequency, indicated by the yellow arrow). On probe-alone trials, the masker tone is omitted.
(C) Left: Single unit raster to the masker (M, gray) and probe (P, yellow) as a function of masker frequency. Inset: spike waveform (scale bar, 1 ms). Right: responses (mean ± SEM) to the masker and probe as a function of masker frequency. Blue diamonds, significantly suppressed probe responses; yellow arrow, probe frequency.
(D) 64% of units were suppressed.
(E) As (C) for a forward-facilitated unit. Gold diamonds, facilitated probe responses.
(F) 13% of units were facilitated.
(G) As (C) for a unit with a mixed effect. Blue diamonds, suppressed responses; gold diamond, facilitated response.
(H) 3% of units showed mixed effects.
(I) As (C) for an unaffected unit.
(J) 20% of units were unaffected by the masker.
Stimulus history had a variety of effects on the response to the probe tone. Most units (43 of 67, n = 20 mice) exhibited forward suppression (i.e., their probe responses were suppressed by at least one masker tone; Figures 1C and 1D). Fewer units (9 of 67) showed forward facilitation (i.e., the probe response was enhanced by at least one masker tone; Figures 1E and 1F). An even smaller portion of units (2 of 67) showed mixed effects—both forward suppression and forward facilitation (Figures 1G and 1H). The remainder (13 of 67) were unaffected by the presence of a preceding masker stimulus (Figures 1I and 1J).
Altering Inhibition Changes the Quality of Forward Interactions
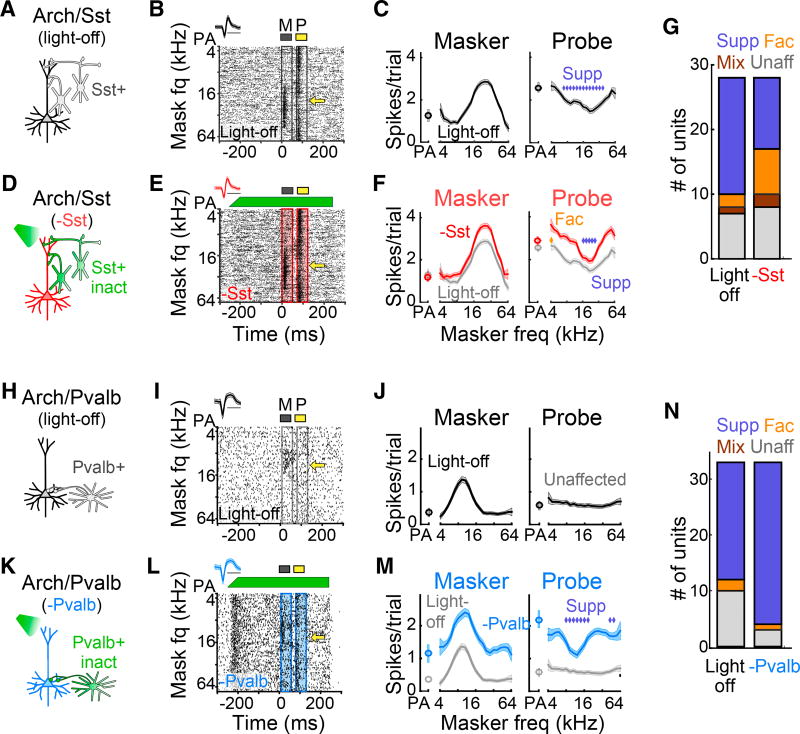
To test whether intracortical synaptic inhibition contributes to forward suppression, we used mouse crosses in which we could optogenetically inactivate either somatostatin-positive (Sst+) or parvalbumin-positive (Pvalb+) interneurons (Ai35/Sst-Cre and Ai35/PV-Cre; Experimental Procedures). On random trials, we inactivated either of the two cell types by shining green light on the surface of the AC while recording from single units. To avoid direct effects of the optogenetic inactivation in our analysis, we excluded units for which green light significantly suppressed masker-evoked firing rates (Ai35/Sst-Cre, 2 of 30; Ai35/PVCre, 4 of 37).
Inactivating Sst+ interneurons (n = 11 mice) considerably altered the influence of prior stimuli on responses to the probe tone (Figure 2). Among forward-suppressed units, two-thirds became either facilitated (4 of 18), mixed (2 of 18), or unaffected (6 of 18), whereas the other third (6 of 18) remained purely suppressed. Neurons unaffected by a masker under the light off condition became either suppressed (3 of 7), facilitated (2 of 7), or remained unaffected (2 of 7). In total, the result of inactivating Sst+ cells was a net decrease in the number of suppressed units and a net increase in the number of facilitated, mixed, and unaffected units (Figure 2G). In contrast, inactivating Pvalb+ interneurons in Ai35/Pvalb-Cre mice (n = 9) did not alter the quality of forward interactions in most forward-suppressed units; only one unit became unaffected, whereas the majority (20 of 21) remained purely suppressed. Interestingly, the majority of unaffected units (7 of 10) became forward-suppressed with inactivation of Pvalb+ cells. Overall, inactivating Pvalb+ cells resulted in a net increase in the number of suppressed units and a net decrease in the number of facilitated and unaffected units (Figure 2N). These distinct effects of Sst+ and Pvalb+ interneuron inactivation were not explained by differences in overall firing rate changes because inactivation of Sst+ and Pvalb+ interneurons produced similar changes in spontaneous (rank-sum test, p = 0.88), masker-evoked (rank-sum test, p = 0.63), and probe-evoked (rank-sum test, p = 0.23) firing rates (Figure S1). These results demonstrate that intracortical inhibition does sculpt the influence of stimulus history on neural responses and that Sst+ and Pvalb+ interneurons contribute to forward suppression in qualitatively different ways.
Figure 2. Inactivation of Sst+ versus Pvalb+ Interneurons Differentially Alters the Quality of Forward Interactions.
(A) Schematic of the circuit during light-off trials.
(B) Single unit response raster from light-off trials. Yellow arrow, probe frequency. Inset: spike waveform (scale bar, 1 ms).
(C) Responses (mean ± SEM) to the masker and probe as a function of masker frequency in light-off trials. Blue diamonds, significantly suppressed probe responses.
(D) Schematic of the circuit during light-on trials, in which Sst+ cells are inactivated with green light.
(E) As (B) for light-on trials. Green bar, light duration and power.
(F) As (C) for Sst+ inactivation trials (red) versus light-off trials (gray). Sst+ inactivation produces a mixture of suppression and facilitation. Blue diamonds, suppressed probe responses; gold diamonds, facilitated probe responses.
(G) Proportion of suppressed, facilitated, mixed, and unaffected units without (left) and with (right) inactivation of Sst+ cells.
(H–M) As (A)–(F) for a single unit in which inactivation of Pvalb+ cells (blue) reveals forward suppression. As (A)–(F): circuit schematic in light-off (H) and light-on (K) trials, single unit response raster for light-off (I) and light-on (L) trials, and masker and probe responses for light-off (J) and light-on (M) trials, for a single unit inwhich inactivation of Pvalb+ cells (blue) reveals forward suppression.
(N) As (G) for inactivation of Pvalb+ cells.
See also Figures S1 and S2.
Altering Inhibition Changes the Relative Strength and Spectral Dependence of forward Suppression
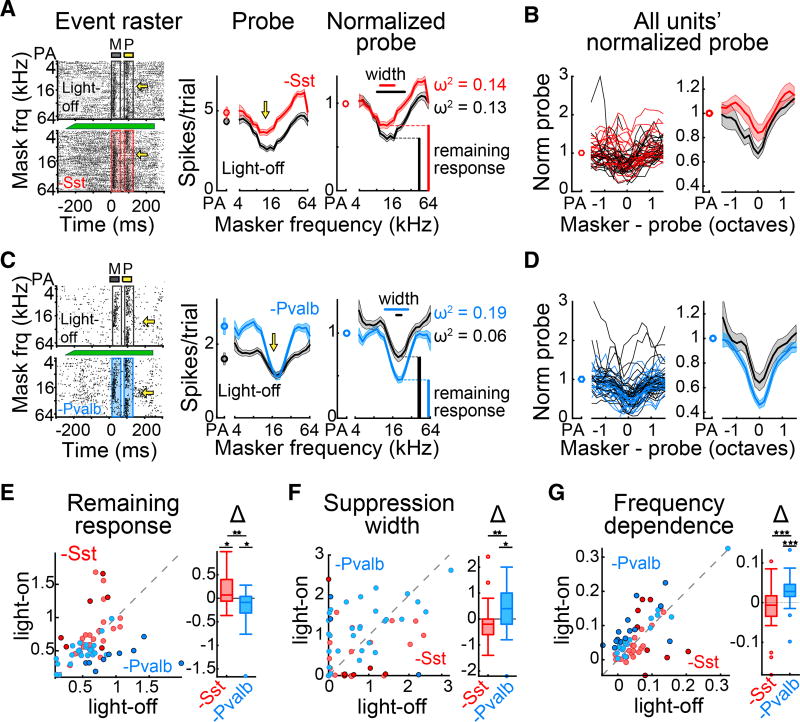
To quantitatively compare the strengths of forward suppression with and without interneuron inactivation, we separately normalized light-off and light-on suppression curves by dividing each by the light-off or light-on probe-alone response, respectively (Figures 3A and 3C). We found that inactivating Sst+ interneurons produced normalized suppression curves that were shifted upward compared with those in light-off trials (n = 28 units from 11 mice; Figure 3B). In contrast, normalized suppression curves with inactivation of Pvalb+ interneurons were shifted downward and appeared less variable (n = 33 units from 9 mice; Figure 3D).
Figure 3. Inactivation of Sst+ versus Pvalb+ Interneurons Differentially Alters the Strength and Shape of Forward Interactions.
(A) Left: example single unit responses to masker and probe stimuli without (top) and with (bottom) inactivation of Sst+ cells. Center: Responses (mean ± SEM) to the probe as a function of masker frequency without (black) and with (red) inactivation of Sst+ cells. Right: normalized probe responses without (black) and with (red) inactivation of Sst+ cells.
(B) All single units’ normalized probe responses (left) and unit-averaged probe responses (right) as a function of masker-probe distance without (black) and with (red) inactivation of Sst+ cells.
(C) As (A) for an example single unit with inactivation of Pvalb+ interneurons (blue).
(D) As (B) for all units with inactivation of Pvalb+ interneurons.
(E–G) Remaining response at probe frequency (E), suppression width (F), and frequency dependence (G) without versus with inactivation of Sst+ (red) or Pvalb+ (blue) interneurons. Darker circles represent units with significant effects. Box and whisker plots show distribution of effect sizes (*p < 0.05, **p < 0.01, ***p < 0.001).
See also Figure S3.
To quantify these effects, we measured three aspects of units’ suppression curves (Figures 3A and 3C). Remaining response at probe frequency was measured as the normalized firing rate of the probe response when the masker and probe had the same frequency. Suppression width was quantified as the range of masker frequencies, in octaves, that suppressed the probe response relative to the probe-alone response. Frequency dependence of suppression was defined as the proportion of variance in response to the probe that was explained by the frequency of the masker (ω2; Experimental Procedures).
Inactivation of Sst+ cells significantly increased remaining responses at probe frequency (signed-rank test, p = 0.037; Figure 3E) but did not change suppression widths (signed-rank test, p = 0.11; Figure 3F) or the proportion of variance explained by masker frequency (signed-rank test, p = 0.25; Figure 3G), suggesting that Sst+ inactivation weakens the strength of forward suppression but does not alter how it depends on masker frequency. On the other hand, inactivation of Pvalb+ interneurons significantly decreased remaining responses at probe frequency (signed-rank test, p = 0.016; Figure 3E), increased suppression widths (signed-rank test, p = 0.02; Figure 3F), and increased the proportion of variance explained by the masker frequency (signed-rank test, p = 8.2 × 10−5; Figure 3G), suggesting that Pvalb+ inactivation both increases the strength of forward suppression and increases its dependence on masker frequency.
These effects are not completely explained by changes in response to the masker tone or by changes in ongoing firing rate: selecting subsets of trials from the light-off and light-on conditions with identical numbers of spikes (in response to the masker or during baseline periods, respectively) did not greatly alter the effects of inactivating Sst+ or Pvalb+ interneurons on probe responses (Figure S3).
Short-Term Synaptic Facilitation and Depression Differentially Shape Forward Suppression in a Network Model
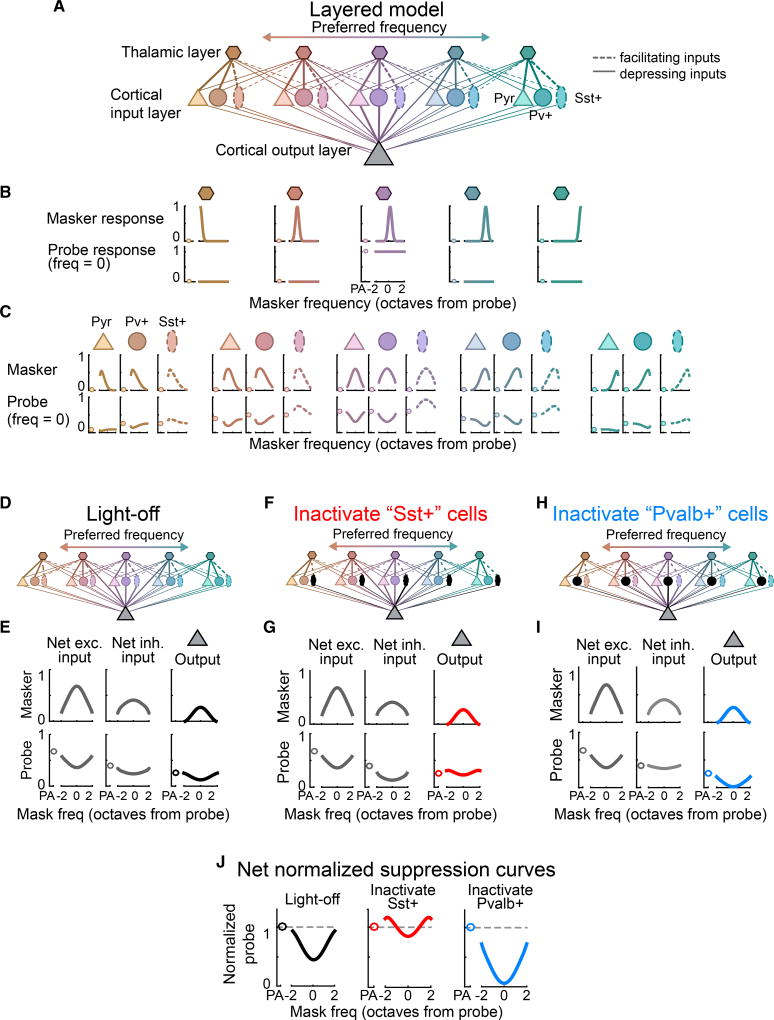
In vitro, Sst+ cells receive mostly facilitating inputs, whereas Pvalb+ cells receive mostly depressing inputs (Beierlein et al., 2003; Takesian et al., 2013; Tan et al., 2008)—a potential mechanism for their distinct effects on forward suppression. Thus, we modeled the influences of interneuron populations with either depressing or facilitating inputs on responses in a two-tone paradigm. We used a linear threshold firing rate model containing three layers of neurons (Experimental Procedures; Figure 4A). The first layer contains “thalamic” neurons that provide depressing input to both “pyramidal” neurons and to Pvalb+ interneurons (Figure 4A, solid lines) and facilitating input to Sst+ interneurons (Figure 4A, dotted lines). Thus, responses of the pyramidal and Pvalb+ neurons to the probe tone are forward-suppressed, but responses to Sst+ cells are forwardfacilitated (Figure 4C, bottom row). All cells in the thalamic and cortical input layers then provide depressing input to the cortical output neuron (Figure 4D) so that the net excitatory and net inhibitory inputs to the cortical output neuron show forward suppression (Figure 4E), resulting in forward suppression of the cortical output neuron’s normalized suppression curve (Figure 4J).
Figure 4. Short-Term Synaptic Dynamics Produce Diverse Effects on Forward Suppression.
(A) Schematic of the layered linear threshold model. Solid lines, depressing inputs; dotted lines, facilitating inputs (only to Sst+ cells).
(B) Top row: responses of each thalamic neuron to masker tones as a function of frequency (units, octaves from the probe frequency). Bottom row: responses of each thalamic neuron to probe-alone (circles) and masked-probe trials as a function of masker frequency (lines).
(C) As (B) for neurons in the second layer, which receive either depressing inputs (Pyr and Pvalb+) or facilitating inputs (Sst+).
(D) Schematic of a network with both Sst+ and Pvalb+ interneurons (no inactivation).
(E) Net excitatory input (left), net inhibitory input (center), and output (right) of the cortical output neuron without inactivation of interneurons.
(F and G) As (D) and (E) with inactivation of Sst+ interneurons (inhibition from depressing Pvalb+ cells remains).
(H and I) As (F) and (G) with inactivation of Pvalb+ interneurons (inhibition from facilitating Sst+ cells remains).
(J) Normalized suppression curves for the output neuron without inactivation, with inactivation of Sst+ interneurons, and with inactivation of Pvalb+ interneurons.
To model the effects of inactivating either interneuron population, we removed either facilitating Sst+ interneurons (Figure 4F) or depressing Pvalb+ interneurons (Figure 4H) from the network. Removing Sst+ interneurons produced forward suppression in the net inhibitory input to the cortical output neuron and resulted in inhibition that was greatest at the edges of the suppression curve (Figure 4G, center). This reduced the probe-alone response relative to the rest of the suppression curve (Figure 4G, right), resulting in overall weakened forward suppression and facilitation of edge probe responses in the cortical output neuron (Figure 4J, center). These results mirrored the observed effects of inactivating Sst+ neurons (Figures 2 and 3). On the other hand, removing Pvalb+ cells produced relatively little forward suppression in the net inhibitory drive to the cortical output neuron, resulting in inhibition that was strong and flat across tone frequency (Figure 4I, center). This flat inhibition strengthened forward suppression evenly across masker frequency, resulting in strong forward suppression that retained its dependence on masker frequency (Figure 4J, right), similar to the observed effects of inactivating Pvalb+ cells.
DISCUSSION
In the AC of awake mice, we find that forward suppression is shaped by synaptic inhibition: reducing inhibition from Sst+ interneurons weakens the strength of forward suppression, whereas reducing inhibition from Pvalb+ interneurons strengthens forward suppression and enhances its dependence on tone frequency. Using a model, we show that these differences arise naturally from the differences in synaptic dynamics of the inputs onto Pvalb+ versus Sst+ cells.
This role for inhibition has not been observed before. For example, Wehr and Zador (2005) showed that inhibitory conductances in AC do not last longer than 100 ms, suggesting that inhibition cannot explain the long-lasting neural and behavioral effects of forward suppression. We instead propose that short-term synaptic plasticity at interneurons’ inputs, rather than lingering inhibition from the masker tone, mediates the strength and frequency dependence of forward suppression. A more recent study (Yao et al., 2015) infused GABA receptor antagonists into the AC and found no attenuation of forward suppression. However, such antagonists act upon all sources of synaptic inhibition and may conflate the distinct effects of interneuron types observed in the current study. However, optogenetic manipulations have their own caveats, including counterintuitive network effects (Phillips and Hasenstaub, 2016; Seybold et al., 2015) and complex interactions among interneuron networks (Pfeffer et al., 2013). Thus, interneuron inactivation, especially when prolonged, likely results in both indirect and direct effects of inhibition.
How can inactivation of Sst+ and Pvalb+ interneurons produce different effects on forward suppression? One possibility is that manipulating inhibition has an indirect effect on forward suppression by changing ongoing pyramidal cell activity (e.g., through synaptic depression or spike frequency adaptation). Counter to this possibility, Sst+ and Pvalb+ inactivation similarly increased local network activity (Figure S1) but differentially altered forward suppression. Another potential mechanism, modeled here, is that Sst+ and Pvalb+ interneurons are recruited by feedforward inputs with different dynamics. In both somatosensory and auditory cortical slices, inputs onto Sst+ interneurons facilitate and inputs onto Pvalb+ cells depress when stimulated at rates similar to the onset asynchrony of the tones presented in the current study (10–60 Hz; Beierlein et al., 2003; Takesian et al., 2013; Tan et al., 2008). Thus, Sst+ cells may be robustly and persistently activated during repeated sensory stimulation, allowing them to directly suppress their targets across multiple stimulations. Therefore, when their activity is suppressed, forward suppression weakens. Pvalb+ interneurons, on the other hand, may be activated only transiently at the onset of sensory stimulation, which may limit their ability to suppress their targets across repeated stimulations. Thus, Pvalb+ interneurons may only be available to suppress responses to successive stimuli when weakly activated by the first stimulus (for instance, when the masker stimulus lies near the edges of their receptive fields), and removing this inhibition may influence the stimulus specificity of forward suppression. Differential effects of Pvalb+ and Sst+ interneurons on the frequency dependence of forward suppression may also reflect differences in the spectral breadth of their inputs (i.e., whether they pool input from the local network) or in the extent of their connectivity onto target cells.
Together, these results offer potential mechanisms by which inhibition from distinct interneurons may differentially influence temporal processing and the behaviors it supports. For example, decreased forward suppression may be important for detecting gaps in noise—an important component of interpreting speech in noise—because it has recently been shown that increasing cortical responses after brief gaps (which would effectively reduce forward suppression) increases the ability to detect gaps (Weible et al., 2014).
EXPERIMENTAL PROCEDURES
Protocols
All experiments were performed in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Francisco.
Animals
Adult mice (male or female) were housed under a 12 hr/12 hr light/dark cycle and used for experiments between the ages of 6 and 12weeks. Food and waterwere provided ad libitum. To target either Sst+ or Pvalb+ cells for optogenetic manipulation, we crossed Sst-IRES-Cre or Pvalb-IRES-Cre knockin lines to the Cre-dependent archaerhodopsin line Ai35 (Supplemental Experimental Procedures).
Surgeries
Mice were anesthetized with 3% isoflurane, maintained with 1.25%–2% isoflurane, and given pre- and post-operative multimodal analgesia. A custom metal head bar was fixed to the right temporal skull with dental cement. Silicone elastomer was placed over the exposed bone. After 2–5 days of recovery, a craniotomy was drilled above the right auditory cortex, new silicone elastomer was placed over the exposed brain, and mice recovered for 1–3 hr before recordings.
Data Acquisition and Stimuli
During recordings, mice were head-fixed above an air-floated spherical treadmill (Niell and Stryker, 2010; Supplemental Experimental Procedures). The sound pressure from the treadmill was maintained at or below 45 dB and had spectral power mainly at frequencies below 4 kHz. Sounds were presented through a free-field speaker (ES1, Tucker-Davis) directed toward the mouse’s left ear. All sound envelopes were applied with 2-ms linear ramps. Best frequencies (BFs) were determined using 50-ms tones. The BF of the recording site at 10–15 dB above threshold was used as the frequency of the probe tone for subsequent forward suppression experiments. Only units for which the probe frequency was within 0.5 octaves of the BF were analyzed.
On randomly interleaved trials, green light was shined directly above the surface of the auditory cortex through a 400-micron fiber. Light turned on 250 ms before sound onset, and the power linearly ramped upward for 50 ms before reaching maximum (10–15 mW). After sound offset, the light remained on for 120 ms.
Single-Unit Tuning and Suppression Curves
Neural events were thresholded and sorted using custom software. For each single unit (n = 81), we constructed 1-ms binned peri-stimulus time histograms (PSTHs) by collapsing events across masker frequency for light-off trials. Single units were defined as tone-responsive when the activity during the masker tone (50 ms) was significantly (α = 0.01, rank-sum test) greater than in the 50 ms before masker onset. For all tone-responsive single units (n = 77), we defined the masker response latency as the time at which masker-evoked activity crossed 3 SDs above spontaneous activity. Frequency tuning curves were calculated as the average firing rate as a function of masker frequency during the 50-ms period after masker response latency. Single units were defined as tuned when the tuning curve was significantly (α= 0.05, Kruskal-Wallis test) modulated by frequency under the light-off condition.
For all tuned units (n = 67), we then generated suppression curves separately for light-on and light-off conditions as the average firing rate as a function of masker frequency during the 50-ms period after probe response onset (taken to be the same as masker response onset). Normalized suppression curves were generated by dividing light-on and light-off suppression curves by the probe-alone response under the light-on and light-off conditions, respectively.
Measuring Forward Suppression
Suppression curves were classified separately for light-off and light-on conditions. Units were suppressed when at least one of the masker stimuli significantly (α = 0.01, rank-sum test) suppressed the probe response relative to the probe-alone response, facilitated when at least one masker stimulus significantly (α = 0.01, rank-sum test) increased the probe response relative to the probe-alone response, mixed when they exhibited both suppression and facilitation, or unaffected when masker stimuli had no effect on the response to the probe. Frequency dependence (ω2) was calculated by using a one-way ANOVA between the probe response and masker frequency to compute the sum of squares between masker frequencies (SSfreq), the total sum of squares (SStotal), the mean square error (MSerror), and the degrees of freedom (df). ω2 was then defined as follows:
Multilayered Model
We built a three-layered linear threshold model as in Phillips et al. (2017) (Supplemental Experimental Procedures). The first layer contains thalamic neurons, which respond to tones of varying frequency in a Gaussian fashion. Thalamic neurons synapse onto three different types of cells in the cortical (second) layer: pyramidal cells, Sst+ cells, and Pvalb+ cells, through Gaussian connectivity functions. Synapses onto pyramidal cells and Pvalb+ cells are modeled as depressing, whereas the synapses onto Sst+ cells are modeled as facilitating. Thalamic neurons in the first layer and cortical cells in the second layer all synapse onto a “cortical output” cell in the third layer through separately weighted Gaussian connectivity functions.
Supplementary Material
Highlights.
Cortical inhibition contributes to forward suppression in the auditory cortex
Inactivation of Sst+ cells attenuates the strength of forward suppression
Inactivation of Pvalb+ cells alters the spectral dependence of forward suppression
In a model, short-term synaptic plasticity accounts for these differences
Acknowledgments
The research was supported by NIH Grant RO1 DC014101 (to A.R.H.) and DC02260 (to C.E.S.), Hearing Research Inc. (San Francisco), the Klingenstein Foundation, the J.C. and E. Coleman Memorial Fund, and NSF PHY-1125915.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2017.07.001.
AUTHOR CONTRIBUTIONS
Conceptualization and Writing – Review & Editing, A.R.H., C.E.S., and E.A.K.P. Data Curation, Formal Analysis, Methodology, Software, Validation, and Visualization: A.R.H. and E.A.K.P. Investigation and Writing – Original Draft Preparation, E.A.K.P. Funding Acquisition, Project Administration, and Supervision, A.R.H.
References
- Abolafia JM, Vergara R, Arnold MM, Reig R, Sanchez-Vives MV. Cortical auditory adaptation in the awake rat and the role of potassium currents. Cereb. Cortex. 2011;21:977–990. doi: 10.1093/cercor/bhq163. [DOI] [PubMed] [Google Scholar]
- Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB. Possible Principles Underlying the Transformation of Sensory Messages. In: Rosenblith WA, editor. Sensory Communication. Cambridge, MA: MIT Press; 1961. pp. 217–234. [Google Scholar]
- Bayazitov IT, Westmoreland JJ, Zakharenko SS. Forward suppression in the auditory cortex is caused by the Ca(v)3.1 calcium channel-mediated switch from bursting to tonic firing at thalamocortical projections. J. Neurosci. 2013;33:18940–18950. doi: 10.1523/JNEUROSCI.3335-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Brosch M, Schreiner CE. Time course of forward masking tuning curves in cat primary auditory cortex. J. Neurophysiol. 1997;77:923–943. doi: 10.1152/jn.1997.77.2.923. [DOI] [PubMed] [Google Scholar]
- Calford MB, Semple MN. Monaural inhibition in cat auditory cortex. J. Neurophysiol. 1995;73:1876–1891. doi: 10.1152/jn.1995.73.5.1876. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O, Hellweg FC, Schreiner C. Thalamocortical transformation of responses to complex auditory stimuli. Exp. Brain Res. 1980;39:87–104. doi: 10.1007/BF00237072. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kuwada S, Kim DO, Parham K, Batra R. Responses of neurons to click-pairs as simulated echoes: auditory nerve to auditory cortex. J. Acoust. Soc. Am. 1999;106:3460–3472. doi: 10.1121/1.428199. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Häusser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J. Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- Malone BJ, Semple MN. Effects of auditory stimulus context on the representation of frequency in the gerbil inferior colliculus. J. Neurophysiol. 2001;86:1113–1130. doi: 10.1152/jn.2001.86.3.1113. [DOI] [PubMed] [Google Scholar]
- Miller LM, Escabí MA, Read HL, Schreiner CE. Spectrotemporal receptive fields in the lemniscal auditory thalamus and cortex. J. Neurophysiol. 2002;87:516–527. doi: 10.1152/jn.00395.2001. [DOI] [PubMed] [Google Scholar]
- Nelson SB. Temporal interactions in the cat visual system. I. Orientation-selective suppression in the visual cortex. J. Neurosci. 1991;11:344–356. doi: 10.1523/JNEUROSCI.11-02-00344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips EA, Hasenstaub AR. Asymmetric effects of activating and inactivating cortical interneurons. eLife. 2016;5 doi: 10.7554/eLife.18383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips EAK, Schreiner CE, Hasenstaub AR. Diverse effects of stimulus history in waking mouse auditory cortex. J. Neurophysiol. 2017 doi: 10.1152/jn.00094.2017. Published online May 31, 2017. http://dx.doi.org/10.1152/jn.00094.2017. [DOI] [PMC free article] [PubMed]
- Schreiner C. Post-stimulatory effects in the medial geniculate body of guinea pigs. In: Syka J, Aitkin L, editors. Neuronal Mechanisms of Hearing. New York: Plenum Press; 1981. pp. 191–196. [Google Scholar]
- Seybold BA, Phillips EA, Schreiner CE, Hasenstaub AR. Inhibitory Actions Unified by Network Integration. Neuron. 2015;87:1181–1192. doi: 10.1016/j.neuron.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ. Temporal and spatial integration in the rat SI vibrissa cortex. J. Neurophysiol. 1985;54:615–635. doi: 10.1152/jn.1985.54.3.615. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J. Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sharma N, Sanes DH. Hearing loss differentially affects thalamic drive to two cortical interneuron subtypes. J. Neurophysiol. 2013;110:999–1008. doi: 10.1152/jn.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AYY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J. Neurophysiol. 2004;92:630–643. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- Tan Z, Hu H, Huang ZJ, Agmon A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc. Natl. Acad. Sci. USA. 2008;105:2187–2192. doi: 10.1073/pnas.0710628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Farkas D, Nelken I. Multiple time scales of adaptation in auditory cortex neurons. J. Neurosci. 2004;24:10440–10453. doi: 10.1523/JNEUROSCI.1905-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov IO, Galazjuk AV. Formation of spike response to sound tones in cat auditory cortex neurons: interaction of excitatory and inhibitory effects. Neuroscience. 1991;43:307–321. doi: 10.1016/0306-4522(91)90295-y. [DOI] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Simada J. Auditory temporal masking: an electrophysiological study of single neurons in the cat’s cochlear nucleus and inferior colliculus. Jpn. J. Physiol. 1971;21:537–549. doi: 10.2170/jjphysiol.21.537. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Weible AP, Moore AK, Liu C, DeBlander L, Wu H, Kentros C, Wehr M. Perceptual gap detection is mediated by gap termination responses in auditory cortex. Curr. Biol. 2014;24:1447–1455. doi: 10.1016/j.cub.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. J. Neurophysiol. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Yao JD, Bremen P, Middlebrooks JC. Emergence of Spatial Stream Segregation in the Ascending Auditory Pathway. J. Neurosci. 2015;35:16199–16212. doi: 10.1523/JNEUROSCI.3116-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.