Abstract
Lung cancer is the most common solid tumor and the leading cause of cancer-related mortality worldwide. miR-21 is one of the most commonly observed aberrant miRNAs in human cancers. However, the biological roles of miR-21 in glucose metabolism of non-small cell lung cancer (NSCLC) cells remain unknown. In the present study, our findings demonstrated that miR-21 promoted glucose uptake and increased TXNIP expression. miR-21 increased lactate generation and decreased oxygen consumption in NSCLC cells. Moreover, we found that miR-21 promoted glycolysis and decreased OXPHOS. Mechanistically, fructose-1,6-biphosphatase (FBP1) was a direct target of miR-21 and observed a negative correlation between miR-21 and FBP1 in NSCLC samples. Restoring FBP1 expression reversed the effects induced by miR-21 overexpression in NSCLC cells. Together, our findings suggest the critical role of miR-21 in glucose metabolism through suppression of FBP1 in NSCLC cells. miR-21 may be a potential target of NSCLC treatment.
Keywords: miR-21, glucose, metabolism, FBP1
Introduction
Lung cancer is the most common solid tumor and the leading cause of cancer-related mortality worldwide, accounting for more than 1.4 million deaths per year [1,2]. Non-small cell lung cancer (NSCLC), including squamous cell carcinoma, adenocarcinoma, adenosquamous cell carcinoma and large cell carcinoma, accounts for approximately 80% cases [3]. Despite the recent advancements of the early detection of the disease and early diagnosis and treatment, the prognosis of patients with NSCLC remains unsatisfied. High mortality is mainly due to tumor heterogeneity, metastasis and the development of resistance to therapies [4,5]. Thus, the identification of novel treatment strategies is critical and essential for lung cancer management. Moreover, there is an urgent need to find accurate biomarkers which could detect lung cancer with high sensitivity and specificity in the early state of disease.
With great progressions of genome and transcriptome sequencing technologies, many novel non-protein-coding transcripts were identified. About 80% of the genome is transcribed into transcripts, but only 2% of the genome codes for protein [6]. Among these numerous non-protein-coding transcripts, MicroRNAs (miRNAs) are a recently discovered class of non-coding small RNA molecules approximately 22 nucleotides in length that play important roles in cell differentiation, proliferation, apoptosis, and metabolism by inhibiting target genes [7,8]. Each miRNA has the ability to target multiple genes within a pathway makes miRNAs one of the most abundant classes of regulatory genes in humans, regulating up to 30% of human protein coding genes [9]. miR-21 is one of the most commonly observed aberrant miRNAs in human cancers and is one of the first miRNAs to be described as an oncomir. The miR-21 is up-regulated in multiple solid tumors, including lung cancer, breast cancer, and pancreatic cancer, and is overexpressed in most cancer tissues, suggesting a close relationship between miR-21 expression and tumor development [10]. miR-21 posttranscriptionally downregulates the expression of the tumor suppressor PTEN and subsequently, stimulates growth and invasion in NSCLC [11]. Moreover, inhibition of miR-21 expression reduces proliferation, migration, and invasion of A549 cells by upregulating the expression of the programmed cell death protein 4 (PDCD4) [12].
Glucose homeostasis is reciprocally controlled by the catabolic glycolysis/oxidative phosphorylation (OXPHOS) and the anabolic gluconeogenesis pathway. Otto Warburg noticed that some tumor cells preferentially metabolized glucose to lactate in the presence of ample oxygen, a process called aerobic glycolysis [13]. Activation of several oncogenes contributes to the Warburg effect in tumor cells, such as AKT1 and Myc. Although metabolism plays a fundamental role in essentially every function of a cell, little is known about the role of miR-21 in cell’s glucose metabolism during NSCLC development. In the present study, we performed the gain- and loss-of-function experiments to investigate the biological roles of miR-21 in glucose metabolism. The molecular mechanisms by which miR-21 exerts its biological roles were also explored.
Materials and methods
Cell culture and tissue specimens
NSCLC and adjacent normal tissue samples were obtained with informed consent under institutional review board-approved protocols. The samples were collected between July 2010 and June 2017 at the Department of Critical Care Medicine of Cangzhou central hospital, Heibei province China. The NSCLC cases selected were based on a clear pathological diagnosis, follow-up data, and had not received previous local or systemic treatment. This study was approved by the institute research ethics committee of Cangzhou central hospital. A549 and 95D cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS).
RNA isolation and quantitative real-time PCR (qPCR)
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, California, USA). cDNA was synthesised with the PrimeScript RT reagent Kit (Promega, Madison, WI, USA). Real-time PCR was carried out using an ABI StepOne Plus real-time PCR system (Applied Biosystems, Foster City, California, USA).
Lentivirus production and transduction
Virus particles were harvested 48 h after pCDH-CMV-miR-21 transfection with the packaging plasmid pRSV/pREV, pCMV/pVSVG and pMDLG/pRRE into 293FT cells using Lipofectamine 2000 reagent (Invitrogen). A549 cells were infected with recombinant lentivirus-transducing units plus 8 mg/ml Polybrene (Sigma, St Louis, Missouri, USA).
Oligonucleotide transfection
MiR-21 inhibitor was synthesized by Genepharma (Shanghai, China). Oligonucleotide transfection was performed with Lipofectamine 3000 reagent (Invitrogen).
Luciferase reporter assay
The putative miR-21 binding site at the 3’-UTR of fructose-1,6-biphosphatase (FBP1) mRNAs was cloned downstream of the cytomegalovirus (CMV) promoter in a pMIR-REPORT vector (Ambion, Carlsbad, CA, USA). Two mutant constructs were generated by either deletion or mutation.
The firefly luciferase construct was cotransfected with a control Renilla luciferase vector into A549 cells in the presence of either miR-21 or miR-control. A dual luciferase assay (Promega) was performed 48 h after transfection. The experiments were performed independently in triplicate.
Western blot
Cells were lysed by RIPA buffer (Beyotime) supplemented with protease inhibitors (Roche). 30 μg protein samples were separated on a SDS-PAGE, and transfered into PVDF membranes (Millipore), blocked with 5% BSA for 1 hour at room temperature, and immunoblotted with primary antibodies (GAPDH antibody, Rabbit, Santa Cruz; TXNIP antibody, Rabbit, Santa Cruz; FBP1 antibody, Rabbit, Abcam) overnight at 4°C. After the incubation with the corresponding secondary antibodies conjugated to horseradish peroxidase for 1 hour, the signals of the membranes were detected by ECL Substrate (Millipore).
Metabolic assays
Glucose uptake and lactate production were measured by assay kits from BioVision (San Francisco, CA). Oxygen consumption was determined using the Seahorse Extracellular Flux (XF-96) analyzer (Seahorse Bioscience, Chicopee, MA). To allow comparison between experiments, data are presented as OCR in pMol/min/104 cells. Basal OCR were measured four times and plotted as a function of cells with and without treatment under the basal condition followed by the sequential addition of oligomycin (1 mg/ml) and FCCP (1 mM) as indicated. The progress curve is annotated to show the relative contribution of basal, ATP-linked and maximal oxygen consumption after the addition of FCCP, and the reserve capacity of the cells.
For the [U-13C6]-glucose tracer experiment, Vector- and anti-miR-21-expressing 95D cells were cultured in the DMEM medium with glucose replaced by 0.1% 13C6-glucose for 24 hr. Polar metabolites were extracted from cells and media using the acetonitrile/water/chloroform partitioning and 10% trichloracetic acid methods, respectively. The extracts were subjected to 1D 1H and 1H(13C) HSQC NMR and GC-MS analysis, as previously described [14].
Statistical analysis
Experiments were repeated three times. Results are expressed as mean ± SD as indicated. An independent Student’s t test was performed to analyze the assay results. A p value < 0.05 was considered statistically significant.
Results
miR-21 promotes glucose uptake in NSCLC cells
To reveal the effect of miR-21 on glucose metabolism in NSCLC, A549 cells were infected with miR-21 (Figure 1A), and 95D cells were transfected with anti-miR-21 (Figure 1B). We first measured glucose uptake and found that miR-21 overexpression significantly increased glucose uptake in A549 cells (Figure 1C), whereas knockdown of miR-21 suppressed glucose uptake in 95D cells (Figure 1D). Thioredoxin-interacting protein (TXNIP) is commonly used as an intracellular glucose sensor. We thus examined TXNIP induction by depleting glucose for 12 hr, followed by glucose stimulation for additional 3 hr. TXNIP was robustly induced in NSCLC cells. TXNIP was markedly stimulated by miR-21 overexpression A549 cells (Figure 1E), while knockdown of miR-21 suppressed TXNIP expression in 95D cells (Figure 1F). These results indicate that miR-21 is critical in promoting glucose uptake.
Figure 1.
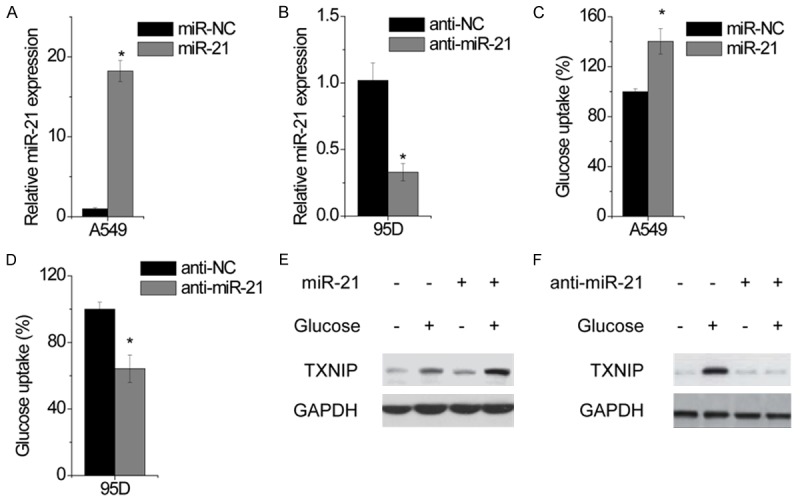
miR-21 promotes glucose uptake in NSCLC Cells. A549 cells were transfected with miR-negative control (miR-NC) or miR-21 for 24 hr. The relative expression of miR-21 was determined by qPCR. 95D cells were transfected with anti-negative control (anti-NC) or anti-miR-21 for 24 hr. The relative expression of miR-21 was detected by qPCR. Glucose uptake was measured in control and miR-21-overexpressing A549 cells. Glucose uptake was measured in control and miR-21-knockdown 95D cells. Cells were deprived for glucose for 12 hr followed by glucose stimulation for additional 3 hr. TXNIP expression was examined in control and miR-21-overexpressing A549 cells by western blotting. Cells were deprived for glucose for 12 hr followed by glucose stimulation for additional 3 hr. TXNIP expression was examined in control and miR-21-knockdown 95D cells by western blotting. Data are shown as mean ± SD in three separate experiments in triplicate. *p < 0.05.
miR-21 increases lactate generation and decreases oxygen consumption in NSCLC cells
To examine whether miR-21 changes glucose metabolism from glycolysis/oxidative phosphorylation (OXPHOS) to aerobic glycolysis, we measured lactate production and found that miR-21-overexpressed A549 cells produced more lactate than control cells (Figure 2A), whereas miR-21-knockdown 95D cells had less lactate production (Figure 2B). We then investigated the effect of miR-21 on cell growth under different oxygen conditions. At normoxic condition (21% oxygen), miR-21 overexpression induced a minor promotion in cell growth in A549 cells (Figure 2C). Similarly, miR-21 overexpression induced a minor suppression in cell growth in 94D cells (Figure 2D). However, under hypoxic condition (0.1% oxygen), miR-21 overexpression induced a drastic growth promotion in A549 cells (Figure 2C), whereas miR-21 inhibition significantly enhanced hypoxia-mediated growth inhibition in 95D cells (Figure 2D), suggesting that the effect of miR-21 on cell growth depends on oxygen. We thus examined oxygen consumption rate (OCR). We found that the basal OCR significantly decreased in miR-21 overexpressed A549 cells (Figure 2E), whereas miR-21-silencing 95D cells displayed an increased in basal OCR (Figure 2F). Similar results were obtained in the analysis of ATP-linked and maximal OCR (Figure 2E and 2F).
Figure 2.
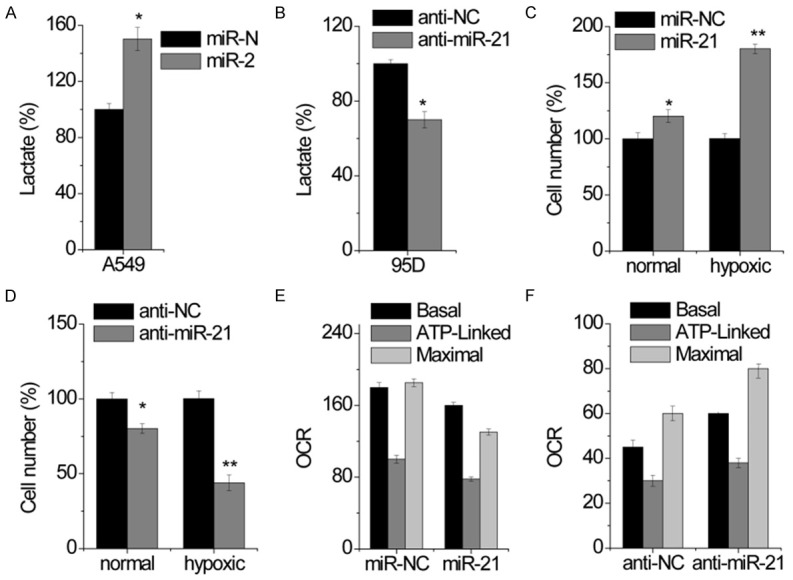
miR-21 increases lactate generation and decreases oxygen consumption in NSCLC cells. A. Lactate excretion was measured in control and miR-21-overexpressing A549 cells. B. Lactate excretion was measured in control and miR-21-knockdown 95D cells. C. Cell growth under normal and hypoxic condition was measured by cell-count assay for 2 days. Data are presented as a percentage of miR-NC control values for A549 cells. D. Cell growth under normal and hypoxic condition was measured by cell-count assay for 2 days. Data are presented as a percentage of anti-NC control values for 95D cells. E. Oxygen consumption was measured in control and miR-21-overexpressing A549 cells. F. Oxygen consumption was measured in control and miR-21-knockdown 95D cells. Data are shown as mean ± SD in three separate experiments in triplicate. *p < 0.05, **p < 0.01.
miR-21 promotes glycolysis and decreases OXPHOS
We further investigated the metabolic fate of [U-13C]-glucose in control and miR-21 knockdown 95D cells by using stable isotope-resolved metabolomics (SIRM). Nuclear magnetic resonance (NMR) analysis of the culture media demonstrated a reduced 13C6-glucose uptake in miR-21 silenced cells (Figure 3A). NMR analysis of 13C-labeled metabolites in cell extracts also indicated a reduced 13C3-lactate in miR-21-silenced cells (Figure 3B), consisting of reduced 13C3-lactate excretion into the media. Interestingly, miR-21 inhibition also reduced 13C abundance in the ribosyl unit of ribonucleotides and derivatives, such as adenine nucleotides (AXP), uracil nucleotides (UXP), NAD+ and UDP-glucose (UDPG) (Figure 3B), suggesting that the pentose phosphate pathway (PPP), which is responsible for generating ribose-5-phosphate for the synthesis of ribonucleotides and NADPH production, is inhibited. The decreased level of 13C-UDPG in miR-21-silenced 95D cells suggests that the glycosylation process is reduced. The same cell extracts were further analyzed by GC-MS to quantify 13C-metabolites involved in glycolysis and the TCA cycle (Figure 3C). We found that the production of the m3 or 13C3-isotopologues of glycerol-3-phosphate (G3P) and serine were significantly reduced in miR-21-silenced cells, which is again consistent with attenuated glycolytic activity. In line with the observations that miR-21 inhibition increased OXPHOS, the production of 13C2 or m2-succinate, fumarate, and malate (markers of the first turn of the TCA cycle) as well as the 13C4- or m4-citrate (markers of the second turn of the TCA cycle) were increased in miR-21-silenced 95D cells (Figure 3C). Together, these results indicate that miR-21 promotes glycolytic flux, reduces biosynthesis and enhances OXPHOS.
Figure 3.
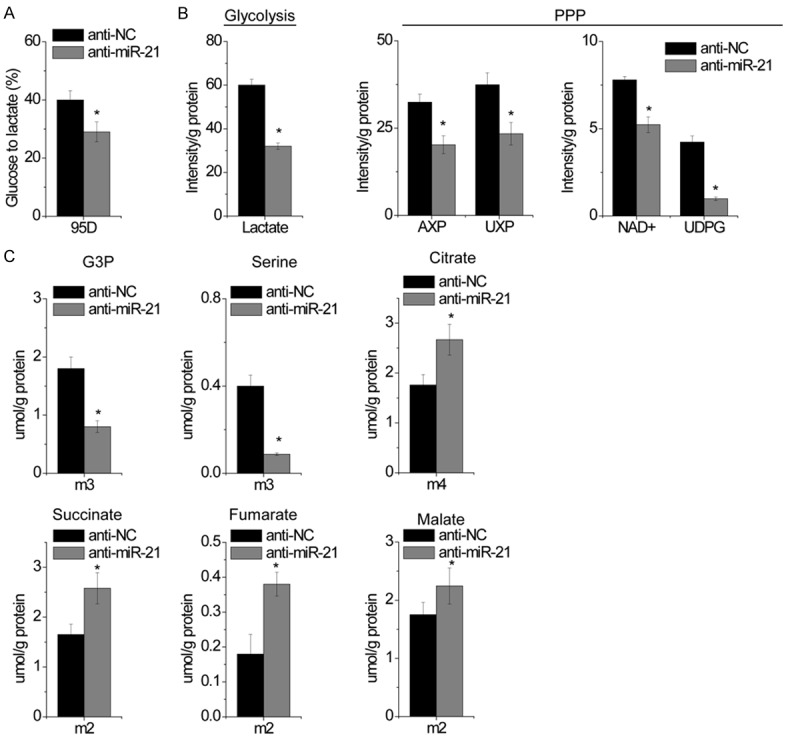
miR-21 promotes glycolysis and decreases OXPHOS. A. The conversion of 13C6-Glucose to 13C3-lactate were measured by 1D 1H NMR analysis of the media of anti-NC and miR-21-knockdown 95D cells grown in 13C6 Glucose. B. A pair of representative 1D 1H(13C) HSQC NMR spectra show the changes in 13C abundance (represented by the intensity of 13C-attached 1H peaks) of various assigned metabolites elicited by miR-21 knockdown in 95D cells. The relative 13C abundance of indicated metabolites from cell extracts was quantified from their HSQC peak intensity. AXP, adenine nucleotides; UXP, uracil nucleotides; UDPG, UDP-glucose. C. The doubly 13C labeled TCA cycle metabolites are derived from the first turn of the TCA cycle while the quadruply 13C labeled citrate is produced from the second turn of the cycle. The levels of several indicated 13C isotopologues of glycolytic and TCA cycle metabolites were obtained from the GC-MS analysis of the same cell extracts as in B. Data are shown as mean ± SD in three separate experiments in triplicate. *p < 0.05.
FBP1 is a direct target of miR-21
To clarify the molecular mechanisms of miR-21 on glucose metabolism of NSCLC cells, we searched for the target mRNAs using TargetScan. Fructose-1,6-biphosphatase (FBP1) was selected as one of the candidate targets of miR-21, based on putative target sequences at 187-194 bp of FBP1 3’UTR (Figure 4A). To further confirm targeting of FBP1 by miR-21, luciferase activity assay was performed. Through the luciferase activity assay, we found that A549 cells transfected with miR-21 significantly inhibited wild-type FBP1 3’UTR reporter activity (Figure 4B), while had no inhibitory effect on the mutant-type FBP1 3’UTR reporter activity (Figure 4B). In addition, the mRNA and protein levels of FBP1 were detected in NSCLC cells transfected miR-21 or miR-NC. Our results showed that miR-21 overexpression significantly attenuated the mRNA and protein levels of FBP1 in A549 cells (Figure 4C and 4D). On the contrary, inhibition of miR-21 increased both FBP1 mRNA and protein expression (Figure 4E and 4F). Above results implied that miR-21 suppressed FBP1 expression by binding to 3’UTR of FBP1 mRNA.
Figure 4.
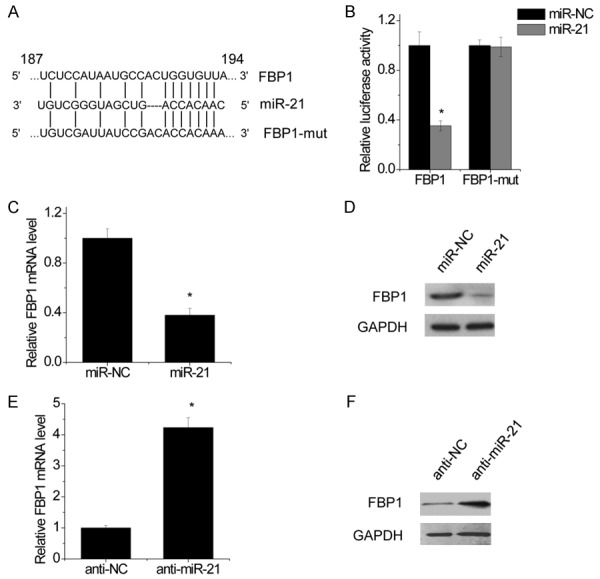
FBP1 is a direct target of miR-21. Schematic illustration of the predicted miR-21-binding sites in FBP1 3’UTR. FBP1 was a target of miR-21. MiR reporter constructs, containing a wild-type and mutant FBP1 3’UTR, were co-transfected into A549 cells which were transfected by miR-NC or miR-21. Data are presented as the relative ratio of firefly luciferase activity to renilla luciferase activity. FBP1 mRNA level after miR-21-induced expression in A549 cells was examined by real-time PCR. FBP1 protein level after miR-21-induced expression in A549 cells was examined by western blot. FBP1 mRNA level after transfection of miR-21 inhibitor in A549 cells was examined by real-time PCR. FBP1 mRNA level after transfection of miR-21 inhibitor in A549 cells was examined by western blot. Data are shown as mean ± SD in three separate experiments in triplicate. *p < 0.05.
FBP1 was down-regulated and negatively correlated with miR-21 in NSCLC tissues
To further explore the pathological relationship between miR-21 and FBP1, we examined the expression of miR-21 and FBP1 mRNA in 75 NSCLC tissues and corresponding nontumor tissues. The results showed that miR-21 was much higher in NSCLC tissues, whereas FBP1 was significantly lower in NSCLC samples than in corresponding nontumor tissues (Figure 5A and 5B). Furthermore, Spearman’s correlation analysis showed a negative correlation between miR-21 and FBP1 expression in OS tissues (r=-0.5219, p < 0.0001; Figure 5C).
Figure 5.
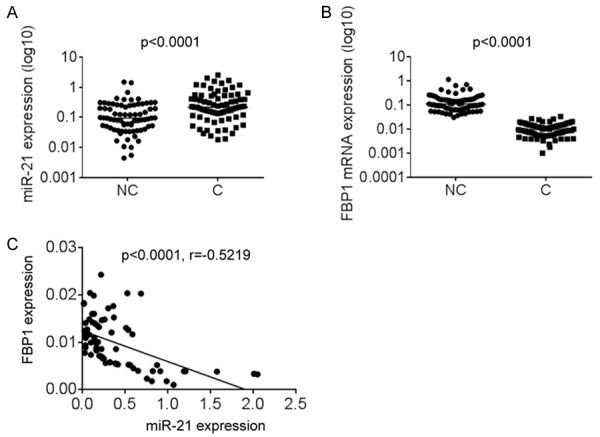
FBP1 was down-regulated and negatively correlated with miR-21 in NSCLC tissues. The miR-21 expression in 60 pairs of lung cancer tissues (C) and adjacent noncancerous lung tissues (NC) was measured using qPCR. The FBP1 mRNA expression in 60 pairs of lung cancer tissues (C) and adjacent noncancerous lung tissues (NC) was measured using qPCR. The correlation between miR-21 and FBP1 expression in 60 lung cancer tissues. Data are shown as mean ± SD in three separate experiments in triplicate. *p < 0.05.
Restoring FBP1 expression reverses the effects induced by miR-21 overexpression in NSCLC cells
To evaluate if FBP1 is responsible for the functional effect of miR-21 in NSCLC cells, some rescue experiments were performed. miR-21-overexpressing A549 cells were transfected with FBP1 (Figure 6A). We found that FBP1 attenuated glucose uptake mediated by miR-21 overexpression (Figure 6B), and decreased the lactate generation induced by miR-21 overexpression in A549 cells (Figure 6C). Moreover, our results showed that under hypoxic condition (0.1% oxygen), overexpression of FBP1 could abolish the promotion of growth induced by miR-21 overexpression in A549 cells (Figure 6D). These results indicated that miR-21 exerts it biological roles in NSCLC by targeting FBP1.
Figure 6.
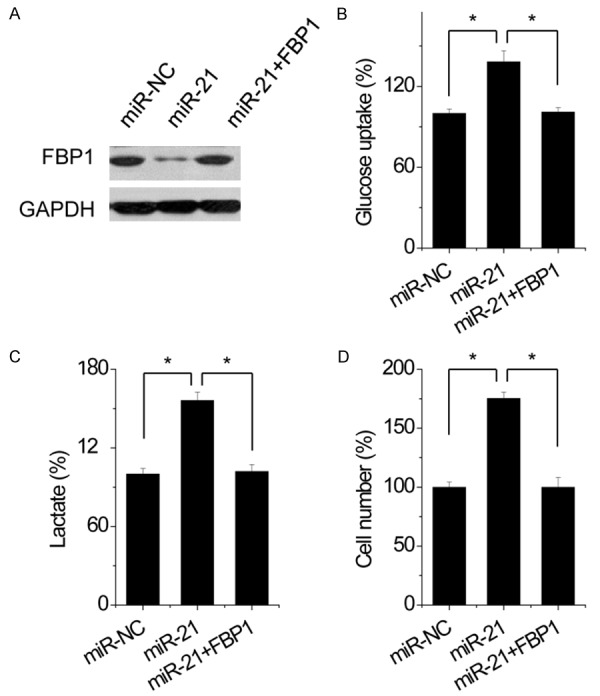
Restoring FBP1 expression reverses the effects induced by miR-21 overexpression in A549 cells. A549 cells were transfected with miR-21. Subsequently, FBP1 was transfected into miR-21-A549 cells. The expression of FBP1 was shown by Western blot assay. Glucose uptake was measured in indicated cells. Lactate excretion was measured in indicated cells. Cell growth under hypoxic condition was measured by cell-count assay for 2 days. Data are presented as a percentage of miR-NC control values for 95D cells. Data are shown as mean ± SD in three separate experiments in triplicate. *p < 0.05.
Discussion
Our study provides several insights into the relationship between microRNA and NSCLC. First, miR-21-mediated suppression of FBP1 is essential to trigger glycolytic reprogramming and results in several metabolic benefits in NSCLC (Figure 7): (1) increase glucose uptake as evidenced by elevated TXNIP after stimulation, (2) increased glycolytic intermediates for biosynthesis (such as PPP, G3P, and serine), (3) maintenance of ATP production under hypoxia.
Figure 7.
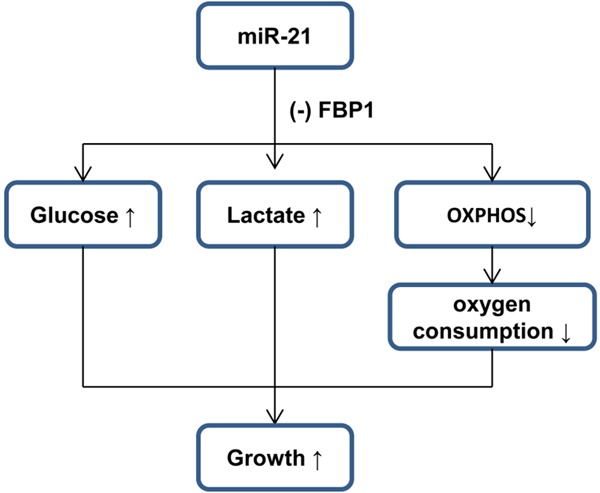
A proposed model to illustrate the repression of FBP1 by miR-21 in NSCLC, which results in the switch to aerobic glycolysis.
Accumulating evidences have been suggested that miRNAs function as either tumor suppressors or oncogenes by regulating various biological processes of cancer cells, such as cell proliferation, apoptosis, migration and invasion. In NSCLC, some miRNAs, such as members of the let-7 family, miR-126, miR-145, miR-144 or miR-34a, have been identified as tumor suppressors [15,16], while miR-17-92, miR-21, miR-221, miR-222 and miR-31 were found to act as oncogenes in NSCLC [17-19], suggesting that miRNAs act as new direction in NSCLC diagnosis and treatment. Upregulation of miR-21 has been reported in several type cancers [20-22]. It has been showed that miR-21 could promote cancer cell proliferation, colony formation, migration and invasion, as well as inhibited cell apoptosis by targeting multiple genes, such as PTEN [23,24]. However, little is known about the role of miR-21 in glucose metabolism. In the present study, we found that miR-21 promoted glucose uptake and glycolysis, increased lactate generation, and decreases oxygen consumption and OXPHOS. In addition, we identified that miR-21 directly targeted the 3’UTR of FBP1 and suppressed its expression. In addition, we also found that restoring FBP1 reversed the effects of miR-21 on glucose metabolism in NSCLC cells. These results provided new insights into NSCLC research and therapeutic strategies.
FBP1 is a key regulatory enzyme during the process of gluconeogenesis, which that can block the glycolysis, by converting 1,6-fructose diphosphate to fructose-6-phosphate [25]. It has been reported that deletion of FBP1 expression is found in liver, gastric and colon cancers, which can accelerate glucose uptake and glycolysis [26,27]. Compelling evidence indicates that increased FBP1 levels predicts a better outcome in various neoplastic disease, including kidney carcinoma [28], lung carcinoma [29], suggesting that FBP1 plays a vital role in dominating glucose metabolism in cancers. It has been reported that FBP1 was epigentically silenced by DNA methylation, histone de acetylation and histone methylation. However, whether FBP1 is regulated by microRNA remains unknown. In our study, for the first, we demonstrated that FBP1 was a direct target of miR-21. FBP1 expression level was downregulated, and negatively correlated with miR-21 expression levels in NSCLC tissues. Moreover, FBP1 overexpression reversed the effects on glucose metabolism mediated by miR-21 overexpression in NSCLC cells. These findings suggest that miR-21 regulates glucose metabolism by repressing FBP1 expression.
In this study, we demonstrated that miR-21 promoted glucose uptake and glycolysis, increased lactate generation, and decreases oxygen consumption and OXPHOS through targeting FBP1. Moreover, miR-21 is dramatically upregulated, while FBP1 is significantly decreased in NSCLC tissues. These data suggested that miR-21-FBP1 axis might serve as a new therapy target for NSCLC.
Disclosure of conflict of interest
None.
References
- 1.Mao Y, Yang D, He J, Krasna MJ. Epidemiology of Lung Cancer. Surg Oncol Clin N Am. 2016;25:439–445. doi: 10.1016/j.soc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Tan DS, Yom SS, Tsao MS, Pass HI, Kelly K, Peled N, Yung RC, Wistuba II, Yatabe Y, Unger M, Mack PC, Wynes MW, Mitsudomi T, Weder W, Yankelevitz D, Herbst RS, Gandara DR, Carbone DP, Bunn PA Jr, Mok TS, Hirsch FR. The International Association for the Study of Lung Cancer consensus statement on optimizing management of EGFR mutation positive non-small cell lung cancer: status in 2016. J Thorac Oncol. 2016;11:946–63. doi: 10.1016/j.jtho.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Zheng M. Classification and Pathology of Lung Cancer. Surg Oncol Clin N Am. 2016;25:447–468. doi: 10.1016/j.soc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Bansal P, Osman D, Gan GN, Simon GR, Boumber Y. Recent advances in targetable therapeutics in metastatic non-squamous NSCLC. Front Oncol. 2016;6:112. doi: 10.3389/fonc.2016.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352:169–175. doi: 10.1126/science.aaf2784. [DOI] [PubMed] [Google Scholar]
- 6.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Roder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigo R, Gingeras TR. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Mol Cancer. 2016;15:43. doi: 10.1186/s12943-016-0530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Andres-Pablo A, Morillon A, Wery M. LncRNAs, lost in translation or licence to regulate? Curr Genet. 2017;63:29–33. doi: 10.1007/s00294-016-0615-1. [DOI] [PubMed] [Google Scholar]
- 9.Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38(Suppl):S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- 10.Li T, Li D, Sha J, Sun P, Huang Y. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochem Biophys Res Commun. 2009;383:280–285. doi: 10.1016/j.bbrc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC) Clin Chim Acta. 2010;411:846–852. doi: 10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Meng H, Peng Q, Yang X, Gan R, Zhao L, Chen Z, Lu J, Meng QH. Downregulation of microRNA-21 expression restrains non-small cell lung cancer cell proliferation and migration through upregulation of programmed cell death 4. Cancer Gene Ther. 2015;22:23–29. doi: 10.1038/cgt.2014.66. [DOI] [PubMed] [Google Scholar]
- 13.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 14.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung MC, Evers BM, Zhou BP. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Liang H, Liao Z, Wang Y, Hu X, Chen X, Xu L, Hu Z. miR-203 enhances let-7 biogenesis by targeting LIN28B to suppress tumor growth in lung cancer. Sci Rep. 2017;7:42680. doi: 10.1038/srep42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inamura K. Diagnostic and therapeutic potential of microRNAs in lung cancer. Cancers (Basel) 2017;9 doi: 10.3390/cancers9050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legras A, Pecuchet N, Imbeaud S, Pallier K, Didelot A, Roussel H, Gibault L, Fabre E, Le Pimpec-Barthes F, Laurent-Puig P, Blons H. Epithelial-to-mesenchymal transition and microRNAs in lung cancer. Cancers (Basel) 2017;9 doi: 10.3390/cancers9080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guinot A, Oeztuerk-Winder F, Ventura JJ. miR-17-92/p38alpha dysregulation enhances wnt signaling and selects lgr6+ cancer stemlike cells during lung adenocarcinoma progression. Cancer Res. 2016;76:4012–4022. doi: 10.1158/0008-5472.CAN-15-3302. [DOI] [PubMed] [Google Scholar]
- 19.Alamolhodaei NS, Behravan J, Mosaffa F, Karimi G. MiR 221/222 as new players in tamoxifen resistance. Curr Pharm Des. 2016;22:6946–6955. doi: 10.2174/1381612822666161102100211. [DOI] [PubMed] [Google Scholar]
- 20.Markou A, Zavridou M, Lianidou ES. miRNA-21 as a novel therapeutic target in lung cancer. Lung Cancer (Auckl) 2016;7:19–27. doi: 10.2147/LCTT.S60341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J. Considering exosomal miR-21 as a biomarker for cancer. J Clin Med. 2016;5 doi: 10.3390/jcm5040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrovic N. miR-21 might be lnvolved in breast cancer promotion and invasion rather than in Initial events of breast cancer development. Mol Diagn Ther. 2016;20:97–110. doi: 10.1007/s40291-016-0186-3. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Zhang B, Chen G, Wu W, Zhou L, Shi Y, Zeng Q, Li Y, Sun Y, Deng X, Wang F. Targeting miR-21 with sophocarpine inhibits tumor progression and reverses epithelial-mesenchymal transition in head and neck cancer. Mol Ther. 2017;25:2129–2139. doi: 10.1016/j.ymthe.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang H, Xie J, Zhang M, Zhao Z, Wan Y, Yao Y. miRNA-21 promotes proliferation and invasion of triple-negative breast cancer cells through targeting PTEN. Am J Transl Res. 2017;9:953–961. [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, He C, Li Z, Wang Z, Zhang Q. FBP1 modulates cell metabolism of breast cancer cells by inhibiting the expression of HIF-1alpha. Neoplasma. 2017;64:535–542. doi: 10.4149/neo_2017_407. [DOI] [PubMed] [Google Scholar]
- 26.Chen M, Zhang J, Li N, Qian Z, Zhu M, Li Q, Zheng J, Wang X, Shi G. Promoter hypermethylation mediated downregulation of FBP1 in human hepatocellular carcinoma and colon cancer. PLoS One. 2011;6:e25564. doi: 10.1371/journal.pone.0025564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Wang Y, Li QG, Xue JJ, Wang Z, Yuan X, Tong JD, Xu LC. Downregulation of FBP1 Promotes Tumor Metastasis and Indicates Poor Prognosis in Gastric Cancer via Regulating Epithelial-Mesenchymal Transition. PLoS One. 2016;11:e0167857. doi: 10.1371/journal.pone.0167857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alderton GK. Tumorigenesis: FBP1 is suppressed in kidney tumours. Nat Rev Cancer. 2014;14:575. doi: 10.1038/nrc3810. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Wang J, Xing H, Li Q, Zhao Q, Li J. Down-regulation of FBP1 by ZEB1-mediated repression confers to growth and invasion in lung cancer cells. Mol Cell Biochem. 2016;411:331–340. doi: 10.1007/s11010-015-2595-8. [DOI] [PubMed] [Google Scholar]


