Abstract
Exosomes are nano-vesicles transporting bioactive material between cells. This study explored the prognostic association of exosomal TGF-β1 with lymph node (LN) metastasis of gastric cancer (GC). TGF-β1 expressions in the exosomes isolated from the gastroepiploic veins of 61 GC patients analyzed by ELISA. The regulatory T (Treg) cells in celiac LNs of gastric cancer analyzed by immunohistochemistry. Exosomal TGF-β1 expression and the ratio of Treg cells in draining LNs were both significantly associated with pathological stages and LN metastasis of gastric cancer. Besides, the exosomal TGF-β1 expression and Treg proportion in LN were also significantly correlated in gastric cancer patients. Recombinant TGF-β1 and exosomes isolated from GC patients were used to induce FOXP3+ Treg cells from naïve T cells in vitro. Compared to the control, recombinant TGF-β1 induced more CD25 (41%), FOXP3 (19%) and CTLA-4 (47%), while reduced CD45RA expression by 38% in primary naïve T cell cultures (p<0.01). Exosomes treatment induced more CD25 and 45% higher CTLA-4 expression, and increased 29% higher of CD45RA-negative cells than recombinant TGF-β1 did (p<0.01). Adding TGF-β1 neutralizing antibody partially abrogated the effects of exosomes on Treg induction. Our study showed exosomal TGF-β1 related to lymph node metastasis and the ratio of Treg cells in lymph nodes of gastric cancers. Exosomes from gastric cancer patients could induce Treg cells formation through the effect of TGF-β1.
Keywords: Exosomes, TGF-β1, lymph node, regulatory T cell, gastric cancer
Introduction
Exosomes are nano-sized membrane vesicles derived from endosomal origin of multi-vesicular bodies and constitutively released by fusion with the cell membrane [1]. Exosomes composes specific proteins, mRNA, and microRNA et al [1,2]. The most abundant proteins in exosome belong to the tetraspanins family, such as CD63, used as the marker of isolated exosomes [1]. Exosomes are found to mediate inter-cellular communication through the transfer of their cargo.
Cancer cells secrete exosomes that aid transforming the microenvironments suitable for their metastasis. Melanoma-derived exosomes could enhance metastasis by increasing pro-angiogenic cells in bone marrow [3]. Two proposed mechanisms explain tumor-derived exosomes could facilitate metastasis: (1) exosomes remodel the extracellular matrix to enhance recruitment of hematopoietic cells [4] or motility of tumor cells [5]; (2) exosomes transfer molecules to modulate the immunity [6,7]. For example, tumor-derived exosomes educate metastatic niche through activating regulatory T-cells (Treg) which efficiently blunt T cell-, NK cell-, and dendritic cells-mediated immune responses [8].
Lymphatic metastasis is the most common form of metastasis in gastric cancer (GC) [9], though the causes of favoring lymphatic spread is not known yet. The incidence of lymphatic metastases increases with deeper tumor invasion. The metastasis of gastric cancer cells to lymph nodes (LNs) usually follows the order, along peri-gastric regions to the branches of celiac trunk. Skipped LN metastasis is rare (2-4% only) [9]. Radical lymph nodes dissection showed its effect in reducing the recur of gastric cancer [10]. However, 5-year recurrence -free survival for node-positive GC is only 53% [11,12].
Transforming growth factor-β1 (TGF-β1) is a cytokine capable of inducing naïve T cells’ transition to FOXP3+ regulatory T cells [13,14]. Regulatory T cells (Treg) are a subpopulation of T cells that mediate immunosuppressive effect, helping cancer cells to evade the immune surveillance of the host. We propose that gastric cancer cells secrete exosomes which modulate the immune surveillance in lymphatic microenvironment. We demonstrated that TGF-β1 expression in exosomes related to LN metastasis of GC patients, and the exosomes induced Treg formation through the effect of TGF-β1.
Materials and methods
Human samples collection
The patients, aged between 20 to 85 year-old, diagnosed to have primary gastric cancers were included in this study. The patients who had other malignancies or received chemotherapies before were excluded from the analysis. All of the patients received surgical treatment for gastric cancer, and those patients with pathological stage IV received salvage chemotherapies. The paired gastric tissue, lymph nodes (LN) and peripheral blood harvested from patients who underwent surgery for gastric adenocarcinoma at the Departments of Surgery, National Taiwan University Hospital (NTUH). Institutional review board of the NTUH ethics committee approved the use of human samples in this study, and written consents obtained from patients and healthy donors before the sampling.
Isolation of exosomes
The peripheral blood samples from the gastroepiploic vein were centrifuged at 500×g for 5 minutes, 3,000×g for 20 minutes and 12,000×g for 20 minutes separately to eliminate cell debris and apoptotic bodies, and subsequently pelleted in 100,000×g for 70 minutes (using Beckman 70.1 TI rotor). The pellet was then washed with PBS and centrifuged again at 100,000×g to remove protein contaminants [3]. Exosome pellets were re-suspended in 100 μl of cold PBS and stored at -80°C until later use.
Immuno-EM
The details were listed in the supplement. The isolated exosomes were fixed in glutaformaldehyde and dispensed onto copper grids. Grids were stained with 1% (w/v) filtered uranyl acetate. For immunogold analysis, exosomes loaded onto grids were permeabilized with Triton X-100 and incubated with anti-CD63 (Genetex) antibody. The grids were stained with gold-labelled secondary goat anti-rabbit antibody (Aurion), and imaged under a Hitachi H-7100 transmission electron microscope at acceleration voltage of 100 kV.
Nanoparticle tracking analysis
The Nanoparticle Tracking Analysis utilizes the properties of light scattering to characterize particle size distribution and quantitated by NanoSight LM10 system (Malvern Instruments) equipped with a 405-nm blue laser. Exosome samples (10 uL) were diluted in PBS to reach recommended concentration (1×108 to 1×109 particles/mL). Scattered light of exosomes is visualized by 20× magnification microscope. By analyzing the Brownian movement of exosomes, the diameters of exosome were calculated by NTA 2.3 Analytical software.
Western blot analysis
Protein samples were separated by SDS-PAGE, blocked and incubated overnight at 4°C with the following primary antibodies: 1:1000 anti-CD63 (GeneTex), 1:1000 anti-CD9 (Abcam) and 1:1000 anti-HSC70 (Abcam). Afterward, horseradish peroxidase (HRP) -conjugated secondary antibodies were incubated at room temperature. Membranes were washed with TBS-T after antibody incubations. Blots were developed with Luminata Crescendo Western HRP substrate (Millipore) and detected with X-films. The amount of exosomes used for western blotting was 15 ug.
Immunohistochemistry
The lymph nodes at the root of celiac trunk (celiac nodes) microscopically confirmed as non-metastatic in H&E stain were selected for immune-staining of FOXP3. Paraffin sections (5 µm) were deparaffinized, followed by antigen retrieval. Sections were incubated with 0.3% H2O2 for 10 minutes to block endogenous peroxidase activity. After being rinsed in phosphate-buffered saline (PBS), the sections were blocked with 5% bovine serum albumin (BSA) in PBS for 1 hour and reacted with the primary antibodies (in PBS/1% BSA): 10 µg/ml anti-FOXP3 antibody (BioLegend) or 5 µg/mL anti-TGF beta antibody (Abcam) overnight at 4°C. Signals were visualized employing UltraVision Quanto Detection System (Thermo). All sections were counterstained with hematoxylin.
Images of immunostained sections of lymph nodes were taken under 40× magnification. Three paracortical regions that contained the highest Treg densities were selected, and the total lymphocyte and FOXP3+ counts were calculated. We divided the number of FOXP3+ cells by the total number of lymphocyte count to obtain the proportion of Treg cells located in the regional lymph node.
Fluorescence-activated cell sorting (FACS) analysis
The cells were stained with PE/Cy7 anti-human CD25, FITC anti-human CD45RA, anti-human APC CTLA-4, and PE anti-human FOXP3 (BioLegend). The fixation, permeation and staining were performed according to the manufacturer’s protocol (FOXP3 Fixation/Permeabilization kit, eBioscience). IgG-matched isotypic controls were used in each experiment. BD FACSCanto II (BD Biosciences) was used for data acquisition and analyzed with FlowJo software (Tree Star, Inc.).
T-Cell culture
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation over Ficoll-Paque PLUS (GE Healthcare) gradient at 400×g for 30 min followed by a PBS washing step. CD4+ CD45RA+ naïve T cells were purified using EasySep™ human CD4+ T cell enrichment kit (Stemcell Technologies). Purified cells were then seeded at a concentration of 2×106 cells/ml with RPMI-1640 culture medium containing 10% heat-inactivated fetal bovine serum (GeneDireX), 100 U/ml penicillin-streptomycin (HyClone), 1 mM sodium pyruvate (HyClone), 2 mM L-glutamine (HyClone), 1× non-essential amino acids (Hyclone), 50 μM 2-mercaptoethanol (G-Bioscience), 25 mM HEPES, 2 μg/mL mouse anti-human CD28 (BioLegend) and 5 ng/mL IL-2 (Peprotech) in 96-wells plates that were pre-coated (4°C, O/N) with 10 μg/mL of mouse anti-human CD3 monoclonal antibody (BioLegend). For Treg induction, 5 ng/mL of Recombinant Human Transforming Growth Factor-β1 (rhTGF-β1, R&D Systems) or 60 μg/mL of GC-derived exosome (containing~0.135 ng/ml TGF-β1) was added in the culture media. After seeding, the plate was centrifuged (500×g, 5 min) and incubated at 37°C and 5% CO2 for 3 days. On day 3 post plating, half of the original media were replaced with fresh media. On day 6 post plating, cells were analyzed by flow cytometry to determine whether differentiation has taken place.
Statistics
All values and bars are presented in the form of mean ± SEM. Student’s t test and one-way ANOVA were employed for comparison among groups. Spearman ρ tests were utilized for comparison of exosomal TGF-β1 expression with percentage of FOXP3+ cells in LN.
Results
Characterization of purified exosomes from gastric cancer patients
The expressions of 3 exosomal markers, CD9, CD63 and HSC70, on the isolated exosomes were analyzed by Western blot. The exosomes from different stages of gastric cancer patients expressed all three markers (Figure 1A). Morphologically, the isolated exosomes exhibited a typical spherical shape with a lipid bilayer membrane (Supplementary Figure 1A), and were labelled with CD63 and gold-conjugated antibody (immuno-EM, Figure 1B). The size distribution of the isolated particles measured by NTA (Supplementary Figure 1B) was approximately 100 nm, which is within the typical size range of exosomes (30~150 nm). The above analysis demonstrated that exosomes were successfully isolated from the peripheral blood of gastric cancer patients.
Figure 1.
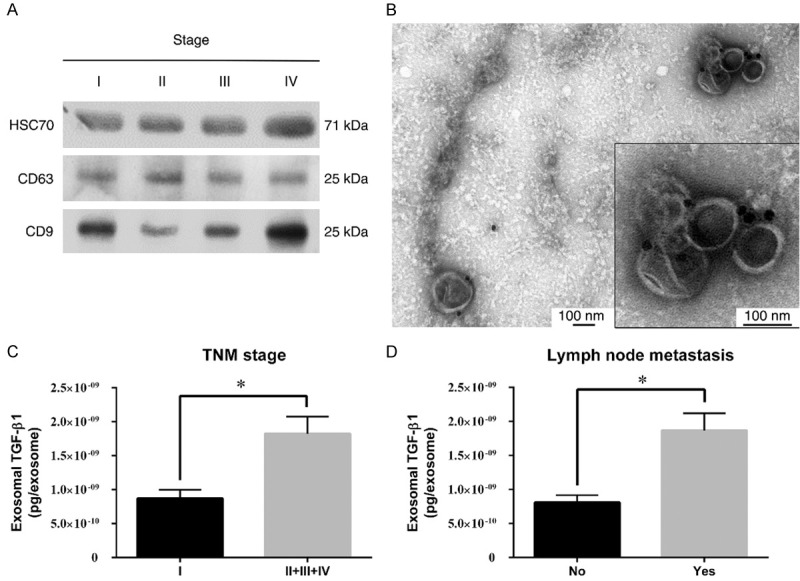
Verification and clinical correlation of exosomes from GC patients. A. Representative western blotting of HSC70, CD63 and CD9 in the plasma exosomes of gastric cancer patients (stage I to IV, as indicated). B. Immunogold staining of exosome samples shows positive CD63 expression on exosomal membrane at 50,000× magnifications. Scale bar: 100 nm. C. The expression of exosomal TGF-β1 in GC patients with different TNM stages. D. TGF-β1 expression level in LN metastasis-negative and LN metastasis-positive groups.
The expression of TGF-β1 in plasma exosomes of gastric cancer patients
The amount of TGF-β1 in the isolated exosomes from 61 GC patients was determined by ELISA. There was no difference in the concentration of exosomes isolated from different stages of GC patients (Supplementary Figure 3). The concentration of TGF-β1 per exosome was calculated and correlated with the clinicopathological characteristics of GC patients (Table 1). The expression of exosomal TGF-β1 in GC patients did not correlate with age, gender, location, tumor size, Borrmann type, differentiation, Lauren classification, tumor depth or distant metastasis. However, higher exosomal TGF-β1 expression was significantly associated with TNM stage (P=0.03) and LN metastasis (P=0.01). Gastric cancer patients of advanced stages (TNM stages 2, 3, 4) shown higher exosomal TGF-β1 versus those with stage 1 disease (Figure 1C). The patients with LN metastasis exhibited a twofold of TGF-β1 expressions in exosomes versus those without LN metastasis (Figure 1D). These results indicated that increased exosomal TGF-β1 expression was associated with advanced stages and LN metastasis in GC patients.
Table 1.
Exosomal TGFβ-1 expression and clinicopatholgical correlation in 61 GC patients
| Factor | Case number | Expression of TGF-β1 in exosomes (pg/10^10 exosomes) | P-value |
|---|---|---|---|
| All patients | 61 | ||
| Age (years) | 0.27 | ||
| ≤60 | 29 | 14.1±2.6 | |
| >60 | 32 | 17.5±2.9 | |
| Gender | 0.57 | ||
| Female | 27 | 16.9±3.2 | |
| Male | 34 | 15.1±2.5 | |
| Tumor size (cm) | 0.29 | ||
| <3 | 27 | 13.7±2.3 | |
| ≥3 | 34 | 17.6±3.0 | |
| Location | 0.77 | ||
| Proximal+middle | 26 | 16.4±3.6 | |
| Distal | 35 | 15.5±2.2 | |
| Borrmann Type | 0.48 | ||
| I+II | 26 | 13.3±2.2 | |
| III+IV | 35 | 17.8±3.0 | |
| Differentiation | 0.33 | ||
| Well+Moderate | 40 | 16.7±2.6 | |
| Poor | 21 | 14.4±3.1 | |
| Lauren classification | 0.63 | ||
| Intestinal type | 23 | 18.4±3.7 | |
| Diffuse+Mixed type | 38 | 14.4±2.3 | |
| Tumor depth | 0.06 | ||
| T1+T2 | 22 | 12.7±2.7 | |
| T3+T4 | 39 | 17.7±2.7 | |
| Lymph node metastasis | 0.01 | ||
| No | 16 | 8.1±1.1 | |
| Yes | 45 | 18.7±2.5 | |
| Distant metastasis | 0.24 | ||
| No | 54 | 16.2±2.1 | |
| Yes | 7 | 13.4±6.6 | |
| TNM stage | 0.03 | ||
| I | 15 | 8.7±1.3 | |
| II+III+IV | 46 | 18.2±2.5 |
The proportion of Treg in draining LNs of gastric cancers
GC patients with LN metastasis had higher expression of exosomal TGF-β1. We propose that exosomes harboring TGF-β1 are able to induce a more Treg cells transition in the draining lymph node of GC. The proportion of FOXP3+ Treg cells in LNs at the root of celiac trunk of GC patients were analyzed by IHC staining. The FOXP3+ Treg cells were present in the paracortical region of LN (Figure 2A). The proportion of FOXP3+ Treg cells in the para-cortical area was correlated with clinicopathological characteristics of GC patients. The proportion of FOXP3+ Treg cells was not correlated with age, gender, location, differentiation, Lauren’s classification or distant metastasis (Table 2). However, a higher ratio of FOXP3+ Treg cells in draining LNs was significantly correlated with advanced stages (P=0.01) (Figure 2B) and LN metastasis of GC patients (P=0.04) (Figure 2C).
Figure 2.
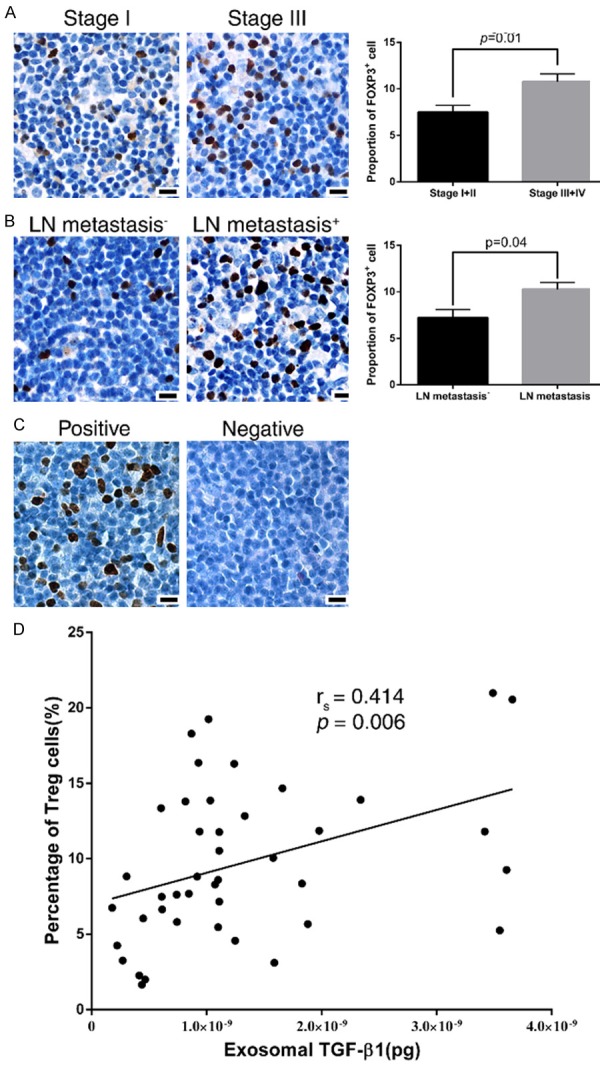
FOXP3+ Treg cells in lymph nodes and clinical correlation of gastric cancer A. Representative immunohistochemical staining showing FOXP3+ Treg cells in the paracortical region of the draining LN in different groups of patients. Bars: 50 μm. B. The percentage of FOXP3+ cells in draining LN were correlated with pathological stages of GC patients. C. The percentage of FOXP3+ cells in draining LN were correlated with the status of lymph node metastasis in GC patients. D. Correlation between exosomal TGF-β1 and Treg cell percentage in the draining LNs.
Table 2.
The correlation between FOXP3+ cells proportion in draining LN and clinicopathological factors in 61 GC patients
| Factor | Case number | Percentage of FOXP3+ cells in paracortical region | P-value |
|---|---|---|---|
| All patients | 61 | ||
| Age (years) | 0.62 | ||
| ≤60 | 27 | 9.9±1.0 | |
| >60 | 34 | 9.2±0.8 | |
| Gender | 0.81 | ||
| Female | 33 | 9.7±0.9 | |
| Male | 28 | 9.4±0.8 | |
| Tumor size(cm) | 0.03* | ||
| <3 | 38 | 7.8±0.9 | |
| ≥3 | 23 | 10.6±0.8 | |
| Location | 0.35 | ||
| Non-distal | 24 | 9.0±1.1 | |
| Distal | 37 | 9.9±0.8 | |
| Borrmann Type | 0.01* | ||
| I+II | 29 | 7.9±0.7 | |
| III+IV | 32 | 11.0±0.9 | |
| Differentiation | 0.87 | ||
| Well+Moderate | 20 | 9.7±1.1 | |
| Poor | 41 | 9.4±0.8 | |
| Lauren classification | 0.14 | ||
| Intestinal type | 22 | 10.6±1.1 | |
| Diffuse+Mixed type | 39 | 8.9±0.7 | |
| Tumor depth | 0.00* | ||
| T1+T2 | 26 | 7.5±0.7 | |
| T3+T4 | 35 | 11.0±0.9 | |
| Lymph node metastasis | 0.04* | ||
| No | 15 | 7.2±0.9 | |
| Yes | 46 | 10.0±0.8 | |
| Distant metastasis | 0.96 | ||
| No | 55 | 9.5±0.7 | |
| Yes | 6 | 9.7±2.1 | |
| TNM stage | 0.01* | ||
| I+II | 23 | 7.5±0.7 | |
| III+IV | 38 | 10.8±0.8 |
Significant difference between groups.
The calculated coefficient (rs) of Treg proportion in LNs and exosomal TGF-β1 level in the GC patients was 0.414 (P=0.006) (Figure 2D), which indicated that advanced gastric cancer expressing high TGF-β1 in exosomes also expressed high proportion of Treg in their draining LNs.
Induction of Tregs from naïve T cells by exosomes from GC patients
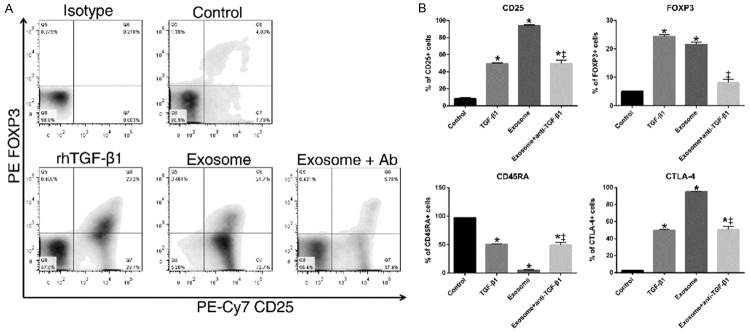
TGF-β1 induces the transformation of Tregs with the phenotypic marker of FOXP3 [14,15]. FOXP3 is a transcriptional factor and delineates the immune-regulatory function of Tregs [16]. We hypothesized that plasma exosomes from GC patients may induce Treg cells formation through the effect of TGF-β1. Compared to the control group, rhTGF-β1 induced more CD25 (41%), FOXP3 (19%) and CTLA-4 (47%) expressions, while attenuating CD45RA expression by 38% (p<0.01). Similarly, exosomes treatment increased CD25, FOXP3, and CTLA-4 expression and decreased CD45RA expression in primary naïve CD4+ T cell cultures (Figure 3). Besides, exosomes treatment induced more CD25 and CTLA-4 expression (45% higher), and reduced more CD45RA+ (by 29%) than rhTGF-β1 did (p<0.01). The addition of TGF-β1 neutralizing antibody partially abrogated the effects of exosomes on Treg induction from naïve T cells. CTLA-4 was another Treg marker and considered responsible for immunosuppressive effect of Tregs. Increased FOXP3 and decreased CD45RA expression indicates the successful differentiation from naïve T cells into Tregs. These results showed that the GC exosomes carrying TGF-β1 were able to induce the differentiation of CD25+CTLA4+FOXP3+ Tregs from naïve T cells, which indicated the possibility that increased Tregs in the draining LNs of GC was associated with the exosomal TGF-β1.
Figure 3.
GC exosomes induces CD25+CTLA4+FOXP3+ Treg cells Naïve T cells from healthy donors were cultured with rhTGF-β1 or GC patient-derived exosomes. After treatment, cells were stained and analyzed by flow cytometry. A. Representative density plot of FOXP3 expression in cultured cells. B. Quantitative analysis of CD25, CD45RA, FOXP3 and CTLA-4 expression in cultured cells. (*p<0.01 compared to control, †p<0.01 compare to TGF-β1, and ‡p<0.01 compare to exosome).
Discussion
Our study showed that (1) The expression of exosomal TGF-β1 in GC patients was associated with pathological stages and LN metastasis; (2) The proportion of FOXP3+ Treg cells in draining LN was correlated with pathological stages and LN metastasis; (3) The expression of exosomal TGF-β1 was correlated with FOXP3+ Treg cells in draining LNs; (4) Exosomes from GC patients could transform naïve T cells into FOXP3+ Treg cells in vitro, and the effect was reduced by TGF-β1 neutralizing antibody. The above results indicate the exosomal TGF-β1 is associated with gastric cancer progression, and exosomes from GC patients are capable of modulating the immune surveillance.
With the development of biotechnology, novel biomarkers for gastric cancer’s progression, including microRNAs, hypo-methylation of DNA is explored [17]. Exosomes are microvesicles released by cells into the circulation. Tumor-derived exosomes could transfer messenger biomaterials between cancer cells and their pre-metastatic niche. Therefore, the components carried by exosomes could be used as biomarkers. It has been shown that the level of macrophage migration inhibitory factor in exosomes is associated with progression of pancreatic ductal carcinoma [4]. Higher levels of miR-1290 and miR-375 in plasma exosomes were significantly associated with poor overall survival in prostate cancers [18]. Our study revealed that exosomal TGF-β1 was associated with LN metastasis of GC patients.
Recurrence in the lymphatics are common after curative surgery in gastric cancers [11], indicating that micro-metastasis to LNs beyond surgical dissection occurs. Lymph nodes are secondary lymphoid organs specialized in anti-tumor immune surveillance. For the circulating tumor cells to lodge and proliferate, the immunities at the microenvironment (LNs) has to be modulated [19]. Sabotage the anti-tumor immunity by recruiting immune-suppressive regulatory T cells is one of the proposed paradigms [7]. Previous reports showed that the proportion of Treg cells in draining LNs was correlated with prognosis of gastric cancers [20,21]. However, the mechanistic explanations about Treg induction in LNs remain unknown. Our study showed the TGF-β1 carried in exosomes was correlated with the Treg proportion in draining LNs. Besides, exosome from GC patients could induce Treg formation through TGF-β1 in a primary T cell culture, which suggested that exosomes might precondition the lymph node immunity by transporting cytokines. The messenger roles of exosomes in modulating LN microenvironment have been demonstrated that melanoma-derived exosomes traveled to the sentinel lymph nodes and facilitated LN metastasis of melanoma in an animal model [22]. The injection of exosomes from a cell line with highly-lymphatic metastasis potential promoted lymph node metastasis of gastric cancer cells in vivo [23].
TGF-β1 is an immunosuppressive cytokine produced by immune and tumor cells [24]. Intra-tumoral TGF-β1 expression was predictive of gastric cancer progression [25,26] or associated with neoplastic transformation [27]. However, the relationship between serum TGF-β1 level and clinicopathological characters of gastric cancers were controversial [27-29]. Our study showed that the level of TGF-β1 per exosome maybe a better predictive parameter for lymph node metastasis of gastric cancers.
There are unsolved problems in our study. First, the uptake and the effects of exosomes on the development of lymphocytes in draining LN remains to be examined in an in vivo model. Researchers found that distinct integrin profiles expressed on exosomes predicted the metastatic sites [6]. Whether there exist specific lymphotropic profiles in exosomes deserves further studies. Second, other cargos in the exosomes might modulate the immune surveillance in lymphatic microenvironment as well. As shown, TGF-β1 neutralizing antibody did not completely abolish the effect of exosomes on Treg induction, which indicated that components other than TGFβ-1 in exosomes also have immune-modulatory functions. Third, the free TGF-β in plasma measured by ELAS was 1,957±78 pg/ml, and the remaining TGF-β in plasma after exosome purification is 1,628±245 pg/ml. Namely, the amount of TGF-β in the exosomes accounted for about 17% of total TGF-β secreted. Considering the uptake efficiency of recipient cells and degradation of cytokines in circulation, the exosomes might work in a dominant way with only a little TGF-β carried. Forth, we did not examine the dose effects of exosomes on Treg induction from different stages of GC. It is important to know whether exosomes from early stages also have the same induction capability.
In summary, our study showed that TGF-β1 carried by exosomes are associated with lymph node metastasis of gastric cancer. Exosomes derived from gastric cancer patients could modulate the immune surveillance through the induction of regulatory T cells.
Acknowledgements
The authors acknowledge statistical assistance by Chin-Hao Chang and Jia-Chun He, the Taiwan Clinical Trial Statistical Center, Training Center, and Pharmacogenomics Laboratory (founding grant: MOST 104-2325-B-002-032) and the Department of Medical Research in NTUH. The authors acknowledge the grant support from the Excellent Translational Medicine Research Projects of National Taiwan University College of Medicine, and National Taiwan University Hospital (106C101-33). This study was supported by Ministry of Science and Technology R.O.C. (106-2320-B-002-034).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 3.Peinado H, Alečković M, Lavotshkin S, Lyden D, et al. Melanoma exosomes educate bone marrow progenitor cells toward a prometastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Lyden D, et al. Pancreatic cancer exosomes initiate premetastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–828. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mu W, Rana S, Zöller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15:875–887. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 7.Poschke I, Mougiakakos D, Kiessling R. Camoutage and sabotage: tumor escape from the immune system. Cancer Immunol Immunother. 2011;60:1161–1171. doi: 10.1007/s00262-011-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer: General pattern in 1931 patients. Ann Surg. 1989;210:596–602. doi: 10.1097/00000658-198911000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346–51. doi: 10.1002/bjs.1800820321. [DOI] [PubMed] [Google Scholar]
- 11.Sasako M, Sakuramoto S, Katai H, Kinoshita T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011;29:4387–93. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 12.Noh SH, Park SR, Bang YJ, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year followup of an open-label, randomized phase 3 trial. Lancet Oncol. 2014;15:1389–96. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor FOXP3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–43. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, Zhang H, Ding Y, Bromberg JS. TGF-beta induces Foxp3+ T-regulatory cells from CD4+ CD25- precursors. Am J Transplant. 2004;4:1614–27. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 16.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 17.Jin Z, Jiang W, Wang L. Biomarkers for gastric cancer: Progression in early diagnosis and prognosis (Review) Oncol Lett. 2015;9:1502–1508. doi: 10.3892/ol.2015.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, Wang D, See W, Costello BA, Quevedo F, Tan W, Nandy D, Bevan GH, Longenbach S, Sun Z, Lu Y, Wang T, Thibodeau SN, Boardman L, Kohli M, Wang L. Exosomal miR-1290 and miR-375 as prognostic markers in castrationresistant prostate cancer. Eur Urol. 2015;67:33–41. doi: 10.1016/j.eururo.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu S, Cochran AJ, Huang RR, Morton DL, Maecker HT. Immune responses in the draining lymph nodes against cancer: Implications for immunotherapy. Cancer Metastasis Rev. 2006;25:233–42. doi: 10.1007/s10555-006-8503-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee HE, Park DJ, Kim WH, Kim HH, Lee HS. High FOXP3+ regulatory T-cell density in the sentinel lymph node is associated with downstream non-sentinel lymph-node metastasis in gastric cancer. British Journal of Cancer. 2011;105:413–419. doi: 10.1038/bjc.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashimura S, Saze Z, Terashima M, Soeta N, Ohtani S, Osuka F, Kogure M, Gotoh M. CD83(+) dendritic cells and Foxp3(+) regulatory T cells in primary lesions and regional lymph nodes are inversely correlated with prognosis of gastric cancer. Gastric Cancer. 2012;15:144–53. doi: 10.1007/s10120-011-0090-9. [DOI] [PubMed] [Google Scholar]
- 22.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Li C, Trojanowicz B, Li X, Shi D, Zhan C, Wang Z, Chen L. CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric Cancer. 2016;19:754–66. doi: 10.1007/s10120-015-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factorbeta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vagenas K, Spyropoulos C, Gavala V, Tsamandas AC. TGFbeta1, TGFbeta2 and TGFbeta3 protein expression in gastric carcinomas: correlation with prognostics factors and patient survival. J Surg Res. 2007;139:182–188. doi: 10.1016/j.jss.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Ananiev J, Gulubova M, Tchernev G, Penkova M, Miteva R, Julianov A, Manolova I. Relation between transforming growth factor-beta1 expression, its receptor and clinicopathological factors and survival in HER2-negative gastric cancers. Wien Klin Wochenschr. 2011;123:668–673. doi: 10.1007/s00508-011-0078-9. [DOI] [PubMed] [Google Scholar]
- 27.Ma GF, Miao Q, Zeng XQ, Luo TC, Ma LL, Liu YM, Lian JJ, Gao H, Chen SY. Transforming growth factor-β1 and -β2 in gastric precancer and cancer and roles in tumor-cell interactions with peripheral blood mononuclear cells in vitro. PLoS One. 2013;8:e54249. doi: 10.1371/journal.pone.0054249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suda A, Saito N, Seshimo A, Kameoka S, Kobayashi M. Examination of transforming growth factor beta1 expression in the serum and tumor tissue of gastric cancer. Int Surg. 2009;94:182–188. [PubMed] [Google Scholar]
- 29.Li X, Yue ZC, Zhang YY, Bai J, Meng XN, Geng JS, Fu SB. Elevated serum level and gene polymorphisms of TGF-beta1 in gastric cancer. J Clin Lab Anal. 2008;22:164–171. doi: 10.1002/jcla.20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.