Abstract
MicroRNAs play key roles during various crucial cell processes, such as proliferation, migration, and invasion. In addition, microRNAs have been shown to possess oncogenic and tumor suppressive functions in human cancers. Increasing evidence has clarified that miR-149-3p, a novel cancer-related microRNA, plays an important role in suppression of proliferation, migration, and invasion; however, the effect and mechanisms underlying the miR-149-3p effect in bladder cancer (BCa) remain unclear. In the current study we found that the increased expression of miR-149-3p significantly suppressed cell proliferation, migration, and invasion ability in BCa. The suppressive effect was related to S100A4. A further investigation showed that miR-149-3p negatively regulated S100A4, as verified by the luciferase reporter assay. Furthermore, our study showed that S100A4 mediated the anti-metastatic effects of miR-149-3p on proliferation, migration, and invasion of BCa cells. Analysis of a xenograft mouse model showed that miR-149-3p expression significantly decreased tumor growth by targeting S100A4. Taken together, these data indicate that S100A4 promotes cell growth, migration, and invasion and can by reversed by miR-149-3p in BCa.
Keywords: Bladder cancer, S100A4, miR-149-3p, proliferation, migration
Introduction
Urinary bladder cancer (BCa) is a prevalent urogenital malignant tumor which causes an estimated 380,000 new cases and 150,000 deaths annually. BCa is among the fifth most common malignancies worldwide [1,2]. The annual incidence of new BC cases has declined over the last 10 years, but the fatality rate has remained stable, especially for transitional cell carcinoma (TCC) [3]. Conventional cytotoxic chemotherapy, such as platinum-based combination chemotherapy, is commonly used as a main regimen for advanced stages of TCC, which currently provides a potential cure only in select patients and is underpowered [4,5]. In addition, the frequent emergence of drug resistance due to tumor heterogeneity and serious side effects from current treatment regimens make treatment outcomes unsatisfactory [6]. Increasing evidence has shown that chemotherapy resistance is related to tumor metastasis [7-9]. Thus, it is of great significance to understand the molecular mechanisms underlying the metastasis and proliferation of BCa.
MicroRNA (miR) is a short non-coding RNA sequence that plays an important role in adjusting gene expression. MicroRNA binds to the 3’ untranslated region (UTR) of target messenger RNAs (mRNAs) and affects the corresponding transcription process [10-12]. Emerging evidence has established that miRNAs are actively involved in a number of different cellular processes, such as cell proliferation, invasion, and migration [13-16]. Recent studies have suggested that miR-149-3p may play an important role in tumor suppression in various cancer types, such as pancreatic and gastric cancer [17,18]. The role of miR-149-3p in BCa remains unclear.
S100A4 is a member of the S100 protein family, which is associated with cell differentiation, cell motility, and transcriptional regulation [19,20]. Additional studies showed that S100A4 is highly expressed in metastatic tumor cell lines and the expression of S100A4 relative to several malignancies, including bladder, colorectal, breast, and thyroid cancers, and plays a role in tumor aggressiveness [21-26]; however, the exact pathologic role of S100A4 in bladder cancer remains unclear. Bioinformatics analysis showed that miR-149-3p can target the 3’UTR of S100A4. Thus, this study aimed to clarify the regulatory effect of miR-149-3p in S100A4-mediated bladder cancer progression.
The present study demonstrated that up-regulation of the expression of miR-149-3p can significantly suppress the proliferation, invasion, and migration in vitro and suppresses tumor growth of BCa cells in vivo. Additionally, miR-149-3p is capable of down-regulating the expression by targeting S100A4 and binding to the 3’-UTRs. Over-expression of S100A4 can restore the phenotypes of BCa cell lines and inhibit the suppressive effect of miR-149-3p. Finally, the present study demonstrated that miR-149-3p has a tumor suppressive role in BCa progression.
Materials and methods
Ethics statement
All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals, and all experiments were approved and performed according to the guidelines of the Ethics Committee of Tongren Hospital of Shanghai Jiaotong University School of Medicine. All surgical procedures were performed under anesthesia, and every effort was made to minimize suffering. Rats were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg).
Reagents
Cell lines and cell culture
Two human bladder cancer cell lines (UM-UC-3) were purchased from the American Type Culture Collection (Manassas, VA, USA). All of the cell lines were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum in a humidified atmosphere with 5% CO2 at 37°C.
In vivo tumor model
Male BALB/C nude mice at 6 weeks of age were randomly divided into two groups (five mice per group). miR-149-3p mimics or miR-control stable transfection UM-UC-3 cells were suspended (1×106 cells/mL) in 100 μL of PBS were subcutaneously injected into the flanks of nude mice. Tumor growth was examined every 5 days. After 30 days, tumor samples were carefully removed and weighed.
Cell viability assay
A Cell Counting Kit-8 (CCK8) was used to assess cell viability. Cells (1×104) were seeded into a 96-well plate and incubated overnight in the previously described conditions. The cells were pre-treated with sterile water or 10 mM NAC for 2 h, then with 0.1% DMSO or 40 µM wogonin for 24 h. Then, the medium was removed and the cells were washed three times with PBS. DMEM (90 µl) and CCK8 (10 µl) were subsequently added to each well and incubated for 1.5 h at 37°C. A microplate reader was used to measure the optical density (OD) at 450 nm.
Transfection of miR-149-3p mimics and western blotting analyses
The miR-149-3p mimics and corresponding negative control (miR-NC) were purchased from GenePharma (Shanghai, China). The mimics or control oligonucleotides were transfected into UM-UC-3 cells at a final concentration of 100 nM. The transfected cells were collected 48 h post-transfection. Proteins were resolved in an SDS/PAGE gel and subjected to immunoblot analysis using monoclonal antibodies against S100A4, E-cadherin, vimentin, or GAPDH (Abcam, Cambridge, MA, USA). All antibodies were used at 1 μg/ml in PBS with 5% dried milk. Detection by enzyme-linked chemiluminescence was performed according to the manufacturer’s protocol.
Luciferase reporter assay
To construct luciferase reporter vectors, the 3’-UTR of S100A4 cDNA fragments containing the predicted potential miR-149-3p binding sites were amplified by PCR and sub-cloned downstream of the luciferase gene in the PYr-MirTarget luciferase vector (Ambion, Inc., Austin, TX, USA). The 3’-UTR of S100A4 containing the binding sites for miR-149-3p was amplified from a cDNA library with the following primers: forward, 5’-CTCGAGGTCTGCCAGCTGGGGCCCTCCCT-3’ and reverse, 5’-GCGGCCGCCTTCCAAGAATCTTTATTG-3’. The mutant 3’-UTR of S100A4, in which 8 nucleotides were mutated in the binding sites, was amplified using the following primer sequences: forward, 5’-CTCGAGGTCTGCCGAGTGGGGCGGCTGCT-3’ and reverse, 5’-GCGGCCGCCTTCCAAGAATCTTTATTG-3’.
For luciferase assays, HEK293T cells were cultured in 24-well plates and co-transfected with 50 ng of the corresponding vectors containing firefly luciferase together with 25 ng of miR-149-3p or control. Transfection was performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). At 48 h post-transfection, relative luciferase activity was calculated by normalizing the Firefly luminescence to the Renilla luminescence using a Dual-Luciferase Reporter Assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Real-time quantitative PCR analysis
Total RNA was isolated using TRIzol reagent (Invitrogen). Reverse transcription was carried out using a Rt-PCR system (Promega, Shanghai, China). Real-time quantitative Rt-PCR analysis was performed in a 20 μl final reaction volume using SYBR Green I Supermix (Takara, Dalian, China) according to the manufacturer’s protocol. All reactions were performed in triplicate on an iCycler IQ multicolor Detection System (BioRad, Hercules, CA, USA) with the following cycling parameters: 95°C for 10 s, followed by 40 cycles of 94°C for 15 s, annealing at 55°C for 30 s, and final extension at 70°C for 30 s. All quantifications were normalized to the level of human U6 snRNA in the reaction. The comparative threshold cycle (CT [DDCT]) method, which compares differences in CT values between common reference RNA and target gene RNA, was used to obtain the relative fold changes in gene expression. miR-149-3p and S100A4 primers for PCR were designed by GenePharma Co., Ltd. (Shanghai, China). The results are expressed as the mean ± SE.
Lentiviral vector construction and infection
Human S100A4 full-length cDNA was generated by PCR using forward primer (5’-CCACCGGTATGGCGTGCCCTCTGGAG-3’) and reverse primer (5’-CGGCTAACTCATTTCTTCCTGGGCTG-3’). The amplified fragment was then sub-cloned into pGC-FU-EGFP-3FLAG plasmid (GeneChem, Shanghai, China) after AgeI/NheI restriction enzyme digestion to produce pGC-FU-NEDD4-1-3FLAG.
siRNA specifically targeted to S100A4 (forward, 5’-GCCAUGAUGUGUAACGAAUUU-3’; reverse, AUUCGUUACACAUCAUGGCUU) were purchased from GenePharma Co., Ltd. Cells were plated in six-well plates and grown to ~80% confluence before transient transfections with siRNAs (100 pmol per well) were performed using Lipofectamine® 2000 (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. After a 48-h transfection, cells were collected for other experiments.
Colony formation assay
UM-UC-3 cells were harvested 24 h after transfection with RNA duplexes (50 nM of NC or miR-149-3p mimics). All of the cells were resuspended in DMEM medium supplemented with 10% FBS and seeded in six-well plates at a density of 400 cells per well. After 10 days of culture under standard conditions, the colonies on the plates were fixed with absolute methanol for 15 min and stained with 0.1% crystal violet for 20 min. The colonies with diameters > 2 mm were counted.
Wound healing assay
UM-UC-3 cells transfected with corresponding vectors were seeded in six-well plates to form the single confluent cell layer. The wounds were made with 100 μl tips in the confluent cell layer. Zero and 12 h after wound scratching, the width of the wound was photographed with a phase contrast microscope.
Transwell migration and invasion assay
The migratory and invasive ability of bladder cancer cells were evaluated with Transwell chambers (BD Biosciences, Franklin Lakes, NJ, USA). Cells (5-10×104) were suspended in 100 μl of medium without serum and seeded into the upper chamber, and the lower chamber was filled with 20% FBS to induce bladder cancer cell migration or invasion through the membrane. Matrigel (1:6 dilution; BD Biosciences) was added on the upper chamber for the invasion assay. Twenty-four hours later, cells with crystal violet (MedChem Express, Shanghai, China) staining that migrated or invaded across the Transwell membrane were counted under optical microscope.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation (SD). One-way ANOVA was carried out for multiple comparisons using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). P-values ≤ 0.05 indicate a statistically significant difference.
Results
miR-149-3p inhibits the proliferation and mobility of BCa cells
Increased cancer cell proliferation and mobility is an important reason for the metastasis and recurrence of human cancers [27,28]. Previous results have demonstrated that the expression of miR-149-3p has tumor suppression effects in many human cancers, including pancreatic and gastric cancers [17,18]. The effect of miR-149-3p in regulating the progress of BCa cells is unclear. To identify the regulatory effect of miR-149-3p, UM-UC-3 cells were used with or without transfection with miR-149-3p-NC or miR-149-3p mimics vector. After a 48-h transfection, the expression of miR-149-3p in UM-UC-3 cells was measured by Rt-PCR. The results showed that miR-149-3p expression was increased significantly (Figure 1A). CCK-8 assays showed that the expression of miR-149-3p significantly suppressed the growth of cultured BCa cells from 48 h (Figure 1B). The colony formation rates of BCa cells after the overexpression of miR-149-3p were clearly low in contrast with cells transfected with NC (Figure 1C and 1D). The wound healing assays showed that miR-149-3p overexpression notably reduced cell migration in UM-UC-3 cells (Figure 1E). Transwell assays revealed that ectopic expression of miR-149-3p significantly reduced the number of migrated and invaded UM-UC-3 cells (Figure 1F-H). Thus, miR-149-3p has an anti-metastatic and proliferation role in BCa cells.
Figure 1.
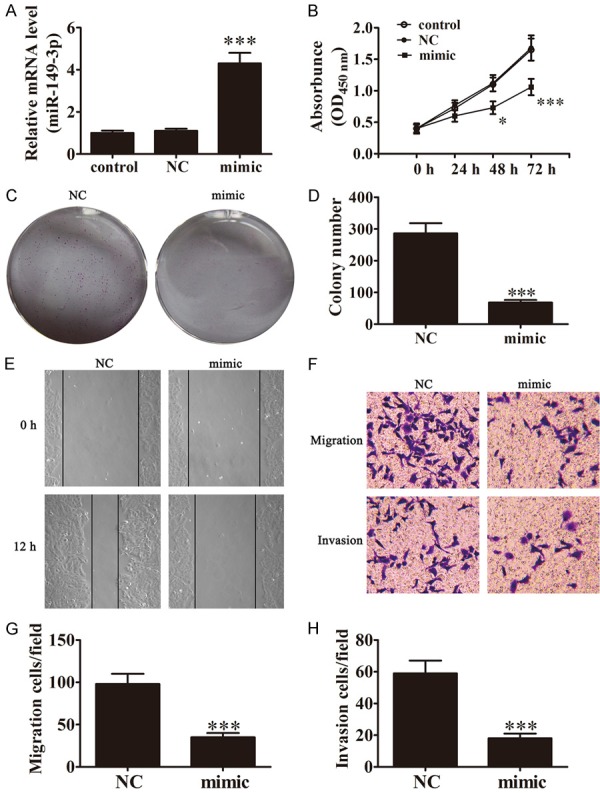
Expression of miR-149-3p inhibits the proliferation, invasion, and migration of BCa cells. A. Expression of miR-149-3p in UM-UC-3 cells was measured by Rt-PCR after transfection with miR-149-3p mimics for 48 h (n=3). Data are presented as the mean ± SD (***P < 0.001 versus control group). B. The CCK-8 assay showed that the relative viability of UM-UC-3 cells treated with miR-149-3p mimics was significantly lower than NC-treated cells (n=5). Data are presented as the mean ± SD (*P < 0.05, ***P < 0.001 versus control group). C and D. Colony formation assay show that the colony formation rates of miR-149-3p mimic-transfected UM-UC-3 cells were lower in contrast with NC-transfected cells (n=5). Data are presented as the mean ± SD (***P < 0.001 versus NC group). E. Wound healing assays indicated that miR-149-3p over0expression reverses the migration of UM-UC-3 cells. F-H. Transwell assays confirmed that miR-149-3p over-expression inhibited migration and invasion of UM-UC-3 cells (n=5). Data are presented as the mean ± SD (***P < 0.001 versus NC group).
Down-regulation of S100A4 expression phenocopied the effect of miR-149-3p
To identify if S100A4 expression has an anti-tumor effect in BCa, we transfected UMUC-3 cells with siS100A4, a small interfering RNA against S100A4, to specifically knock down the expression of S100A4. After a 48-h transfection, the expression of S100A4 was measured by western blot and Rt-PCR. The results showed that transfection of siS100A4 remarkably suppressed expression of S100A4 (Figure 2A and 2B). CCK-8 assays showed that siS100A4 significantly suppressed the growth of cultured UMUC-3 cells after 48 h as the result of over-expression of miR-149-3p (Figure 1C). The colony formation rates of BCa cells after down-regulation of S100A4 were obviously low in contrast with cells transfected with NC (Figure 1D and 1E). The wound healing assays showed that siS100A4 significantly reduced cell migration in UM-UC-3 cells (Figure 1F). Transwell assays showed that knock-down of S100A4 significantly reduced the number of migrated and invaded UM-UC-3 cells (Figure 1G-I). Thus, knock-down of S100A4 expression exerts a similar anti-metastatic and proliferation effect in BCa cells as over-expression of miR-149-3p, suggesting some internal relationships between miR-149-3p and S100A4.
Figure 2.
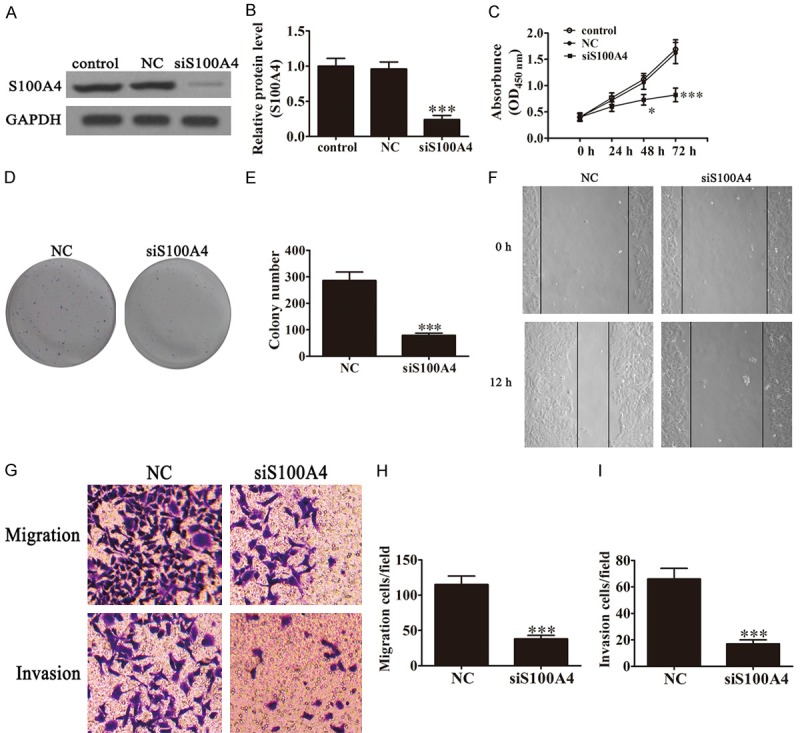
Down-regulation of S100A4 phenocopied the effect of miR-149-3p. (A and B) Expression of S100A4 in UM-UC-3 cells was measured by western blot (A) and Rt-PCR (B) after transfection with siRNA against S100A4 (n=5). Data are presented as the mean ± SD (***P < 0.001 versus control group). (C) The CCK-8 assay showed that the relative viabilities of UM-UC-3 cells were significantly decreased after down-regulating the expression of S100A4 (n=5). Data are presented as the mean ± SD (*P < 0.05, ***P < 0.001 versus control group). (D and E) Colony formation assay showed that the colony formation rates of UM-UC-3 cells were decreased after transfection with siS100A4 (n=5). Data are presented as the mean ± SD (***P < 0.001 versus NC group). (F) Wound healing assays indicated that suppression of S100A4 expression reverses the migration of UM-UC-3 cells. (G-I) Transwell assays confirmed that down-regulation of S100A4 expression inhibited the migration and invasion of UM-UC-3 cells (n=5). Data are presented as the mean ± SD (***P < 0.001 versus NC group).
Ectopic expression of S100A4 reversed the anti-tumorous effect of miR-149-3p
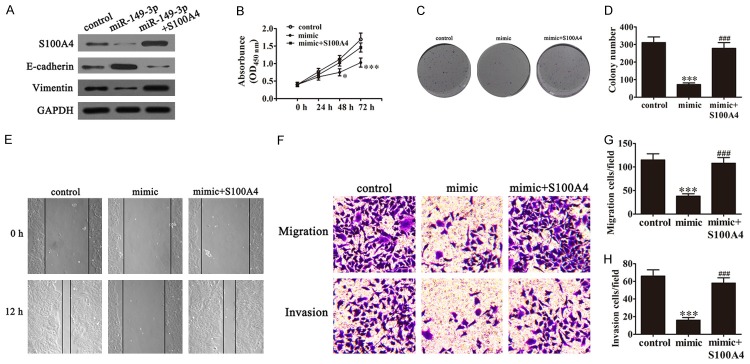
In order to identify the effect of S100A4 to miR-149-3p-induced growth inhibition of BCa cells, we transfected UM-UC-3 cells with miR-149-3p mimics and S100A4 over-expression vector alone or in combination. Western blot showed that the expression of miR-149-3p suppressed S100A4 expression. After transfection, S100A4 over-expression vector reversed the inhibitory effect of miR-149-3p to S100A4 expression. The results also showed that over-expression of miR-149-3p decreased vimentin expression, and increased E-cadherin expression in UM-UC-3 cells, which play an important role in mediating BCa migration and invasion. Over-expression of S100A4 reversed miR-149-3p-mediated migration and invasion of protein expression (Figure 3A). CCK-8 assays showed that over-expression of S100A4 significantly removed the inhibitory effect of miR-149-3p to the growth suppression of UMUC-3 cells (Figure 3B). The results of colony formation showed that over-expression of S100A4 reversed the inhibitory effect of miR-149-3p to UMUC-3 cell proliferation in vitro (Figure 3C and 3D). The wound healing assays (Figure 1E) and Transwell assays (Figure 1F-H) also showed that over-expression of S100A4 reversed the inhibitory effect of miR-149-3p to cell migration and invasion in UM-UC-3 cells. Taken together, we found that over-expression of S100A4 reversed the suppression of cell proliferation and metastasis caused by miR-149-3p.
Figure 3.
Ectopic expression of S100A4 restores the phenotypes of BCa cell lines transfected with miR-149-3p. A. Western blot shows the expression of S100A4, E-cadherin, and vimentin in UM-UC-3 cells after ectopic expression of S100A4. B. The CCK-8 assay shows that S100A4 rescues the capacity of relative viabilities decreased in UM-UC-3 cells after up-regulation of miR-149-3p expression (n=5). Data are presented as the mean ± SD. *P < 0.05 (***P < 0.001 versus control group). C and D. Colony formation assay showed that the colony formation rates of UM-UC-3 cells recovered with up-regulation of S100A4 expression after transfection with miR-149-3p mimics (n=5). Data are presented as the mean ± SD (***P < 0.001 versus control group; ###P < 0.001 versus mimic group). E. Wound healing assays indicated that the expression of S100A4 reversed the inhibitory effect of miR-149-3p on UM-UC-3 cell migration. F-H. Transwell assays confirmed that up-regulation of S100A4 expression reverses the inhibitory effect of miR-149-3p on UM-UC-3 cell migration (F and G) and invasion (F and H).
miR-149-3p regulates the expression of S100A4 by directly targeting the 3’-UTR
Knowing the basic mechanism by which miRNAs regulate mRNA expression, we determined the exact targets of miR-149-3p responsible for the anti-tumor effect. By using an online bioinformatics database (TargetScan, http://www.targetscan.org/), we found a broadly-conserved binding site between 3’UTR of S100A4 and miR-149-3p (Figure 4A). A mutated version of the S100A4 3’-UTR was generated in which 10 complementary nucleotides in the binding site were altered (Figure 4B) and this mutated construct was fused to the luciferase coding region (PYr-RGS-17 3’-UTR) and co-transfected into HEK293T cells along with miR-149-3p mimics (Figure 4C). Determination of the relative luciferase activity revealed that when the wild-type S100A4 3’-UTR was co-transfected with miR-149-3p mimics, S100A4 expression was significantly decreased compared to co-transfection with control miRNA. This effect was not observed, however, when the mutant S100A4 3’-UTR construct was used for co-transfection, indicating the specific targeting and suppression of the 3’-UTR of S100A4 by miR-149-3p. Western blot was used to check the levels of S100A4 expression after transfection with a miR-149-3p mimic or miR-203-NC. The expression of S100A4 were significantly suppressed in UM-UC-3 cells with miR-149-3p overexpression (Figure 4D).
Figure 4.
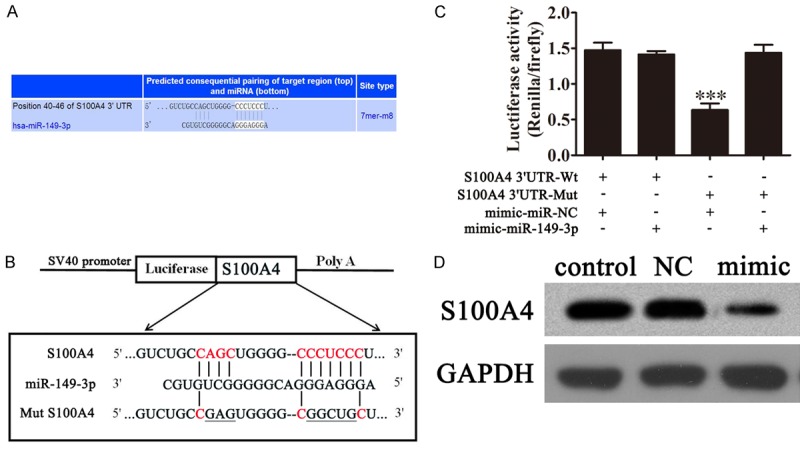
S100A4 is a potential target of miR-149-3p. A, B. Complementary sequences between miR-149-3p and 3’-UTR of S100A4 mRNA were obtained using publicly available algorithms. The mutated version of the USP22 3’-UTR is also shown. C. The 3’-UTR of S100A4 was fused to the luciferase coding region (PYr-RGS-17 3’-UTR) and co-transfected into HEK293T cells with miR-149-3p mimics to confirm that S100A4 is the target of miR-149-3p. The PYr-RGS-17 3’-UTR and miR-149-3p mimic constructs were co-transfected into HEK293T cells with a control vector and the relative luciferase activity was determined 48 h after transfection. The data are presented as the mean ± SD (***P<0.001 versus the control). D. Western blot analysis of the effect of S100A4 expression on UM-UC-3 cells after transfection with miR-149-3p mimics (n=5). Levels of GAPDH expression were detected as an endogenous control.
miR-149-3p expression inhibits tumor growth in vivo
To determine whether or not miR-149-3p is involved in tumorigenesis in vivo, a xenograft tumor model was used in nude mice. One hundred microliters (1×106 cells/mL) of UM-UC-3 cells transfected with miR-149-3p mimics or miR-149-3p-NC were subcutaneously injected into the flanks of nude mice. Compared with the miR-149-3p-NC group, the miR-149-3p over-expression group showed dramatically reduced tumor volume and weight during the 30 days after the tumors became palpable (Figure 5A-C). Western blot showed that S100A4 expression in tumor tissue from the miR-149-3p over-expression group was significantly decreased compared with the miR-149-3p-NC group (Figure 5D), suggesting that miR-149-3p inhibits BCa proliferation by targeting S100A4 in vivo.
Figure 5.
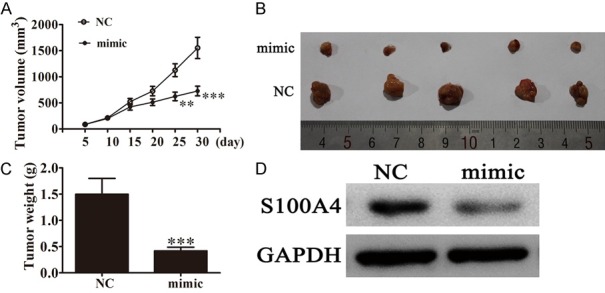
The expression of miR-149-3p suppressed tumor growth of bladder cancer in a xenograft model. A. Growth curves for tumor volumes in xenografts of nude mice were established based on the tumor volume measured every 5 days until 30 days (n=5; *P < 0.05, **P < 0.01, ***P < 0.001 versus NC group). B. Photographs of tumor tissue with different groups on day 30. C. Tumor weights of different groups on day 30 (n=5; ***P < 0.001 versus NC group). D. S100A4 level in tumor tissues of different groups was determined by western blot (n=5). Level of GAPDH expression was detected as an endogenous control.
Discussion
Emerging evidence has confirmed that miRNAs play a critical role in carcinogenesis and cancer progression [29,30]. Individual miRNAs have been ascribed oncogenic and tumor suppressor functions [31-33], and aberrant miRNA expression has been implicated in many malignancies, including BCa [34-37]. Profiling and functional studies have demonstrated potential novel prognostic value for a number of miRNAs in BCa. Previous research has shown that the role of miR-149-3p (also known as miR-149) is different with respect to the progression of different types of tumors, include colonic carcinoma, myeloid leukemia, hepatocellular carcinoma, and renal cell carcinoma [38-41]. Ectopic expression of miR-149 represses metastasis of hepatocellular carcinoma by targeting the actin-regulatory protein, PPM1F [42]. In colorectal cancer, miR-149 significantly suppresses cell migration and invasion by directly targeting forkhead box transcription factor FOXM1 [43]. Here, we report that miR-149-3p expression significantly inhibits cell proliferation, migration, and invasion in BCa cells. Furthermore, we also verified that S100A4 is a direct target gene of miR-149-3p in BCa cells.
S100A4 is a member of the S100 family of calcium-binding proteins that is directly involved in tumor metastasis. S100A4 is over-expressed in most malignancies, such as breast [44], thyroid [26], pancreatic [45], gastric [46] and bladder cancers [22,47]. S100A4 expression is correlated with metastasis and patient outcome, suggesting that enhanced S100A4 expression contributes to the manifestation of a metastatic phenotype. Based on the experimental and clinicopathologic evidence, S100A4 has emerged as a promising therapeutic target for BCa [21,48,49].
In summary, our research revealed that miR-149-3p may function as a tumor suppressor in the UM-UC-3 cell line by targeting S100A4. Therefore, up-regulation of S100A4 promotes BCa cell proliferation, invasion, and migration. Silencing of S100A4 significantly reduces cell invasiveness. Moreover, our results showed that S100A4 may function as an oncogene in the development of BCa. Therefore, miR-149-3p may serve as a therapeutic target for BCa treatment; however, because each miRNA may regulate many target genes that can affect carcinogenesis in different ways, more detailed molecular and cellular mechanisms underlying the action of miR-149-3p targets are warranted.
Acknowledgements
This study was supported by the Shanghai Science and Technology Commission Scientific Research Project (15ZR1425500).
Disclosure of conflict of interest
None.
References
- 1.Chen H, Lin YW, Mao YQ, Wu J, Liu YF, Zheng XY, Xie LP. MicroRNA-449a acts as a tumor suppressor in human bladder cancer through the regulation of pocket proteins. Cancer Lett. 2012;320:40–47. doi: 10.1016/j.canlet.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205. doi: 10.1016/j.eururo.2005.04.006. discussion 205-206. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Zhou J, Wu K, Huang J, Ding Y, Yun EJ, Wang B, Ding C, Hernandez E, Santoyo J, Chen H, Lin H, Sagalowsky A, He D, Hsieh JT. Targeting XBP1-mediated beta-catenin expression associated with bladder cancer with newly synthetic Oridonin analogues. Oncotarget. 2016;7:56842–56854. doi: 10.18632/oncotarget.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Froehner M, Koch R, Heberling U, Novotny V, Oehlschlaeger S, Hubler M, Baretton GB, Hakenberg OW, Wirth MP. Decreased overall and bladder cancer-specific mortality with adjuvant chemotherapy after radical cystectomy: multivariable competing risk analysis. Eur Urol. 2016;69:984–987. doi: 10.1016/j.eururo.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 7.Deng G, Zeng S, Ma J, Zhang Y, Qu Y, Han Y, Yin L, Cai C, Guo C, Shen H. The anti-tumor activities of Neferine on cell invasion and oxaliplatin sensitivity regulated by EMT via Snail signaling in hepatocellular carcinoma. Sci Rep. 2017;7:41616. doi: 10.1038/srep41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villegas-Pineda JC, Toledo-Leyva A, Osorio-Trujillo JC, Hernandez-Ramirez VI, Talamas-Rohana P. The translational blocking of alpha5 and alpha6 integrin subunits affects migration and invasion, and increases sensitivity to carboplatin of SKOV-3 ovarian cancer cell line. Exp Cell Res. 2017;351:127–134. doi: 10.1016/j.yexcr.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Ding K, Yuan Y, Chong QY, Yang Y, Li R, Li X, Kong X, Qian P, Xiong Z, Pandey V, Ma L, Wu Z, Lobie PE, Zhu T. Autocrine prolactin stimulates endometrial carcinoma growth and metastasis and reduces sensitivity to chemotherapy. Endocrinology. 2017;158:1595–1611. doi: 10.1210/en.2016-1903. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, Zhuang Q, Cui L. MiR-194 inhibits cell proliferation and invasion via repression of RAP2B in bladder cancer. Biomed Pharmacother. 2016;80:268–275. doi: 10.1016/j.biopha.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Shen F, Wang C, Lu W, Wei J, Shang A. MiR-1-3p inhibits the proliferation and invasion of bladder cancer cells by suppressing CCL2 expression. Tumour Biol. 2017;39:1010428317698383. doi: 10.1177/1010428317698383. [DOI] [PubMed] [Google Scholar]
- 14.Lu S, Wang MS, Chen PJ, Ren Q, Bai P. miRNA-186 inhibits prostate cancer cell proliferation and tumor growth by targeting YY1 and CDK6. Exp Ther Med. 2017;13:3309–3314. doi: 10.3892/etm.2017.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Shi C, Cao L, Zhong L, Wang D. MicroRNA-320a is downregulated in non-small cell lung cancer and suppresses tumor cell growth and invasion by directly targeting insulin-like growth factor 1 receptor. Oncol Lett. 2017;13:3247–3252. doi: 10.3892/ol.2017.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan L, Wang Y, Liang J, Liu Z, Sun X, Cai K. MiR-301b promotes the proliferation, mobility and epithelial-to-mesenchymal transition of bladder cancer cells by targeting EGR1. Biochem Cell Biol. 2017;95:571–577. doi: 10.1139/bcb-2016-0232. [DOI] [PubMed] [Google Scholar]
- 17.Si L, Xu L, Yin L, Qi Y, Han X, Xu Y, Zhao Y, Liu K, Peng J. Potent effects of dioscin against pancreatic cancer via miR-149-3P-mediated inhibition of the Akt1 signalling pathway. Br J Pharmacol. 2017;174:553–568. doi: 10.1111/bph.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao D, Jia Z, You L, Wu Y, Hou Z, Suo Y, Zhang H, Wen S, Tsukamoto T, Oshima M, Jiang J, Cao X. 18beta-glycyrrhetinic acid suppresses gastric cancer by activation of miR-149-3p-Wnt-1 signaling. Oncotarget. 2016;7:71960–71973. doi: 10.18632/oncotarget.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002;7:d1356–1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- 20.Ebralidze A, Tulchinsky E, Grigorian M, Afanasyeva A, Senin V, Revazova E, Lukanidin E. Isolation and characterization of a gene specifically expressed in different metastatic cells and whose deduced gene product has a high degree of homology to a Ca2+-binding protein family. Genes Dev. 1989;3:1086–1093. doi: 10.1101/gad.3.7.1086. [DOI] [PubMed] [Google Scholar]
- 21.Sagara Y, Miyata Y, Iwata T, Kanda S, Hayashi T, Sakai H, Kanetake H. Clinical significance and prognostic value of S100A4 and matrix metalloproteinase-14 in patients with organconfined bladder cancer. Exp Ther Med. 2010;1:27–31. doi: 10.3892/etm_00000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Zhao Z, Ma Y, Wang H, Ma J, He X, Zhang D. Combined expression of S100A4 and Annexin A2 predicts disease progression and overall survival in patients with urothelial carcinoma. Urol Oncol. 2014;32:798–805. doi: 10.1016/j.urolonc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Bansal N, Gupta AK, Gupta A, Sankhwar SN, Mahdi AA. Serum-based protein biomarkers of bladder cancer: a pre- and post-operative evaluation. J Pharm Biomed Anal. 2016;124:22–25. doi: 10.1016/j.jpba.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Mudduluru G, Ilm K, Fuchs S, Stein U. Epigenetic silencing of miR-520c leads to induced S100A4 expression and its mediated colorectal cancer progression. Oncotarget. 2017;8:21081–21094. doi: 10.18632/oncotarget.15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egeland EV, Boye K, Park D, Synnestvedt M, Sauer T, Naume B, Borgen E, Maelandsmo GM. Prognostic significance of S100A4-expression and subcellular localization in early-stage breast cancer. Breast Cancer Res Treat. 2017;162:127–137. doi: 10.1007/s10549-016-4096-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, Yu M, Hao F, Dong A, Chen D. Knockdown of S100A4 blocks growth and metastasis of anaplastic thyroid cancer cells in vitro and in vivo. Cancer Biomark. 2016;17:281–291. doi: 10.3233/CBM-160640. [DOI] [PubMed] [Google Scholar]
- 27.Wu D, Niu X, Tao J, Li P, Lu Q, Xu A, Chen W, Wang Z. MicroRNA-379-5p plays a tumor-suppressive role in human bladder cancer growth and metastasis by directly targeting MDM2. Oncol Rep. 2017;37:3502–3508. doi: 10.3892/or.2017.5607. [DOI] [PubMed] [Google Scholar]
- 28.Arlt F, Stein U. Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol. 2009;41:2356–2359. doi: 10.1016/j.biocel.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 30.Piao Y, Piao M, Ryu KH. Multiclass cancer classification using a feature subset-based ensemble from microRNA expression profiles. Comput Biol Med. 2017;80:39–44. doi: 10.1016/j.compbiomed.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Qiu Z, Guo W, Wang Q, Chen Z, Huang S, Zhao F, Yao M, Zhao Y, He X. MicroRNA-124 reduces the pentose phosphate pathway and proliferation by targeting PRPS1 and RPIA mRNAs in human colorectal cancer cells. Gastroenterology. 2015;149:1587–1598. e1511. doi: 10.1053/j.gastro.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto K, Seki N, Matsushita R, Yonemori M, Yoshino H, Nakagawa M, Enokida H. Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Br J Cancer. 2016;115:354–363. doi: 10.1038/bjc.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu T, Qin L, Zhu Z, Wang X, Liu Y, Fan Y, Zhong S, Zhang X, Xia L, Xu C, Shen Z. MicroRNA-31 functions as a tumor suppressor and increases sensitivity to mitomycin-C in urothelial bladder cancer by targeting integrin alpha5. Oncotarget. 2016;7:27445–27457. doi: 10.18632/oncotarget.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong DA, Green BB, Seigne JD, Schned AR, Marsit CJ. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol Cancer. 2015;14:194. doi: 10.1186/s12943-015-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrew AS, Marsit CJ, Schned AR, Seigne JD, Kelsey KT, Moore JH, Perreard L, Karagas MR, Sempere LF. Expression of tumor suppressive microRNA-34a is associated with a reduced risk of bladder cancer recurrence. Int J Cancer. 2015;137:1158–1166. doi: 10.1002/ijc.29413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X, Du L, Wang L, Li J, Liu Y, Zheng G, Qu A, Zhang X, Pan H, Yang Y, Wang C. Serum microRNA expression signatures identified from genome-wide microRNA profiling serve as novel noninvasive biomarkers for diagnosis and recurrence of bladder cancer. Int J Cancer. 2015;136:854–862. doi: 10.1002/ijc.29041. [DOI] [PubMed] [Google Scholar]
- 37.Jia AY, Castillo-Martin M, Bonal DM, Sanchez-Carbayo M, Silva JM, Cordon-Cardo C. MicroRNA-126 inhibits invasion in bladder cancer via regulation of ADAM9. Br J Cancer. 2014;110:2945–2954. doi: 10.1038/bjc.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian P, Yan L. Inhibition of MicroRNA-149-5p induces apoptosis of acute myeloid leukemia cell line THP-1 by targeting fas ligand (FASLG) Med Sci Monit. 2016;22:5116–5123. doi: 10.12659/MSM.899114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin L, Zhang YD, Chen ZY, Chen Y, Ren CP. The clinicopathological significance of miR-149 and PARP-2 in hepatocellular carcinoma and their roles in chemo/radiotherapy. Tumour Biol. 2016;37:12339–12346. doi: 10.1007/s13277-016-5106-y. [DOI] [PubMed] [Google Scholar]
- 40.Zhang G, Liu X, Li Y, Wang Y, Liang H, Li K, Li L, Chen C, Sun W, Ren S, Zhu P, Zhang L. EphB3-targeted regulation of miR-149 in the migration and invasion of human colonic carcinoma HCT116 and SW620 cells. Cancer Sci. 2017;108:408–418. doi: 10.1111/cas.13161. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Jin L, Li Y, Liu J, Yang S, Gui Y, Mao X, Nie G, Lai Y. Tumor suppressor miR-149-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Mol Med Rep. 2016;13:5386–5392. doi: 10.3892/mmr.2016.5205. [DOI] [PubMed] [Google Scholar]
- 42.Luo G, Chao YL, Tang B, Li BS, Xiao YF, Xie R, Wang SM, Wu YY, Dong H, Liu XD, Yang SM. miR-149 represses metastasis of hepatocellular carcinoma by targeting actin-regulatory proteins PPM1F. Oncotarget. 2015;6:37808–37823. doi: 10.18632/oncotarget.5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu K, Liu X, Mao X, Xue L, Wang R, Chen L, Chu X. MicroRNA-149 suppresses colorectal cancer cell migration and invasion by directly targeting forkhead box transcription factor FOXM1. Cell Physiol Biochem. 2015;35:499–515. doi: 10.1159/000369715. [DOI] [PubMed] [Google Scholar]
- 44.Ismail TM, Bennett D, Platt-Higgins AM, Al-Medhity M, Barraclough R, Rudland PS. S100A4 elevation empowers expression of metastasis effector molecules in human breast cancer. Cancer Res. 2017;77:780–789. doi: 10.1158/0008-5472.CAN-16-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang S, Zheng J, Huang Y, Song L, Yin Y, Ou D, He S, Chen X, Ouyang X. Impact of S100A4 expression on clinicopathological characteristics and prognosis in pancreatic cancer: a metaanalysis. Dis Markers. 2016;2016:8137378. doi: 10.1155/2016/8137378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S, Chen D, Shen W, Chen L, Yu A, Fu H, Sun K, Sun X. EZH2 mediates the regulation of S100A4 on E-cadherin expression and the proliferation, migration of gastric cancer cells. Hepatogastroenterology. 2015;62:737–741. [PubMed] [Google Scholar]
- 47.Ismail MF, El Boghdady NA, Shabayek MI, Awida HA, Abozeed H. Evaluation and screening of mRNA S100A genes as serological biomarkers in different stages of bladder cancer in Egypt. Tumour Biol. 2016;37:4621–4631. doi: 10.1007/s13277-015-4264-7. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto K, Irie A, Satoh T, Ishii J, Iwabuchi K, Iwamura M, Egawa S, Baba S. Expression of S100A2 and S100A4 predicts for disease progression and patient survival in bladder cancer. Urology. 2007;70:602–607. doi: 10.1016/j.urology.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Davies BR, O’Donnell M, Durkan GC, Rudland PS, Barraclough R, Neal DE, Mellon JK. Expression of S100A4 protein is associated with metastasis and reduced survival in human bladder cancer. J Pathol. 2002;196:292–299. doi: 10.1002/path.1051. [DOI] [PubMed] [Google Scholar]