Abstract
The functional phenotypes (M1 and M2) of tumor-associated macrophages (TAMs) are influenced by the tumor microenvironment (TME) and contribute greatly to the development of non-small cell lung cancer (NSCLC). However, the molecular mechanisms for TAM polarization remain unclear. Angiopoietin-like protein 2 (Angptl2) is involved in tumor progression. In this study, Angptl2 expression was aberrantly increased in NSCLC cells and positively correlated with TAM infiltration, tumor size and poor patient survival. Moreover, in vitro tumor cell-macrophage co-culture and recombinant protein stimulation revealed that Angptl2 fostered the M2 polarization of TAMs through the p65 nuclear factor-kappa B (NF-ĸB) pathway. In addition, Angptl2-promoted TAM enhanced proliferation, invasion, and migration of NSCLC cells and the tube formation of human umbilical vein endothelial cells (HUVECs). In vivo, TAM depletion inhibited the tumor growth induced by Angptl2. Here, for the first time, we determined that Angptl2 promoted the M2 polarization of TAMs and enhanced NSCLC progression. Interfering with Angptl2 might be an effective strategy for reprogramming TAM polarization in NSCLC, providing a promising therapy for NSCLC treatment.
Keywords: Angptl2, M2 macrophages, NSCLC, NF-ĸB, tumor progression
Introduction
Lung cancer is the leading cause of death from cancer worldwide. Based on histology, approximately 80% of lung cancers are non-small cell lung cancer (NSCLC) [1]. The incidence of NSCLC is increasing annually because of growing smoking rates and environmental pollution. Most NSCLC cases are diagnosed at a late stage. Despite advances in treatment, its prognosis is still poor. Therefore, the identification of molecular mechanisms underlying its metastasis and progression can improve the effects of NSCLC therapies.
Chronic inflammation plays roles at different stages of cancer development [2]. As a secreted inflammatory glycoprotein, Angptl2 is structurally similar to angiopoietin and belongs to the Angiopoietin-like proteins (Angptls) family [3]. Physical Angptl2 signaling maintains tissue homeostasis via adaptive inflammation [4], whereas over activation of Angptl2 signaling causes pathological tissue remodeling [5,6]. Angptl2 is highly expressed in various cancer types, including NSCLC [7-11], and facilitates cancer metastasis by promoting tumor cell invasion and angiogenesis [12,13]. Previous studies have focused on how tumor cell-derived Angptl2 directly affected tumor progression. Whether the action of Angptl2 on stromal cells is involved in Angptl2-driven tumor behaviors remains unknown.
Macrophages that infiltrate tumor tissues are referred to as tumor-associated macrophages (TAMs) and are prominent in the stromal compartment. They have two well-established phenotypes, classically (M1)- and alternatively (M2)-activated macrophages [14,15], which play different functions in tumor progression. M1 macrophages are potent effector cells that kill microorganisms and are viewed as anti-neoplastic [16]. They primarily produce pro-inflammatory cytokines, such as tumor necrosis factor-α (TNFα) and interleukin (IL) 12 [17]. M2 macrophages are immunosuppressive and tumor promotive. They produce immunosuppressive, cell growth and angiogenesis factors, such as IL10 and vascular endothelial growth factors (VEGFs) [18,19]. TAMs reprogram their functional phenotype in response to cues from the tumor microenvironment (TME), such as tumor-derived signals and tissue-specific signals [20,21]. Thus, the TAM phenotype rather than the number of TAMs regulates tumorigenesis [22].
Both Angptl2 and TAMs are involved in carcinogenesis and tumor development. Tumor cell-derived Angptl2 increases tumor cell motility and angiogenesis, further accelerates metastasis and shortens survival periods in tumor cell-implanted mouse models [13]. Tumor cell-derived Angptl2 enhances tumor cell invasion and tumor cell intravasation of blood vessels by increasing the expression and activity of matrix metalloproteinases (MMPs), such as MMP-1 and MMP-9 in osteosarcoma cells [23]. In addition, TAMs promote tumor progression by secreting platelet derived growth factor (PDGF) and releasing various angiogenesis modulating enzymes, including MMP-2, MMP-9, and cyclooxygenase-2 [24,25]. Moreover, Angptl2 recruits macrophages in adipose tissue inflammation [26]. These studies suggest that Angptl2 may influence TAM function.
Our data indicate that NSCLC cell-derived Angptl2 induced TAM M2 polarization, which in turn fostered NSCLC progression, suggesting that targeting Angptl2 can contribute to NSCLC treatment.
Materials and methods
Ethical statement
This study was carried out in accordance with ethical standards and was approved by the Institutional Review Board of the Jinan Central Hospital Authority.
Patients and samples
A total of eighty-one tumor specimens from patients undergoing surgical resections for NSCLC at Jinan Central Hospital Affiliated to Shandong University, China, were collected. None of the patients underwent chemo- or radiotherapy prior to surgical resection. Of all the patients included in the study, 58 were male, and 23 were female, and the average patient age was 61.8 years at the time of operation. Of these 81 tumors, 43 were adenocarcinomas, 34 were squamous cell carcinoma, and 4 were other types of tumors. The patients were classified according to the Union for International Cancer Control, 7th edition, staging system for NSCLC.
Immunohistochemical (IHC) staining
Human primary NSCLC tissue and murine transplanted tumor specimens were paraffin-embedded and sectioned. After the sections were de-paraffinized and rehydrated, antigen retrieval was performed by heating the specimens in 10 mmol/L sodium citrate (pH 6.0) for 10 min in a microwave oven. After naturally cooling the sections, they were incubated in 3% H2O2 for 10 min and then blocked in 10% goat serum at room temperature for 1 h. Anti-Angptl2 polyclonal rabbit antibody (1:50 dilution; Abcam, Cambridge, UK), anti-CD68 mouse monoclonal antibody (1:400 dilution; Abcam), anti-CD34 rabbit monoclonal antibody (1:250 dilution; Abcam), anti-F4/80 rat monoclonal antibody (1:50 dilution; Abcam) were added and incubated with the tissues overnight at 4°C. To detect the primary antibody, the sections were incubated with the Elivision Plus Polymer Horseradish Peroxidase (Mouse/Rabbit) IHC Kit (25 min) and streptavidin-conjugated peroxidase (25 min) at room temperature. Finally, sections were visualized with 3,3’-diaminobenzidine solution (MXB) and counterstained with hematoxylin. Negative controls were prepared using normal mouse and rabbit IgG instead of the primary antibody. All the staining results were scored by two independent pathologists. For Angptl2, the proportion score represented the estimated fraction of positive tumor cells (0=none; 1=less than 25%; 2=25-75%; 3=greater than 75%); the intensity score represented the estimated average staining intensity of positive tumor cells (0=none; 1=weak; 2=intermediate; 3=strong); and the overall amount of protein is presented as the total score of the proportion and intensity scores (ranges=0-9). Angptl2 expression levels were defined by a final score, either a low expression level (score <3) or a high expression level (score ≥3). The numbers of CD68+ macrophages in five, high-power fields of view of the tumors and stroma were counted (×200).
Cell culture
THP-1 human monocytes and human umbilical vein endothelial cells (HUVECs) were purchased from the Cell Bank, Institute of Biochemistry and Cell Biology, China Academy of Sciences (Shanghai, China). NSCLC cell lines (A549, H1299, H1650, H1975, and H226) and the lung/brunch normal epithelial cell line, BEAS-2B, were purchased from the Cell Resource Center of the Chinese Academy of Sciences (Beijing, China). THP-1, H226, H1299, H1650 and H1975 cells were cultured in RPMI 1640 medium (HyClone, South Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, HyClone), 20 μg/mL penicillin, and 20 μg/mL streptomycin (Sigma, St.Louis, MO, USA); HUVECs, A549 and BEAS-2B cells were cultured in DMEM (HyClone) supplemented with 10% FBS (HyClone). All cells were cultured at 37°C in a humidified atmosphere of 5% CO2 in air.
Cell treatments
For experiments, THP-1 cells were seeded in 6-well plates and differentiated into macrophages by adding 100 ng/ml phorbol 12-myristate 13-acetate (PMA, Abcam) for 72 h. Angptl2 fusion protein (Proteintech, Wuhan, China), pyrimidine dithiocarbamate (PDTC) (Abcam) and AS1517499 (Axon Medchem, The Netherlands) were all purchased from commercial sources. For treatment with inhibitors, the cells were pretreated with either 100 nM AS1517499, 100 μM PDTC, or 0.1% DMSO 30 min before stimulation with rAngptl2.
Cell transfections
Lentivirus carried overexpression Angptl2 were built by Genechem Co. (Shanghai, China). H1299 cells were inoculated in 6-well plates 24 h prior to infection. For infection, 1 ml of fresh medium containing 10 μl of lentivirus (1×109 TU/ml) was added to each well, and after 72 h, the infection rate was observed using a fluorescence microscope, and Angptl2 expression was detected by RT-PCR and western blot. Plasmid shAngptl2 (Genepharma Co., Shanghai, China) was transfected into A549 cells; non-targeting plasmid (shNC) was used as a negative control. X-tremeGENE HP Reagents (Roche, Basel, Switzerland) were used according to the manufacturer’s instructions. A549 cells (2×105) were transfected with 2 μg of plasmid in 6-well plates. The interference sequences are listed in Table 1.
Table 1.
Interference sequences targeting Angptl2
| shRNA name | Sense (5’-3’) | Anti-sense (5’-3’) |
|---|---|---|
| shAngptl2 | CGCAAAGTCTTTGCAGAAT | ATTCTGCAAAGACTTTGCG |
| shNC | TTCTCCGAACGTGTCACGT | ACGTGACACGTTCGGAGAA |
Co-culture experiments
THP-1-derived macrophages were plated into 6-well dishes (2×106 cells per well) in the culture media. A549-shNC, A549-shAngptl2, H1299-MOCK, and H1299-Angptl2 cells (1.5 to 2×106 cells per insert) were plated directly on Transwell inserts (0.4 mm, Corning, New York, USA) in culture medium. Prior to co-culture, lung cancer cells and macrophages were washed with RPMI containing 0.1% bovine serum albumin (BSA). After the last wash, the appropriate basal medium was added to the macrophages, and inserts containing lung cancer cells were placed in each well.
Western blot
The proteins were separated on a 10% SDS-PAGE gel and transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Subsequently, the membrane was blocked and incubated overnight at 4°C with the primary antibody, which included anti-Angptl2 pAb (1:800, Abcam), anti-phospho-p65 (1:2000, Abcam), anti-p65 pAb (1:1000, Santa Cruz), anti-phospho-Erk pAb (1:800, Abcam), anti-Erk mAb (1:800, Abcam), anti-phospho-Akt mAb (1:1000, Proteintech), anti-Akt mAb (1:1000, Proteintech), anti-phospho-p38 pAb (1:800, Abcam), anti-p38 mAb (1:800, Abcam), anti-phospho-Stat6 pAb (1:800, Abcam) and anti-Stat6 mAb (1:800, Abcam). Afterwards, the blots were labeled for 1 h with HRP-conjugated secondary antibodies (1:5000, Proteintech). Finally, the blots were exposed to the ChemiDoc™ XRS+ system (Bio-Rad, Hercules, CA, USA). The same membrane was probed for GAPDH (1:10000, Proteintech) as a loading control.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s protocol. cDNAs were synthesized from total RNA (2 μg) using oligodT primers with the reverse transcription kit (Fermentas, Burlington, Ontario, Canada). RT-PCR involved the ABI7500 sequence detector (Applied Biosystems, CA, USA). The forward and reverse primer sequences are listed in Table 2. All mRNA that was detected was normalized to GAPDH expression. Gene expression for RT-PCR was calculated as the change relative to the control (2-ΔΔCt).
Table 2.
Primer sequences for RT-PCR
| Forward (5’-3’) | Reverse (5’-3’) | |
|---|---|---|
| Angptl2 | GCGACCAGAGACACGACC | CAGGCGGAAACTGGCGTATT |
| IL10 | GCTGTCATCGATTTCTTCCC | CTCATGGCTTTGTAGATGCCT |
| Arg1 | CACTCCCCTGACAACCAGCT | AGGACACAGGTTGCCCATG |
| IL12 | AGGGCCGTCAGCAACATG | TCTTCAGAAGTGCAAGGGTAAAATTC |
| TNFα | ATGAGAAGTTCCCAAATGGCC | ACGTGGGCTACAGGCTTGTC |
| GAPDH | ACCCACTCCTCCACCTTTGA | GTCCACCACCCTGTTGCTGTA |
Flow cytometry
Samples were incubated with FITC-CD206 (BD Biosciences, San Jose, CA, USA)/APC-CD14 (BD Biosciences), PE-conjugated CD209 (BD Biosciences)/APC-CD14 (BD Biosciences) antibodies according to the manufacturers’ instructions. Flow cytometry was performed using a Guava® easyCyte 6HT-2L (Millipore), and the results were analyzed with guavaSoft™ 3.1.1 software (Millipore). For each sample, at least 1×104 cells should be analyzed.
Cell proliferation assay
Cell proliferation was assessed with the CCK8 (Cell Counting Kit-8) assay. Cells were plated in 96-well culture plates at 1×103 cells per well containing 0.2 mL of different kinds of conditioned media (CM). After incubation for 24 h, 48 h and 72 h, 10 µl of CCK8 solution was added to each well, incubated for 4 h at 37°C, and then measured at 450 nm. Assays were repeated at least three times.
Wound-healing assay
Cells were seeded at a density of 1×105 cells/mL in 6-well plates and incubated until a 90% density. Then, a scratch wound was made across the center of the monolayer of cells in each well using a sterile 200 μl pipette tip. This was followed by incubation in the indicated CM for 24 hours. Images of the cells that had migrated into the cell-free scratch wound area were acquired and the migration distance was measured under an inverted microscope. The scratch wound widths were determined by the relative percentage compared to untreated control cells.
Transwell assay
Matrigel (BD Transduction Laboratories, San Jose, USA) was melted and stored at 4°C overnight the day before the experiment. A pipette tip was pre-cooled in ice-cold distilled water for 0.5 h before the experiment, and the ECM gel was diluted in a 1:8 ratio with serum-free medium. Then, 100 μl of Matrigel was added to the upper chambers; the entire process was performed on ice. Then, the cells were placed in an incubator at 37°C for 5 h, after which 105 cells in the logarithmic growth phase/mL were added to each well for a total volume of 200 μl. Then, 500 μl of the indicated CM was added to the lower chamber. After culturing for 24 h, the medium was discarded, and a cotton swab was used to gently remove cells from the upper layer of the Transwell. The Transwell membrane was fixed with methanol for 20 min, washed with PBS 3 times, and then stained with 0.1% crystal violet for 20 min after airing. The invasive cell numbers from 5 fields of view (×200) (upper and lower, left and right, middle) were counted under a microscope. The mean value for each was calculated and statistically analyzed. The cells in each group were treated in triplicate and the experiments were repeated three times.
Cell migration
Cell migration was determined using Transwell insert chambers (8-mm pore size; Corning). Approximately 3×104 THP-1-derived cells were added to the upper chamber. Indicated CM was added to the lower chamber as a chemoattractant. After incubating at 37°C for 24 h, the migrated cells were fixed with 20% methanol, stained with 0.1% crystal violet (Invitrogen), and the number of cells in three fields (×200) of each sample was counted. The mean number of migrated cells was calculated.
Tube formation assay
HUVECs (2×104 cells/well) were seeded into a 96-well plate that had been pre-coated with 50 μl of Matrigel and cultured with CM. The formation of tube-like structures was monitored by microscopic observation (×50). The average number of tubules was calculated from three fields in each sample.
In vivo animal study
Female nude BALB/c mice, aged 6-8 weeks, were purchased from Beijing HFK Bioscience (Beijing, China). Selective macrophage depletion, by means of the macrophage suicide technique utilizing liposome-mediated intracellular delivery of dichloromethylene-biphosphonate is a well-established experimental protocol [27,28]. Lentivirus carrying overexpressed Angptl2-infected H1299 cells (1×106) were inoculated into the subcutaneous left axillary of the mice (n=5), and cancer progression was evaluated. Seven days after the tumor cell inoculations, the mice were injected with Clophosome-clodronate liposomes (CCL; FormuMax Scientific, USA) or control neutral liposomes (CNL; FormuMax Scientific, USA) at initial doses of 0.2 ml per mouse, followed by 0.1 ml per mouse twice a week for 4 weeks.
Tumor sizes were monitored every 7 days using calipers and tumor volumes were calculated according to the formula: L×S2×0.5, (L: longest diameter; S: shortest diameter of tumor). Microvessel density (MVD) was counted in high-power fields (HPFs) (×400). Each tissue section was assigned 5 fields, the average of which was calculated as the MVD [29]. The number of cells positive for F4/80+ was also counted in 5 HPFs of the transplanted tumor tissues.
Statistical analysis
All data were expressed as the mean ± SD. Cell experiments were performed in triplicate and a minimum of three independent experiments were evaluated. Differences were assessed for statistical significance using GraphPad Prism and SPSS. The statistical significance of differences between groups was determined by Student’s t test. A p-value <0.05 was considered statistically significant.
Results
Highly expressed Angptl2 in human NSCLC samples correlated with TAM infiltration, tumor size and overall survival
NSCLC samples were evaluated for Angptl2 expression by IHC staining. The positive expression of Angptl2 protein showed brownish yellow to brown particles, mainly expressed in the cytoplasm of NSCLC cells (Figure 1A). There were 58 cases (58/81, 71.6%) showing Angptl2-positive expression in NSCLC tissues, but only 16 cases (16/54, 29.6%) showing Angptl2-positive expression in adjacent normal tissues. A significant difference in the Angptl2-positive rate was found between NSCLC tissues and adjacent normal tissues (**p<0.01, Figure 1B).
Figure 1.
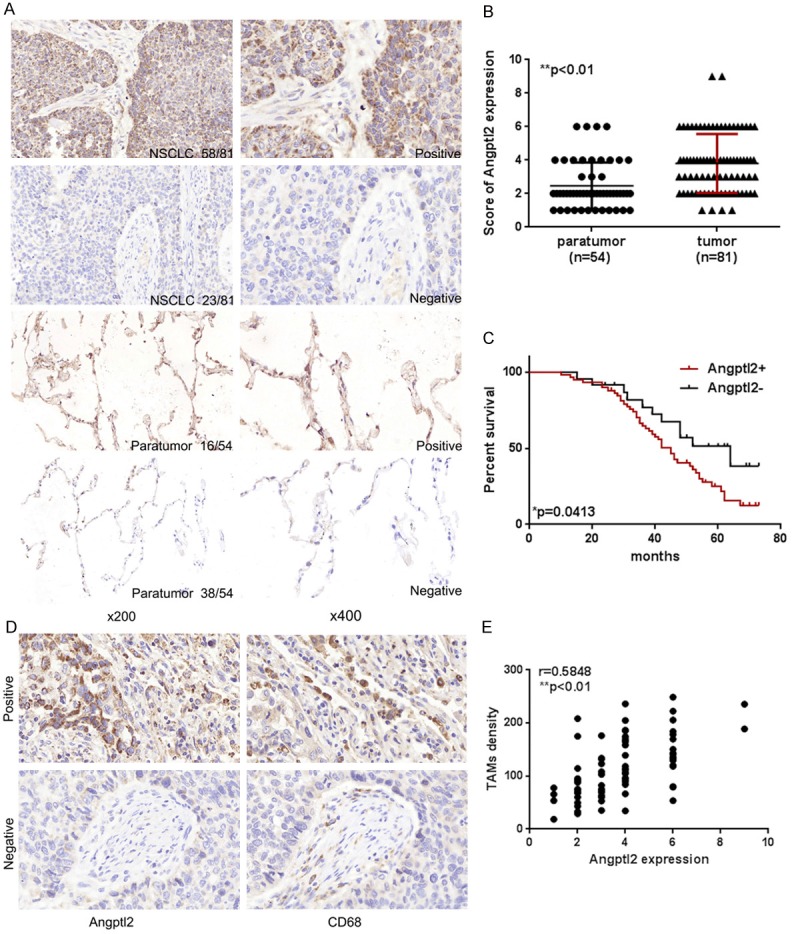
Angptl2 expression in NSCLC samples. A. IHC images of Angptl2 expression in NSCLC tissues and in paratumor lung tissues. Positive Angptl2 expression was identifiable by brown staining in the cytoplasm of primary NSCLC cells and surrounding pulmonary epithelial cells (magnification of ×200 and ×400). B. Comparison of Angptl2 expression in primary NSCLC tissues and paratumor lung tissues. C. Survival analysis of NSCLC patients between the Angptl2-positive group and the Angptl2-negative group in NSCLC tissues was performed by Kaplan-Meier survival analysis. D. Angptl2 expression and CD68+ cells in the same specimen (magnification, ×400). CD68 was used as a pan-macrophage marker. E. The correlation between intratumoral Angptl2 expression and TAM density was analyzed. r=0.5848, Pearson’s correlation. *p<0.05, **p<0.01.
The association between Angptl2 expression in NSCLC tumor tissues and clinical pathological characteristics is depicted in Table 3. Expression of Angptl2 was positively correlated with tumor size (*p=0.022) and higher pathological stage (*p=0.037). Moreover, Kaplan-Meier survival analysis showed that the levels of Angptl2 negatively correlated with the overall survival (OS) of NSCLC patients (Figure 1C).
Table 3.
Correlations between Angptl2 expression and the clinicopathological parameters of 81 patients with NSCLC
| Characteristics | Angptl2 protein expression | p-value | |
|---|---|---|---|
|
| |||
| Negative | Positive | ||
| Age (years) | |||
| >60 | 13 | 32 | 0.912 |
| ≤60 | 10 | 26 | |
| Sex | |||
| Male | 17 | 41 | 0.772 |
| Female | 6 | 17 | |
| Smoking history (years) | |||
| <30 | 8 | 30 | 0.168 |
| ≥30 | 15 | 28 | |
| Tumor size (cm) | |||
| ≤5 | 13 | 17 | 0.022* |
| >5 | 10 | 41 | |
| Histological type | |||
| Squamous | 10 | 24 | 0.089 |
| Adenocarcinoma | 10 | 33 | |
| Other | 3 | 1 | |
| TNM stage groupings | |||
| I/II | 11 | 14 | 0.037* |
| III/IV | 12 | 44 | |
p<0.05, statistically significant difference.
To explore the effects of tumor cell-derived Angptl2 on TAMs, we analyzed the relationship between the density of TAMs (CD68+) and Angptl2 expression in NSCLC tissues (Figure 1D). A Pearson correlation analysis showed that Angptl2 expression was positively correlated with TAM density (r=0.5848, **p<0.01, Figure 1E).
NSCLC cell-derived Angptl2 promoted M2 polarization of TAMs in vitro
M2 TAMs and Angptl2 both promote cancer progression; polarized phenotypes, rather than the infiltration of macrophages, play a key role in NSCLC progression [22]; based on our results, we hypothesized that Angptl2 was involved in TAM polarization during the development of NSCLC.
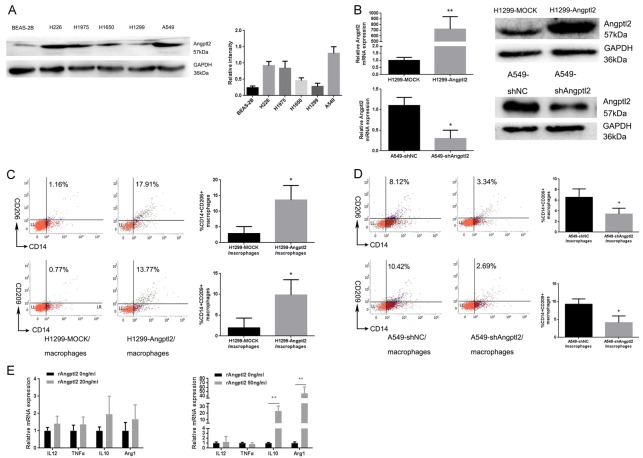
We detected expression of Angptl2 protein in all five tumor cell lines. H1650 and H1299 cells had low Angptl2 expression, A549, H226 and H1975 had high Angptl2 expression, and BEAS-2B cells had the lowest Angptl2 expression (Figure 2A). Then, Angptl2 expression was downregulated in A549 cells (A549-shAngptl2), which typically have high endogenous Angptl2 expression, while H1299, which has low endogenous Angptl2 expression, was forced to express exogenous Angptl2 (H1299-Angptl2) (Figure 2B). Transwell co-culture experiments were performed for 24 h with THP-1-derived macrophages and NSCLC cell lines. Transwell co-culture of H1299-Angptl2 with THP-1-derived macrophages led to an increased percentage of CD206+ and CD209+ macrophages compared with H1299-MOCK macrophages (CD206 and CD209 are both believed to be markers for M2 macrophages), as detected by flow cytometry (Figure 2C). Macrophages co-cultured with A549-shAngptl2 cells had lower percentage of CD206+ and CD209+ macrophages than those co-cultured with the A549-shNC cells (Figure 2D).
Figure 2.
Angptl2 induced M2 macrophage polarization in vitro. A. Angptl2 protein expression in all five NSCLC cell lines and the normal lung/bronchus epithelial cell line, BEAS-2B, as measured by western blot; GAPDH was used as a loading control. B. Angptl2 expression in lentivirus-infected H1299 cells and shRNA-transfected A549 cells was detected by RT-PCR and western blot. C. The percentage of CD14+/CD206+ and CD14+/CD209+ cells, as detected by flow cytometry, from THP-1-derived macrophages were co-cultured with H1299-MOCK cells or H1299-Angptl2 cells for 24 hours. D. The percentage of CD14+/CD206+ and CD14+/CD209+ cells detected by flow cytometry from THP-1-derived macrophages co-cultured with A549-shNC or A549-shAngptl2 cells for 24 hours. E. Relative IL10, Arg1, IL12 and TNFα mRNA expression, as detected by RT-PCR, in THP-1-derived cells in the presence or absence of rAngptl2 (20 ng/ml and 50 ng/ml) for 24 hours. Relative mRNA expression levels are presented as fold inductions. *p<0.05, **p<0.01. IL10 and Arg1 are M2 macrophage markers; IL12 and TNFα are M1 macrophage markers. The same volume of PBS was added to both the 0 ng/ml rAngptl2 and control groups.
To further confirm these results, we used recombinant protein Angptl2 (rAngptl2) to detect its function. The concentration of Angptl2 in NSCLC patient serum is 8.38 ± 1.73 ng/ml [30]. Thus, it is reasonable for us to assume that the concentration of Angptl2 was higher in the TME than in the serum. We chose 20 ng/ml and 50 ng/ml rAngptl2 to stimulate THP-1-derived macrophages. RT-PCR showed that the mRNA expression of the M2 markers IL10 and Arg1 increased after 50 ng/ml rAngptl2 stimulation (Figure 2E).
Induction of M2 polarization of TAMs by Angptl2 was dependent on p65 NF-ĸB signaling
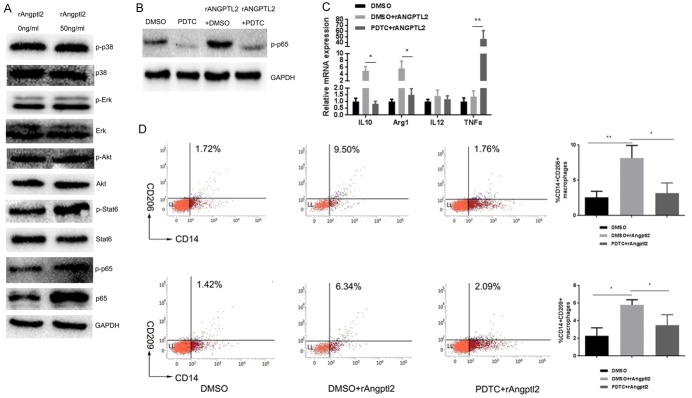
We detected certain intracellular pathways reported to mediate macrophage polarization [31-33]. rAngptl2 stimulation increased phosphorylation of Stat6 and p65 (Figure 3A). We used selective inhibitors to explore which pathways were involved in Angptl2 promoted M2 macrophage polarization: AS1517499 (Stat6 inhibitor) and PDTC (NF-ĸB inhibitor). As shown in Figure 3B, 3C, PDTC treatment attenuated Angptl2-induced M2 markers mRNA expression detected by RT-PCR, while AS1517499 did not significantly change the M2 markers mRNA expression significantly (data not shown), suggesting that the p65 NF-ĸB pathway might be mediate Angptl2-induced TAMs M2 polarization.
Figure 3.
Angptl2 promoted M2 macrophage polarization via the p65 NF-κB signaling pathway. A. Western blot of phospho-p38 MAPK, p38 MAPK, phospho-Erk, Erk, phospho-Akt, Akt, phospho-Stat6, Stat6, phospho-p65, p65 NF-κB and GAPDH in THP-1-derived macrophages with or without the addition of rAngptl2 (50 ng/ml, 24 hours). B. Western blot of p-p65 NF-κB in THP-1-derived cells with or without pretreatment of PDTC (100 μM in DMSO for 30 min) before the addition of rAngptl2. C. RT-PCR analysis of M1/M2 markers in THP-1-derived cells with or without pretreatment of PDTC before the addition of rAngptl2. D. Flow cytometry analysis of THP-1-derived cells with or without PDTC pretreatment before the addition of rAngptl2. The percentage of CD14+/CD206+ and CD14+/CD209+ cells and quantitative data are shown. *p<0.05, **p<0.01.
To further confirm these results, flow cytometry was used to detect the percentage of CD206+ and CD209+ macrophages influenced by the pathway inhibitors. The results showed the percentage of CD206+ and CD209+ macrophages induced by rAngptl2 were both impaired by PDTC (Figure 3D).
Angptl2-promoted TAMs enhanced the proliferation, invasion, and migration of NSCLC cells and the tube formation of HUVECs in vitro
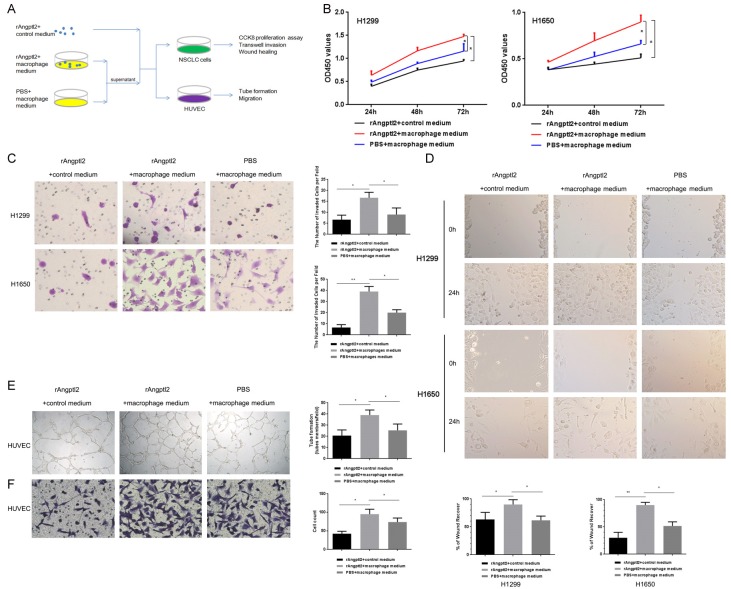
On the basis of the findings that NSCLC cell-derived Angptl2 promoted M2 polarization of TAMs (Figure 2), we further explored the biological function of Angptl2-promoted M2 TAMs on the progression of NSCLC in vitro, as outlined schematically in Figure 4A.
Figure 4.
Angptl2-promoted macrophages enhanced the proliferation, invasion and migration of NSCLC cells and the tube formation and migration of HUVECs in vitro. A. A schematic representation of the experimental approach used in this part. B. NSCLC cells were incubated with the indicated CMs for 24 h, 48 h, and 72 h. The CCK8 assay was used to evaluate proliferation. C. NSCLC cells were incubated with the indicated CMs in 24-well Transwell chambers for 24 h, and the invasive cells were stained with crystal violet. Representative images were taken at a magnification of 200×. The number of invasive cells is shown. D. A wound-healing assay was used to examine the effects of the indicated CMs on NSCLC cell migration. Representative images were taken at a magnification of 100×. Quantitative data are shown. E. HUVECs in the presence of the indicated CMs for 12 h formed capillary-like structures. Representative images were taken at a magnification of 50×. Quantitative data are shown. F. Transwell migration experiments of HUVECs were performed with the indicated CMs for 24 h. Representative images were taken at a magnification of 200×. Quantitative data are shown. *p<0.05; **p<0.01. (rAngptl2+control medium: 50 ng/ml rAngptl2 was added to complete macrophage culture medium; rAngptl2+macrophage medium: THP-1-derived macrophages were cultured with 50 ng/ml rAngptl2 for 24 h, the supernatant was collected; PBS+macrophage medium: THP-1-derived macrophages were cultured with the same amount of PBS to rAngptl2 as the control group for 24 h, the supernatant was collected).
We examined whether Angptl2-promoted M2 macrophages had the ability to enhance NSCLC cell proliferation, invasion and migration. To address this question, a CCK8 proliferation test, a Transwell invasion assay and a wound-healing assay were performed. As shown in Figure 4B-D, after 24 h of culture in the indicated CMs, the proliferation, invasion and migration of NSCLC cells in the Angptl2-promoted macrophage group was highest.
HUVECs were seeded in a 96-well plate pre-coated with Matrigel and formed capillary-like structures in the presence of different macrophage supernatants. The network of tube-like structures in the Angptl2-promoted macrophages group was more extensive than that of the other two groups (Figure 4E). We also examined the migration of HUVECs under various kinds of CM. CM from macrophages stimulated by Angptl2 significantly enhanced the migration of HUVECs (Figure 4F).
NSCLC cell-derived Angptl2 promoted NSCLC tumor growth in the presence of TAMs in vivo
We established tumor transplant mouse model with Angptl2 up-regulated NSCLC cells. Intravenous tail injections of CCL were used to deplete macrophages, and CNL served as control [27]. Tumor volume and weight in the Angptl2 up-regulated group were significantly higher than the control group, and both were reduced in the CCL group compared with the CNL control group (Figure 5A, 5B).
Figure 5.
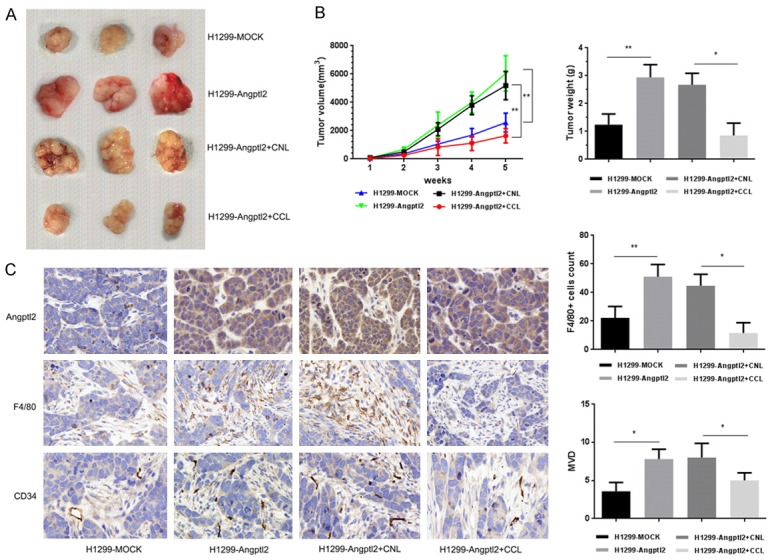
Angptl2 induced NSCLC tumor growth in the presence of TAMs in vivo. A. Groups of female nude mice (n=5) were injected with 1×106 H1299-MOCK cells or H1299-Angptl2 lung cancer cells in subcutaneous of the left axillary. Seven days after tumor cell inoculation, the mice were injected with CCL or CNL, at an initial dose of 0.2 ml per mouse, followed by 0.1 ml per mouse twice a week for 4 weeks. B. Weekly tumor size and weight. Data are presented as the mean ± SD. C. IHC staining of Angptl2, F4/80 and CD34 in xenograft tissues. Representative images (×400). Quantitative results of TAM density and MVD in primary xenograft tissues are shown. Data are presented as the mean ± SD, *p<0.05; **p<0.01.
Higher Angptl2 expression was related to enhanced MVD; depletion of macrophages impaired the enhancement (Figure 5C), which indicated the importance of TAMs in pro-tumor functions related to Angptl2.
Thus, the results from animal models confirmed our observations in vitro and lend further support to our hypothesis that TAMs are involved in Angptl2-driven NSCLC progression via M2 polarization.
These data suggest that Angptl2 promotes M2 polarization of TAMs through the NF-ĸB pathway in NSCLC and further promotes tumor progression.
Discussion
Tumor cell products, such as IL10, colony stimulating factor (CSF)-1 and extracellular matrix components stimulate macrophage differentiation into M2 macrophages that have the capacity to promote cancer [34]. Cancer cell-derived TGF-β affects stromal cells in a paracrine fashion, leading to fibroblast activation and enhances tumor growth [35]. These studies suggest that tumor cell-produced factors promote tumor progression by influencing the functional phenotypes of stromal cells.
Here, for the first time, we showed that tumor cell-derived Angptl2 promoted NSCLC progression by inducing M2 polarization of TAMs. The direct link between Angptl2 and the M2 polarization of TAMs in NSCLC was suggested by several pieces of evidence in our studies. First, Angptl2 expression was positively related to TAM infiltration and correlated with disease progression as defined by tumor size, tumor grades and the patients’ overall survival. M2 TAMs were also reported to be closely related to the poor survival of NSCLC patients, which suggested that Angptl2-related NSCLC promotion may be related to M2 macrophages. Second, we used both co-culture and recombinant protein stimulation systems to identify how Angptl2 influenced macrophage polarization by detecting the subtype markers. A co-culture of the macrophages with NSCLC cells with various Angptl2 expression levels was performed to simulate the interaction between tumor cells and macrophages in the TME. A recombinant protein stimulation experiment was performed to clarify how different concentrations of Angptl2 affected macrophage polarization. Higher concentrations of Angptl2 promoted macrophage M2 polarization more strongly. Angptl2 is an inflammatory factor that enhances immune factor production in adipose tissue [26]. Based on our results, its influence on macrophage polarization might depend on its concentration and the surrounding microenvironment; this hypothesis needs more exploration. Third, tumor cell growth and migration and tumor angiogenesis are important events that occurred in the TME that influence NSCLC development. We detected functions of Angptl2 promoted macrophages in NSCLC cells and HUVECs in vitro. Angptl2-promoted macrophages enhanced the proliferation, invasion and migration of NSCLC cells and the tube formation of HUVECs, which indicates that Angptl2-promoted TAMs promote NSCLC progression. Finally, we used a macrophage depletion mouse model to clarify the importance of macrophages in Angptl2-related NSCLC progression in vivo. Up-regulated Angptl2 in the mouse tumor transplant model resulted in increased tumor growth and MVD, while up-regulated Angptl2 tumors with depleted TAMs did not show increased tumor growth or MVD. Collectively, our data strongly suggest that Angptl2 induces TAMs M2 polarization and further promotes NSCLC progression.
To minimize the direct stimulatory effects of Angptl2 on NSCLC cells in this study, we used complete medium with the same amount of rAngptl2 (50 ng/ml) as the control group for CM from rAngptl2-treated macrophages. CM from Angptl2-promoted macrophages enhanced cancer cell proliferation, migration and invasion and tube formation and migration abilities of HUVECs more strongly than rAngptl2 itself. Furthermore, our in vivo experiments showed that depleting mouse macrophages reduced Angptl2-induced xenograft growth, which clearly supports the conclusion that TAMs contribute to Angptl2-induced NSCLC progression.
Our data suggest that p65 NF-ĸB signaling mediated the Angptl2-induced M2 polarization of TAMs. The NF-ĸB pathway is a classical mediator of inflammation. Hepatoma-derived toll-like receptor (TLR) 2-related ligands stimulate M2 macrophage differentiation by controlling NF-ĸB RELA/p65 protein homeostasis through selective autophagy [36]. The different responses in NF-ĸB signaling activation may be due to the dynamic process of cancer development. Additionally, there may be other mechanisms in the NF-ĸB activation pathway, such as autophagy, which require further study.
In conclusion, the data presented in this study suggest that Angptl2 enhances the M2 polarization of TAMs in NSCLC, which in turn fosters the NSCLC progression. Our findings highlight the potential role of Angptl2 in the promotion of TAM M2 polarization and provide a new target for NSCLC therapy.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81372334) and the Project of Jinan Youth Team for Technological Innovation (Grant no. 2010-1). We are very grateful to Professor Yihai Cao (Department of Microbiology, Tumor and Cell Biology, Karolinska Institute) for providing guidance on the experimental design.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santulli G. Angiopoietin-like proteins: a comprehensive look. Front Endocrinol (Lausanne) 2014;5:4. doi: 10.3389/fendo.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim I, Moon SO, Koh KN, Kim H, Uhm CS, Kwak HJ, Kim NG, Koh GY. Molecular cloning, expression, and characterization of angiopoietinrelated protein. angiopoietin-related protein induces endothelial cell sprouting. J Biol Chem. 1999;274:26523–26528. doi: 10.1074/jbc.274.37.26523. [DOI] [PubMed] [Google Scholar]
- 5.Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178–188. doi: 10.1016/j.cmet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 7.Carbone C, Piro G, Fassan M, Tamburrino A, Mina MM, Zanotto M, Chiao PJ, Bassi C, Scarpa A, Tortora G, Melisi D. An angiopoietin-like protein 2 autocrine signaling promotes EMT during pancreatic ductal carcinogenesis. Oncotarget. 2015;6:13822–13834. doi: 10.18632/oncotarget.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ide S, Toiyama Y, Shimura T, Kawamura M, Yasuda H, Saigusa S, Ohi M, Tanaka K, Mohri Y, Kusunoki M. Angiopoietin-Like protein 2 acts as a novel biomarker for diagnosis and prognosis in patients with esophageal cancer. Ann Surg Oncol. 2015;22:2585–2592. doi: 10.1245/s10434-014-4315-0. [DOI] [PubMed] [Google Scholar]
- 9.Aoi J, Endo M, Kadomatsu T, Miyata K, Nakano M, Horiguchi H, Ogata A, Odagiri H, Yano M, Araki K, Jinnin M, Ito T, Hirakawa S, Ihn H, Oike Y. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011;71:7502–7512. doi: 10.1158/0008-5472.CAN-11-1758. [DOI] [PubMed] [Google Scholar]
- 10.Masuda T, Endo M, Yamamoto Y, Odagiri H, Kadomatsu T, Nakamura T, Tanoue H, Ito H, Yugami M, Miyata K, Morinaga J, Horiguchi H, Motokawa I, Terada K, Morioka MS, Manabe I, Iwase H, Mizuta H, Oike Y. ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling. Sci Rep. 2015;5:9170. doi: 10.1038/srep09170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L, Ge C, Fang T, Zhao F, Chen T, Yao M, Li J, Li H. ANGPTL2 promotes tumor metastasis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:396–404. doi: 10.1111/jgh.12702. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Yu X, Xie J, Zhan M, Yu Z, Xie L, Zeng H, Zhang F, Chen G, Yi X, Zheng J. ANGPTL2/LILRB2 signaling promotes the propagation of lung cancer cells. Oncotarget. 2015;6:21004–21015. doi: 10.18632/oncotarget.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endo M, Nakano M, Kadomatsu T, Fukuhara S, Kuroda H, Mikami S, Hato T, Aoi J, Horiguchi H, Miyata K, Odagiri H, Masuda T, Harada M, Horio H, Hishima T, Nomori H, Ito T, Yamamoto Y, Minami T, Okada S, Takahashi T, Mochizuki N, Iwase H, Oike Y. Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical driver of metastasis. Cancer Res. 2012;72:1784–1794. doi: 10.1158/0008-5472.CAN-11-3878. [DOI] [PubMed] [Google Scholar]
- 14.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 15.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 16.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 17.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hume DA. The Many Alternative Faces of Macrophage Activation. Front Immunol. 2015;6:370. doi: 10.3389/fimmu.2015.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 20.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 21.Rahat MA, Bitterman H, Lahat N. Molecular mechanisms regulating macrophage response to hypoxia. Front Immunol. 2011;2:45. doi: 10.3389/fimmu.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Cao J, Ma S, Dong R, Meng W, Ying M, Weng Q, Chen Z, Ma J, Fang Q, He Q, Yang B. Tumor hypoxia enhances Non-Small Cell Lung Cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget. 2014;5:9664–9677. doi: 10.18632/oncotarget.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odagiri H, Kadomatsu T, Endo M, Masuda T, Morioka MS, Fukuhara S, Miyamoto T, Kobayashi E, Miyata K, Aoi J, Horiguchi H, Nishimura N, Terada K, Yakushiji T, Manabe I, Mochizuki N, Mizuta H, Oike Y. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin alpha5beta1, p38 MAPK, and matrix metalloproteinases. Sci Signal. 2014;7:ra7. doi: 10.1126/scisignal.2004612. [DOI] [PubMed] [Google Scholar]
- 24.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumorassociated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki Y, Ohta M, Desai D, Figueiredo JL, Whelan MC, Sugano T, Yamabi M, Yano W, Faits T, Yabusaki K, Zhang H, Mlynarchik AK, Inoue K, Mizuno K, Aikawa M. Angiopoietin Like Protein 2 (ANGPTL2) Promotes Adipose Tissue Macrophage and T lymphocyte Accumulation and Leads to Insulin Resistance. PLoS One. 2015;10:e0131176. doi: 10.1371/journal.pone.0131176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 28.Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80:802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 29.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Jiang H, Zhu L, Wang P, Liu S, Xiao X, Yu H, Dong W. Diagnostic and prognostic value of serum angiopoietin-like protein 2 in patients with non-small cell lung cancer. Clin Lab. 2017;63:59–65. doi: 10.7754/Clin.Lab.2016.160528. [DOI] [PubMed] [Google Scholar]
- 31.Zawistowska-Deniziak A, Basalaj K, Strojny B, Mlocicki D. New data on human macrophages polarization by hymenolepis diminuta Tapeworm-An in vitro Study. Front Immunol. 2017;8:148. doi: 10.3389/fimmu.2017.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JW, Kwon MJ, Kim IH, Kim YM, Lee MK, Nam TJ. Pyropia yezoensis glycoprotein promotes the M1 to M2 macrophage phenotypic switch via the STAT3 and STAT6 transcription factors. Int J Mol Med. 2016;38:666–674. doi: 10.3892/ijmm.2016.2656. [DOI] [PubMed] [Google Scholar]
- 33.Yan W, Liu X, Ma H. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut. 2015;64:1593–1604. doi: 10.1136/gutjnl-2014-307671. [DOI] [PubMed] [Google Scholar]
- 34.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 35.Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, Howell A, Aquila S, Ando S, Martinez-Outschoorn U, Sotgia F, Lisanti MP. Metabolic reprogramming of cancer-associated fibroblasts by TGF-beta drives tumor growth: connecting TGF-beta signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012;11:3019–3035. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang CP, Su YC, Lee PH, Lei HY. Targeting NFKB by autophagy to polarize hepatoma-associated macrophage differentiation. Autophagy. 2013;9:619–621. doi: 10.4161/auto.23546. [DOI] [PMC free article] [PubMed] [Google Scholar]