Abstract
Interleukin-32 theta (IL-32θ) is newly identified isoform of IL-32 which plays a vital role in inflammatory responses. Like IL-32α and IL-32β, IL-32θ isoform acts as an intracellular inflammatory modulator. It results in reduction of IL-1β production by attenuating the expression of PU.1 and inhibition of monocytes differentiation into macrophages. IL-32θ hinders TNF-α expression by inhibiting p38 MAPK and inhibitor of κB (IκB) as well. It also reserved STAT3-ZEB1 pathway leading to the inhibition of epithelial-mesenchymal transition (EMT) and stemness. Hence, it can be concluded that IL-32θ is an anti-inflammatory cytokine that can act as a tumor suppressor and can play vital role in colon cancer therapies. IL-32θ also plays a crucial role in immune system responses and cellular differentiation during disease pathogenesis. To our best knowledge this is the first ever review to condense the importance, precise mode of action in disease progression and latent remedial implications of IL-32θ in several inflammatory disorders.
Keywords: Cancer, cellular differentiation, IL-32θ, inflammation, remedial, tumor suppression
Introduction
A number of biological processes like development, differentiation and activity of immune cells are regulated by the specific soluble proteins known as cytokines [1]. Cytokines are produced and responded by almost all cells [2]. Any alteration in production and activity of these cytokines leads to the onset of various autoimmune disorders (inflammatory bowel disease, gastric cancer etc) and autoimmunity [1]. Several studies proved that cytokines exercise a pivotal job in the development and progression of multiple ailments like crohn’s disease, inflammation and rheumatoid arthritis besides considered as important component that may prove to be effective therapeutic targets [3,4]. Numerous growth factors and cytokines are involved in injury induced neural damage and repair as well as orchestrate cellular behavior in cornea healing [5,6]. Cytokines have been reported to be useful in HIV treatment therapies as well as in therapies that enable immune system to identify and destroy cancerous cells [7-9]. Therefore, cytokines are helpful in immunogenic tumors and hematologic malignancies treatment.
Broadly cytokines can be classified into various protein families on the basis of structural homology i.e. hemopoietic cytokines, TNF family and IL-1 and related proteins. The hemopoietic cytokine family is a large cytokine group containing helical bundles and collectively play vital and varied role in regulation of immune system [10].
Interleukins (ILs) are cytokines that act as messenger molecules transmitting signals between cells of immune system. They are secreted by lymphocytes and macrophages and their production is initiated in response to infection and injury. Interleukins activity has been reported to influence cells of the immune system along with the tissues and organs i.e. liver and brain [11]. They are of various types ranging from IL-1 to IL-37 [12].
Interleukin-32 (IL-32) is a recently reported interleukin, encoded by a gene present on human chromosome 16 p13.3, consisting of eight coding regions (exons). Pathogenesis of various disorders is mediated by IL-32 that involves interplay among body cells and immune system [13]. IL-32 induces the expression of various inflammatory cytokines and macrophage inflammatory protein-2 (MIP-2) which are known to be involved in progression of various inflammatory diseases and pathogenesis including cancer, inflammatory bowel disease (IBD), rheumatoid arthritis, gastric inflammation and chronic obstructive pulmonary disease (COPD) [14].
Review
IL-32 exhibits six isomeric forms (α, β, γ, δ, ε, and ζ) that arise from alternative splicing of mRNA [15-17]. Among the variants, IL-32α is the most abundant protein secreted by natural killer (NK) cells, T cells, monocytes, epithelia and endothelial cells. It acts as an important effector and mediator of abnormal immune responses that lead to the auto immune responses and multiple inflammatory disorders including COPD, IBD, rheumatoid arthritis etc. [13]. IL-32β was reported to influence the secretion and expression of an anti-inflammatory cytokine i.e. IL-10 [18-22]. Besides, previously reported six isoforms of IL-32, three more isoforms (η, θ, s) are newly reported. It has been reported that IL-32θ interacts with IL-32β and ceases IL-10 production [19].
IL-32θ in myeloid cell differentiation
Myeloid cell differentiation is a strictly controlled process regulated by a number of cytokines and some transcription factors because any interruption in myeloid lineage differentiation results in blood cancer and immune problems [23]. Lineage commitment is under tight regulation of GM-CSF and some other key interleukins [24-26]. Previously, most of the studies involving IL-32 were carried out to understand its proinflammatory activities in innate immune response. However, some studies have been carried out recently focusing the involvement of IL-32 in apoptosis and metastasis. Furthermore, its level has been reported as a potent diagnostic marker of gastric cancer [27-30]. Since the time of its discovery, progress in the study of the relationship of IL-32 with cell differentiation is quite slow. IL-32 by thymic stromal lymphopoietin provokes monocytic differentiation in macrophages [31]. Moreover, it synergistically causes differentiation of osteoclasts along with IL-17 which is a key regulator of osteoclastogenesis in vitro [32,33]. IL-32γ has also been reported to be involved in dendritic cell maturation by inducing IL-12 and IL-6 expression [34]. IL-32α by restraining PU.1 expression in a STAT3-dependent manner was shown to be involved in differentiation of THP-1 cells [35]. Cell differentiation by all other isoforms of IL-32 is still unclear, and it seems that myeloid differentiation depends on each isoforms of IL-32. It has been previously reported that IL-32θ expression leads to a reduction of IL-1β production by attenuating phosphorylation of PU.1 [36] (Figure 1). PU.1 is an important element of Ets family of transcription factors that not only regulates the expression of various macrophage-specific genes viz, glycoprotein pDP4 [37], CD11b [38], CD18 [39] but is also required for monocytic differentiation [40-42]. Therefore it could be expected that IL-32θ may suppress PU.1 expression by binding to a distal enhancer on the PU.1 promoter region, as PU.1 executes auto-regulatory functions [43]. Similarly, CCAAT-enhancer-binding protein α (C/EBPα) is also an important transcription factor like PU.1 that is significantly involved in differentiation of monocytes into macrophage [44]. C/EBPα induces PU.1 expression via binding to distal enhancer of PU.1 [45]. Kim MS et al., (2015) reported the involvement of IL-32θ in the inhibition of phorbol 12-myristate 13-acetate (PMA)-induced monocytic differentiation in both THP-1 and HL-60 cell lines. Even macrophage cell surface markers, CD11b, CD18, and CD36 expression has been repressed by IL-32θ. Expression of cell cycle related factors was neither affected by IL-32β nor by IL-32θ, thus, it has no contributions towards PMA-induced cell cycle arrest. IL-32θ attenuates PU.1 expression, thus in THP-1/IL-32θ cells transitory expression of both C/EBPα and PU.1 results in rescuing the differentiation defect acting synergistically [46]. Thus, IL-32θ inhibits monocytic differentiation by suppressing PU.1 expression. Various attempts i.e. ‘differentiation therapy’ have been made in the past to treat myeloid leukemia [47,48]. IL-32θ is a significant contributor and may prove to be a useful therapeutic tool in treating myeloid differentiation-mediated diseases.
Figure 1.
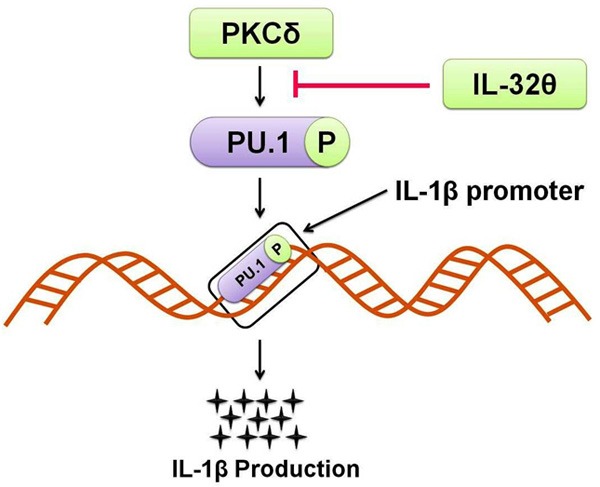
How IL-32θ inhibits IL-1β production: IL-32θ attenuates IL-1β production by inhibiting the PKCδ interaction to PU.1. As IL-32θ inhibits PKCδ mediated PU.1 phosphorylation it ultimately prevents IL-1β transcription and production.
IL-32θ in acute myeloid leukemia (AML)
AML, a malignancy of hematopoietic stem cells, is characterized by a reduced production of normal blood cells as well as a buildup of dysfunctional myeloid cells [49]. A number of factors of varying nature including carcinogens [50], mutations [51,52] and radiation [53] are responsible for AML. AML is regulated by a network of cytokines regarding propagation, apoptosis, and differentiation of leukemic cells [54]. Level of various cytokines in patients of AML is reported to be higher than that of healthy individuals. Cytokines produced in leukemogenesis are TNF-α, IL-6, and IL-1β which induce AML blast growth via colony stimulating factor (CSF)-induced clonogenicity in vitro [55]. In contrast to it, cytokines involved in development and differentiation of AML cells are downregulated by IL-10 [56]. In a study, NF-κB has been reported as an active constituent whose level is fairly maintained in AML patients in RelA/p50 and p50/p50 complexes [57]. A consistent higher level of TNF-α results in persisting proliferation and is maintained by NF-κB activation in AML blasts [58]. Certain hematologic diseases like AML oftenly develop because of an altered profile of cytokines [59,60]. TNF-α expression in leukemia blasts has been reported to be much higher in patients with AML than in cells of healthy persons [61]. In hematopoiesis, TNF-α is pleiotropic in action as it increases the expression of IL-1β and GM-CSF [62], induces apoptosis in leukemic cells [63] and results in senescence as well as chromosomal instability [64]. Leukemic blasts of AML normally show a highly activated and over-expressed PKCs level [65]. In various cell types, TNF-α gene transcription is regulated by PKC via PKC signaling pathways [66]. Rottlerin (a PKCδ-specific inhibitor) not only influences the synthesis of a number of cytokines but restrains TNF-α production as well [67]. It has been attempted to prevent AML tumorigenesis in some recent trials by targeting PKC-mediated signal transduction pathways [68]. PKCs mostly trigger p38 MAPK and NF-κB signaling either directly or indirectly [69-71]. Activation of p38, in Mo7e human megakaryoblastic leukemia cell line, partially supports NF-κB (p65) transcriptional activation function [72]. Furthermore, upon regulation by p38, NF-κB transcriptional activation via p65 phosphorylation leads to TNF-α production [73]. Various molecules, regardless of p38 association with NF-κB, involved in PKC signaling may be positive regulators of TNF-α gene expression [74]. In a study, NF-κB via autocrine TNF-α secretion constitutively activated AML [58]. On the basis of above mentioned observations, it may be proposed that TNF-α production is provoked by complex signaling pathways that require the activation of PKC, p38 MAPK and NF-κB either individually or together. Various isoforms of IL-32 have been named for their involvement in a number of inflammatory disorders that are characterized by upregulation of proinflammatory cytokines [75]. In contrast to it, some recent studies have reported IL-32 as an intracellular intermediary molecule as it interacts with many other molecules [35,76,77]. Regulation of a spectrum of pro- and anti-inflammatory cytokines including IL-1β, IL-6, and IL-10 in the cells associated with PKC isoforms by IL-32 has been reported previously [18,36,78]. As IL-32θ inhibit the IL-1β and CCL-5 expression which via PKCδ association and regulation of its downstream signals is involved in the pathogenesis of inflammatory diseases, considered as an anti-inflammatory component. Furthermore, IL-32θ by attenuating PU.1 expression suppresses monocytic differentiation into macrophage [46]. Kim MS et al., (2015) reported the expression of endogenous IL-32θ in 38% of the patients suffering from AML compared to healthy individuals. TNF-α level was not found to be increased with IL-32θ expression. PMA-induced TNF-α production was reported to be attenuated by IL-32θ. IL-32θ inhibited activity of nuclear translocation of NF-κB (a key positive regulator of TNF-α expression) by inhibiting phosphorylation of p38 MAPK. Hence, it may prove as an inhibitor of TNF-α in AML patients [79] (Figure 2).
Figure 2.
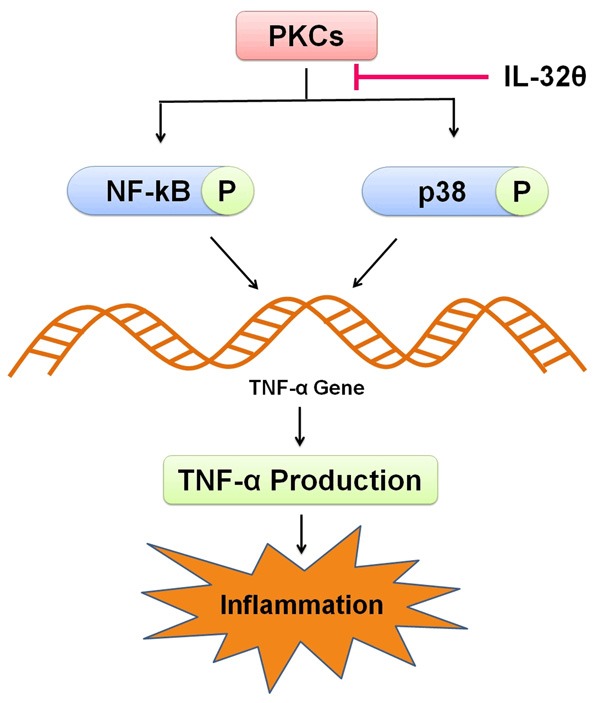
Attenuation of TNF-α production by IL-32θ. PKCs promote TNF-α production through p38 MAPK and NF-κB signaling. As IL-32θ prevents nuclear translocation of NF-κB by inhibiting p38 MAPK, it ultimately prevents inflammation by inhibiting TNF-α production.
IL-32θ in colon cancer
Colon cancer is the 2nd largest cause of cancer-related mortality. Some cancer cells are resistant to current therapies i.e. cancer stem cells (CSCs) [80]. Entire tumor population has been reported to be recapitulated by CSCs in vitro and in vivo [81]. There has been growing evidence of the presence of CSCs in breast [82], colon [80] ovarian [83] and brain cancer [84]. They have been associated with recurrence, progression and drug resistance of cancer [85]. Like undifferentiated hematopoietic stem cells, CSCs also express markers of stem cells [84] and show self-renewal ability [86]. Such an ability is responsible for promoting tumorigenesis [87]. Constant activation of STAT3 has been reported to be responsible for perpatuation of colon cancer [88,89] and colon cancer-initiating cells [90]. STAT3 is one of the significant signaling pathways among various oncogenic pathways in cancer. Several researchers have previously tried to elaborate abnormalities in this pathway during colon cancer [91,92]. However, the exact mechanism involved in STAT3 inhibition during colon cancer is still unclear. The fate of CSCs is determined by both intrinsic and extrinsic pathways viz, cytokine networks [85,93]. In breast cancer, IL-6 and IL-8 enhance CSC self-renewal [94,95] while in colon cancer, stemness and invasiveness of CSCs is stimulated by IL-1β [96]. Similarly, IL-32 has also been reported to regulate various types of cancer. IL-32α has been reported to be involved in the development of hepatocellular carcinoma [29] but in contrast, it has also been reported to repress colorectal cancer [97]. Furthermore, IL-32β has been reported to inactivate NF-κB and STAT3 pathways as well as found to promote cytotoxic lymphocyte activation and stimulate breast cancer cells migration as well [98,99]. Role of IL-32θ in colon cancer is still to be elucidated. IL-32θ has sequence homologies with IL-32β but lacks exon 6 [100]. Bak Y et al., (2016) reported suppression of IL-32θ mRNAs expression in colon cancer patients in tumor regions. IL-32θ leads to repression of invasive and migratory potential of HT29 colon cancer cells by inhibiting epithelial-mesenchymal transition (EMT). IL-32θ amends genes of CSCs responsible for sphere formation and expression of stemness. IL-32θ tends to inhibit transcription of Bmi1 and ZEB1 (downstream factors) by directly binding to STAT3 and inhibiting nuclear translocation. IL-32θ stops key factors of EMT and inhibits stemness by inhibiting STAT3-ZEB1 pathway (Figure 3). They concluded that IL-32θ may be a tumor suppressor and may probably be used in near future as therapy for colon cancer [101].
Figure 3.
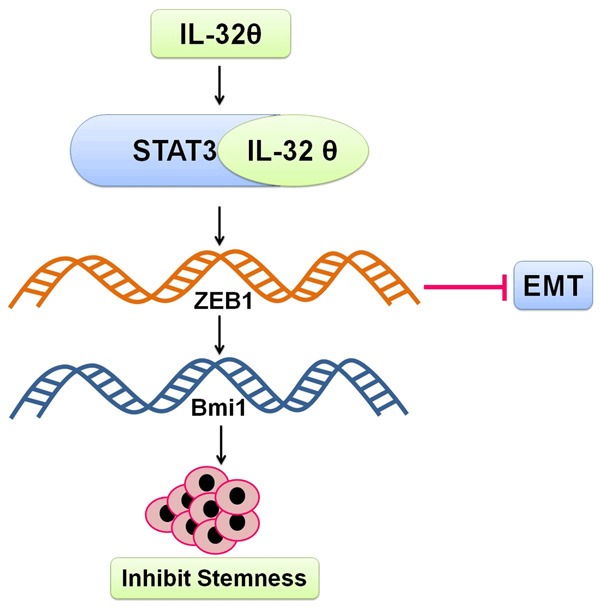
Inhibition of stemness and EMT by IL-32θ. IL-32θ interacts with STAT3 pathway to suppress ZEB1 and Bmi1 transcription and ultimately prevents stemness and EMT in colon cancer cells.
IL-32θ in IL-1β production
Immune system responses to various types of stimuli have been reported to be mediated by human myelomonocytes THP-1 cells. The isomers of IL-32 were typically characterized as being pro-inflammatory cytokines in the immune system [102,103]. The isomers of IL-32β induce anti-inflammatory cytokine IL-10 [18]. It can therefore be suggested that IL-32 performs both anti- and pro-inflammatory effects on cells of immune system. Moreover, its isomers interact with other molecules via their binding motifs and display intercellular activity [18]. IL-32 isomers interact particularly with heterogeneous PKC isomers that lead to the pro- and anti-inflammatory cytokines production [78,104].
Various immune system responses were mediated by novel PKC family via pro-inflammatory cytokines production [105-107]. PKC-α and PKC-δ induced toll like receptor (TLR)-2/4 expression that leads to the high glucose induced-immuneresponses [108]. PKC-δ also involved in increasing IL-2 production by expressing themselves in lymphoid cells. It has also been reported to be involved in cytokine production and peri-bronchiolar cell proliferation in lungs of PKC-δ knockout mice [109,110].
PKC-δ binds to the PU.1 and activates it resulting in the phosphorylation of its trans-activator domain [111]. PU.1 may bind with multiple target promoters and is involved in various immune responses [112,113]. IL-1β also acts as transcription key regulator because PU.1 has an ability to bind to the consensus sequence of IL-1β promoter region [113,114]. PU.1 along with other transcription factors including interferon regulatory factor (IRF)-4/8 and C/EBPβ regulates IL-1β transcription in human monocytes [113-115].
IL-32θ also acts as a strong intracellular modulator as it reduces the production of IL-1β in THP-1 human myelomonocytes by binding to the PKCδ that ultimately suppresses interaction between the PU.1 and PKCδ. Consequently, it leads to the inhibition of PKCδ-mediated PU.1 phosphorylation and hence inhibits IL-1β production (Figure 1). These findings suggest that IL-32θ plays a key role as intracellular modulator in the production of cytokines [36].
IL-32θ down regulates CCL5 expression
Activated CCL5 genes encode a normal T cell-expressed and secreted (RANTES) protein that expresses during late activation of T-cell [116]. CCL5 belongs to CC chemokine family which has two pairs of adjoining cysteine residues locating nearby their amino terminus. Members of this family of chemokines show achemotactic effect on T cells, monocytes, basophils and eosinophils [117-121]. CCL5 expression has been found in different types of cells such as epithelial cells, vascular smooth muscle cells (VSMCs), monocytes, macrophages and fibroblasts [116,122-124]. CCL5 was reported to be involved in onset of a number of inflammatory disorders [125,126].
Signal transducers and activators of transcription (STAT) are triggered by several cytokines, kinases and growth factors. Seven STAT family members including (STAT 1, 2, 3, 4, 5A, 5B and 6) have been identified [127-130]. Constitutive activation of STAT leads to the onset of cancer and suppresses immune system [130,131]. Receptor associated tyrosine kinase family including JAK activates STATs by phosphorylating the target portion on carboxylterminus that causes dimerization and translocation of STAT3 and binding to target genes DNA as well [132,133]. In comparison to other transcription factors STAT proteins contain Src homology (SH2) domain that acts as receptor-binding domain and facilitates dimerization of STAT proteins [134]. STAT3 can be phosphorylated on both Ser727 and Tyr705 residues and it was found that Tyr705 is highly important for the activation of STAT3 whereas Ser727’s role in activation of STAT3 is not clear [135-138].
IL-32θ blocks the CCL5 signaling by altering phosphorylation of STAT3 on Ser727. Upon stimulation of PMA, IL-32θ interacts with PKCδ and form a trimeric complex with it and STAT3. This trimeric complex facilitates the induction of IL-32θ and phosphorylation of STAT3 on Ser727 controlled by PKCδ. IL-32θ activity was mediated by PKCδ that leads to the inactivation of STAT3. Physical interaction of IL-32θ with PKCδ and STAT3 is phosphorylated at Ser727 which inhibit its trans-activating activity leading to the blockade of STAT3-mediated expression of CCL5. These findings confirmed that IL-32θ interacts with PMA-activated PKCδ that results in phosphorylation of STAT3 at Ser727. IL-32θ-mediated phosphorylation of STAT3 on Ser727, delays its ability to bind to DNA and hinders transcription of CCL5 gene [100]. Even under PMA stimulation, IL-32θ has an ability to inhibit translocation of STAT3 because the transcriptional activation of the CCL5 promoter takes place by binding of STAT3 to CCL5 promoter regulatory element [122]. Furthermore, it was reported that unphosphorylated STAT3 compete with IκB and forms a transcriptional complex with unphosphorylated NF-κB to initiate expression of CCL5 [116].
Therefore, it is concluded that in signaling pathway of IL-32θ, PKCδ acts as a serine kinase of STAT3 that leads to the activation of phosphorylation of STAT3, ultimately leading to inhibition of CCL5 expression.
Conclusion
In the light of the foregoing literature cited on IL-32θ, it can be concluded that IL-32θ plays a crucial role in immune system responses and cellular differentiation during disease pathogenesis as well as tumor suppression. Therefore, in future, it can be used in cancer therapies. However, more critical and extensive clinical investigations are highly recommended for a better and deeper understanding of possible therapeutic applications. To our best knowledge this is the first ever review to condense the role, exact mechanism of pathogenesis and latent therapeutic uses of IL-32θ in several inflammatory disorders.
Acknowledgements
The authors are thankful to the Vice chancellor of University of the Punjab, Lahore, Pakistan for providing financial support for the accomplishment of this review.
Disclosure of conflict of interest
None.
References
- 1.O’Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 2.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 3.Nazemian V, Shadnoush M, Manaheji H, Zaringhalam J. Probiotics and inflammatory pain: a literature review study. Middle East J Rehabil Health. 2016;3:e36087. [Google Scholar]
- 4.Pizarro TT, Cominelli F. Cytokine therapy for Crohn’s disease: advances in translational research. Annu Rev Med. 2007;58:433–444. doi: 10.1146/annurev.med.58.121205.100607. [DOI] [PubMed] [Google Scholar]
- 5.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 6.Schroeter M, Jander S. T-cell cytokines in injury-induced neural damage and repair. Neuromole Med. 2005;7:183–195. doi: 10.1385/NMM:7:3:183. [DOI] [PubMed] [Google Scholar]
- 7.Kalaaji AN. Cytokine therapy in advanced melanoma. J Drugs Dermatol. 2007;6:374–378. [PubMed] [Google Scholar]
- 8.Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss’s soft tissue tumors. Elsevier Health Sciences; 2007. [Google Scholar]
- 9.Kim B, Ayran JC, Sagar SG, Adman ET, Fuller SM, Tran NH, Horrigan J. New human immunodeficiency virus, Type 1 reverse transcriptase (HIV-1 RT) mutants with increased fidelity of DNA synthesis accuracy, template binding, and processivity. J Biol Chem. 1999;274:27666–27673. doi: 10.1074/jbc.274.39.27666. [DOI] [PubMed] [Google Scholar]
- 10.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta-1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 11.Gleeson M. Interleukins and exercise. J Physiol. 2000;529:1. doi: 10.1111/j.1469-7793.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of antiinflammatory cytokines. Nat Immunol. 2012;13:925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khawar B, Abbasi MH, Sheikh N. A panoramic spectrum of complex interplay between the immune system and IL-32 during pathogenesis of various systemic infections and inflammation. Eur J Med Res. 2015;20:1–7. doi: 10.1186/s40001-015-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khawar MB, Abbasi MH, Sheikh N. IL-32: a novel pluripotent inflammatory interleukin, towards gastric inflammation, gastric cancer, and chronic rhino sinusitis. Mediat Inflamm. 2016;2016:8413768. doi: 10.1155/2016/8413768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, Choi WS, Kim BK, Lee CK, Yoon DY. Identification of the most active interleukin-32 isoform. Immunology. 2009;126:535–542. doi: 10.1111/j.1365-2567.2008.02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, Izuhara K. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 17.Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci U S A. 2006;103:3316–3321. doi: 10.1073/pnas.0511206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JW, Choi SC, Cho MC, Kim HJ, Kim JH, Lim JS, Kim SH, Han JY, Yoon DY. A proinflammatory cytokine interleukin-32beta promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology. 2009;128:e532–e540. doi: 10.1111/j.1365-2567.2008.03025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JW, Park YS, Lee DH, Kim MS, Bak Y, Ham SY, Park SH, Kim H, Ahn JH, Hong JT. Interaction network mapping among IL-32 isoforms. Biochimie. 2014;101:248–251. doi: 10.1016/j.biochi.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Kim YG, Lee CK, Kim SH, Cho WS, Mun SH, Yoo B. Interleukin-32gamma enhances the production of IL-6 and IL-8 in fibroblast-like synoviocytes via Erk1/2 activation. J Clin Immunol. 2010;30:260–267. doi: 10.1007/s10875-009-9360-2. [DOI] [PubMed] [Google Scholar]
- 21.Yagi Y, Andoh A, Imaeda H, Aomatsu T, Ohsaki R, Inatomi O, Bamba S, Tsujikawa T, Shimizu T, Fujiyama Y. Interleukin-32alpha expression in human colonic subepithelial myofibroblasts. Int J Mol Med. 2011;27:263. doi: 10.3892/ijmm.2010.575. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama M, Niki Y, Kawasaki T, Takeda Y, Horiuchi K, Sasaki A, Okada Y, Umezawa K, Ikegami H, Toyama Y. Enhanced susceptibility to lipopolysaccharide-induced arthritis and endotoxin shock in interleukin-32 alpha transgenic mice through induction of tumor necrosis factor alpha. Arthritis Res Ther. 2012;14:1. doi: 10.1186/ar3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Evans CA, Pierce A, Winter SA, Spooncer E, Heyworth CM, Whetton AD. Activation of granulocyte-macrophage colony-stimulating factor and interleukin-3 receptor subunits in a multipotential hematopoietic progenitor cell line leads to differential effects on development. Blood. 1999;94:1504–1514. [PubMed] [Google Scholar]
- 25.Just U, Stocking C, Spooncer E, Dexter TM, Ostertag W. Expression of the GM-CSF gene after retroviral transfer in hematopoietic stem cell lines induces synchronous granulocytemacrophage differentiation. Cell. 1991;64:1163–1173. doi: 10.1016/0092-8674(91)90271-y. [DOI] [PubMed] [Google Scholar]
- 26.Reddy VA, Iwama A, Iotzova G, Schulz M, Elsasser A, Vangala RK, Tenen DG, Hiddemann W, Behre G. Granulocyte inducer C/EBPalpha inactivates the myeloid master regulator PU. 1: possible role in lineage commitment decisions. Blood. 2002;100:483–490. doi: 10.1182/blood.v100.2.483. [DOI] [PubMed] [Google Scholar]
- 27.Ishigami S, Arigami T, Uchikado Y, Setoyama T, Kita Y, Sasaki K, Okumura H, Kurahara H, Kijima Y, Harada A. IL-32 expression is an independent prognostic marker for gastric cancer. Med Oncol. 2013;30:1–5. doi: 10.1007/s12032-013-0472-4. [DOI] [PubMed] [Google Scholar]
- 28.Sakitani K, Hirata Y, Hayakawa Y, Serizawa T, Nakata W, Takahashi R, Kinoshita H, Sakamoto K, Nakagawa H, Akanuma M. Role of interleukin-32 in Helicobacter pylori-induced gastric inflammation. Infect Immun. 2012;80:3795–3803. doi: 10.1128/IAI.00637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang YH, Park MY, Yoon DY, Han SR, Lee CI, Ji NY, Myung PK, Lee HG, Kim JW, Yeom YI. Dysregulation of overexpressed IL-32alpha in hepatocellular carcinoma suppresses cell growth and induces apoptosis through inactivation of NF-κB and Bcl-2. Cancer Lett. 2012;318:226–233. doi: 10.1016/j.canlet.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CY, Wang CS, Tsai MM, Chi HC, Cheng WL, Tseng YH, Chen CY, Lin CD, Wu JI, Wang LH. Interleukin-32 increases human gastric cancer cell invasion associated with tumor progression and metastasis. Clin Cancer Res. 2014;20:2276–2288. doi: 10.1158/1078-0432.CCR-13-1221. [DOI] [PubMed] [Google Scholar]
- 31.Jeong HJ, Nam SY, Oh HA, Han NR, Kim YS, Moon PD, Shin SY, Kim MH, Kim HM. Interleukin-32-induced thymic stromal lymphopoietin plays a critical role in macrophage differentiation through the activation of caspase-1 in vitro. Arthritis Res Ther. 2012;14:R259. doi: 10.1186/ar4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon YM, Yoon BY, Her YM, Oh HJ, Lee JS, Kim KW, Lee SY, Woo YJ, Park KS, Park SH. IL-32 and IL-17 interact and have the potential to aggravate osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther. 2012;14:R246. doi: 10.1186/ar4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mabilleau G, Sabokbar A. Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PLoS One. 2009;4:e4173. doi: 10.1371/journal.pone.0004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung MY, Son MH, Kim SH, Cho D, Kim TS. IL-32gamma induces the maturation of dendritic cells with Th1-and Th17-polarizing ability through enhanced IL-12 and IL-6 production. J Immunol. 2011;186:6848–6859. doi: 10.4049/jimmunol.1003996. [DOI] [PubMed] [Google Scholar]
- 35.Kang JW, Park YS, Kim MS, Lee DH, Bak Y, Ham SY, Song YS, Hong JT, Yoon DY. IL-32alpha down-regulates beta2 integrin (CD18) expression by suppressing PU. 1 expression in myeloid cells. Cell Signal. 2014;26:1514–1522. doi: 10.1016/j.cellsig.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Kim MS, Kang JW, Lee DH, Bak Y, Park YS, Song YS, Ham SY, Oh DK, Hong J, Yoon DY. IL-32theta negatively regulates IL-1beta production through its interaction with PKCdelta and the inhibition of PU. 1 phosphorylation. FEBS Lett. 2014;588:2822–2829. doi: 10.1016/j.febslet.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Rosenbauer F, Wagner K, Zhang P, Knobeloch KP, Iwama A, Tenen DG. pDP4, a novel glycoprotein secreted by mature granulocytes, is regulated by transcription factor PU. 1. Blood. 2004;103:4294–4301. doi: 10.1182/blood-2003-08-2688. [DOI] [PubMed] [Google Scholar]
- 38.Pahl HL, Scheibe RJ, Zhang DE, Chen HM, Galson DL, Maki RA, Tenen DG. The protooncogene PU. 1 regulates expression of the myeloid-specific CD11b promoter. J Biol Chem. 1993;268:5014–5020. [PubMed] [Google Scholar]
- 39.Rosmarin AG, Caprio D, Levy R, Simkevich C. CD18 (beta 2 leukocyte integrin) promoter requires PU. 1 transcription factor for myeloid activity. Proc Natl Acad Sci U S A. 1995;92:801–805. doi: 10.1073/pnas.92.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terry RL, Miller SD. Molecular control of monocyte development. Cell Immunol. 2014;291:16–21. doi: 10.1016/j.cellimm.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jego G, Lanneau D, De Thonel A, Berthenet K, Hazoumé A, Droin N, Hamman A, Girodon F, Bellaye PS, Wettstein G. Dual regulation of SPI1/PU. 1 transcription factor by heat shock factor 1 (HSF1) during macrophage differentiation of monocytes. Leukemia. 2014;28:1676–1686. doi: 10.1038/leu.2014.63. [DOI] [PubMed] [Google Scholar]
- 42.de la Rica L, Rodriguez-Ubreva J, Garcia M, Islam AB, Urquiza JM, Hernando H, Christensen J, Helin K, Gomez-Vaquero C, Ballestar E. PU. 1 target genes undergo Tet2-coupled demethylation and DNMT3b-mediated methylation in monocyte-to-osteoclast differentiation. Genome Biol. 2013;14:R99. doi: 10.1186/gb-2013-14-9-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okuno Y, Huang G, Rosenbauer F, Evans EK, Radomska HS, Iwasaki H, Akashi K, Moreau-Gachelin F, Li Y, Zhang P. Potential autoregulation of transcription factor PU. 1 by an upstream regulatory element. Mol Cell Biol. 2005;25:2832–2845. doi: 10.1128/MCB.25.7.2832-2845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, D’Costa J, Civin CI, Friedman AD. C/EBPalpha directs monocytic commitment of primary myeloid progenitors. Blood. 2006;108:1223–1229. doi: 10.1182/blood-2005-12-008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeamans C, Wang D, Paz-Priel I, Torbett BE, Tenen DG, Friedman AD. C/EBPalpha binds and activates the PU. 1 distal enhancer to induce monocyte lineage commitment. Blood. 2007;110:3136–3142. doi: 10.1182/blood-2007-03-080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim MS, Kang JW, Park YS, Lee DH, Bak Y, Kwon T, Yoon DY. IL-32theta inhibits monocytic differentiation of leukemia cells by attenuating expression of transcription factor PU. 1. Oncotarget. 2015;6:4394. doi: 10.18632/oncotarget.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowak D, Stewart D, Koeffler HP. Differentiation therapy of leukemia: 3 decades of development. Blood. 2009;113:3655–3665. doi: 10.1182/blood-2009-01-198911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sell S. Leukemia: stem cells, maturation arrest and differentiation therapy. Tumor Biol. 2006;27:14–14. doi: 10.1385/SCR:1:3:197. [DOI] [PubMed] [Google Scholar]
- 49.Foon KA, Gale RP, Todd RF 3rd. Recent advances in the immunologic classification of leukemia. Semin Hematol. 1986;23:257–283. [PubMed] [Google Scholar]
- 50.Austin H, Delzell E, Cole P. Benzene and leukemia. A review of the literature and a risk assessment. Am J Epidemiol. 1988;127:419–439. doi: 10.1093/oxfordjournals.aje.a114820. [DOI] [PubMed] [Google Scholar]
- 51.Horwitz M, Goode EL, Jarvik GP. Anticipation in familial leukemia. Am J Hum Genet. 1996;59:990–8. [PMC free article] [PubMed] [Google Scholar]
- 52.Evans DI, Steward JK. Down’s syndrome and leukaemia. Lancet. 1972;300:1322. doi: 10.1016/s0140-6736(72)92704-3. [DOI] [PubMed] [Google Scholar]
- 53.Yoshinaga S, Mabuchi K, Sigurdson AJ, Doody MM, Ron E. Cancer risks among radiologists and radiologic technologists: review of epidemiologic studies 1. Radiology. 2004;233:313–321. doi: 10.1148/radiol.2332031119. [DOI] [PubMed] [Google Scholar]
- 54.Ryningen A, Wergeland L, Glenjen N, Gjertsen BT, Bruserud O. In vitro crosstalk between fibroblasts and native human acute myelogenous leukemia (AML) blasts via local cytokine networks results in increased proliferation and decreased apoptosis of AML cells as well as increased levels of proangiogenic Interleukin 8. Leukemia Res. 2005;29:185–196. doi: 10.1016/j.leukres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Oster W, Cicco NA, Klein H, Hirano T, Kishimoto T, Lindemann A, Mertelsmann RH, Herrmann F. Participation of the cytokines interleukin 6, tumor necrosis factor-alpha, and interleukin 1-beta secreted by acute myelogenous leukemia blasts in autocrine and paracrine leukemia growth control. J Clin Invest. 1989;84:451–457. doi: 10.1172/JCI114186. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Westermann F, Kube D, Haier B, Bohlen H, Engert A, Zuehlsdorf M, Diehl V, Tesch H. Interleukin 10 inhibits cytokine production of human AML cells. Ann Oncol. 1996;7:397–404. doi: 10.1093/oxfordjournals.annonc.a010607. [DOI] [PubMed] [Google Scholar]
- 57.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 58.Kagoya Y, Yoshimi A, Kataoka K, Nakagawa M, Kumano K, Arai S, Kobayashi H, Saito T, Iwakura Y, Kurokawa M. Positive feedback between NF-κB and TNF-alpha promotes leukemia-initiating cell capacity. J Clin Invest. 2014;124:528–542. doi: 10.1172/JCI68101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao XZ, Bi S, Copra H, Devemy E, Venugopal P, Li B, Hsu WT, Loew J, Galvez A, Gregory S. Cytokine gene activity in AML cells in vivo in patients. Leukemia Res. 1998;22:429–438. doi: 10.1016/s0145-2126(98)00024-1. [DOI] [PubMed] [Google Scholar]
- 60.Lowenberg B, Salem M, Delwel R. Effects of recombinant multi-CSF, GM-CSF, G-CSF and M-CSF on the proliferation and maturation of human AML in vitro. Blood Cells. 1987;14:539–549. [PubMed] [Google Scholar]
- 61.Wakamiya N, Stone R, Takeyama H, Spriggs D, Kufe D. Detection of tumor necrosis factor gene expression at a cellular level in human acute myeloid leukemias. Leukemia. 1989;3:51–56. [PubMed] [Google Scholar]
- 62.Murohashi I, Rodriguez-Cimadevilla JC, Hoang T. Tumor necrosis factor-a enhances cytokine production by AML blasts. Ann N Y Acad Sci. 1991;628:148–150. doi: 10.1111/j.1749-6632.1991.tb17232.x. [DOI] [PubMed] [Google Scholar]
- 63.Testa U, Grignani F, Samoggia P, Zanetti C, Riccioni R, Coco FL, Diverio D, Felli N, Passerini CG, Grell M. The PML/RARalpha fusion protein inhibits tumor necrosis factor-alpha-induced apoptosis in U937 cells and acute promyelocytic leukemia blasts. J Clin Invest. 1998;101:2278–89. doi: 10.1172/JCI1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beyne-Rauzy O, Recher C, Dastugue N, Demur C, Pottier G, Laurent G, Sabatier L, Mansat-De Mas V. Tumor necrosis factor alpha induces senescence and chromosomal instability in human leukemic cells. Oncogene. 2004;23:7507–7516. doi: 10.1038/sj.onc.1208024. [DOI] [PubMed] [Google Scholar]
- 65.Nishikawa M, Shirakawa S. The expression and possible roles of protein kinase C in haematopoietic cells. Leuk Lymphoma. 1992;8:201–211. doi: 10.3109/10428199209054906. [DOI] [PubMed] [Google Scholar]
- 66.Chung IY, Kwon J, Benveniste EN. Role of protein kinase C activity in tumor necrosis factor-alpha gene expression. Involvement at the transcriptional level. J Immunol. 1992;149:3894–3902. [PubMed] [Google Scholar]
- 67.Kontny E, Kurowska M, Szczepanska K, Maslinski W. Rottlerin, a PKC isozyme-selective inhibitor, affects signaling events and cytokine production in human monocytes. J Leukocyte Biol. 2000;67:249–258. doi: 10.1002/jlb.67.2.249. [DOI] [PubMed] [Google Scholar]
- 68.Ruvolo PP, Zhou L, Watt JC, Ruvolo VR, Burks JK, Jiffar T, Kornblau S, Konopleva M, Andreeff M. Targeting PKC-mediated signal transduction pathways using enzastaurin to promote apoptosis in acute myeloid leukemiaderived cell lines and blast cells. J Cell Biochem. 2011;112:1696–1707. doi: 10.1002/jcb.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim JS, Park ZY, Yoo YJ, Yu SS, Chun JS. p38 kinase mediates nitric oxide-induced apoptosis of chondrocytes through the inhibition of protein kinase C zeta by blocking autophosphorylation. Cell Death Differ. 2005;12:201–212. doi: 10.1038/sj.cdd.4401511. [DOI] [PubMed] [Google Scholar]
- 70.Greene MW, Ruhoff MS, Burrington CM, Garofalo RS, Orena SJ. TNFalpha activation of PKCdelta, mediated by NFκB and ER stress, cross-talks with the insulin signaling cascade. Cell Signal. 2010;22:274–284. doi: 10.1016/j.cellsig.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 71.Naitoh K, Yano T, Miura T, Itoh T, Miki T, Tanno M, Sato T, Hotta H, Terashima Y, Shimamoto K. Roles of Cx43-associated protein kinases in suppression of gap junction-mediated chemical coupling by ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2009;296:H396–H403. doi: 10.1152/ajpheart.00448.2008. [DOI] [PubMed] [Google Scholar]
- 72.Liu RY, Fan C, Liu G, Olashaw NE, Zuckerman KS. Activation of p38 mitogen-activated protein kinase is required for tumor necrosis factor-alpha-supported proliferation of leukemia and lymphoma cell lines. J Biolo Chem. 2000;275:21086–21093. doi: 10.1074/jbc.M001281200. [DOI] [PubMed] [Google Scholar]
- 73.Olson CM, Hedrick MN, Izadi H, Bates TC, Olivera ER, Anguita J. p38 mitogen-activated protein kinase controls NF-κB transcriptional activation and tumor necrosis factor alpha production through RelA phosphorylation mediated by mitogen-and stress-activated protein kinase 1 in response to Borrelia burgdorferi antigens. Infect Immun. 2007;75:270–277. doi: 10.1128/IAI.01412-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoareau L, Bencharif K, Rondeau P, Murumalla R, Ravanan P, Tallet F, Delarue P, Cesari M, Roche R, Festy F. Signaling pathways involved in LPS induced TNFalpha production in human adipocytes. J Inflamm. 2010;7:1. doi: 10.1186/1476-9255-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Kang JW, Park YS, Lee DH, Kim MS, Bak Y, Park SH, Ham SY, Yang Y, Hong JT, Yoon DY. Interleukin-32delta interacts with IL-32beta and inhibits IL-32beta-mediated IL-10 production. FEBS Lett. 2013;587:3776–3781. [PubMed] [Google Scholar]
- 77.Heinhuis B, Koenders MI, van den Berg WB, Netea MG, Dinarello CA, Joosten LA. Interleukin 32 (IL-32) contains a typical alphahelix bundle structure that resembles focal adhesion targeting region of focal adhesion kinase-1. J Biol Chem. 2012;287:5733–5743. doi: 10.1074/jbc.M111.288290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang JW, Park YS, Lee DH, Kim Jh, Kim MS, Bak Y, Hong J, Yoon DY. Intracellular interaction of interleukin (IL)-32alpha with protein kinase C-epsilon (PKC-epsilon) and STAT3 protein augments IL-6 production in THP-1 promonocytic cells. J Biol Chem. 2012;287:35556–35564. doi: 10.1074/jbc.M112.400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim MS, Kang JW, Jeon JS, Kim JK, Kim JW, Hong J, Yoon DY. IL-32theta gene expression in acute myeloid leukemia suppresses TNF-alpha production. Oncotarget. 2015;6:40747. doi: 10.18632/oncotarget.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 81.Jordan CT. Cancer stem cells: controversial or just misunderstood? Cell Stem Cell. 2009;4:203–205. doi: 10.1016/j.stem.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 85.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 86.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 87.Sastry KS, Al-Muftah MA, Li P, Al-Kowari MK, Wang E, Chouchane AI, Kizhakayil D, Kulik G, Marincola FM, Haoudi A. Targeting proapoptotic protein BAD inhibits survival and self-renewal of cancer stem cells. Cell Death Differ. 2014;21:1936–1949. doi: 10.1038/cdd.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Somsouk M. Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma. World J Gastroenterol. 2004;11:007. doi: 10.3748/wjg.v10.i11.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufman R, Huber LA, Zatloukal K. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, Lin J. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011;71:7226–7237. doi: 10.1158/0008-5472.CAN-10-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spano JP, Milano G, Rixe C, Fagard R. JAK/STAT signalling pathway in colorectal cancer: a new biological target with therapeutic implications. Eur J Cancer. 2006;42:2668–2670. doi: 10.1016/j.ejca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 92.Xiong H, Hong J, Du W, Lin YW, Ren Ll, Wang YC, Su WY, Wang Jl, Cui Y, Wang ZH. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem. 2012;287:5819–5832. doi: 10.1074/jbc.M111.295964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 94.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hartman ZC, Yang XY, Glass O, Lei G, Osada T, Dave SS, Morse MA, Clay TM, Lyerly HK. HER2 overexpression elicits a proinflammatory IL-6 autocrine signaling loop that is critical for tumorigenesis. Cancer Res. 2011;71:4380–4391. doi: 10.1158/0008-5472.CAN-11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer. 2012;11:1. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yun HM, Park KR, Kim EC, Han SB, Yoon DY, Hong JT. IL-32alpha suppresses colorectal cancer development via TNFR1-mediated death signaling. Oncotarget. 2015;6:9061. doi: 10.18632/oncotarget.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park JS, Choi SY, Lee JH, Lee M, Nam ES, Jeong AL, Lee S, Han S, Lee MS, Lim JS. Interleukin-32beta stimulates migration of MDA-MB-231 and MCF-7cells via the VEGFSTAT3 signaling pathway. Cell Oncol. 2013;36:493–503. doi: 10.1007/s13402-013-0154-4. [DOI] [PubMed] [Google Scholar]
- 99.Yun HM, Oh JH, Shim JH, Ban JO, Park KR, Kim JH, Lee DH, Kang JW, Park YH, Yu D. Antitumor activity of IL-32beta through the activation of lymphocytes, and the inactivation of NF-κB and STAT3 signals. Cell Death Dis. 2013;4:e640. doi: 10.1038/cddis.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bak Y, Kang JW, Kim MS, Park YS, Kwon T, Kim S, Hong J, Yoon DY. IL-32theta downregulates CCL5 expression through its interaction with PKCdelta and STAT3. Cell Signal. 2014;26:3007–3015. doi: 10.1016/j.cellsig.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 101.Bak Y, Kwon T, Hong J, Yu DY, Yoon DY. IL-32theta inhibits stemness and epithelial-mesenchymal transition of cancer stem cells via the STAT3 pathway in colon cancer. Oncotarget. 2016;7:7307. doi: 10.18632/oncotarget.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moschen AR, Fritz T, Clouston AD, Rebhan I, Bauhofer O, Barrie HD, Powell EE, Kim SH, Dinarello CA, Bartenschlager R. Interleukin-32: a new proinflammatory cytokine involved in hepatitis C virus-related liver inflammation and fibrosis. Hepatology. 2011;53:1819–1829. doi: 10.1002/hep.24285. [DOI] [PubMed] [Google Scholar]
- 103.Felaco P, Castellani ML, De Lutiis MA, Felaco M, Pandolfi F, Salini V, De Amicis D, Vecchiet J, Tete S, Ciampoli C. IL-32: a newly-discovered proinflammatory cytokine. J Biol Regul Homeost Agents. 2008;23:141–147. [PubMed] [Google Scholar]
- 104.Kang JW, Park YS, Kim MS, Lee DH, Bak Y, Ham SY, Park SH, Hong JT, Yoon DY. Interleukin (IL)-32beta-mediated CCAAT/enhancer-binding protein alpha (C/EBPalpha) phosphorylation by protein kinase C delta (PKCdelta) abrogates the inhibitory effect of C/EBP alpha on IL-10 production. J Biol Chem. 2013;288:23650–23658. doi: 10.1074/jbc.M113.465575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Min JK, Kim YM, Kim SW, Kwon MC, Kong YY, Hwang IK, Won MH, Rho J, Kwon YG. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-κB activation in endothelial cells. J Immunol. 2005;175:531–540. doi: 10.4049/jimmunol.175.1.531. [DOI] [PubMed] [Google Scholar]
- 106.Bennasser Y, Bahraoui E. HIV-1 Tat protein induces interleukin-10 in human peripheral blood monocytes: involvement of protein kinase C-beta II and-delta. FASEB J. 2002;16:546–554. doi: 10.1096/fj.01-0775com. [DOI] [PubMed] [Google Scholar]
- 107.Tiwari RL, Singh V, Singh A, Barthwal MK. IL-1R-associated kinase-1 mediates protein kinase Cdelta-induced IL-1beta production in monocytes. J Immunol. 2011;187:2632–2645. doi: 10.4049/jimmunol.1002526. [DOI] [PubMed] [Google Scholar]
- 108.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes Mechanism of activation. Diabetes. 2008;57:3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Isakov N. Regulation of Immune system cell functions by protein kinase C. Frontiers E-books; 2014. Involvement of distinct PKC gene products in T cell functions. [Google Scholar]
- 110.Shukla A, Lounsbury KM, Barrett TF, Gell J, Rincon M, Butnor KJ, Taatjes DJ, Davis GS, Vacek P, Nakayama KI. Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase C-delta knockout mice. Am J Pathol. 2007;170:140–151. doi: 10.2353/ajpath.2007.060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamdorf M, Berger A, Schule S, Reinhardt J, Flory E. PKC-delta Induced PU. 1 phosphorylation promotes hematopoietic stem cell differentiation to dendritic cells. Stem Cells. 2011;29:297–306. doi: 10.1002/stem.564. [DOI] [PubMed] [Google Scholar]
- 112.Kamath MB, Houston IB, Janovski AJ, Zhu X, Gowrisankar S, Jegga AG, DeKoter RP. Dose-dependent repression of T-cell and natural killer cell genes by PU. 1 enforces myeloid and B-cell identity. Leukemia. 2008;22:1214–1225. doi: 10.1038/leu.2008.67. [DOI] [PubMed] [Google Scholar]
- 113.Stopka T, Amanatullah DF, Papetti M, Skoultchi AI. PU. 1 inhibits the erythroid program by binding to GATA-1 on DNA and creating a repressive chromatin structure. EMBO J. 2005;24:3712–3723. doi: 10.1038/sj.emboj.7600834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marecki S, Riendeau CJ, Liang MD, Fenton MJ. PU. 1 and multiple IFN regulatory factor proteins synergize to mediate transcriptional activation of the human IL-1beta gene. J Immunol. 2001;166:6829–6838. doi: 10.4049/jimmunol.166.11.6829. [DOI] [PubMed] [Google Scholar]
- 115.Yang Z, Wara-aswapati N, Chen C, Tsukada J, Auron PE. NF-IL6 (C/EBPbeta) vigorously activates il1b gene expression via a Spi-1 (PU. 1) protein-protein tether. J Biol Chem. 2000;275:21272–21277. doi: 10.1074/jbc.M000145200. [DOI] [PubMed] [Google Scholar]
- 116.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NF-κB. Gene Dev. 2007;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 118.Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dahinden CA, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, Minty A, Baggiolini M. Monocyte chemotactic protein 3 is a most effective basophil-and eosinophil-activating chemokine. J Exp Med. 1994;179:751–756. doi: 10.1084/jem.179.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schober A, Manka D, Von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 122.Kovacic JC, Gupta R, Lee AC, Ma M, Fang F, Tolbert CN, Walts AD, Beltran LE, San H, Chen G. Stat3-dependent acute Rantes production in vascular smooth muscle cells modulates inflammation following arterial injury in mice. J Clin Invest. 2010;120:303–314. doi: 10.1172/JCI40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yun JJ, Fischbein MP, Laks H, Irie Y, Espejo ML, Fishbein MC, Berliner JA, Ardehali A. Rantes production during development of cardiac allograft vasculopathy. Transplantation. 2001;71:1649–1656. doi: 10.1097/00007890-200106150-00026. [DOI] [PubMed] [Google Scholar]
- 124.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein tolllike receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, Balkwill FR. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 2003;63:8360–8365. [PubMed] [Google Scholar]
- 126.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 127.Horvath CM. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem Sci. 2000;25:496–502. doi: 10.1016/s0968-0004(00)01624-8. [DOI] [PubMed] [Google Scholar]
- 128.Akira S. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells. 1999;17:138–146. doi: 10.1002/stem.170138. [DOI] [PubMed] [Google Scholar]
- 129.Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1420. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 130.Haura EB, Turkson J, Jove R. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 131.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 132.Stahl N, Farruggella TJ, Boulton TG, Zhong Z. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–53. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 133.Bromberg J, Darnell JE. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2474. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 134.Ihle JN, Hawkins PT, Parker PJ. Janus kinases in cytokine signalling [and Discussion] . Philos Trans R Soc Lond B Biol Sci. 1996;351:159–166. doi: 10.1098/rstb.1996.0012. [DOI] [PubMed] [Google Scholar]
- 135.Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE. Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gartsbein M, Alt A, Hashimoto K, Nakajima K, Kuroki T, Tennenbaum T. The role of protein kinase C delta activation and STAT3 Ser727 phosphorylation in insulin-induced keratinocyte proliferation. J Cell Sci. 2006;119:470–481. doi: 10.1242/jcs.02744. [DOI] [PubMed] [Google Scholar]
- 137.Lim CP, Cao X. Serine phosphorylation and negative regulation of Stat3 by JNK. J Biol Chem. 1999;274:31055–31061. doi: 10.1074/jbc.274.43.31055. [DOI] [PubMed] [Google Scholar]
- 138.Turkson J, Bowman T, Adnane J, Zhang Y, Djeu JY, Sekharam M, Frank DA, Holzman LB, Wu J, Sebti S. Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol Cell Biol. 1999;19:7519–7528. doi: 10.1128/mcb.19.11.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]


