Abstract
It is reported that long noncoding RNAs (lncRNAs) were expressed aberrantly in cartilage of osteoarthritis (OA). Current evidence indicates that lncRNAs not only serve as positive or negative regulators of OA, but also crosstalk with multiple potential targets to impact on the critical events in OA process. This review summarized the lncRNAs identified in OA to date, discussed their influence on the survival of chondrocytes and synoviocytes, arthritis-associated factors, and angiogenesis, and indicated the potential in diagnosis, therapy, and prognosis.
Keywords: Long noncoding RNA, osteoarthritis, cartilage
Introduction
Articular cartilage homeostasis is tightly orchestrated and maintained in a balance between anabolic and catabolic processes by the activity of the chondrocytes. When joints are subjected to altered loading caused by malalignment or trauma [1], catabolism often predominates and causes joint abnormalities, including synovitis, cartilage degradation, subchondral bone sclerosis, and osteophyte formation, which can be defined as osteoarthritis (OA) collectively [2]. In 1990, OA was estimated as the eighth leading non-fatal burden of life all over the world, whereas in 2000 it became the sixth [3,4]. The pathogenesis of OA is complex and involves interplay of multiple factors such as genetic predisposition, altered mechanical loading, and the imbalance between anabolic and catabolic factors. It is thought that the imbalance plays a major role in cartilage degradation in OA [5].
Given that articular cartilage is normally avascular and has little intrinsic regenerative capacity, OA is a challenging disease to treat. There have been no effective therapies discovered to ameliorate or stop OA progression. Recent studies have shed light on the connection between noncoding RNAs (ncRNAs) and OA development [6]. The ncRNAs can be broadly divided into small ncRNAs and long ncRNAs (lncRNAs) [7]. It is known that lncRNAs, which are mRNA-like and more than 200 nucleotides in length, are transcribed in mammalian genomes pervasively [8]. In the past decade, the lncRNAs emerge as novel regulators of numerous biological processes where they serve as guides, signals, decoys, and scaffolds [9,10], and have effects on a broad spectrum of development and diseases [11-17].
LncRNAs have been reported to play critical roles in the development of bone and cartilage tissue [18,19]. It aroused interest in aberrant expression of lncRNAs in OA cartilage which might influence the balance between anabolic and catabolic phase of joint cartilage. It is suggested that lncRNAs could be applied for diagnosis and prognosis, and could serve as a personalized therapeutic biomarker to impede, stop, and even reverse OA progression [20-22]. In the current review, we mainly summarized and emphasized the roles of lncRNAs in the OA progression, and harnessed them for the treatment of OA.
LncRNAs regulate the fate of cells
Table 1 summarized several lncRNAs that played demonstrated roles in the fate of chondrocytes and synoviocytes. These representative lncRNAs were selected to illustrate the diverse targets and mechanisms of lncRNAs in the regulation of chondrocytes and synoviocytes survival (Table 1, Figure 1).
Table 1.
Selected lncRNAs identified to date in the regulation of the chondrocytes and synoviocytes survival
| LncRNA | Proposed mechanism of action | Reference |
|---|---|---|
| GAS5 | Suppress miR-21 expression to inhibit the autophagic response and stimulate apoptosis | [30] |
| PCGEM1 | Sponge for miR-770 to regulate synoviocytes proliferation, apoptosis, and autophagy | [40] |
| UFC1 | Interact with miR-34a to promote chondrocytes proliferation and inhibit apoptosis | [37] |
| uc.343 | Cis-regulate HOXC8 to impact chondrocytes cycle | [22] |
Abbreviations: GAS5, Growth arrest-specific 5; PCGEM1, prostate cancer gene expression marker 1.
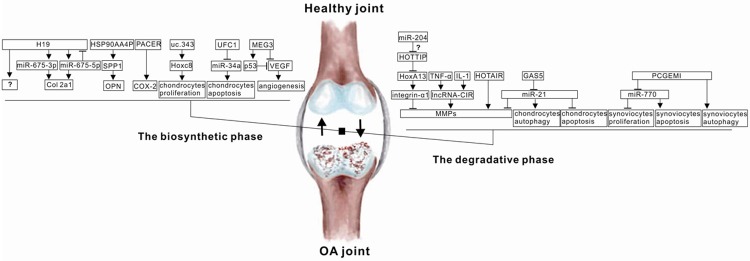
Figure 1.
Schematic mode of lncRNAs and signaling pathways involved in the OA process. LncRNA stimulated or inhibited diverse targets to impact on the balance between the biosynthetic phase and the degradative phase of joint cartilage.
Chondrocytes
Recently, many studies reported both apoptotic and non-apoptotic cell death in OA chondrocytes [23]. It is demonstrated that chondrocyte death is responsible for the severity of cartilage degradation [24-27], suggesting that apoptosis could be of diagnostic valve and be a potent option for the new therapeutic target.
It is known that Growth Arrest-Specific 5 (GAS5) is expressed in various tissues with multiple splice isoforms differentially and widely [28], and the inhibition of GAS5 will suppress cell apoptosis [29]. Several studies revealed that the expression level of GAS5 was significantly upregulated in OA chondrocytes, and RNA FISH analysis showed that GAS5 was positioned at nucleus and cytoplasm in OA chondrocytes but merely at nucleus in healthy chondrocytes [30].
MiR-21 is one kind of well-known onco-microRNAs. It has been shown experimentally that miR-21 targeted numerous genes involved in tumor growth and metastasis, for example, inhibiting tumor suppressors, such as PTEN and PDCD4 in gastric cancer [31], Tipe2 in immune diseases [32], and methionine adenosyltransferase in hepatoma cells [33]. It is demonstrated that miR-21 was suppressed in OA patients and the modulation of miR-21 influenced apoptosis and autophagy of OA chondrocytes [30].
It is observed that miR-21 was upregulated and GAS5 was downregulated in breast tumor tissues, indicating a negative correlation between GAS5 and miR-21 in several breast cancer cell lines [34]. Song et al observed a reciprocal repression of GAS5 and miR-21 during OA pathogenesis. The upregulation of GAS5 decreased the level of miR-21 expression significantly and regulated cartilage degradation [30]. It is supposed that GAS5 regulated cell survival by acting as the sponge of miR-21 and thereby contributed to the pathogenesis of OA. However, the possible inter-regulatory network between miR-21 and GAS5 has not been well studied.
Previous studies have shown that lncRNA UFC1 regulated cell survival positively and was expr-essed aberrantly in colorectal cancer and liver cancer [35,36]. Recently, functional studies demonstrated UFC1 functioned as the promotor of proliferation and the inhibitor of apoptosis of chondrocytes, and it is reported that the expression of UFC1 was downregulated in OA cartilage [37]. It is known that UFC1 could interact with miR-34a in OA chondrocytes. MiR-34a could stimulate apoptosis of OA chondrocytes, while silencing miR-34a could reduce chondrocytes apoptosis effectively [38]. This observation suggests that the interplay between UFC1 and miR-34a could regulate the survival of chondrocytes, and restoring the expression of UFC1 has the potential to relieve or stop cartilage degradation.
It is shown that Homeobox gene C8 (Hoxc8) knock-down chondrocytes appeared to be with prolonged duration and delayed exit from M-phase [39], which implicated that Hoxc8 could control cell cycles to affect the proliferation of chondrocytes and cartilage development at this critical time point. LncRNA uc.343 was reported to reside upstream of Hoxc8 and cis-regulate Hoxc8. LncRNA uc.343 was upregulated in OA cartilage and was correlated with Hoxc8 positively in SW1353 cells treated with IL-1β [22]. These results provide evidence that uc.343 might target Hoxc8 to regulate chondrocytes cycle progression.
Synoviocytes
Hyperplasia of synoviocytes is a hallmark of OA and the fibroblast-like synoviocytes can secrete proinflammatory cytokines to degrade cartilage [40]. It is well-known that prostate cancer gene expression marker 1 (PCGEM1) was overexpressed in prostate cancer. Overexpression of PCGEM1 decreased doxorubicin-induced expression of p53 and p21Waf1/Cip1, and suppressed apoptosis in LNCaP cells [41]. LncRNA PCGEM1 was also overexpressed in OA synoviocytes. Overexpression of PCGEM1 boosted proliferation of synoviocytes, activated beclin-1, and depressed PARP and caspase-9 [40]. MiR-770 is reported to suppress synoviocytes proliferation and stimulate synoviocytes apoptosis significantly, and the level of miR-770 decreased in OA synoviocytes. It is demonstrated that PCGEM1 suppressed miR-770 by direct binding in synoviocytes, and led to the hyperplasia of synoviocytes [40].
LncRNAs regulate arthritis-associated factors
Table 2 summarized several lncRNAs that targeted the arthritis-associated factors. These representative lncRNAs were selected to illustrate the diverse signaling pathways and mechanisms of lncRNAs in the regulation of arthritis-associated factors (Table 2, Figure 1).
Table 2.
Selected lncRNAs identified to date in the regulation of arthritis-associated factors
| LncRNA | Proposed mechanism of action | Reference |
|---|---|---|
| HOTAIR | Upregulate MMPs expression | [6] |
| HOTTIP | Inhibit HoxA13/integrin-α1 signaling pathway to promote cartilage degradation | [7] |
| GAS5 | Suppress miR-21 expression to upregulate MMPs expression | [30] |
| IncRNA-CIR | Upregulate MMP-13 expression | [21] |
| HSP90AA4P | Regulate SPP1/OPN pathway | [22] |
| PACER | Positively regulate COX-2 production | [60] |
| Encode miR-675-3p and miR-675-5p; | ||
| H19 | Negatively regulated by miR-675-5p; | [20,63] |
| Modulate Col2a1 expression |
Abbreviations: HOTAIR, HOX transcript antisense RNA; MMPs, Matrix metalloproteinase; HOTTIP, HoxA distal transcript antisense RNA; IncRNA-CIR, Cartilage Injury Related lncRNA; SPP1, Secreted phosphoprotein 1; OPN, osteopontin; PACER, p50-associated cyclooxygenase 2-extragenic RNA; COX-2, Cyclooxygenase 2.
MMPs
Matrix metalloproteinases (MMPs) are well-known factors responsible for cartilage degradation. Hox transcript antisense intergenic RNA (HOTAIR) was upregulated in knee OA cartilage as well as the synovial fluid of temporomandibular joint (TMJ) OA cartilage, according to microarray analysis [6]. IL-1β treatment of TMJ condylar cartilage enhanced the expression of MMP-1, MMP-3, and MMP-9 dramatically, whereas the effects were reversed by HOTAIR knockdown [42], indicating that HOTAIR functioned as a regulator of MMPs.
HoxA distal transcript antisense RNA (HOTTIP), locating in 5’ end of the HoxA cluster, encodes the lncRNA which could suppress HoxA-13 [43]. In OA chondrocytes, HOTTIP was upregulated significantly, while HoxA-13 was downregulated. In addition, it is reported that HoxA-13 could regulate integrin-α1 positively [7]. Overexpression of integrin-α1 subunit could promote chondrogenesis, whereas integrin-α1 knockdown could increase MMP-2 synthesis and contribute to cartilage degradation at younger mice [44]. Therefore, HOTTIP may serve as the promotor of cartilage degradation via inhibiting the HoxA-13/integrin-α1 signaling pathway. It is shown that miR-204 suppressed HOTTIP expression in hepatocellular carcinoma [45], and it arouses the interest whether miR-204 targets HOTTIP and negatively regulates HOTTIP in cartilage, which remains further studied.
It is reported that miR-21 interacted with MMPs by indirect targeting. In laryngeal squamous cell carcinoma, miR-21 was relevant to cell migration and tumorigenicity via regulation of MMP-2 expression [46]. The expression levels of miR-21 in cerebral ischemia and renal fibrosis were associated with the regulation of MMP-9 [47,48]. RECK and TIMP3, the major inhibitors of MMPs, have been known as targets of miR-21 in glioma cells [49]. The downregulation of miR-21 increased the expression level of MMP-13 significantly in OA chondrocytes [30]. The overexpression of GAS5 in chondrocytes in vitro led to the increment of MMPs, and GAS5 acted as a negative regulator of miR-21, indicating that GAS might serve as a sponge of miR-21 to regulate cartilage degradation. However, the specific inter-regulatory network between miR-21 and GAS5 remains to be further elucidated.
Cartilage Injury Related lncRNA (IncRNA-CIR), a vimentin pseudogene, was upregulated in OA cartilage and could induce degradation of cartilage extracellular matrix in vitro. It is reported that knockdown of IncRNA-CIR increased expression of cartilage associated genes (collagens I/II and aggrecan), while its overexpression caused the increment of MMP-13. TNF-α and IL-1, two critical mediators of OA, could stimulate the expression of lncRNA-CIR [21]. Overall, lncRNA-CIR plays a key role in the pathogenesis of OA, but the precise molecular mechanisms need to be deciphered.
OPN
Secreted phosphoprotein 1 (SPP1) deficient mice were apt to developing OA [50]. SPP1 encoded osteopontin (OPN). OPN is a well-characterized regulator of cartilage mineralization [51]. It is found that OPN was distributed at pericelluar sites in cartilage [52], while OPN was upregulated in osteoarthritic cartilage [53] and promoted pathologic mineralization [54]. SPP1 resided at upstream of HSP90AA4P and served as the cis-regulated target of HSP90AA4P. It is reported that HSP90AA4P was downregulated in OA cartilage [22]. These evidences lead to speculation that HSP90AA4P might function as a protector of cartilage by SPP1/OPN pathway.
COX-2
Cyclooxygenase 2 (COX-2) plays a crucial role in regulating the arachidonic acid pathway and prostaglandin E2 production [55], which is presumed to stimulate inflammation and pain in OA cartilage [56,57]. The expression of COX-2 was significantly lower in late OA than that in early OA [58], indicating it may play different roles in different stages of OA. It is shown that inhibitors of COX-2 delayed the resolution of inflammation [59]. LncRNA p50-associated COX-2 extragenic RNA (PACER) is positioned adjacent to COX-2 and is reported to positively regulate COX-2 production [60]. It has been shown that PACER was induced in OA chondrocytes by various proinflammatory cytokines [61], indicating that PACER was a key regulator in the inflammatory response of joint cartilage. However, lncRNA expression of chondrocytes responding to proinflammatory stimuli was rapid and transient. In knee and hip OA cartilage, PACER was reported to be downregulated [61], suggesting a pathologic reduction in the ability of the cartilage tissue to resolve aberrant inflammation.
Col II
The IncRNA H19 expressed abundantly in embryonic tissue of endodermal and mesodermal origin and diverse tumors [62,63]. H19 generated miR-675-5p and miR-675-3p, whereas miR-675-5p suppressed the expression of H19 [64], which made a self-regulatory feedback. It is shown that inhibition of H19 downregulated COL2a1, and overexpression of H19 upregulated COL2a1, while overexpression of miR-675 could rescue COL2a1 in H19-depleted chondrocytes [65], indicating H19 regulated COL2a1 which was mediated by miR-675. However, less is known about the direct target of miR-675 for repressing COL2a1.
The expression of H19 and miR-675 were upregulated under anabolic conditions and downregulated under catabolic conditions [20,65], suggesting that H19 and/or miR-675 might be of diagnostic value as metabolic indicators of OA. However, H19 and miR-675 were expressed higher in cartilage tissue of knee OA according to cDNA array analysis [20]. It raises a question of whether the upregulation of H19 and/or miR-675 in OA chondrocytes functions as a compensatory effort in extracellular matrix synthesis and matrix destruction antagonism during OA development. Interestingly, the variation of miR-675 regulation was more than four-fold below that of H19, inferring that only a fraction of H19 was degraded to provide miR-675.
LncRNAs regulate angiogenesis
Cartilage is normally avascular, and the invasion of blood vessels is an essential step in ossifications. OA is a disease closely associated with angiogenesis. Many studies highlighted the importance of angiogenesis in OA as well as its contribution to progressive joint damage [66]. Vascular endothelial growth factor (VEGF) is a crucial mediator of angiogenesis. It has been shown that VEGF could regulate hypertrophic cartilage remodeling and vascular invasion into growth plate cartilage [67], and the vasculature offers a conduit to recruit cells that involved in cartilage resorption and bone deposition [68]. Therefore, the inhibition of angiogenesis presents a novel therapeutic approach to reduce inflammation and pain in OA.
Maternally expressed gene 3 (MEG3) is a maternally expressed lncRNA and a tumor suppressor gene [69]. It is suggested that MEG3 may inhibit tumor progression through inhibiting angiogenesis [70]. Recently, a study reported that the expression of IncRNA MEG3 was decreased in OA and that its expression levels were reversely associated with VEGF levels [71]. It has been indicated that MEG3 stimulated p53-mediated transcriptional activation [72,73]. P53 could reversely regulate VEGFA transcription by binding to the transcription factor Sp1 sites on the VEGFA promoter [74]. These results above indicate that MEG3 inhibited angiogenesis by means of p53 pathways (Figure 1). However, the detailed mechanisms by which MEG3 inhibits angiogenesis remain to be elucidated.
Summary
LncRNA regulates the OA progression through sophisticated and multi-layered influences on the balance between the biosynthetic phase and the degradative phase (Figure 1). In this review, we summarized the roles of lncRNAs played in the survival of chondrocytes and synoviocytes, arthritis-associated factors, and angiogenesis. These evidences points out lncRNAs as new regulators in OA, which are likely to be the diagnostic, therapeutic, and prognostic biomarkers.
However, enormous challenges need to be overcome before the clinical use. Most studies presented the differences of expression of lncRNAs between OA cartilage and normal cartilage [6], and several lncRNAs were tested and verified by Polymerase chain reaction (PCR) (summarized in Table 3), while there were a tiny fraction of evidence confirming the mechanisms. It is highlighted that one lncRNA has multiple potential targets, which might coordinate or antagonize each other’s functions. The crosstalk between lncRNAs and targets might depend on the tissue source and the stage of OA process, which increases the difficulties in predicting the prognosis and the side effects of lncRNA-based therapies. In spite of these difficulties, the development of lncRNA-based diagnosis, therapy, and prognosis, after being validated the efficacy and safety in animals, will be seen in next few years, and could be applied to OA clinically and other conditions associated with chronic inflammation.
Table 3.
Selected lncRNAs upregulated or downregulated in the OA cartilage
| Upregulated in OA cartilage | Downregulated in OA cartilage | |||
|---|---|---|---|---|
| H19 | GAS5 | BX096395 | MEG3 | UFC1 |
| HOTAIR | HOTTIP | BI015463 | PACER | AK289744 |
| RP11-445H22.4 | CTD2574D22.4 | uc.277 | HSP90AA4P | AL137603 |
| PMS2L2 | PCGEM1 | RP11-195E11.3 | RP11-396J17.1 | AP003175.1 |
| IncRNA-CIR | uc.343 | BC044611 | BQ045000 | RP11-102H24.1 |
| AK054860 | AL359062 | AL832767 | RP11-464O2.5 | |
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81470722 and No. 81201379).
Disclosure of conflict of interest
None.
References
- 1.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB. Articular cartilage degradation in osteoarthritis. HSS J. 2012;8:7–9. doi: 10.1007/s11420-011-9250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Evidence-based health policy--lessons from the global burden of disease study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 4.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing D, Liang JQ, Li Y, Lu J, Jia HB, Xu LY, Ma XL. Identification of long noncoding RNA associated with osteoarthritis in humans. Orthop Surg. 2014;6:288–293. doi: 10.1111/os.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D, Song J, Han J, Kim Y, Chun CH, Jin EJ. Two non-coding RNAs, MicroRNA-101 and HOTTIP contribute cartilage integrity by epigenetic and homeotic regulation of integrin-alpha1. Cell Signal. 2013;25:2878–2887. doi: 10.1016/j.cellsig.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 11.Tye CE, Gordon JA, Martin-Buley LA, Stein JL, Lian JB, Stein GS. Could lncRNAs be the missing links in control of mesenchymal stem cell differentiation? J Cell Physiol. 2015;230:526–534. doi: 10.1002/jcp.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–R161. doi: 10.1093/hmg/ddq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipovich L, Johnson R, Lin CY. MacroRNA underdogs in a microRNA world: evolutionary, regulatory, and biomedical significance of mammalian long non-protein-coding RNA. Biochim Biophys Acta. 2010;1799:597–615. doi: 10.1016/j.bbagrm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:925–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques-Rocha JL, Samblas M, Milagro FI, Bressan J, Martínez JA, Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015;29:3595–3611. doi: 10.1096/fj.14-260323. [DOI] [PubMed] [Google Scholar]
- 18.Hassan MQ, Tye CE, Stein GS, Lian JB. Noncoding RNAs: epigenetic regulators of bone development and homeostasis. Bone. 2015;81:746–756. doi: 10.1016/j.bone.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh NP, Anderson BA, Guilak F, McAlinden A. Emerging roles for long noncoding RNAs in skeletal biology and disease. Connect Tissue Res. 2017;58:116–141. doi: 10.1080/03008207.2016.1194406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steck E, Boeuf S, Gabler J, Werth N, Schnatzer P, Diederichs S, Richter W. Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med. 2012;90:1185–1195. doi: 10.1007/s00109-012-0895-y. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Zhang X, Dai L, Hu X, Zhu J, Li L, Zhou C, Ao Y. Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheum. 2014;66:969–978. doi: 10.1002/art.38309. [DOI] [PubMed] [Google Scholar]
- 22.Fu M, Huang G, Zhang Z, Liu J, Zhang Z, Huang Z, Yu B, Meng F. Expression profile of long noncoding RNAs in cartilage from knee osteoarthritis patients. Osteoarthritis Cartilage. 2015;23:423–432. doi: 10.1016/j.joca.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Sharif M, Whitehouse A, Sharman P, Perry M, Adams M. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis Rheum. 2004;50:507–515. doi: 10.1002/art.20020. [DOI] [PubMed] [Google Scholar]
- 24.Blanco FJ, Guitian R, Vázquezmartul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with the initiation and severity of articular cartilage degradation. Int J Rheum Dis. 2011;14:191–198. doi: 10.1111/j.1756-185X.2010.01578.x. [DOI] [PubMed] [Google Scholar]
- 27.Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, Tenen A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy-a review. BMC Musculoskelet Disord. 2016;17:230. doi: 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raho G, Barone V, Rossi D, Philipson L, Sorrentino V. The gas 5 gene shows four alternative splicing patterns without coding for a protein. Gene. 2000;256:13–17. doi: 10.1016/s0378-1119(00)00363-2. [DOI] [PubMed] [Google Scholar]
- 29.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrestand starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song J, Ahn C, Chun CH, Jin EJ. A long noncoding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J Orthop Res. 2014;32:1628–1635. doi: 10.1002/jor.22718. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Zhou L, Li Y, Lin S, Tomuleasa C. MicroRNA-21 stimulates gastric cancer growth and invasion by inhibiting the tumor suppressor effects of programmed cell death protein 4 and phosphatase and tensin homolog. J BUON. 2014;19:228–236. [PubMed] [Google Scholar]
- 32.Ruan Q, Wang P, Wang T, Qi J, Wei M, Wang S, Fan T, Johnson D, Wan X, Shi W, Sun H, Chen YH. MicroRNA-21 regulates T-cell apoptosis by directly targeting the tumor suppressor gene Tipe2. Cell Death Dis. 2014;5:e1095. doi: 10.1038/cddis.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo TF, Tsai WC, Chen ST. MicroRNA-21-3p, a berberine-induced miRNA, directly downregulates human methionine adenosyltransferases 2A and 2B and inhibits hepatoma cell growth. PLoS One. 2013;8:e75628. doi: 10.1371/journal.pone.0075628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C, Xu M, Wu F, Mo YY. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013;20:1558–1568. doi: 10.1038/cdd.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao C, Sun J, Zhang D, Guo X, Xie L, Li X, Wu D, Liu L. The long intergenic noncoding RNA UFC1, a target of MicroRNA 34a, interacts with the mRNA stabilizing protein HuR to increase levels of beta-catenin in HCC cells. Gastroenterology. 2015;148:415–426. e18. doi: 10.1053/j.gastro.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Yu T, Shan TD, Li JY, Huang CZ, Wang SY, Ouyang H, Lu XJ, Xu JH, Zhong W, Chen QK. Knockdown of linc-UFC1 suppresses proliferation and induces apoptosis of colorectal cancer. Cell Death Dis. 2016;7:e2228. doi: 10.1038/cddis.2016.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang G, Wu Y, Xu D, Yan X. Long noncoding RNA UFC1 promotes proliferation of chondrocyte in osteoarthritis by acting as a sponge for miR-34a. DNA Cell Biol. 2016;35:691–695. doi: 10.1089/dna.2016.3397. [DOI] [PubMed] [Google Scholar]
- 38.Abouheif MM, Nakasa T, Shibuya H, Niimoto T, Kongcharoensombat W, Ochi M. Silencing microRNA-34a inhibits chondrocyte apoptosis in a rat osteoarthritis model in vitro. Rheumatology. 2010;49:2054–2060. doi: 10.1093/rheumatology/keq247. [DOI] [PubMed] [Google Scholar]
- 39.Kamel S, Kruger C, Salbaum JM, Kappen C. Morpholino-mediated knockdown in primary chondrocytes implicates Hoxc8 in regulation of cell cycle progression. Bone. 2009;44:708–716. doi: 10.1016/j.bone.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang Y, Song J, Kim D, Ahn C, Park S, Chun CH, Jin EJ. PCGEM1 stimulates proliferation of osteoarthritic synoviocytes by acting as a sponge for miR-770. J Orthop Res. 2015;34:412–418. doi: 10.1002/jor.23046. [DOI] [PubMed] [Google Scholar]
- 41.Fu X, Ravindranath L, Tran N, Petrovics G, Srivastava S. Regulation of apoptosis by a prostate-specific and prostate cancer-associated noncoding gene, PCG EM1. DNA Cell Biol. 2006;25:135–141. doi: 10.1089/dna.2006.25.135. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Wang P, Jiang P, Lv Y, Dong C, Dai X, Tan L, Wang Z. Upregulation of lncRNA HOTAIR contributes to IL-1β-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. Gene. 2016;586:248–253. doi: 10.1016/j.gene.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, Zhao X, Zhou Y, Liu Y, Zhou Q, Ye H, Wang Y, Zeng J, Song Y, Gao W, Zheng S, Zhuang B, Chen H, Li W, Li H, Li H, Fu Z, Chen R. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J Transl Med. 2015;13:84. doi: 10.1186/s12967-015-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zemmyo M, Meharra EJ, Kuhn K, Creighton-Achermann L, Lotz M. Accelerated, agingdependent development of osteoarthritis in alpha1 integrin-deficient mice. Arthritis Rheum. 2003;48:2873–2880. doi: 10.1002/art.11246. [DOI] [PubMed] [Google Scholar]
- 45.Ge Y, Yan X, Jin Y, Yang X, Yu X, Zhou L, Han S, Yuan Q, Yang M. MiRNA-192 [corrected] and miRNA-204 directly suppress lncRNA HOTTIP and interrupt GLS1-mediated glutaminolysis in hepatocellular carcinoma. PLoS Genet. 2015;11:e1005726. doi: 10.1371/journal.pgen.1005726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren J, Sun Y, Zhao X, Wang X, Feng J, Li D, Liu M, Zhu D. Downregulation of miR-21 regulates MMP-2 expression and suppress migration of Laryngeal squamous cell carcinoma. Head Neck Oncol. 2012;4:65. [Google Scholar]
- 47.Deng X, Zhong Y, Gu L, Shen W, Guo J. MiR-21 involve in ERK-mediated upregulation of MMP9 in the rat hippocampus following cerebral ischemia. Brain Res Bull. 2013;94:56–62. doi: 10.1016/j.brainresbull.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Gao Y, Ma M, Li M, Zou D, Yang J, Zhu Z, Zhao X. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochem Biophys. 2013;67:537–546. doi: 10.1007/s12013-013-9539-2. [DOI] [PubMed] [Google Scholar]
- 49.Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsui Y, Iwasaki N, Kon S, Takahashi D, Morimoto J, Matsui Y, Denhardt DT, Rittling S, Minami A, Uede T. Accelerated development of aging-associated and instability-induced osteoarthritis in osteopontin-deficient mice. Arthritis Rheum. 2009;60:2362–2371. doi: 10.1002/art.24705. [DOI] [PubMed] [Google Scholar]
- 51.Giachelli C, Speer M, Li X, Rajachar R, Yang H. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res. 2005;96:717–722. doi: 10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- 52.Parikh A, Lee G, Tchivilev I, Graff R. A neocartilage ideal for extracellular matrix macromolecule immunolocalization. Histochem Cell Biol. 2003;120:427–434. doi: 10.1007/s00418-003-0580-x. [DOI] [PubMed] [Google Scholar]
- 53.Pullig O, Weseloh G, Gauer S, Swoboda B. Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biol. 2000;19:245–255. doi: 10.1016/s0945-053x(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 54.Rosenthal AK, Gohr CM, Uzuki M, Masuda I. Osteopontin promotes pathologic mineralization in articular cartilage. Matrix Biol. 2007;26:96–105. doi: 10.1016/j.matbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegle I, Klein T, Backman JT, Saal JG, Nusing RM, Fritz P. Expression of cyclooxygenase 1 and cyclooxygenase 2 in human synovial tissue: differential elevation of cyclooxygenase 2 in inflammatory joint diseases. Arthritis Rheum. 1998;41:122–129. doi: 10.1002/1529-0131(199801)41:1<122::AID-ART15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 56.Pelletier JP, Boileau C, Brunet J, Boily M, Lajeunesse D, Reboul P, Laufer S, Martel-Pelletier J. The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone. 2004;34:527–538. doi: 10.1016/j.bone.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Raynauld JP, Martel-Pelletier J, Bias P, Laufer S, Haraoui B, Choquette D, Beaulieu AD, Abram F, Dorais M, Vignon E, Pelletier JP Canadian Licofelone Study Group. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Ann Rheum Dis. 2009;68:938–947. doi: 10.1136/ard.2008.088732. [DOI] [PubMed] [Google Scholar]
- 58.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010;184:6418–6426. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krawczyk M, Emerson BM. p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-kB complexes. Elife. 2014;3:e01776. doi: 10.7554/eLife.01776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pearson MJ, Philp AM, Heward JA, Roux BT, Walsh DA, Davis ET, Lindsay MA, Jones SW. Long intergenic noncoding RNAs mediate the human chondrocyte inflammatory response and are differentially expressed in osteoarthritis cartilage. Arthritis Rheumatol. 2016;68:845–856. doi: 10.1002/art.39520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goshen R, Rachmilewitz J, Schneider T, de-Groot N, Ariel I, Palti Z, Hochberg A. The expression of the H-19 and IGF-2 genes during human embryogenesis and placental development. Mol Reprod Dev. 1993;34:374–379. doi: 10.1002/mrd.1080340405. [DOI] [PubMed] [Google Scholar]
- 63.Lustig O, Ariel I, Ilan J, Lev-Lehman E, De-Groot N, Hochberg A. Expression of the imprinted gene H19 in the human fetus. Mol Reprod Dev. 1994;38:239–246. doi: 10.1002/mrd.1080380302. [DOI] [PubMed] [Google Scholar]
- 64.Liang W, Fu W, Wang Y, Sun Y, Xu L, Wong C, Chan K, Li G, Waye MM, Zhang J. H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Sci Rep. 2016;6:20121. doi: 10.1038/srep20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dudek KA, Lafont JE, Martinez-Sanchez A, Murphy CL. Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J Biol Chem. 2010;285:24381–24387. doi: 10.1074/jbc.M110.111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 67.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 68.Harper J, Klagsbrun M. Cartilage to bone--angiogenesis leads the way. Nat Med. 1999;5:617–618. doi: 10.1038/9460. [DOI] [PubMed] [Google Scholar]
- 69.Benetatos L, Vartholomatos G, Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int J Cancer. 2011;129:773–779. doi: 10.1002/ijc.26052. [DOI] [PubMed] [Google Scholar]
- 70.Gordon FE, Nutt CL, Cheunsuchon P, Nakayama Y, Provencher KA, Rice KA, Zhou Y, Zhang X, Klibanski A. Increased expression of angiogenic genes in the brains of mouse Meg3-null embryos. Endocrinology. 2010;151:2443–2452. doi: 10.1210/en.2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su W, Xie W, Shang Q, Su B. The long noncoding RNA MEG3 is downregulated and inversely associated with VEGF levels in osteoarthritis. Biomed Res Int. 2015;2015:356893. doi: 10.1155/2015/356893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y, Zhong Y, Wang Y, Zhang X, Batista DL, Gejman R, Ansell PJ, Zhao J, Weng C, Klibanski A. Activation of p53 by MEG3 noncoding RNA. J Biol Chem. 2007;282:24731–24742. doi: 10.1074/jbc.M702029200. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48:45–53. doi: 10.1530/JME-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61:6952–6957. [PubMed] [Google Scholar]