Abstract
Cytoskeletal proteins and associated regulatory proteins are essential for maintaining cell structure and growth. β-actin is a major component of the cytoskeleton, and β-actin remodeling is involved in lymphocyte migration, infiltration and apoptosis. However, little is known about whether changes in β-actin expression affect lymphocyte cell fate, particularly during acute rejection after liver transplantation in a rat model. In our studies, grafts were harvested on days 5, 7 or 9 after xenogeneic rat liver transplantation. The acute rejection grade was histopathologically evaluated. Recipient-derived CD8+ T lymphocytes gradually infiltrated into liver allografts in cases of severe acute rejection. The apoptotic rate of CD8+ T lymphocytes peaked on day 7 and then decreased. Moreover, changes in β-actin expression were consistent with the apoptotic rate of CD8+ T lymphocytes in both allografts and peripheral blood based on western blotting and immunohistochemistry results. Additionally, jasplakinolide (an actin-stabilizing drug) evoked CD8+ T lymphocyte apoptosis. In conclusion, our study is the first to describe the fluctuating expression levels and dynamics of the cytoskeletal protein β-actin and its potential roles in the pathogenesis of acute rejection following rat liver transplantion. Our results enhance the understanding of the roles of CD8+ T lymphocytes during acute rejection and suggest that β-actin regulation leads to apoptosis.
Keywords: Acute rejection, liver transplantation, CD8+ T lymphocyte, β-actin
Introduction
Since Starzl conducted the first liver transplantation (LT) operation, improved surgical techniques and effective immunosuppression have allowed LT to become the primary treatment for patients with end-stage liver disease [1,2]. According to certain reports from large transplant centers, the 5-year survival rate for recipients after LT is more than 70%, and the 10-year survival rate reaches 60% [3]. Although the liver is an immunologically privileged organ, the acute rejection (AR) reaction remains a major problem that seriously affects allograft function and recipient survival. Approximately four percentage of death is directly caused by AR, particularly during the first few weeks after operation [4,5]. The application of immunosuppressive protocols can not reduce the incidence of graft loss caused by chronic rejection and may lead to serious complications, including life-threatening infections, renaltoxicity and cancer. Thus, there is a critical need to further explore the mechanisms of rejection after LT.
AR constitutes a post-transplantation reaction characterized by varying degrees of mononuclear cell infiltration into the allograft interstitium [6,7]. CD8+ T lymphocytes are attracted by cytokines secreted in the graft, such as IFN-γ and TNF-α; these lymphocytes infiltrate into the graft and become activated, accompanied by the apoptosis of parenchymal cells, which playimportant roles in liver graft AR [8]. According to recent studies, there is a relationship between apoptosis of CD8+ T lymphocytes and the AR reaction after LT. As described by Miwa Morita et al., the apoptosis of infiltrating CD8+ T lymphocytes tips the immunological balance toward tolerance in a mouse LT model, while IFN-γ knockout in recipient mice leads to lower rates of CD8+ T lymphocyte apoptosis, accompanied by serious AR [9]. However, the exact mechanism underlying CD8+ T lymphocyte apoptosis during acute allograft rejection remains elusive.
Apoptosis is a precise process of programmed cell death that leads to characteristic cell changes and death [10]. A growing body of literature suggests that apoptosis plays a essential part in various biological processes, such as organismal evolution, inner environmental stability and immune system regulation [11]. Two classical pathways mediate cell apoptosis: the death receptor (TNFR, Fas, TRAILR)-mediated extrinsic pathway and the mitochondria-mediated intrinsic pathway [12]. Many proteins participate in and co-regulate the apoptotic process [13]. β-actin, a major component of the cytoskeleton, plays an significant role in many cellular functions, including signal-response coupling, cell motility, endocytosis, growth and organelle trafficking, via filament remodeling and is associated with the regulation and triggering of apoptosis. For example, increasing β-actin stability induces apoptosis in M. avium-infected macrophages [14]. In addition, β-actin mediates TNF-α-induced apoptosis by binding to and stabilizing TNFR2 [15,16]. However, the relationships between β-actin expression as well as remodeling and CD8+ T lymphocyte cell fate following LT have not been previously investigated.
Given the importance of AR reaction in clinical trials and the interest in β-actin, we established a LT model in rats (divided into two groups, homogenic vs allogeneic) and observed the ability of increased β-actin expression to regulate infiltration and migration of CD8+ T lymphocytes into allografts prior to post-transplantation day 7. Then, β-actin expression decreased, accompanied with the reduced apoptotic rate CD8+ T lymphocytes, thus promoting the acute liver graft rejection reaction. Additionally, the disruption of β-actin dynamics by jasplakinolide (JASP), an actin-stabilizing drug, led to higher rate of CD8+ T lymphocyte apoptosis in vitro.
Materials and methods
Animals
Specific pathogen-free male Lewis (LW) and male Brown Norway (BN) rats (8 to 12 weeks old, 200 to 250 g in weight) were purchased from Beijing Vital River Company, China. All rats were housed at the Institute of Laboratory Animal Science, Jinan University, at 21°C and under a 12/12-hour light/dark cycle with ad libitum access to sterile water and standard pellet chow under standardized environmental conditions.
Protocol to construct an AR model
An orthotopic LT (OLT) model without hepatic artery reconstruction was created as previously described [17]. Under isoflurane gas anesthesia, liver grafts were obtained and immediately stored in UW (University of Wisconsin) solution at 4°C. OLT was performed using the “two-cuff” method. Rats were divided into two groups: 1) a control group and 2) an AR reaction group. In the control group (homogenic group), both donors and recipients were BN rats. In the AR reaction group (allogenic group), recipients were BN rats, and donors were LW rats. Some of the rats in each group were sacrificed on days 5, 7, and 9 after LT according to experimental design via anesthesia overdose, respectively. All procedures complied with the “Guide for the Care and Use of Laboratory Animals” from the National Institutes of Health (NIH).
Liver function tests
Blood samples from each group were collected from a retrobulbar vessel on days 5, 7 and 9 post-operation, respectively. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL) were detected to evaluate the liver function of recipient rats using an automatic chemical analyzer (Hitachi 7600-100, Tokyo, Japan) as reported previously [18].
Cytokine detection using an enzyme-linked immunosorbent assay (ELISA)
Serum concentrations of IL-2, IFN-γ, TNF-α and IL-10 in each group were measured using a multiplex ELISA assay kit (R&D Systems, Minneapolis, USA) according to the manufacturer’s protocol. Procedures were performed as previously described [19]. Fluorescence intensity was quantified, and the data were analyzed using a Q-analyzer (RayBiotech, Norcross, GA, USA). All samples were measured in duplicate.
Total RNA extraction and quantitative real-time fluorescent polymerase chain reaction
The mRNA of liver tissue was extracted and reverse-transcribed into cDNA as previously described [20]. Briefly, total mRNA was isolated from frozen liver samples using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. Reverse transcription of total mRNA and real-time PCR were performed using an Alpha Unit Block Assembly for the PTC DNA Engine System and an iCycler IQ Multicolor Real-Time Detection System (Bio-Rad, CA, USA) according to the manufacturer’s instructions for the SYBR Prime Script Reverse Transcription PCR Kit (Roche, Kyoto, Japan). PCR was conducted using an LC480 RT-PCR System (Roche, Basel, Switzerland) with the following parameters: the polymerase was activated by pre-incubation at 95°C for 10 minutes, followed by 40 amplification cycles of 95°C for 15 seconds and 57°C for 60 seconds. GAPDH was used as an internal control in each reaction. Melting curves were constructed, and PCR amplification specificity was evaluated by determining whether only 1 peak was present.
Primers were designed according to the cDNA sequences of GAPDH, β-actin, cofilin, IL-2, IL-10, TNF-α and IFN-γ in the NCBI database and were synthesized by TaKaRa Biotechnology (Shanghai, China). The sequences are listed in Table 1.
Table 1.
Sequences of primers used for quantitative real-time polymerase chain reaction
| mRNAs | Primers | |
|---|---|---|
| IL-2 | Forward | 5’-GCAGGCCACAGAATTGAAAC-3’ |
| Reverse | 5’-CCAGCGTCTTCCAAGTGAA-3’ | |
| IFN-γ | Forward | 5’-CAGGCCACAGAATTGAAAC-3’ |
| Reverse | 5’-CCAGCGTCTTCCAAGTGAA-3’ | |
| TNF-α | Forward | 5’-ACCTTATCTACTCCCAGGTTCT-3’ |
| Reverse | 5’-GGCTGACTTTCTCCTGGTATG-3’ | |
| Fas | Forward | 5’-TTTCCTCAGTCTTCCGCTATTT-3’ |
| Reverse | 5’-GACGCCTCAGTTCACAGTATTA-3’ | |
| IL-10 | Forward | 5’-CCTCCTGGTCAGCAAGAAATAG-3’ |
| Reverse | 5’-GGGCTATTCCAGAGCACATTAG-3’ | |
| Gelsolin | Forward | 5’-TTGAGCCTCCTTCCTTTGTG-3’ |
| Reverse | 5’-CATTGGTAGCTCTGGTCCTTAC-3’ | |
| Cofilin1 | Forward | 5’-AGGAGATTCTGGTAGGAGATGT-3’ |
| Reverse | 5’-GGTCTCATAGGTTGCGTCATAG-3’ | |
| GAPDH | Forward | 5’-ACTCCCATTCTTCCACCTTTG-3’ |
| Reverse | 5’-CCCTGTTGCTGTAGCCATATT-3’ |
IL-2, interleukin-2; IL-10, interleukin-10; TNF-α, tumor necrosis factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blotting to detect caspase-3, β-actin and cofilin in liver tissue
Equal concentrations of protein extracted from liver tissue (quantitated using a Micro BCA Protein Assay) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were incubated with rabbit anti-rat caspase-3, cofilin-1, β-actin and GAPDH antibodies at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG for 1 hour. Membranes were developed with a Super Signal West Pico Chemiluminescent Substrate Kit (Pierce, Rockford, USA) and finally analyzed using a Bio-Image Analysis System (Syngene, Frederick, MD, USA).
Liver histological examination
Liver tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin for 24 hours using standard histological procedures. The sections were cut at a thickness of 4 μm, affixed to slides and stained with hematoxylin and eosin (H&E) for histological examination. The degree of AR was assessed by a pathologist using the Banff scheme to calculate the rejection activity index (RAI) [21] based on three individual characteristics (venous endothelial inflammation, bile duct damage and portal inflammation).
Immunohistochemical staining of liver tissues
Immunohistochemical staining was performed according to the manufacturer’s instructions to evaluate the relationship between β-actin and CD8+ T cell apoptosis. In brief, the immunohistochemical staining procedure was followed: liver paraffin-embedded slides were routinely deparaffinized and rehydrated, antigen retrieval was performed in 10 mmol/L sodium citrate buffer (pH 6.0), and slides were blocked with 5% BSA for 30 minutes. Then, the sections were incubated with primary antibodies at 4°C overnight. The sections were washed twice with phosphate-buffered saline (PBS) and incubated with HRP-linked secondary antibody (DAKO, USA) for 30 minutes at room temperature. Finally, the sections were stained with 3,3’-diaminobenzidine (DAB; DAKO) and counterstained with hematoxylin. Cells containing brown granules in the cytoplasm or cell membrane were considered positive. Antibodiesod β-actin and CD8 were used in this experiment.
Apoptosis assay
TdT-mediated dUTP-X nick end labeling (TUNEL) staining was performed on paraffin-embedded tissue sections using a Cell Death Kit (KeyGEN BioTECH, Jiangshu, China) according to the manufacturer’s instructions. Terminal deoxynucleotidyl transferase, a marker of apoptosis, was not present in the negative control group but was present in the positive control group. All slides were reviewed by a pathologist in a blinded manner, and the number of apoptotic cells was quantified in 10 random fields under a light microscope (original magnification: ×200).
Immunofluorescent staining of CD8 and β-actin
Immunofluorescent staining was performed to assess CD8+ T lymphocyte infiltration and β-actin expression in CD8+ T cells in liver grafts. Briefly, 4-μm-thick hepatic sections were incubated with monoclonal rabbit anti-β-actin antibodyand mouse anti-CD8 antibody at 4°C overnight andthen washed twice with PBS. Then, the slices were incubated with FITC-conjugated anti-rabbit and PE-conjugated anti-mouse secondary antibodies for 1 hour at 37°C. Finally, the sections were incubated with DAPI (1:500 dilution; Vector Laboratories, CA, USA) in 5% donkey serum and 2% BSA and analyzed using a fluorescence microscope (Leica SR GSD, Leica, German). The number of CD8-positive cells per high-power field (HPF) was counted, and β-actin expression in CD8+ T cells was analyzed (magnification: ×200).
Cell isolation
To isolate cells, anti-coagulated blood was treated with red blood cell (RBC) lysis buffer and washed twice with 5% fetal bovine serum (FBS) in PBS. Peripheral blood mononuclear cells (PBMCs) were separated via Ficoll-Hypaque density gradient centrifugation (Pharmacia, Piscataway, NJ, USA) as previously described [20]. Then, PBMCs were resuspended in 100 µL of PBS containing 10% goat serum.
CD8+ T cells were enriched using magnetic beads as previously described [22]. To purify CD8+ T cells, single-cell suspensions of peripheral blood from recipient rats were stained with biotin-conjugated anti-CD8, washed and stained with anti-biotin magnetic micro beads (Miltenyi Biotec). CD8+ T cell enrichment was performed using a magnetic column. To confirm the purification, CD8+ T cells were stained with PE-conjugated anti-CD8 and then assayed by flow cytometry (mentioned following).
Flow cytometry analysis of T lymphocyte subsets and CD8+ T cell sorting
Cell surface antigens (Ags) were characterized using a standard staining method and measured by flow cytometry. Briefly, single cells in suspension (1×106/mL) were incubated with 4 μg/mL fluorochrome-conjugated monoclonal antibodies (mAbs) for 30 minutes at 37°C in the dark. After staining, cells were washed with 1× PBS to remove excess antibodies. The following mAbs were used: APC-conjugated anti-CD3, FITC-conjugated anti-CD4, PE-conjugated anti-CD8 (BD Pharmingen, San Diego, CA, USA), FITC-conjugated anti-Annexin V and propidium iodide (PI). Specimens were assayed by flow cytometry (Becton-Dickinson Inc., San Jose, CA, USA) according to the manufacturer’s instructions. The data were analyzed using FlowJo software (Digital Biology, OR, USA).
Cell culture
CD8+ T cells isolated by using magnetic beads were cultured in RPMI 1640 medium (Gibco, Grand Island, NY) containing 10% FBS with IL-2 (100 IU/L) and PHA (15 μg/mL) and maintained in a humidified incubator containing 5% CO2 at 37°C. After incubation for 48 hours, CD8+ T cells (1×106/mL) were transferred to 6-well plates. There were two treatment groups: the experimental group was treated with JASP (1 μg/mL, Sigma), and DMSO (v/v, 0.05%) was added to the control group. JASP is an actin-specific reagent that promotes actin polymerization and stabilizes actin filaments. After 48 hours, CD8+ T cells in both groups were collected to analyze apoptotic rate using Annexin V/PI assay by flow cytometry.
Statistical analysis
Quantitative data are presented as the mean ± standard deviation (mean ± SD). Student’s t-test was used to evaluate the statistical significance of differences between groups. Spearman’s test was performed to assess the correlation relationships. Statistical analysis was performed using the statistical software package SPSS 17.0 (SPSS Inc., Chicago, IL, USA). A two-tailed P value of <0.05 indicated a significant difference.
Results
Time course of the pathologic characteristics of liver grafts after transplantation
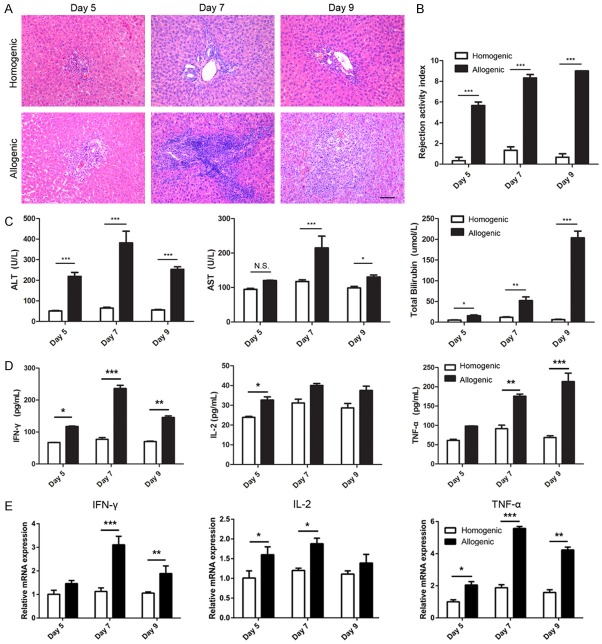
In this study, we established an animal model for AR by transplanting fully allogeneic LW donor livers into BN recipients. Liver allografts exhibited typically histopathological features of AR in this rat strain combination. On day 5 post-transplantation, mononuclear cells began to infiltrate around portal areas, while peripheral regions were almost intact. Large numbers of mononuclear cells infiltrated into not only portal areas but also peripheral regions on day 7 after allo-transplantation. Later, mononuclear cell infiltration into portal and peripheral regions reached its highest levels on day 9 after allo-transplantation. In contrast, assessment of the histopathological features of the homogenic group from day 5 to day 9 post-transplant revealed that mononuclear cell infiltration into portal areas were infrequently accompanied by marginal basal inflammation (Figure 1A). The RAI is a scoring system for evaluating the degree of gross pathological changes. RAI scores of allografts increased progressively from day 5 to day 9 and were higher than those of syngrafts (Figure 1B).
Figure 1.
Time course of pathologic characteristics of liver grafts on days 5, 7, 9 after LT. A: Analysis of pathologic characteristics of allogenic and homogenic groups by H&E staining (original magnification, 200×). B: Bar graph showing the rejection activity index (RAI) results in two groups. C, D: Analysis of serum levels of ALT, AST, TBIL and cytokine (IFN-γ, IL-2 and TNF-α). E: Relative RNA expression for cytokine genesin liver grafts of allogenic and homogenic groups detected by qPCR, GAPDH was used as loading controls. All data representative of three independent experiments and calculated data are shown as mean ± SD. All statistical analyses were performed by student t test, *p<0.05, **p<0.01, ***p<0.001 compared to homogenic group.
Liver function was measured from day 5 to day 9 after LT in both groups. ALT, AST and TBIL levels dramatically increased in the allogeneic group compared with the homogenic group (P<0.001). AST and ALT levels peaked on day 7 after transplantation in the allogeneic group, while TBIL levels rapidly peaked on day 9 (Figure 1C).
Inflammatory cytokine levels in liver grafts and recipient serum after transplantation
The levels of inflammatory cytokines (IFN-γ, IL-2 and TNF-α) in serum and levels in liver graftswere measured by ELISA and qPCR, respectively. The results showed that with the AR reaction in the allogeneic group, serum levels of IFN-γ, IL-2 and TNF-α dramatically increased compared with those in the homogenic group (P<0.05). Interestingly, TNF-α levels increased gradually over time, whereas IFN-γ and IL-2 levels peaked on day 7 after transplantation and declined on day 9 (Figure 1D). Furthermore, the AR reaction caused similar and significant trends form RNA levels of IFN-γ, IL-2 and TNF-α in allografts compared with those in the homogenic group (P<0.05, Figure 1E).
CD8+ T cells infiltration into hepatic tissue after LT
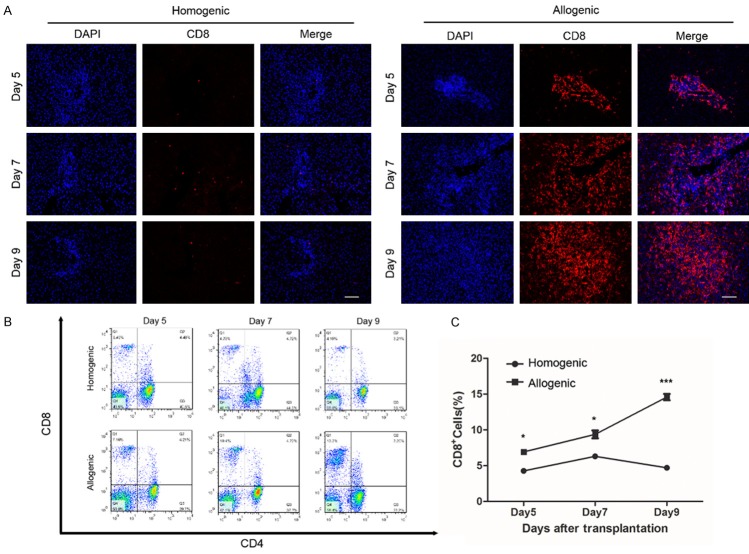
CD8+ T cells in graft tissues were quantified by immunofluorescent staining to evaluate the percentage of CD8+ T cell infiltration. We found that the percentage of CD8+ T cell infiltration increased significantly in the allogeneic group compared with the homogenic group at all three time points. Furthermore, CD8+ T cells progressively increased after transplantation and peaked at day 9 in the allogeneic group (Figure 2A).
Figure 2.
The ratio of CD8+ T cells in liver grafts and peripheral blood on days 5, 7, 9 after LT. A: Immunofluorescent staining for CD8 in liver grafts of allogenic and homogenic groups (original magnification 200×). CD8 antibody was labeled with cyanidin-3 (red) and cell nucleus was labeled DAPI (blue). B, C: Flow cytometry analysis of ratio of peripheral CD8+ T cells in allogenic and homogenic groups. All data representative of three independent experiments and calculated data are shown as mean ± SD. All statistical analyses were performed by student t test, *p<0.05, ***p<0.001 compared to homogenic group.
Moreover, flow cytometry analysis showed that the percentage of CD8+ T cells in peripheral blood was higher in the allogeneic group than in the homogenic group (Figure 2B). Specifically, the population of CD8+ T cells inperipheral blood increased gradually and peaked at day 9. However, in the homogenic group, CD8+ T cells peaked at day 7 and gradually decreased through day 9 (Figure 2C).
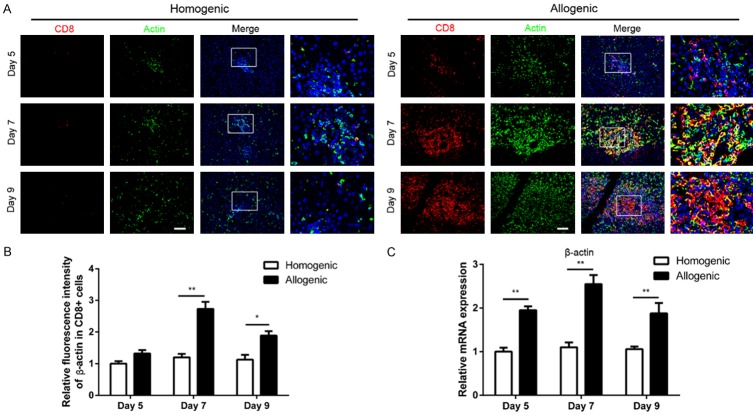
β-actin expression in CD8+ T cells in liver grafts
To evaluate β-actin expression in CD8+ T cells, immunofluorescent staining was performed. Because β-actin is a housekeeping protein, we shortened the staining duration in all liver tissues to analyze its expression. β-actin expression increased in CD8+ T cells in allogenic grafts compared with homogenic grafts (Figure 3A). According to an analysis of relative fluorescent intensity, the β-actin intensity in CD8+ T cells was higher in the allogeneic group than in the homogenic group and peaked at day 7 (Figure 3B). Furthermore, after transplantation, β-actin mRNA levels in liver graft tissues were measured to analyze the effects of AR on β-actin expression by qPCR. The results revealed that β-actin mRNA levels in the allogeneic group peaked at day 7 and were significantly up-regulated (P<0.01) (Figure 3C).
Figure 3.
Alterations of the expression of β-actin in CD8+ T cells in liver grafts on days 5, 7, 9 after LT. A: Triple double fluorescent staining for CD8 (red), β-actin (green) and DAPI (blue) in allogenic group and homogenic group (original magnification 200×). B: Histogram showing relative fluorescence intensity of β-actin expression in CD8+ T cells. C: qPCR was performed to analyze the expression of β-actin in liver tissues, GAPDH was used as loading controls. All data representative of three independent experiments and calculated data are shown as mean ± SD. All statistical analyses were performed by student t test, *p<0.05, **p<0.01 compared to homogenic group.
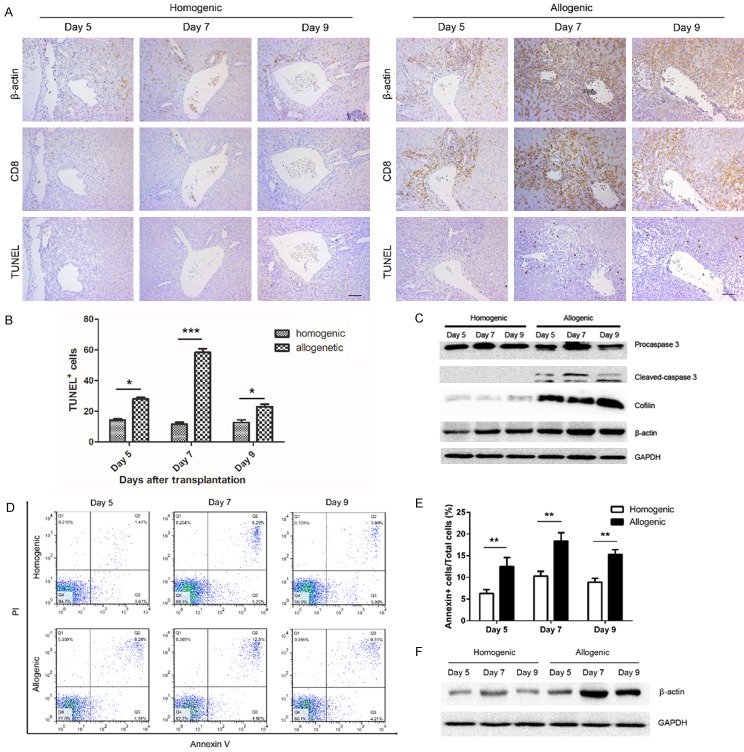
Correlation between β-actin expression and apoptosis in CD8+ T cells in liver grafts and peripheral blood after LT
Given the results shown in Figure 3, in which β-actin expression increased in CD8+ T cells in the allogeneic group, we next evaluated the correlation between β-actin expression and CD8+ T cell apoptosis in both groups by performing immunohistochemical staining and western blot, respectively. CD8+ T cell apoptosis increased with increasing β-actin expression in liver grafts (Figure 4A). And apoptotic rate of CD8+ T cells was higher in the allogeneic group than in the homogenic group (Figure 4B). Next, β-actin, cofilin-1 and caspase-3 protein levels in homogenic or allogeneic liver graft tissues were analyzed by western blotting (Figure 4C). β-actin and cofilin-1 protein levels were markedly increased in the allogeneic group compared with the homogenic group at all three investigated time points (p<0.05), and caspase-3 protein levels, which correlate with apoptosis, were also notably increased (P<0.05).
Figure 4.
Correlation between the expression of β-actin in CD8+ T cells and the counts of apoptosis CD8+ T cells in liver grafts and peripheral blood. A: Immunohistochemical images showing the expression of β-actin in CD8+ T cells and the counts of apoptosis CD8+ T cells in the grafts slices of allogenic and homogenic groups (original magnification 200×). B: Histogram showing the alternated counts of apoptosis CD8+ T cells with time course in the liver slices. C: Western blot was performed to analyze the levels of cofilin, β-actin, cleaved-caspase 3 in the liver grafts at different time point. D: The apoptosis rate of CD8+ T cells isolated by using CD8 magnetic beads from peripheral blood was measured by Annexin V/PI assay. E: Histogram showing that apoptosis rate of CD8+ T cells from allogenic liver allografts was significant greater than homogenic liver grafts at each time point postoperatively. F: The expression of β-actin in CD8+ T cells isolated from peripheral blood were analyze by western blot. All data representative of three independent experiments and calculated data are shown as mean ± SD. All statistical analyses were performed by student t test, *p<0.05, **p<0.01, ***p<0.001 compared to homogenic group.
Flow cytometry was performed to evaluate CD8+ T cell apoptosis in peripheral blood. CD8+ T cell apoptosis was remarkably increased in the allogeneic group compared with the homogenic group at all three investigated time points (Figure 4D). Additionally, CD8+ T cell apoptosis increased progressively and peaked at day 7 (Figure 4E). Next, β-actin protein levels in CD8+ T cells in the peripheral blood were detected by western blotting and found to be clearly higher in the allogenic group than in the homogenic group (P<0.05); a similar trend was observed for CD8+ T cell apoptosis in the peripheral blood in the allogeneic group (Figure 4F).
Correlation analysis
The correlation between β-actin expression and T cell-related cytokine expression (IFN-γ, IL-2 and TNF-α) was investigated in the allogeneic group. Higher β-actin mRNA levels were associated with higher levels of IFN-γ mRNA (Figure 5A), IL-2 mRNA (Figure 5B) and TNF-α mRNA (Figure 5C). Because the RAI measures the severity of the AR reaction following LT, we determined the correlation between β-actin expression and allograft rejection and observed a positive correlation, as shown in Figure 5D.
Figure 5.
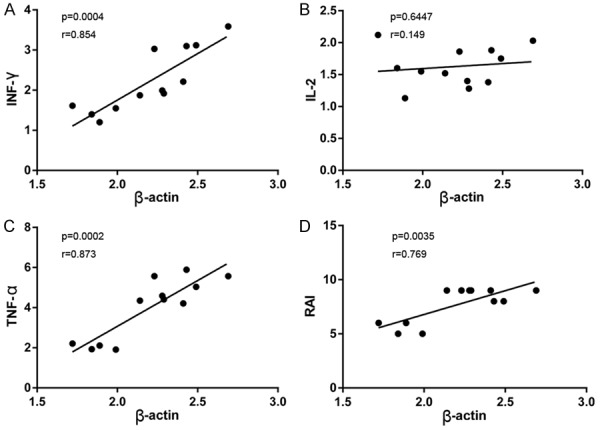
Correlation analysis in allografts during acute rejection episodes. A-C: Correlation between β-actin and the inflammatory cytokines (IFN-γ, IL-2 and TNF-α) mRNA levels; D: Correlation between β-actin mRNA levels and the severity of allograft rejection which was represented as RAI. Statistical analyses were performed by Spearman’s test to assess any correlation. A probability level of P<0.05 was considered statistically significant.
JASP promotes CD8+ T cell apoptosis in vitro
As shown in Figure 4, higher β-actin expression in CD8+ T cells correlated with higher CD8+ T cell apoptosis in liver grafts over time. Therefore, we further investigated the effects of JASP, a reagent that promotes actin polymerization, on cultured CD8+ T cells. CD8+ T cells were collected and incubated with FITC-labeled Annexin V antibodies and PI after culture with JASP for 48 hours. CD8+ T cells were analyzed by flow cytometry to measure CD8+ T cell apoptosis after increased β-actin synthesis. CD8+ T cell apoptosis notably increased after culture with JASP (Figure 6A and 6B, P<0.001). β-actin protein levels in CD8+ T cells were analyzed by western blotting and found to be markedly increased after culture with JASP (Figure 6C, P<0.05).
Figure 6.
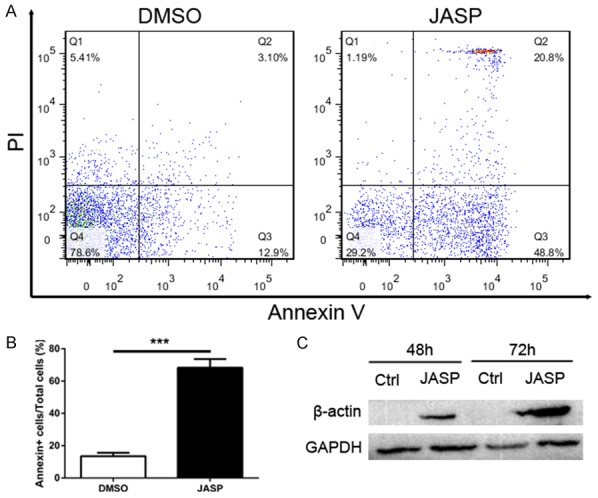
The number of CD8+ T cells undergone apoptosis was significant increased by using actin stabilization drug jasplakinolide. A: CD8+ T cells were treated with DMSO (0.05%) or jasplakinolide (1 μg/mL). Cells were harvested after 48 h incubation to analyze apoptosis rate using Annexin V/PI assay by flow cytometry. B: Histogram showing apoptosis rate of CD8+ T cells in JASP group was significant higher than the control group. C: Expression levels of β-actin protein in control group and JASP group was detected by western blot. All data representative of three independent experiments and calculated data are shown as mean ± SD. All statistical analyses were performed by student t test, ***p<0.001 compared to homogenic group.
Discussion
Acute hepatic rejection is a major cause of graft dysfunction in recipients after LT [23]. However, the mechanisms underlying acute liver allograft rejection have not been well characterized. Here, we used a rat liver allo-transplantation model to investigate whether β-actin expression is involved in AR immune reactions and explore the potential mechanism.
T lymphocytes play a central role in post-transplantation rejection [24,25]. CD8+ T lymphocyte infiltration into solid tissue allografts has been reported in experimental cardiac, skin, kidney and liver transplant models. A decreased CD4/CD8 ratio was observed in allografts due to an increase in CD8+ T lymphocyte number [26]. CD4+ T lymphocytes from recipients are activated following the specific recognition of graft antigens through an interaction between self-MHC and TCR after transplantation; these cells then release a variety of inflammatory cytokines (IFN-γ, IL-2, TNF-α and others), leading to delayed-type hypersensitivity and activating CD8+ T lymphocytes to evoke cytotoxic effects and attack the allograft [27]. However, changes in CD8+ T lymphocyte apoptosis have not been well characterized during acute allograft rejection.
In our LT models, T lymphocyte infiltration increased from day 5 to 9 in allografts, and CD8+ T lymphocyte apoptosis increased to its peak on day 7 and then decreased. Normal T lymphocyte apoptosis is triggered by one of 2 general pathways [28]. In the first pathway, apoptosis is caused by restimulation of the TCR in activated T cells without appropriate co-stimulation, which is termed activation-induced cell death (AICD). The TNF receptor family is the major mediator of this process. The second pathway is induced by “starvation” or the absence of appropriate survival signals, which is termed activated cell-autonomous death (ACAD). An imbalance between pro-apoptotic (Bim) and anti-apoptotic (Bcl-2 and Bcl-XL) proteins in the mitochondria triggers this form of apoptosis. In this study, TNF-α expression was significantly elevated in the allogeneic group compared with the homogenic group. Caspase-3 expression in CD8+ T lymphocytes infiltrating into liver grafts peaked on day 7. Based on these findings, infiltrating CD8+ T lymphocytes after LT underwent apoptosis via the caspase-3-dependent AICD pathway. Recent studies have focused on the relationship between cytokine gene polymorphisms, particularly those in TNF-α, and AR in organ transplantation, suggesting that death receptors and ligands may participate in the AR reaction. As reported by Miwa Morita et al., T cell apoptosis was observed during acute hepatic rejection in a mouse model, which was consistent with our findings. However, the exact mechanism was not clearly illustrated.
Interestingly, in our study, changes in β-actin expression exhibited a trend similar to that of the apoptotic rate of CD8+ T lymphocytes, indicating that β-actin may play an important role in CD8+ T lymphocyte apoptosis in our models. β-actin is an essential component of the cytoskeleton that plays critical roles in a wide range of cellular processes, including cell migration, cell division, and the regulation of gene expression. These functions are attributable to the ability of β-actin to form filaments that rapidly assemble and disassemble according to cellular needs [29]. β-actin not only serves as a cytoskeleton scaffold but also plays a key role in apoptosis. Disruption of the actin cytoskeleton in vascular smooth muscle cells (VSMCs) ultimately leads to apoptosis by releasing a pro-apoptotic factor called Bmf [30]. Moreover, β-actin degradation in human gingival epithelial cells is essential for apoptosis caused by Porphyromonas gingivalis infection [31]. In HL-60 cells, jaspamide increases the induction of apoptosis [32]. In addition to imbalances in β-actin assembly and disassembly, the association of the membrane receptor TNFR2 with β-actin may constitute the potential mechanism by which changes in β-actin expression affect the survival of CD8+ T lymphocytes. However, these findings were confined to in vitro experiments, and until now, there have been little studies investigating the relationship between β-actin and apoptosis in vivo. Interestingly, inflammatory cytokines, such as IFN-γ and TNF-α, and the RAI score positively correlated with β-actin expression, indicating that β-actin expression may be a predictive factor at the beginning of AR episodes.
Based on the similar trends in the apoptotic rate and β-actin expression in CD8+ T lymphocytes, as well as the function of β-actin in cell migration and apoptosis, we hypothesized that the elevated expression of β-actin occurred in response to CD8+ T lymphocyte infiltration into liver allografts during the early post-transplantation phase [33-35]. However, both the increased secretion of TNF-α in the allograft environment and the stabilization of TNFR2 via binding to β-actin may trigger ACAD and account for the high rate of CD8+ T lymphocyte apoptosis in the liver or peripheral blood. In addition, given the lethal effects of an actin-stabilizing drug on CD8+ T lymphocytes, the disruption of β-actin dynamics may be related to apoptosis. At later time points, TNF-α and β-actin expression decreased, accompanied by reduced apoptosis of CD8+ T lymphocytes.
This is the first study to show the importance of altered β-actin expression and dynamics on CD8+ T lymphocyte cell fate after LT, subsequently influencing AR progression. Thus, targeting β-actin might be a new strategy for preventing AR. Considering certain limitations of our study, more research should be performed to validate these findings in the context of human LT.
Acknowledgements
This work was supported by: National Natural Science Foundation of China 81372243, 81570593, 81370575, 81370555. Key Scientific and Technological Projects of Guangdong Province, 2014B020228003, 2014B030301041, 2015B020226004. Natural Science Foundation of Guangdong Province, 2015A030312013. Science and Technology Planning Project of Guangzhou, 2014J4100128, 201400000001-3, 201508020262.
Disclosure of conflict of interest
None.
References
- 1.Dutkowski P, Linecker M, DeOliveira ML, Mullhaupt B, Clavien PA. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148:307–323. doi: 10.1053/j.gastro.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 2.Geissler EK, Schlitt HJ. Immunosuppression for liver trnsplantation. Gut. 2009;58:452–463. doi: 10.1136/gut.2008.163527. [DOI] [PubMed] [Google Scholar]
- 3.O’Grady J. Timing and benefit of liver transplantation in acute liverfailure. J Hepatol. 2014;60:663–670. doi: 10.1016/j.jhep.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Neil DA, Hubscher SG. Current views on rejection pathology in liver transplatation. Transpl Int. 2010;23:971–983. doi: 10.1111/j.1432-2277.2010.01143.x. [DOI] [PubMed] [Google Scholar]
- 5.Germani G, Rodriguez-Castro K, Russo FP, Senzolo M, Zanetto A, Ferrarese A, Burra P. Markers of acute rejection and graft acceptance in liver transplantation. Orld J Gastroenterol. 2015;21:1061–1068. doi: 10.3748/wjg.v21.i4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khatri P, Roedder S, Kimura N, De Vusser K, Morgan AA, Gong Y, Fischbein MP, Robbins RC, Naesens M, Butte AJ, Sarwal MM. A common rejection module (CRM) for acute rejection across multiple organs identifies novel therapeutics for organ tranplantation. J Exp Med. 2013;210:2205–2221. doi: 10.1084/jem.20122709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori DN, Kreisel D, Fullerton JN, Gilroy DW, Goldstein DR. Inflammatory triggers of acute rejection of organ llografts. Immunol Rev. 2014;258:132–144. doi: 10.1111/imr.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin CM, Gill RG. Direct and indirect allograft recognition: pathways dictating graft rejection mechanisms. Cur Opin Organ Transplant. 2016;21:40–44. doi: 10.1097/MOT.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita M, Joyce D, Miller C, Fung JJ, Lu L, Qian S. Rejection triggers liver transplant tolerance: involvement of mesenchyme-mediated immune control mechaisms in mice. Hepatology. 2015;62:915–931. doi: 10.1002/hep.27909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata S, Tanaka M. Programmed cell death and the immue system. Nat Rev Immunol. 2017;17:333–340. doi: 10.1038/nri.2016.153. [DOI] [PubMed] [Google Scholar]
- 11.Feig C, Peter ME. How apoptosis got the immune sysem in shape. Eur J Immunol. 2007;37(Suppl 1):S61–S70. doi: 10.1002/eji.200737462. [DOI] [PubMed] [Google Scholar]
- 12.Spencer SL, Sorger PK. Measuring and modeling apotosis in single cells. Cell. 2011;144:926–939. doi: 10.1016/j.cell.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and atophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Yao Y, Wu J, Deng Z, Gu T, Tang X, Cheng Y, Li G. The mechanism of cytoskeleton protein beta-actin and cofilin-1 of macrophages infected by mycobaterium avium. Am J Transl Res. 2016;8:1055–1063. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Leng Y, Li Z. beta-actin in the signaling of transmembrane TNF-alpha-mediatedcytotoxicity. Methods Mol Biol. 2014;1155:55–68. doi: 10.1007/978-1-4939-0669-7_6. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Xiao L, Zhang H, Liu N, Liu T, Liu L, Hu X, Yan D, Yang K, Yin B, Wang J, Li Q, Li Z. The involvement of beta-actin in the signaling of transmembrane TNF-alpha-medited cytotoxicity. J Leukoc Biol. 2011;89:917–926. doi: 10.1189/jlb.1209812. [DOI] [PubMed] [Google Scholar]
- 17.Kamada N, Calne RY. A surgical experience with five hundred thirty live transplants in the rat. Surgery. 1983;93:64–69. [PubMed] [Google Scholar]
- 18.Selvam NT, Venkatakrishnan V, Dhamodharan R, Murugesan S, Kumar SD. Hepatoprotective activity of methanolic extract of syzygium jambos (Linn. ) leaf against paracetamol itoxicated Wistar albino rats. Ayu. 2013;34:305–308. doi: 10.4103/0974-8520.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M, Dobrinskikh E, Busson P, Polyak SJ, Hirashima M, Rosen HR. A crucial role for Kupffer cellderived galectin-9 in regulation of T cell immunityin hepatitis C infection. PLoS One. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou X, Zhou R, Wei H, Sun R, Tian Z. NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and Kupffer cells in a novel murine natural killer cell-depenent fulminant hepatitis. Hepatology. 2009;49:940–949. doi: 10.1002/hep.22725. [DOI] [PubMed] [Google Scholar]
- 21.Banff schema for grading liver allograft rejection: an internaional consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 22.Pittet MJ, Zippelius A, Speiser DE, Assenmacher M, Guillaume P, Valmori D, Lienard D, Lejeune F, Cerottini JC, Romero P. Ex vivo IFN-gamma secretion by circulating CD8 T lymphocytes: implications of a novel approach for T cell monitoring in infectous and malignant diseases. J Immunol. 2001;166:7634–7640. doi: 10.4049/jimmunol.166.12.7634. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigo E, Lopez-Hoyos M, Corral M, Fabrega E, Fernandez-Fresnedo G, San SD, Pinera C, Arias M. ImmuKnow as a diagnostic tool for predicting infection and acute rejection in adult liver transplant recipients: a systematic eview and meta-analysis. Liver Transpl. 2012;18:1245–1253. doi: 10.1002/lt.23497. [DOI] [PubMed] [Google Scholar]
- 24.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathwas in transplant rejection. N Engl J Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 25.Krensky AM, Weiss A, Crabtree G, Davis MM, Parham P. T-lymphocyte-antigen interactins in transplant rejection. N Engl J Med. 1990;322:510–517. doi: 10.1056/NEJM199002223220805. [DOI] [PubMed] [Google Scholar]
- 26.Takehisa Y, Sakiyama S, Uyama T, Sumitomo M, Tamaki M, Hino H, Takehisa M, Liu M, Kondo K, Monden Y. Progressive increase of CD4(+)/CD45RC(-) lymphocytes after allograft rat lung transplantation: a marker ofacute rejection. J Thorac Cardiovasc Surg. 2002;124:675–683. doi: 10.1067/mtc.2002.126043. [DOI] [PubMed] [Google Scholar]
- 27.Turner DL, Gordon CL, Farber DL. Tissueresident T cells, in sit immunity and transplantation. Immunol Rev. 2014;258:150–166. doi: 10.1111/imr.12149. [DOI] [PubMed] [Google Scholar]
- 28.Krammer PH, Arnold R, Lavrik IN. Life and eath in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 29.Pollard TD, Cooper JA. Actin, a centrl player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang S, Kim K, Noh JY, Jung Y, Bae ON, Lim KM, Chung JH. Simvastatin induces the apoptosis of normal vascular smooth muscle through the disruption of actin integrity via the impirment of RhoA/Rac-1 activity. Thromb Haemost. 2016;116:496–505. doi: 10.1160/TH15-11-0858. [DOI] [PubMed] [Google Scholar]
- 31.Kinane JA, Benakanakere MR, Zhao J, Hosur KB, Kinane DF. Porphyromonas gingivalis influences actin degradation within epithelial cell during invasion and apoptosis. Cell Microbiol. 2012;14:1085–1096. doi: 10.1111/j.1462-5822.2012.01780.x. [DOI] [PubMed] [Google Scholar]
- 32.Cioca DP, Kitano K. Induction of apoptosis and CD10/neutral endopeptidase expression by jspamide in HL-60 line cells. Cell Mol Life Sci. 2002;59:1377–1387. doi: 10.1007/s00018-002-8515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, Wang X, Todd EM, Jaeger ER, Vella JL, Mooren OL, Feng Y, Hu J, Cooper JA, Morley SC, Huang YH. Mst1 kinase regulates the actin-bundling protein L-plastin to promote T cell migration. J Immunol. 2016;197:1683–1691. doi: 10.4049/jimmunol.1600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burkhardt JK, Carrizosa E, Shaffer MH. The actin ctoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 35.Mostowy S, Shenoy AR. The cytoskeleton in cell-autonomous immunity: strucural determinants of host defence. Nat Rev Immunol. 2015;15:559–573. doi: 10.1038/nri3877. [DOI] [PMC free article] [PubMed] [Google Scholar]