Abstract
Our previous reports indicated that (+)-cholesten-3-one induces osteogenic differentiation of bone marrow mesenchymal stem cells (MSCs) by activating vitamin D receptor (VDR). However, whether and how miRNAs modulate osteogenic differentiation induced by (+)-cholesten-3-one have not been explored. In this study, miRNA array profiling and further validation by quantitative real-time PCR revealed that miR-351 was downregulated during (+)-cholesten-3-one-induced osteogenic differentiation of MSCs. Overexpression of miR-351 by miR-351 precursor transfection markedly inhibited the expression of osteoblast-specific genes, such as alkaline phosphatase (ALP), collagen type II, osteopontin (OPN), and runt-related transcription factor 2 (RUNX2), which consequently decreased a number of calcium mineralized nodules. Inhibition of miR-351 function by anti-miR-351 promoted expression of osteoblast-specific genes. Our results suggest that miR-351 is a negative regulator of osteoblast differentiation of MSCs induced by (+)-cholesten-3-one. Target prediction analysis tools and experimental validation by luciferase 3’UTR reporter assay identified VDR as a direct target of miR-351. miR-351 inhibited the expression of the VDR, which played a critical role in the control of osteogenic differentiation of MSCs. Importantly, overexpression of VDR significantly abolished the inhibitory effect of miR-351 on (+)-cholesten-3-one induced osteogenic differentiation. Taken together, our results demonstrate that miR-351 negatively regulates osteoblast differentiation of MSCs induced by (+)-cholesten-3-one through targeting VDR. These findings provid evidence that miR-351 can bea possible therapeutic target for bone repair and regeneration.
Keywords: miR-351, vitamin D receptor, mesenchymal stem cells, osteogenic differentiation
Introduction
Osteoporosis and other bone-related degenerative diseases are mainly due to alterations in osteoblast differentiation of mesenchymal stem cells (MSCs) [1-3]. Understanding the regulatory mechanism of osteoblast differentiation of MSCs is a prerequisite for developing strategies to treat bone loss diseases such as osteoporosis. Transcription factors play critical roles in osteoblast differentiation of MSCs. The activation of lineage-specific transcription factors, RUNX2, Osterix, and Dlx5, is known to be essential for osteoblast differentiation [4]. Vitamin D receptor (VDR), ligand-activated transcription factors, has an important role in the control of osteogenic differentiation of MSCs [5]. Moreover, the epigenetic regulation of the VDR may be key to rejuvenate osteoblastogenesis in MSCs from elders [6]. Our recent report indicated that (+)-cholesten-3-one induces osteogenic differentiation of MSCs by activating VDR [7]. However, the role of epigenetic regulation in the (+)-cholesten-3-one induces osteogenic differentiation of MSCs remains poorly understood.
Recently, miRNAs have emerged as important regulators of osteoblast differentiation of MSCs [8]. Several studies reported that miRNAs target the critical transcription factors involved in osteoblast differentiation of MSCs. miRNAs 133, 375 and 204 reduced osteoblast differentiation by directly targeting RUNX2 in MSCs [9-11]. miRNAs miR-141 and miR-200a were downregulated during osteoblast differentiation and inhibited osteoblastogenesis by targeting Dlx5 [12]. miR-637 suppressed osteoblast differentiation of MSCs by directly targeting Osterix, a key transcription factor of osteoblasts [13]. miR-26a functionally repress osteoblast differentiation by targeting transcription factor Smad1 [14]. miR-146a and miR-144-3p negatively regulates the osteogenesis by targeting Smad4 [15,16]. miR34s inhibit osteoblast differentiation in the mouse by targeting SATB2 [17]. A network connecting RUNX2, SATB2, and the miR-23a cluster regulates the osteoblast differentiation [18,19]. Thus, it has been strongly suggested that the regulation of osteogenic master transcription factors by miRNAs is a notable component of the regulatory machinery. Moreover, the role of miRNAs in targeting VDR remains to be clarified in osteogenic differentiation of MSCs. Here, we used miRNA array profiling and cellular model of osteogenic differentiation of MSCs to further investigate the role of miRNAs in targeting VDR involved in (+)-cholesten-3-one-induced osteogenic differentiation of MSCs.
Methods
Animal and materials
A total of 10 male specific pathogen free Sprague-Dawley rats, aged 4 weeks (180-200 g), were acquired from the Animal Centre of Guangzhou University of Chinese Medicine (Guangzhou, China). All animals received humane care in accordance with the guidelines set out by the Care of Experimental Animals Committee of Guangzhou University of Chinese Medicine. Dulbecco’s modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA); VDR, dimethyl sulphoxide (DMSO) and other chemical reagents were purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany); alizarin red stain solution, alkaline phosphatase (ALP; BA0632), osteopontin (OPN; PB0589), Runt-related transcription factor 2 (RUNX2; BA3613-2), VDR (PB0479) and collagen II (BA0533) antibody were provided by Wuhan Boster Biological Technology, Ltd., (Wuhan, China).
Culture of MSCs
The femur and tibia were harvested from rats, anaesthetized by chloral hydrate (330 mg/kg); Wuhan Boster Biological Technology, Ltd.) and sacrificed by cervical dislocation, in order to collect fresh marrow. The marrow was mixed with complete medium (low glucose DMEM supplemented with 10% FBS) and gradient centrifuged at 900× g for 30 min at room temperature with Percoll at a density of 1.073 g•ml-1. Cells of the appropriate density were collected, washed with PBS three times, manually counted using a light microscope and cultured at a density of 1×106 cm-2 on dishes supplemented with complete medium, in a humidified atmosphere at 37°C and 5% CO2, using an incubator. The medium was refreshed and the suspension cells were removed every three days. Isolated MSCs were confluent after nine days, and were digested using 0.25% trypsin in order to promote separation. Cells were subsequently passaged at a density of 1×104 cm-2 onto dishes. MSCs that had been passaged three times were used for subsequent experiments. The surface antigen identification and differentiation ability of MSCs were verified by our previous study.
miRNA microarray analysis
The MSCs of (+)-cholesten-3-one-induced for 3 days was taken to microRNA microarray assay which was performed at Guangzhou RiboBio Co., Ltd. The experiment contains 4 steps, like pre-hybridization, hybridization, hybridization washing, and imaging. CustomArray™ microarray was assembled with hybridization cap and clips. Firstly, it’s pre-hybridization: The hybridization chambers were filled with nuclease-free water to incubate at 65°C for 10 min, and then bring to room temperature. The pre-hybridization solutions were following filled into the chambers after the water aspirating out of the hybridization chambers, and also kept on incubating at 37°C for 60 min with gentle rotation in the hybridization oven. Secondly, the hybridization was performed: The hybridization solution was prepared like the following steps, total RNA from the MSCs in both groups were extracted with trizol method and labeled with cy3 as fluorescence labeling by ULSTM (UNIVERSAL LINKAGE SYSTEM) notation, and then the solution was denatured at 95°C for 3 min and cooled for 20 seconds on ice to prepare the hybridization steps. The hybridization chambers were filled with the hybridization solution as the followed that the pre-hybridization solution was taken out and mixed gently to incubate at 37°C for 16 hours. Finally, the microarray was rinsed to decrease specific hybridization background, and then covered with the imaging solution and loaded into the GenePix 4000B Microarray Scanner to scan.
Quantitative real-time PCR (qRT-PCR)
The serum miRNAs were enriched using the mirVana microRNA Isolation kit (Applied Biosystems, Foster City, CA, USA) after total RNA was isolated by TRIzol Reagent (Invitrogen). The expression of miR-351 was determined by TaqMan microRNA assay kit especially for miR-351 (Applied Biosystems) according to manufacturer’s instructions. For data analysis, we used the U6 RNA as an endogenous control. Total RNA of MSCs was also extracted using TRIzol Reagent. Expression of ALP, collagen II, OPN, RUNX2 was measured by qRT-PCR. Quantitative RT-PCR reactions were performed using the SYBR Green PCR, included 2-minute incubation at 50°C, then 95°C for 10 minutes; this was followed by a 2-step PCR program, as follows: 95°C for 15 seconds and 60°C for 60 seconds for 40 cycles. β-actin was used as an internal control to normalize for differences in the amount of total RNA in each sample. Fold changes were calculated using the 2-ΔΔCt method. All procedures were repeated three times.
Western blot analysis
MSCs were lysed with RIPA containing protease and phosphatase inhibitors (Roche Applied Science, Mannheim, Germany). Equal amounts of protein were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA), immunoblotted with primary Antibody (Ab) (ALP, OPN, RUNX2, VDR, collagen II (all 1:200) and β-catenin (ab32572; 1:5,000; Abcam, Cambridge, UK), and visualized with horseradish-peroxidase-coupled secondary Ab.
Calcium mineral deposition
MSCs were cultured for 14 days, as described. The level of calcium mineral deposition was revealed using alizarin-red staining (AR-S). Following three weeks in culture, cells were fixed with 70% ethanol, washed five times with deionized water and treated with 40 mM alizarin-red solution for 10 min at pH 4.2. Subsequently, cells were washed with PBS for 15 min before being treated with 10% cetylpyridinium chloride in 10 mM sodium phosphate for 15 min at room temperature. An AR-S standard curve, at an optical density of 540 nm, was used to calculate the AR-S concentration.
Bioinformatic analysis
The bioinformatic prediction was performed using three websites: TargetScan (http://www.targetscan.org), PicTar (http://pictar.mdc-berlin.de) and miRanda (http://www.microrna.org). The consistency of the analyses and predictions on these three websites suggests that they are reliable.
Transfection of MSCs with miR-351 mimics and inhibitor
MSCs were cultured in Gibco DMEM (4500 mg/L glucose) and supplemented with 10% FBS and 1% mixture liquid of penicillin/streptomycin. Cell cultures were incubated at 37°C in a humid 5% CO2/95% air environment. MSCs were plated into 6 well cell culture cluster at a density of 2×104 cells per cm2 and transfected with 100 nM miR-351 mimics or inhibitor for 6 h according to the protocol of Lipofectamine 2000. The cells were collected after the terminal transfection for 24 h with 0.025% trypsin digestion and lysed with RIPA buffer for western blot analysis.
Luciferase reporter assay
Luciferase reporter assay was performed using the Firefly Luciferase Reporter Gene Assay Kit (Beyotime Biotechnology), according to the manufacturer’s instructions. Briefly, wild-type and mutant VDR (without miR-351 binding sites) plasmids were co-transfected with 100 ng of pre-miR-351 or pre-miR-ctrl into MSCs using Lipofectamine 2000 (Invitrogen). pRL-TK that express Renilla luciferase served as an internal reference. Thirty-six hours later, the cell lysates were subjected to the Dual Luciferase Reporter Gene Assay Kit (Beyotime Biotechnology).
Statistical analysis
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was used for the statistical analyses. The data were expressed as mean ± standard deviation. The differences between two groups and among more than two groups were analyzed using Tukey’s test and ANOVA, respectively. P<0.05 was considered to indicate a statistically significant difference. Spearman’s correlation coefficients were used to examine the correlation between miR-351 expression and VDR expression. The plots were made using GraphPad Prism 6.0 (Graph-Pad Software Inc, La Jolla, CA, USA).
Results
MiR-351 is down regulated during MSC osteogenic differentiation
To study the expression of miRNAs during (+)-cholesten-3-one-induced osteoblast differentiation, we treated MSCs with (+)-cholesten-3-one for 3 days. MSCs treated with or without (+)-cholesten-3-one were subjected to microarray analysis to identify differentially expressed miRNAs. Of these, the most interesting was miR-351 as its expression was significantly downregulated during osteoblast differentiation (Figure 1A). These data were further verified by quantitative real-time PCR (qRT-PCR). Consistently, the expression of miR-351 was significantly downregulated in (+)-cholesten-3-one treated MSCs compared with untreated control (Figure 1B).
Figure 1.
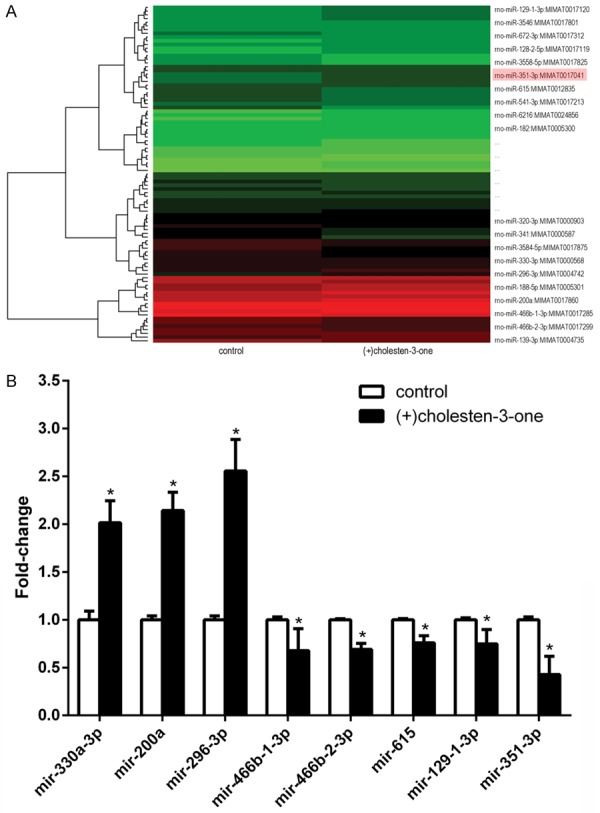
The cluster diagram and bar graph of differential miRNA were illustrated after (+)-cholesten-3-one-induced MSCs osteogenic differentiation for 3 days. A. Compared with control group, there were 52 miRNA up-regulated and 55 down-regulated in (+)-cholesten-3-one induction group. In the microarray cluster diagram, red color represents the miRNAs expression were in relatively high quantity, and green means relatively low. Especially, the data of some miRNA were nearly to limits line. B. We collected significantly (like miR-351-3p), but which we certified with the following qRT-PCR. The fold-change value of miRNA in osteogenic induction group compared with control group. In the total database of miRNA microarray, we made further screening and selected some possible miRNA to identify with qRT-PCR. *p<0.05 versus control group.
MiR-351 negatively regulates osteoblast differentiation of MSCs
To evaluate the role of miR-351 in osteoblast differentiation of MSCs, we transfected MSCs with miR control, mimic miR-351 or anti-miR-351 respectively. Then the cells were cultured with or without (+)-cholesten-3-one for 7 days to induce osteogenic differentiation. Alizarin-red staining assay showed that miR-351 significantly suppressed MSC osteogenic differentiation compared with miR control. However, anti-miR-351 markedly promoted osteogenic differentiation (Figure 2A). The mRNA and protein levels of osteogenesis related markers, such as ALP and collagen II, were tested by qRT-PCR and western blot after miR-351 transfection. qRT-PCR results show that mRNA levels of the osteogenic related markers were declined in the miR-351 group compared with the miR control group, whereas they enhanced in the anti-miR-351 group (Figure 2B). Western blot results also demonstrated that the changes in protein levels were similar to that of the mRNA levels (Figure 2C and 2D). These data suggest that miR-351 negatively regulates osteoblast differentiation of MSCs.
Figure 2.
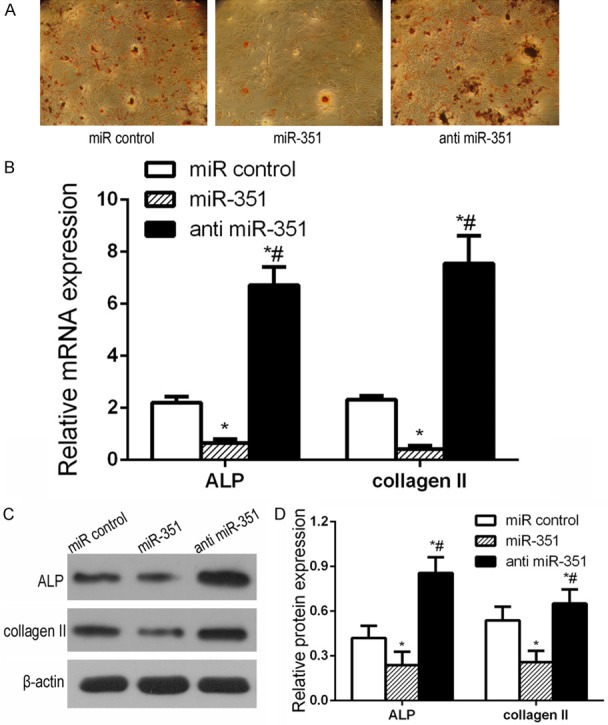
miR-351 negatively regulated MSC osteogenic differentiation. The miR control or miR-351 or anti miR-351 were transfected into MSCs. The mRNA and protein levels of osteogenesis markers (ALP, collagen II) proteins were detected by qRT-PCR and western blot, respectively. A. AR-S indicated the level of calcium mineral deposition. B. qRT-PCR detection of mRNA expression of osteogenic markers in miR-351 or anti miR-351 transfected MSCs during osteogenic differentiation. C. Western blot detection of protein expression of osteogenic markers in miR-351 or anti miR-351 transfected MSC during osteogenic differentiation. D. Comparison of the density between the experimental groups. miR control group: MSCs transfected with miR control; miR-351 group: MSCs transfected with miR-351 mimic; anti miR-351 group: MSCs transfected with anti miR-351. *P<0.05 vs. miR control group. #P<0.05 vs. miR-351 group.
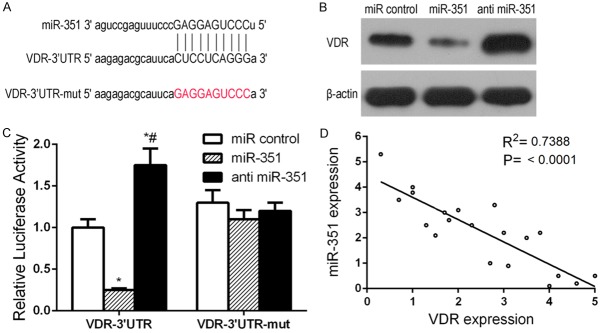
MiR-351 directly targets VDR
To gain insight into the molecular mechanisms by which miR-351 regulates the osteogenic differentiation of MSCs, we predicted the potential targets of miR-351 using TargetScan and found that osteogenic-related genes VDR have miR-351 binding sites in their 3’UTR. To test whether miR-351 directly targets these genes, we constructed luciferase reporters that had either a wild-type (WT) 3’UTR or a 3’UTR containing mutant sequences of the miR-351 binding site. We found that overexpression of miR-351 remarkably inhibited the luciferase reporter activity of the WT VDR 3’UTR, but not that of the mutated 3’UTR or another gene’s 3’UTR (Figure 3A and 3C). These findings indicated that miR-351 may directly regulate VDR expression. Further experiments confirmed that miR-351 overexpression markedly suppressed the expression of VDR, at both the mRNA and protein levels (Figure 3B). Moreover, VDR expression at mRNA and protein levels (Figure 3C) was found to be markedly increased in the presence of anti-miR-351 group compared with miR control group. These results indicate that VDR is the target gene of miR-351.
Figure 3.
miR-351 directly targets VDR. To confirm whether miR-351 targeted the 3’-UTR of VDR, a luciferase activity assay was performed. MSCs were co-transfected with wild type VDR 3’-UTR (VDR-3’-UTR) or mutant VDR 3’-UTR (VDR-3’-UTR-mut) and miR-351 or anti miR-351. The relative luciferase activity was detected. A. The predicted miR-351 target sequence in the 3’UTR of VDR. B. Western blot detection of VDR protein expression in miR-351 or anti miR-351 transfected MSCs during osteogenic differentiation. C. Dual-luciferase reporter assay of MSCs transfected with the VDR-3’UTR reporter and miR-351 or anti miR-351. *P<0.05 vs. miR control. #P<0.05 vs. miR-351 group. D. Correlation analysis of miR-351 and VDR expression.
MiR-351 regulated VDR downstream signaling
To better understand the underlying mechanism of miR-351 regulated VDR downstream signaling in MSCs osteogenic differentiation, we detected the mRNA and protein levels of OPN, RUNX2 by qRT-PCR and western blot after miR-351 transfection at (+)-cholesten-3-one-induced 7 days. qRT-PCR results show that mRNA levels of the OPN and RUNX2 were declined in the miR-351 group compared with the miR control group, whereas they enhanced in the anti-miR-351 group (Figure 4A). Western blot results also demonstrated that the changes in protein levels were similar to that of the mRNA levels (Figure 4B and 4C), suggesting that miR-351 inhibits osteogenic differentiation through VDR downstream signaling.
Figure 4.
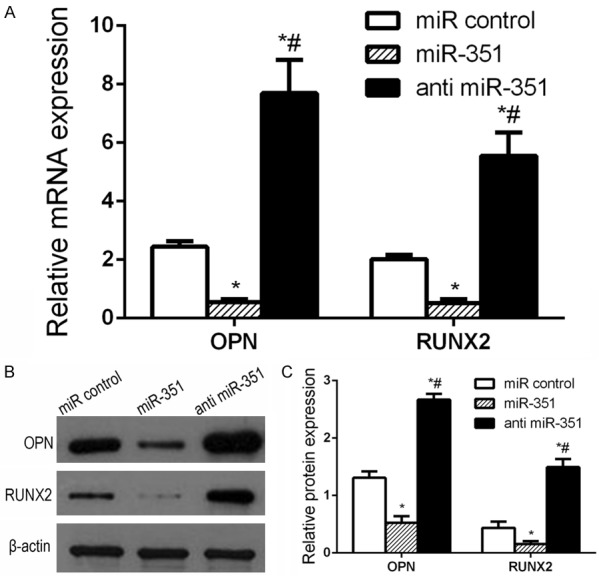
miR-351 regulated VDR downstream signaling. The miR control or miR-351 or anti miR-351 were transfected into MSCs. The mRNA and protein levels of VDR downstream signaling (OPN, RUNX2) proteins were detected by qRT-PCR and western blot, respectively. A. qRT-PCR detection of mRNA expression of osteogenic markers in miR-351 or anti miR-351 transfected MSCs during osteogenic differentiation. *P<0.05 vs. miR control group. #P<0.05 vs. miR-351 group. B. Western blot detection of protein expression of OPN, RUNX2 in miR-351 or anti miR-351 transfected MSC during osteogenic differentiation. C. Comparison of the density between the experimental groups. miR control group: MSCs transfected with miR control; miR-351 group: MSCs transfected with miR-351 mimic; anti miR-351 group: MSCs transfected with anti miR-351. *P<0.05 vs. miR control group. #P<0.05 vs. miR-351 group.
VDR overexpression eliminated the inhibitory effect of miR-351 on osteoblast differentiation
To further understand the functional role of miR-351 on ALP and collange II expression through regulating VDR, we transfected MSCs with miR-351 and VDR under (+)-cholesten-3-one treatment. As shown in Figure 5A, AR-S assay demonstrated that osteogenic differentiation expression was significantly up-regulated after pCMV-VDR transfection compared with (+)-cholesten-3-one group. More calcium mineralized nodules (Figure 5A) were observed in VDR transfected MSCs compared with untransfected control. We also observed that overexpression of VDR remarkably increased the expression of ALP, collange II, but miR-351 suppressed (+)-cholesten-3-one-induced ALP, collange II expression. Moreover, pCMV-VDR transfection also markedly increased ALP, collange II expression, even in the presence of miR-351 overexpression (Figure 5B). In addition, overexpression of VDR could significantly reverse the inhibitory effects of miR-351 overexpression on osteogenic differentiation, restoring the osteogenic markers ALP, collange II expression (Figure 5C). These results indicate that VDR overexpression abolished the inhibitory effect of miR-351 on osteoblast differentiation.
Figure 5.
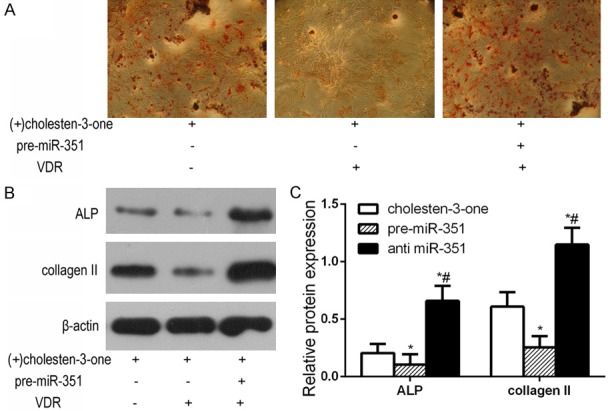
VDR rescues the inhibitory effect of miR-351 on osteoblast differentiation of MSCs. Primary osteoblasts expressing miR-351 were transfected with either the VDR-expressing or control plasmid into MSCs of (+)-cholesten-3-one promotes the osteogenic differentiation. Subsequently, AR-S and Western blot was analyzed. A. AR-S indicated the level of calcium mineral deposition. B. Western blot detection of protein expression of osteogenic markers was analyzed. C. Comparison of the density between the experimental groups. miR control group: MSCs transfected with miR control; miR-351 group: MSCs transfected with miR-351 mimic; anti miR-351 group: MSCs transfected with anti miR-351. *P<0.05 vs. miR control group. #P<0.05 vs. miR-351 group.
Discussion
Osteogenic differentiation of MSCs contributes greatly to bone regeneration. Hence, it is of the utmost importance to explore the positive and negative regulators of osteogenic differentiation of MSCs. The findings will provide promising therapeutic targets for osteoporosis and other degenerative bone diseases. In the previous study, we indicated that steroids in traditional Chinese medicine (TCM) have the therapeutic potential to treat bone disease, (+)-cholesten-3-one induces osteogenic differentiation of MSCs by activating VDR [7]. The aim of this study was further to identify miRNAs regulating osteogenic differentiation of MSCs induced by (+)-cholesten-3-one. The major findings of the present study were: 1) miR-351 is down regulated during (+)-cholesten-3-one induce-MSC osteogenic differentiation; 2) miR-351 is a negative regulator of osteoblast differentiation of MSCs induced by (+)-cholesten-3-one; 3) VDR is a direct target of miR-351. These findings suggest that miR-351-VDR signaling pathway is an important part of the regulatory machinery involved in early osteogenesis, and that the miR-351-VDR signaling pathway might be a key target for drug development in bone repair and regeneration.
An important finding of the present study is that miR-351 is down regulated during (+)-cholesten-3-one induce-MSCs osteogenic differentiation. The previous study reported that miR-351 promoted cellular antiviral activities [20], regulated development of the neural stem cell [21], promoted muscle regeneration [22], regulated two-types of cell death [23] and behaviors of MSCs [24]. However, little is understood about the roles of miR-351 in the osteogenic differentiation of MSCs. We hypothesize that miRNAs targeting the positive regulator during osteogenic differentiation of MSCs are important. In our results, our microarray data and qRT-PCR identified miR-351 to be down-regulated in (+)-cholesten-3-one induce-MSCs osteogenic differentiation. Therefore, we further confirm that miR-351 is a negative regulator of osteoblast differentiation of MSC induced by (+)-cholesten-3-one.
The MSCs used in this study are osteoblasts. As an osteoblast cell line, MSCs have been used in various bone-related studies, including investigations of osteoporosis, bone formation, and osteoblast proliferation. In this study, MSCs were used to evaluate osteogenic differentiation, as assessed by the expression of osteogenic markers and Alizarin Red staining. The increased expression of ALP, collagen II and RUNX2, improved and mineralized nodules all suggest enhanced osteogenic differentiation. miRNAs down-regulate target genes by either promoting mRNA degradation or inhibiting their translation and play significant roles in cell proliferation and differentiation. Moreover, altered miRNA expression has been identified in bone metabolism and other bone diseases. Therefore, it is important to identify specific miRNAs and their targets that are involved in MSCs regulation and investigate the detailed mechanisms of osteogenic differentiation.
Cell fate transition from stem cell to differentiation involves not only positive regulators but also negative regulators that suppress differentiation. More and more miRNAs have been reported to have positive regulation of osteogenic differentiation, including miR-335-5p targeting DDK1 [25], miR-20a/b targeting PPARc [26,27], miR-15 targeting Smurf1 [28], miR-214 down-regulated PTEN [29] and miR-let-7 down-regulate Axin2 in human MSCs [30]. Only a few miRNAs have been reported to suppress osteogenic differentiation [31,32]. In this study, the decrease in the expression of miR-351 during osteoblast differentiation prompted us to test if miR-351 inhibits osteoblast differentiation. We investigated the action of miR-351 in the process of osteoblastogenesis. MiR-351 over-expression inhibited osteoblast differentiation. In contrast, over-expression of anti-miR-351 increased osteoblast differentiation as assessed by ALP, collage II. MiR-351 over-expression also decreased the mineral nodule formation. Our data suggested miR-351 as a negative regulator of osteoblast differentiation of MSCs. These findings may provide a new regulatory role of miR-351 in the process of osteogenic differentiation. In the cell fate-specific type, miR-351 only brake on the osteogenic initiation, because releasing the brake results in enhanced osteogenic differentiation of MSCs.
To study the molecular mechanism by which miR-351 regulates osteoblastogenesis, we searched for potential target genes that have an established function in promoting osteogenesis using target prediction tools like Pictar and Target scan. Interestingly, it was observed that the VDR is one target of miR-351. Our previous study showed that VDR positively regulates osteoblast differentiation induced by (+)-cholesten-3-one, this is the reason why we choose VDR as the target gene. VDR has a key role in the differentiation of MSCs to bone and cartilage [33]. Using a luciferase VDR 3’UTR reporter gene, we show that over-expression of mimic miR-351 suppressed the luciferase activity of the reporter construct. However, this effect was abolished when luciferase reporter containing a mutant 3’UTR of VDR was co-transfected with mimic miR-351, thus confirming the specificity of action. In the present study, we found that miR-351 could significantly inhibit the osteogenic differentiation of MSCs. This study provides evidence that VDR was predicted to be one of the target genes of miR-351 via bioinformatic analysis, and via direct targeting of miR-351 to 3’UTR region of VDR mRNA. Overexpression of VDR markedly reversed the effects of miR-351 overexpression, indicating that VDR was the effector of miR-351 in the osteogenic differentiation of MSCs. Following osteoblast differentiation induced by (+)-cholesten-3-one, miR-351 was significantly down-regulated and VDR was released from miR-351 inhibition, thereby facilitating the escape of MSCs from the quiescent state into osteogenic differentiation.
The results of the present study have several important clinical implications. First, Osteoporosis leads to heavy economic burdens [34]. Understanding of differentiation of MSCs to develop effective drugs for the prevention and treatment of postmenopausal osteoporosis is vital, alterations in the balance between osteoblast and adipocyte differentiation of MSCs can lead to osteoporosis and other bone-related degenerative diseases. The earliest symptoms of Osteoporosis present as a relative deficit. Therefore, sensitive and specific biomarkers for early detection are urgently needed. In this study, we found that miR-351 was decreased significantly in early osteogenic differentiation. Thus, the miR-351 may be potential biomarkers for the early diagnosis of Osteoporosis. Second, currently, there are no therapies to prevent the progression of Osteoporosis. Vitamin D has been used in clinics to stimulate bone formation and treat osteoporosis. However, long-term trials showed that these drugs had no effects on the prevention of hip fracture. The side-effects, such as hypercalcemia, also affect long-term administration [35]. We find that miR-351 functions as a negative regulator of osteogenesis by repressing VDR expression, which in turn, may result in suppression of VDR signaling pathway. Hence, pharmacological inhibition of miR-351 could represent a therapeutic strategy for enhancing bone formation in osteoporosis. Third, our study employing a screen of miRNA array profiling provided an example of highly efficient miRNAs identification and functional dissection of the miRNAs-VDR pathway regulating stem cell fate. Further studies on the identified miRNAs-VDR network will not only help us understand the critical molecular switches on osteogenic differentiations of MSCs but also facilitate the characterization of the miRNAs basis of bone diseases as well as the development of new therapies to treat them.
In conclusion, our study uncovered that miR-351 functions as a negative regulator of osteogenic differentiation of MSCs by repressing VDR expression, which in turn, results in suppression of the VDR signaling pathway. Thus, miR-351 should be considered an important candidate as an osteoblast differentiation of MSCs molecular target for the development of preventive or therapeutic approaches against osteogenic disorders.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (81273896, 81273783 and 81473699), Guangdong Science and Technology Project 2016A050503039. Guangdong Technology Projects of Self-financing Category (grant no. 2014807) and Research Projects of Construction of Chinese Medicine province of Bureau of Traditional Chinese Medicine of Guangdong Province (grant no. 20141084).
Disclosure of conflict of interest
None.
References
- 1.Pietschmann P, Rauner M, Sipos W, Kerschan-Schindl K. Osteoporosis: an age-related and gender-specific disease--a mini-review. Gerontology. 2009;55:3–12. doi: 10.1159/000166209. [DOI] [PubMed] [Google Scholar]
- 2.Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng. 2010;16:445–453. doi: 10.1089/ten.TEB.2009.0825. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa K, Nakashima T, Takeda S. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest. 2010;120:3455–3465. doi: 10.1172/JCI42528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99:1233–1239. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- 5.Olivares-Navarrete R, Sutha K, Hyzy SL. Osteogenic differentiation of stem cells alters vitamin D receptor expression. Stem Cells Dev. 2012;21:1726–1735. doi: 10.1089/scd.2011.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou S, Geng S, Glowacki J. Histone deacetylation mediates the rejuvenation of osteoblastogenesis by the combination of 25(OH) D3 and parathyroid hormone in MSCs from elders. J Steroid Biochem Mol Biol. 2013;136:156–159. doi: 10.1016/j.jsbmb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou QK, Huang YQ, Luo YW, Wang B, Liu YM, Deng RD, Zhang SX, Lai YT, Li WY, Chen DF. (+)-Cholesten-3-one induces osteogenic differentiation of bone marrow mesenchymal stem cells by activating vitamin D receptor. Exp Ther Med. 2017;13:1841–1849. doi: 10.3892/etm.2017.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taipaleenmaki H, Bjerre Hokland L, Chen L, Kauppinen S, Kassem M. Mechanisms in endocrinology: micro-RNAs: targets for enhancing osteoblast differentiation and bone formation. Eur J Endocrinol. 2012;166:359–371. doi: 10.1530/EJE-11-0646. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Hassan MQ, Volinia S. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105:13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du F, Wu H, Zhou Z, Liu YU. microRNA-375 inhibits osteogenic differentiation by targeting runt-related transcription factor 2. Exp Ther Med. 2015;10:207–212. doi: 10.3892/etm.2015.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–364. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh T, Nozawa Y, Akao Y. MicroRNA-141 and -200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J Biol Chem. 2009;284:19272–19279. doi: 10.1074/jbc.M109.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JF, Fu WM, He ML. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol Biol Cell. 2011;22:3955–3961. doi: 10.1091/mbc.E11-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res. 2008;23:287–295. doi: 10.1359/jbmr.071011. [DOI] [PubMed] [Google Scholar]
- 15.Xie Q, Wei W, Ruan J. Effects of miR-146a on the osteogenesis of adipose-derived mesenchymal stem cells and bone regeneration. Sci Rep. 2017;7:42840. doi: 10.1038/srep42840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Geng J, Wei X, Zhang R, Jiang S. MiR-144-3p regulates osteogenic differentiation and proliferation of murine mesenchymal stem cells by specifically targeting Smad4. FEBS Lett. 2016;590:795–807. doi: 10.1002/1873-3468.12112. [DOI] [PubMed] [Google Scholar]
- 17.Wei J, Shi Y, Zheng L. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J Cell Biol. 2012;197:509–521. doi: 10.1083/jcb.201201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan MQ, Gordon JA, Beloti MM, Croce CM, van Wijnen AJ, Stein JL. A network connecting Runx2, SATB2, and the miR-23a~27a~24-2 cluster regulates the osteoblast differentiation program. Proc Natl Acad Sci U S A. 2010;107:19879–19884. doi: 10.1073/pnas.1007698107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu R, Liu W, Li H. A Runx2/miR-3960/miR-2861 regulatory feedback loop during mouse osteoblast differentiation. J Cell Biol. 2011;286:12328–12339. doi: 10.1074/jbc.M110.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Feng R, Huang C, Wang H, Wang J, Zhang Z. MicroRNA-351 regulates TMEM 59 (DCF1) expression and mediates neural stem cell morphogenesis. RNA Biol. 2012;9:292–301. doi: 10.4161/rna.19100. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Melton DW, Gelfond JA, McManus LM, Shireman PK. MiR-351 transiently increases during muscle regeneration and promotes progenitor cell proliferation and survival upon differentiation. Physiol Genomics. 2012;44:1042–1051. doi: 10.1152/physiolgenomics.00052.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato A, Omi T, Yamamoto A, Satake A, Hiramoto A, Masutani M. MicroRNA-351 regulates two-types of cell death, necrosis and apoptosis, induced by 5-fluoro-2’-deoxyuridine. PLoS One. 2016;11:e0153130. doi: 10.1371/journal.pone.0153130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Jiang XL, Yang SC, Lin X, He Y, Yan C. MicroRNAs in the regulation of interfacial behaviors of MSCs cultured on microgrooved surface pattern. Biomaterials. 2011;32:9207–9217. doi: 10.1016/j.biomaterials.2011.08.058. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Tu Q, Bonewald LF, He X, Stein G, Lian J. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res. 2011;26:1953–1963. doi: 10.1002/jbmr.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Zhang JF, Yi C. miRNA-mediated functional changes through co-regulating function related genes. PLoS One. 2010;5:e13558. doi: 10.1371/journal.pone.0013558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JF, Fu WM, He ML, Xie WD, Lv Q, Wan G. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol. 2011;8:829–838. doi: 10.4161/rna.8.5.16043. [DOI] [PubMed] [Google Scholar]
- 28.Vimalraj S, Partridge NC, Selvamurugan N. A positive role of microRNA-15b on regulation of osteoblast differentiation. J Cell Physiol. 2014;229:1236–1244. doi: 10.1002/jcp.24557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao C, Sun W, Zhang P. miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol. 2015;12:343–353. doi: 10.1080/15476286.2015.1017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egea V, Zahler S, Rieth N. Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2012;109:E309–316. doi: 10.1073/pnas.1115083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baglio SR, Devescovi V, Granchi D, Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals osterix regulation by miR-31. Gene. 2013;527:321–331. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Cheng P, Chen C. miR-93/Sp7 function loop mediates osteoblast mineralization. J Bone Miner Res. 2012;27:1598–1606. doi: 10.1002/jbmr.1621. [DOI] [PubMed] [Google Scholar]
- 33.van de Peppel J, van Leeuwen JP. Vitamin D and gene networks in human osteoblasts. Front Physiol. 2014;5:137. doi: 10.3389/fphys.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu B, Ma Y, Yan M. The economic burden of fracture patients with osteoporosis in western China. Osteoporos Int. 2014;25:1853–1860. doi: 10.1007/s00198-014-2699-0. [DOI] [PubMed] [Google Scholar]
- 35.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]