Abstract
Gliomas are one the most prevalent malignant carcinomas of the central nervous system, and angiogenesis plays a critical role in the progression of these blood vessel-rich tumors. HOTAIR, a long non-coding RNA (lncRNA), acts as an oncogene in gliomas; however, its role in glioma angiogenesis remains unclear. In the present study, we identified a pro-angiogenic activity of HOTAIR. Silencing HOTAIR inhibited glioma-induced endothelial cell proliferation, migration, and tube formation. Further studies showed that vascular endothelial growth factor A (VEGFA) was involved in the HOTAIR-induced glioma angiogenesis. Our study also showed that HOTAIR was present in the glioma cell culture supernatant and was protected by membranes, suggesting that HOTAIR may affect glioma angiogenesis not only via regulation of VEGFA expression in the glioma cells, but also by transmission into endothelial cells via glioma cell-derived extracellular vesicles.
Keywords: LncRNA, HOTAIR, glioma, angiogenesis, extracellular vesicles
Introduction
Angiogenesis is a necessary prerequisite for tumor progression and invasion because it fulfills the nutrient and oxygen requirements of the tumors [1,2]. Tumors with diameters of 1~2 mm become dormant in the absence of angiogenesis [3]. Blood vessel-rich human gliomas are the most common and malignant primary brain tumors, and their growth and proliferation are heavily dependent on angiogenesis. Glioma cells promote angiogenesis and microvascular network formation to provide nutrients for tumor invasion and metastasis [4,5]. Therefore, identification of the critical molecules involved in angiogenesis may provide breakthroughs for glioma treatment.
Tumor angiogenesis is a complex multistep process that can be triggered by growth factors such as vascular endothelial growth factor (VEGF) [6] and fibroblast growth factor (FGF) [7], by oncogene activation or anti-oncogene mutations [8,9], and by miRNA deregulation. For example, miR-378 promotes angiogenesis by targeting FUS-1 [10] and miR-130a inhibits the expression of homeobox genes GAX and HOXA5 to promote angiogenesis [11]. A recent study showed that long non-coding RNAs (lncRNAs) play crucial roles in the regulation of gene expression during angiogenesis and tumor aggravation.
The HOX transcript antisense intergenic RNA (HOTAIR), a ~2,000 bp lncRNA transcribed from the HOXC locus, is a “well-established” negative prognostic factor for gliomas. The expression patterns of HOTAIR are closely associated with glioma staging, and its increased expression correlates with tumor progression [12]. HOTAIR interacts with the polycomb repressive complex 2 (PRC2) and miRNAs to promote the malignancy of gliomas [13]. Knockdown of HOTAIR inhibits proliferation, migration, and invasion, promotes apoptosis, and induces cell cycle arrest [14]. However, to the best of our knowledge, there have been no reports regarding the association of HOTAIR with glioma angiogenesis. In the present study, we found that HOTAIR functioned as an angiogenesis activator, and silencing of HOTAIR in glioma cells significantly suppressed tumor angiogenesis in vitro. Moreover, subsequent experiments confirmed that the angiogenic function of HOTAIR in gliomas is mediated not only by the regulation of vascular endothelial growth factor A (VEGFA) expression in glioma cells, but also by direct transmission into endothelial cells via glioma cell-derived vesicles.
Materials and methods
Cell lines
The human brain microvascular endothelial cells (HBMVECs) and the glioblastoma cell line A172 were purchased from the Cell Resource Center of Shanghai Institute of Life Sciences. The cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL, Gaithersburg, Maryland, USA), supplemented with 10% fetal calf serum, penicillin (100 U/mL) and streptomycin (100 mg/mL) at 37°C in 5% CO2.
siRNA, plasmid, and cell transfection
The siHOTAIR and the negative control siRNA (NC) were synthesized by Biomics Biotechnologies Co., Ltd, Nantong, China. The sequences are: siHOTAIR sense, 5’-CCACAUGAACGCCCAGAGAUU-3’; antisense, 5’-AAUCUCUGGGCGUUC AUGUGG-3’; NC sense, 5’-UUCUCCGAACGUGUCACGUTT-3’; antisense, 5’-ACGUGACACGUUCGGAGAATT-3’. VEGFA was overexpressed by cloning the open reading frame of VEGFA into the pcDNA3.1vector backbone. Cells were grown to confluency on 6-well plates and transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Collection of conditional medium (CM)
The supernatant of the A172 cells was collected and centrifuged at 2,000×g for 20 min at 4°C to remove cell debris 48 h after transfection. Then, aliquots of the CM were stored at -80°C until further use.
RNA isolation and real-time reverse transcription-polymerase chain reaction (RT-PCR)
The total RNA from cells was extracted using the TRIzol reagent (Invitrogen, Carlsbad, USA) following the manufacturer’s instructions. The first-strand of cDNA was synthesized using a HiFi-Moloney murine leukemia virus (MMLV) cDNA kit (Beijing ComWin Biotech Co., Ltd. Beijing, China). RT-PCR was conducted using the UltraSYBR mixture (Beijing ComWin Biotech Co., Ltd. Beijing, China). The lncRNA levels were normalized against β-actin levels in cell lysates. RNA in the culture medium was extracted from a fixed volume (600 μL) by the RNApure circulating reagent (Beijing ComWin Biotech Co., Ltd. Beijing, China). The lncRNA levels were normalized against GAPDH levels in the culture medium [15]. The primer sequences for the β-actin gene were 5’-GGCACCACACCTTCTACAAT-3’ (forward) and 5’-GTGGTGGTGAAGCTGTAGCC-3’ (reverse). The primer sequences for GAPDH were 5’-CTCCTCCACCTTGTAGCGTG-3’ (forward) and 5’-CATACCAGGAAATGAGCTTGACAA-3’ (reverse). The primer sequences for HOTAIR were 5’-CAGTGGGGAACTCTGACTCG-3’ (forward) and 5’-GTGCCTGGTGCTCTCTTACC-3’ (reverse). All primers were synthesized by GenScript Co. Ltd. (Nanjing). Data analysis was performed using the 2ΔΔ Ct method. Each sample was analyzed in triplicate.
Western blot analysis
Total protein was extracted from cells using the protein extraction reagent containing 1 mM phenylmethanesulfonyl fluoride (PMSF) (Boster Bioengineering, Wuhan, China). The proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Pall Gelman Laboratory Corporation, Ann Arbor, MI, USA), which was blocked with 1% bovine serum albumin (BSA) overnight at 4°C, followed by successive incubations with primary and secondary antibodies for 2 h each at room temperature. The antibodies for VEGFA and β-actin were obtained from Boster Bioengineering, China. The protein bands were detected by enhanced chemiluminescence (Advansta, Menlo Park, CA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and assessment of doubling time
To determine cell proliferation, the HBMVECs were seeded at a density of 5×103 cells/well in a 96-well plate and incubated for 24 h. The media was subsequently changed to 100% CM and incubated for another 24 h. 10 μL MTT solutions (5 mg/mL) was added to each well and incubated for 4 h at 37°C. After removal of the medium, the formazan crystals were dissolved in 0.15 mL of dimethyl sulfoxide (DMSO). The absorbance of MTT-formazan was measured at 550 nm using a SpectraMaxM3 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The doubling time was calculated using the Patterson formula: Td=Tlg2/lg(Nt/N0), where Td is the doubling time (hours), T is the time taken for cells to proliferate from N0 to Nt (hour), and N is the cell count.
Colony formation assay
For the colony formation assay, the HBMVECs were seeded into 6-well plates at a density of 5×102 cells/well and cultured for further 2 weeks. The cells were then washed twice with phosphate buffered saline (PBS), fixed with 4% paraformaldehyde for 30 min, and stained with Giemsa for 15 min. The colonies were counted using an Eclipse Ti-S inverted microscope (Nikon, Japan).
HBMVEC recruitment assay
A 24-well plate with 8-μm pore size polycarbonate membrane (Corning, USA) was used for the endothelial recruitment assay. The A172 cells were placed in the lower compartments, transfected with siHOTAIR or siNC for 48 h and refreshed with 600 μL serum-free medium before the recruitment experiments. The HBMVECs were then resuspended in 100 μL of serum-free medium and seeded in the upper compartments of the chambers. The cells remaining on the upper surface of the membrane were removed after incubation at 37°C for 12 h. The cells on the lower surface of the membrane were fixed with 4% paraformaldehyde for 30 min and stained with Giemsa for 15 min.
HBMVEC capillary tube formation assay
The HBMVECs (2.5×104 cells) were grown in the absence or presence of 100% CM for 6 h at 37°C in a 96-well plate coated with Matrigel (BD Biosciences, USA). The formation of capillary-like structures was observed under a light microscope. The branch points of the formed tubes, which represent the degree of angiogenesis in vitro, were scanned and quantitated under an Eclipse Ti-S inverted microscope (Nikon, Japan).
Enzyme-linked immunosorbent assay (ELISA)
The VEGFA in the culture supernatants was detected by ELISA (Boster Bioengineering, Wuhan, China) according to the manufacturer’s instructions.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) from at least three separate experiments performed in triplicate, except where otherwise noted. Statistical analysis was performed using one-way analysis of variance (ANOVA) for multiple comparisons and t-tests for comparisons between groups. A P value of less than 0.05 (P<0.05) was considered statistically significant.
Results
siHOTAIR inhibited the pro-angiogenic activity of glioma cells
To explore the effect of HOTAIR on glioma angiogenesis, we knocked down HOTAIR in A172 cells using siRNA, and performed the MTT and colony formation assays and the in vitro endothelial recruitment and capillary tube formation assays. As shown in Figure 1A, the level of HOTAIR expression was significantly reduced 48 h after transfection compared with that of the siRNA NC. We stimulated HBMVECs with supernatant from the siHOTAIR transfected cells (CM), and found that this CM significantly increased the doubling time of the HBMVECs (NC: 21.32±0.65 h; siHOTAIR: 23.22±0.54 h; Figure 1B). The HBMVECs formed fewer colonies after incubation with the CM of the siHOTAIR transfected cells (Figure 1C). Results of both the doubling time and colony formation assays demonstrated the effect of HOTAIR expression in glioma cells on HBMVEC proliferation. Furthermore, the endothelial recruitment assays performed in 24-transwell chambers with 8 μm pore inserts revealed that siHOTAIR significantly suppressed the migratory ability of the HBMVECs (Figure 1D). The tube formation assay showed that the morphological differentiation of HBMVECs was suppressed after incubated with the CM obtained from siHOTAIR transfected A172 cells (Figure 1E). Collectively, these results indicated that downregulation of HOTAIR in glioma cells could inhibit the pro-angiogenic activity in vitro.
Figure 1.
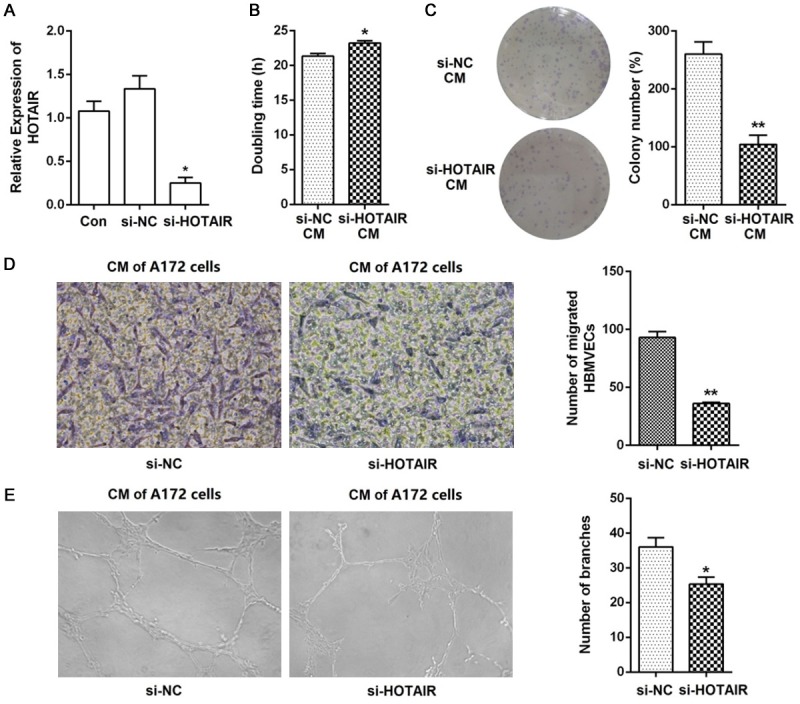
Knockdown of HOTAIR suppressed glioma angiogenesis in vitro. A. HOTAIR was knocked down in A172 cells and real time RT-PCR analysis was performed to detect HOTAIR expression. B. Cell proliferation was measured using the MTT assay and the doubling time was calculated using the Patterson formula. HBMVECs treated for 24 h with the supernatant of A172 siHOTAIR transfectants showed significantly increased doubling time compared to that of the siRNA negative control supernatant. C. HBMVECs treated with A172 siHOTAIR supernatant formed significantly fewer colonies than the cells treated with the siRNA negative control supernatant. D. Migration of HBMVECs was measured using the transwell migration assay (magnification, 200×). The migration ability of HBMVECs was significantly inhibited after incubation with the A172 siHOTAIR supernatant. E. Tube formation was measured by the HBMVEC capillary tube formation assay, and the results were expressed as the number of branches (magnification, 100×). Data represent mean ± SD (N = 3, each). Con: control; si-NC: siRNA negative control; CM: conditional medium; *P<0.05, **P<0.001.
VEGFA is involved in HOTAIR-mediated angiogenesis
VEGFA is one of the most potent factors involved in tumor angiogenesis [16]. Zhang et al. verified that HOTAIR promoted VEGFA transcription by directly targeting the VEGFA promoter [17]. We determined the VEGFA protein level in the A172 cells and culture medium after siHOTAIR transfection to confirm that VEGFA is involved in HOTAIR-mediated glioma angiogenesis. As shown in Figure 2A, the VEGFA levels in the siHOTAIR transfected A172 cells were significantly reduced compared with those transfected with NC. Moreover, the results of the ELISA demonstrated that the secretion of VEGFA decreased in cells treated with the siHOTAIR transfected culture supernatant (Figure 2B).
Figure 2.
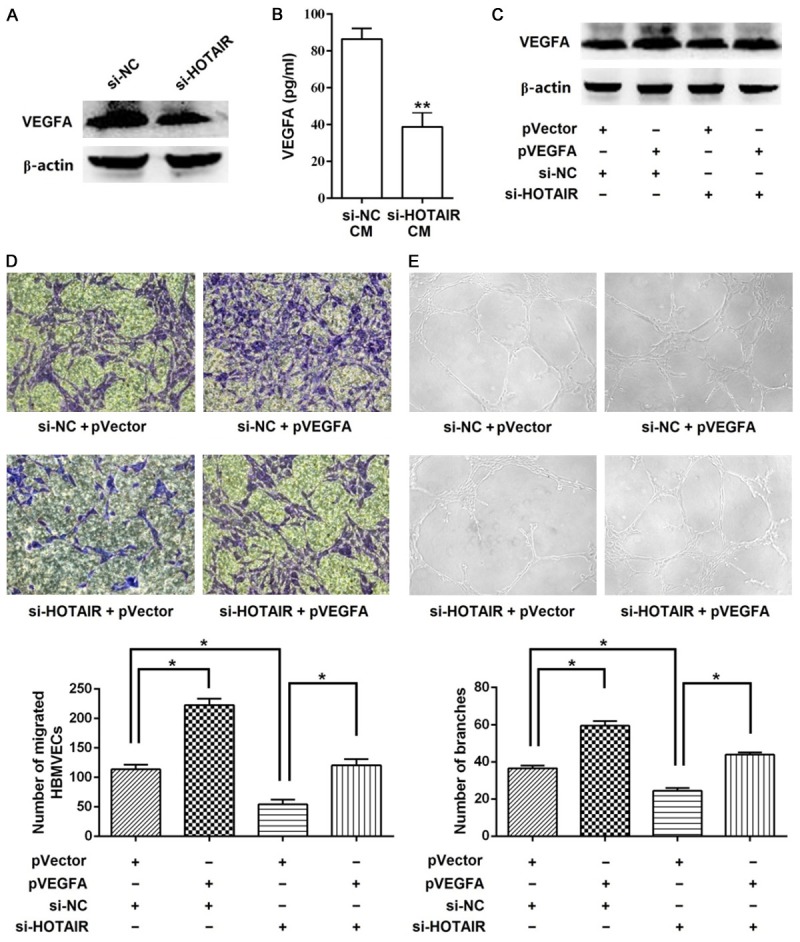
Overexpression of VEGFA attenuates the effect of siHOTAIR. A. Western blot was performed to determine the VEGFA protein level in A172 cells. siHOTAIR suppressed the expression of VEGFA in the A172 cells. B. ELISA was performed to determine the VEGFA protein level in the A172 cell CM. siHOTAIR suppressed the secretion of VEGFA in the A172 cell CM. C. The expression of VEGFA increased in A172 cells infected with the pVEGFA compared with those of cells infected with the empty vector, as measured by western blot. D. CM from A172 cells overexpressing VEGFA attenuated the suppressive effect of siHOTAIR on HBMVEC migration, as measured by the transwell migration assay (magnification, 200×). E. CM from A172 cells overexpressing VEGFA attenuated the suppressive effect of siHOTAIR on HBMVEC tube formation, as measured by the tube formation assay (magnification, 100×). Data represent mean ± SD (N = 3, each). si-NC: siRNA negative control; CM: conditional medium; *P<0.05, **P<0.001.
Next, we overexpressed VEGFA in A172 cells to further confirm that the angiogenic function of HOTAIR in gliomas is mediated by VEGFA. Western blot analysis showed that A172 cells co-transfected with pVEGFA and siNC exhibited elevated VEGFA levels compared with the cells transfected with the empty vector and siNC, and transfection with pVEGFA restored VEGFA levels in the HOTAIR knockdown cells (Figure 2C). Notably, the overexpression of VEGFA in the siHOTAIR transfectants abrogated the inhibitory effects of siHOTAIR on HBMVEC migration and capillary tube formation (Figure 2D and 2E).
HOTAIR is transmitted by A172 cell-derived extracellular vesicles (EVs)
We observed that the level of VEGFA in HBMVECs was significantly reduced after incubation with the CM of the siHOTAIR transfected A172 cells (Figure 3A), indicating that HOTAIR affected not only the proliferation, migration, and tube formation of HBMVECs via induction of VEGFA expression in A172 cells, but also the direct transmission of HBMVECs through CM. To examine whether HOTAIR could be secreted in the extracellular milieu, we extracted RNAs from the cell CM. As shown in Figure 3B, HOTAIR was detectable in both the siHOTAIR and the NC transfected cell CM.
Figure 3.
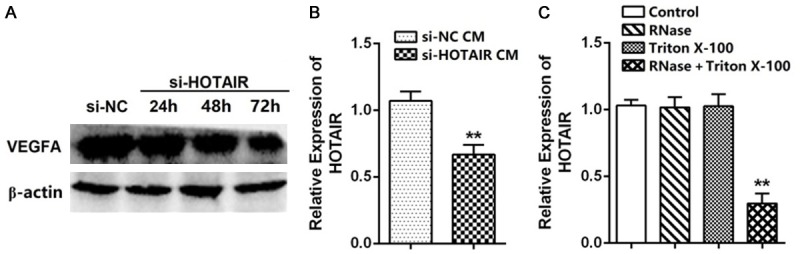
HOTAIR is transmitted by A172 cell-derived vesicles. A. Western blot was performed to analyze the expression of VEGFA in HBMVECs. The VEGFA protein level decreased after incubation with A172 cell CM. B. Real time RT-PCR analysis of HOTAIR in the CM of A172 cells. C. Real time RT-PCR analysis of HOTAIR in the CM of A172 cells treated with RNase, Triton X-100 or a combination of the two for 20 min (N = 3). Data represent mean ± SD (N = 3, each). *P<0.05, **P<0.001.
Recent studies have suggested that some lncRNAs could be transported to target tissues by cell-derived vesicles to regulate tumor progression, including angiogenesis and metastasis. Therefore, we next detected the existing pattern of extracellular HOTAIR. We collected the CM from A172 cells and treated with RNase (2 mg/mL), Triton X-100 (0.1%) or a combination of the two. The RT-PCR results revealed that the levels of HOTAIR in CM were unchanged upon RNase or Triton X-100 treatment, but decreased significantly when treated with RNase and Triton X-100 simultaneously (Figure 3C), indicating that the extracellular HOTAIR was mainly contained in membranous structures instead of being released directly. Collectively, these data suggest that HOTAIR sorted into A172 cell-derived EVs and transmitted to the HBMVECs.
Discussion
Accumulating evidence indicates that ncRNAs, including short ncRNAs (for example, microRNAs and siRNAs) and long ncRNAs (lncRNAs) play an important role in tumor development such as carcinogenesis, angiogenesis, and metastasis [18-20]. LncRNAs, which are ncRNAs ≥ 200 nt in length, are new candidates in molecular diagnosis of cancer. Several cancer-associated lncRNAs have been identified in tissues, cell lines, blood, urine, and saliva from patients with various forms of cancer [21]. HOTAIR is a dysregulated lncRNA, which is frequently observed in multiple types of cancers including gliomas. Previous studies indicated that HOTAIR regulated glioma carcinogenesis and metastasis. Recently, H19 and HULC have been identified as regulators of glioma angiogenesis. H19 promoted glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting miRNA-29a [22]; HULC controls glioma angiogenesis by regulating ESM-1 levels via the PI3K/Akt/mTOR signaling pathway [23]. However, the role of HOTAIR in glioma angiogenesis and its molecular mechanisms have not been reported yet. To investigate the effects of HOTAIR on glioma angiogenesis, we silenced HOTAIR expression in A172 cells and observed that HOTAIR silencing reversed the key processes of glioma angiogenesis such as glioma-induced endothelial cell proliferation, migration, and tube formation.
Angiogenesis is a balanced process controlled by pro- and anti-angiogenic molecules; it is disrupted during tumor development, with a concomitant shift towards pro-angiogenic factors that stimulate uncontrolled and disorganized vascular growth. These molecular factors can be secreted by cancer, endothelial, stromal, and hematopoietic cells, as well as by the extracellular matrix [24]. VEGFA is a well-known pro-angiogenic factor, and accumulating evidence shows that VEGFA is upregulated in gliomas through multiple mechanisms. Low oxygen concentration in growing gliomas upregulates HIF, which increases VEGFA mRNA levels [25]; the brain-derived neurotrophin factor and integrin-linked kinase 1 (ILK1) increases VEGFA expression by stimulating HIF-1 [25,26]; both EGFR signaling and transcription factor FoxM1B stimulate VEGFA levels in gliomas independently of HIF [24,27]; Ido et al. reported that HuR is upregulated in glioblastoma multiforme, which suppresses the post-transcriptional degradation of VEGFA mRNA, thereby contributing to a further increase in VEGFA levels [28]. In addition to these mechanisms, in the present paper, we observed that HOTAIR silencing significantly reduced the expression of VEGFA in glioma cells, and overexpression of the VEGFA abrogated the inhibitory effects of siHOTAIR on glioma-induced endothelial cell migration and capillary tube formation.
Recently, several studies have demonstrated that both normal and tumor cells release EVs as bioactive molecular shuttles to modulate their microenvironment and interact with neighboring cells. EVs can be distinguished into exosomes (EXOs), shedding microvesicles (SMVs), and apoptotic bodies (ABs), depending on their size and origin [29]. Since EVs are verified to be involved in several processes leading to tumor progression, tumor cell derived-EVs have been studied extensively. Tumor cell-derived EVs transmit “messages” to other tumor cells or normal stromal cells to create a microenvironment, support tumor metastasis and drug resistance [30-34], promote tumor cell escape from immunosurveillance and apoptosis [35,36], and stimulate angiogenesis [37-39]. The “messages” in the cargo of EVs includes proteins, lipids, and nucleic acids [40-42], and they are protected from nucleases, proteases, fluctuations in pH and osmolarity, and other environmental factors by membranes. In 2014, Gezer et al. showed that numerous lncRNAs, including HOTAIR, were found in EVs derived from human cervical and breast carcinomas by real time RT-PCR analysis [43]. Although evidence show that glioma cells secrete extracellular vesicles to support tumor progression [44-46], there are no reports of HOTAIR transmission by glioma cell-derived vesicles that stimulate angiogenesis. Our RT-PCR analysis confirmed the presence of HOTAIR in A172 cell CM, and experiments using detergent and RNase indicated that HOTAIR in the CM was protected by membranes. Therefore, we speculated that HOTAIR might be transported into A172 cell-derived EVs. It should be mentioned that HOTAIR could be detected in the CM of siHOTAIR transfected cells, which is consistent with the observation by Gezer et al. that “HOTAIR was highly enriched in EVs even if their expression was very low in cells”. Moreover, our data showed that the level of VEGFA in HBMVECs decreased significantly after incubation with the CM of the siHOTAIR transfected A172 cells, suggesting that HOTAIR not only affected glioma-induced endothelial cell proliferation, migration, and tube formation via regulation of VEGFA expression in A172 cells, but was also involved in angiogenesis by transmission into endothelial cells via A172 cell-derived EVs (Figure 4).
Figure 4.
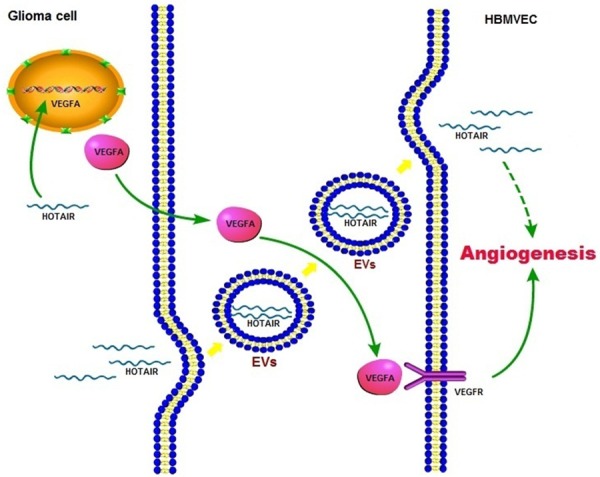
Schematic overview of the HOTAIR enhanced angiogenesis in gliomas. HOTAIR not only affected glioma-induced endothelial cell proliferation, migration, and tube formation via regulation of VEGFA expression in glioma cells, but was also involved in angiogenesis by transmission into endothelial cells via glioma cell-derived EVs.
Altogether, our results revealed the role of HOTAIR in glioma angiogenesis and elucidated its possible molecular mechanisms. Next, we propose to isolate EVs from glioma cell culture supernatant and patient plasma to identify the types of EVs that contain HOTAIR and further investigate the activities of HOTAIR in endothelial cells.
Acknowledgements
This study was supported in part by research fund for National Natural Science Foundation of China (81602624), Project of Science and Technology Department of Zhejiang Province (2013C37011, 2015C37012), The Science and Technology Creative Activity Plan for University Students in Zhejiang Province (2016R410037).
Disclosure of conflict of interest
None.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J, Cole P, Zimmerman S. Tumor behavior in isolated perfused organs: in vitro growth and metastases of biopsy material in rabbit thyroid and canine intestinal segment. Ann Surg. 1966;164:491–502. doi: 10.1097/00000658-196609000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curry RC, Dahiya S, Alva Venur V, Raizer JJ, Ahluwalia MS. Bevacizumab in high-grade gliomas: past, present, and future. Expert Rev Anticancer Ther. 2015;15:387–397. doi: 10.1586/14737140.2015.1028376. [DOI] [PubMed] [Google Scholar]
- 5.Ding H, Shen J, Yang Y, Che Y. Saw palmetto extract inhibits metastasis and antiangiogenesis through STAT3 signal pathway in glioma cell. Evid Based Complement Alternat Med. 2015;2015:926946. doi: 10.1155/2015/926946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 7.El-Kenawi AE, El-Remessy AB. Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. Br J Pharmacol. 2013;170:712–729. doi: 10.1111/bph.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rak J, Filmus J, Finkenzeller G, Grugel S, Marmé D, Kerbel RS. Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Reviews. 1995;14:263–277. doi: 10.1007/BF00690598. [DOI] [PubMed] [Google Scholar]
- 9.Jiang BH, Liu LZ. AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets. 2008;8:19–26. doi: 10.2174/156800908783497122. [DOI] [PubMed] [Google Scholar]
- 10.Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that downregulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JX, Han L, Bao ZS, Wang YY, Chen LY, Yan W, Yu SZ, Pu PY, Liu N, You YP, Jiang T, Kang CS Chinese Glioma Cooperative Group. HOTAIR, a cell cycle-associated long noncoding RNA and a strong predictor of survival, is preferentially expressed in classical and mesenchymal glioma. Neuro Oncol. 2013;15:1595–1603. doi: 10.1093/neuonc/not131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K, Sun X, Zhou X, Han L, Chen L, Shi Z, Zhang A, Ye M, Wang Q, Liu C, Wei J, Ren Y, Yang J, Zhang J, Pu P, Li M, Kang C. Long noncoding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget. 2015;6:537–546. doi: 10.18632/oncotarget.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke J, Yao YL, Zheng J, Wang P, Liu YH, Ma J, Li Z, Liu XB, Li ZQ, Wang ZH, Xue YX. Knockdown of long non-coding RNA HOTAIR inhibits malignant biological behaviors of human glioma cells via modulation of miR-326. Oncotarget. 2015;6:21934–21949. doi: 10.18632/oncotarget.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Occhipinti G, Giulietti M, Principato G, Piva F. The choice of endogenous controls in exosomal microRNA assessments from biofluids. Tumour Biol. 2016;37:11657–11665. doi: 10.1007/s13277-016-5164-1. [DOI] [PubMed] [Google Scholar]
- 16.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling-in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 17.Fu WM, Lu YF, Hu BG, Liang WC, Zhu X, Yang HD, Li G, Zhang JF. Long noncoding RNA Hotair mediated angiogenesis in nasopharyngeal carcinoma by direct and indirect signaling pathways. Oncotarget. 2016;7:4712–4723. doi: 10.18632/oncotarget.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall L, White RJ. Non-coding RNA production by RNA polymerase III is implicated in cancer. Nat Rev Cancer. 2008;8:911–914. doi: 10.1038/nrc2539. [DOI] [PubMed] [Google Scholar]
- 19.Patel JS, Hu M, Sinha G, Walker ND, Sherman LS, Gallagher A, Rameshwar P. Non-coding RNA as mediators in microenvironment-breast cancer cell communication. Cancer Lett. 2016;380:289–295. doi: 10.1016/j.canlet.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi T, Gao G, Cao Y. Long noncoding RNAs as novel biomarkers have a promising future in cancer diagnostics. Dis Markers. 2016;2016:9085195. doi: 10.1155/2016/9085195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia P, Cai H, Liu X, Chen J, Ma J, Wang P, Liu Y, Zheng J, Xue Y. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016;381:359–369. doi: 10.1016/j.canlet.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Zhang X, Qi L, Cai Y, Yang P, Xuan G, Jiang Y. HULC long noncoding RNA silencing suppresses angiogenesis by regulating ESM-1 via the PI3K/Akt/mTOR signaling pathway in human gliomas. Oncotarget. 2016;7:14429–14440. doi: 10.18632/oncotarget.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cea V, Sala C, Verpelli C. Antiangiogenic therapy for glioma. J Signal Transduct. 2012;2012:483040. doi: 10.1155/2012/483040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxiainducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- 26.Tan C, Cruet-Hennequart S, Troussard A, Fazli L, Costello P, Sutton K, Wheeler J, Gleave M, Sanghera J, Dedhar S. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang N, Dai B, Liu M, Sawaya R, Xie K, Huang S. FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells. Cancer Res. 2008;68:8733–8742. doi: 10.1158/0008-5472.CAN-08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ido K, Nakagawa T, Sakuma T, Takeuchi H, Sato K, Kubota T. Expression of vascular endothelial growth factor-A and mRNA stability factor HuR in human astrocytic tumors. Neuropathology. 2008;28:604–611. doi: 10.1111/j.1440-1789.2008.00926.x. [DOI] [PubMed] [Google Scholar]
- 29.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 32.Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, Grau GE. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23:1643–1649. doi: 10.1038/leu.2009.76. [DOI] [PubMed] [Google Scholar]
- 33.Lv MM, Zhu XY, Chen WX, Zhong SL, Hu Q, Ma TF, Zhang J, Chen L, Tang JH, Zhao JH. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 2014;35:10773–10779. doi: 10.1007/s13277-014-2377-z. [DOI] [PubMed] [Google Scholar]
- 34.Chen WX, Cai YQ, Lv MM, Chen L, Zhong SL, Ma TF, Zhao JH, Tang JH. Exosomes from docetaxel-resistant breast cancer cells alter chemosensitivity by delivering microRNAs. Tumour Biol. 2014;35:9649–9659. doi: 10.1007/s13277-014-2242-0. [DOI] [PubMed] [Google Scholar]
- 35.Giusti I, D’Ascenzo S, Millimaggi D, Taraboletti G, Carta G, Franceschini N, Pavan A, Dolo V. Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles. Neoplasia. 2008;10:481–488. doi: 10.1593/neo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 38.Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J, Kim JH, Choi DS, Kim YK, Hwang D, Gho YS. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, Ferrara N. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shifrin DA Jr, Demory Beckler M, Coffey RJ, Tyska MJ. Extracellular vesicles: communication, coercion, and conditioning. Mol Biol Cell. 2013;24:1253–1259. doi: 10.1091/mbc.E12-08-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44:11–19. doi: 10.1007/s12020-012-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gezer U, Özgür E, Cetinkaya M, Isin M, Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int. 2014;38:1076–1079. doi: 10.1002/cbin.10301. [DOI] [PubMed] [Google Scholar]
- 44.Godlewski J, Krichevsky AM, Johnson MD, Chiocca EA, Bronisz A. Belonging to a network--microRNAs, extracellular vesicles, and the glioblastoma microenvironment. Neuro Oncol. 2015;17:652–662. doi: 10.1093/neuonc/nou292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van der Vos KE, Balaj L, Skog J, Breakefield XO. Brain tumor microvesicles: insights into intercellular communication in the nervous system. Cell Mol Neurobiol. 2011;31:949–959. doi: 10.1007/s10571-011-9697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chistiakov DA, Chekhonin VP. Extracellular vesicles shed by glioma cells: pathogenic role and clinical value. Tumour Biol. 2014;35:8425–8438. doi: 10.1007/s13277-014-2262-9. [DOI] [PubMed] [Google Scholar]


