Abstract
One of the features for pancreatic cancer is that it is often resistant to chemotherapy treatment, which is one of the major hindrances in the treatment of this malignancy. Previous studies indicated that the microRNAs (miRNAs) could mediate resistance of tumor cells to chemotherapy drug in the cancer progression. In the present study, we are aimed to examine whether microRNA-429 was involved in mediating the chemo-resistance of pancreatic cancer cells to gemcitabine. Firstly, a gemcitabine-resistant pancreatic cancer cell line (SW1990/GZ) derived from cell line (SW1990) was constructed and found to possess a decreased expression of miR-429 when it is compared to the original cell line. Ectopic expression of miR-429 in SW1990/GZ increased the cellular sensibility to the treatment of gemcitabine, which is coincided with increased expression of PDCD4. As a tumor suppressor, we found that PDCD4 knockdown in SW1990/GZ cells increased its own chemo-resistance to GZ, which indicates PDCD4 also play a regulative role on the GZ-resistance in the pancreatic cancer. To further confirm the function of miR-429 and PDCD4 in gemcitabine-resistant pancreatic cancer, a xenograft nude mouse model was utilized to examine whether miR-429 can restore treatment response of gemcitabine in gemcitabine-resistant xenografts, while protein levels of PDCD4 were up-regulated. Together with those results, these findings collectively provided that miR-429 could enhancer GZ sensitivity via regulation of PDCD4 expression in pancreatic cancer cells, which may offer a novel therapeutic target for the chemotherapy resistance in pancreatic cancer.
Keywords: Pancreatic cancer, chemoresistance, gemcitabine, miR-429, PDCD4
Introduction
Pancreatic cancer, a highly aggressive human cancer, is the sixth cause of cancer-related motality in China [1]. As its early symptoms are not obvious, patients with pancreatic cancer are found more often when they are in the late stage of the disease, and most patients could not accept surgical resection [1,2]. Chemotherapy is the main treatment for the advanced stage of pancreatic cancer [3]. Gemcitabine (GZ) is widely used in the clinical practice as it is one of the first-line chemotherapy drugs for pancreatic cancer [3]. However, due to the reason that the severity of the primary and acquired resistance to gemcitabine in pancreatic cancer, the survival rate of patients treated with gemcitabine was not significantly improved [4]. Thus, the molecular mechanisms of GZ resistance still needs further elucidation and await more investigation.
As a class of regulatory small noncoding RNAs, recent findings indicate that numerous microRNAs (miRNAs) participate in the development and progression of various malignancies [5-8]. Furthermore, miRNAs are also widely reported to be involved in the regulation of chemo-resistance in malignancies [9]. Recently evidence suggests that miR-429 has been deregulated and play a role of tumor suppression in some types of cancer [10]. Moreover, recent reports also implies that miR-429 has been participating in chemoresistance of many tumors, including ovarian cancer [11], colorectal cancer [12]. However, the function of miR-429 in pancreatic cancer remains to be illustrated.
Based on the findings, we hypothesized that miR-429 may also participate in the regulation of chemotherapeutic resistance via its regulation of the PDCD4, and the present study investigated the role of miR-429 and PDCD4 in GZ-resistant pancreatic cancer cells.
Materials and methods
Ethical statement
All protocols were approved by the Institutional Animal Care and Use Committee of Anhui Medical University. All experiments performed followed the guidelines of the Guide for the Care and Use of Laboratory Animals published by the Anhui Province.
Cell culture
SW1990, a cell line of human pancreatic cancer was used in this study and cultured in RPMI-1640 medium (Wisent Inc., St-Bruno, QC, Canada) supplemented with 10% fetal bovine serum (FBS) at 37°C under a humidified atmosphere containing 5% CO2. The parental SW1990 cells were made resistant to GZ by serially escalating doses of gemcitabine. The GZ-resistant cell line was maintained in the presence of GZ (100 μM).
Quantitative real-time PCR (qRT-PCR)
The total RNA was isolated using TRIzol reagent (Invitrogen, CA, USA) from the cells or tissues. For miR-429 and PDCD4 mRNA, qRT-PCR was performed using miRscript SYBR Green PCR Kit and SYBR Green PCR Kit (Takara) according to the manufacturer’s protocol, respectively. Real-time quantitative PCR analysis was performed using a 7500 Real-Time PCR system (Thermo Fisher Scientific, Applied Biosystems). The housekeeping genes GAPDH and U6 were used as endogenous controls for the PCR experiments of mRNA and miRNA, respectively. Primers used for qPCR amplification were as follows [13]: PDCD4 forward, 5’-GGG AGG AGG AAT CGG ACA G-3’, and reverse, 5’-TAT GTT GGG AGG CGT GGC-3’; GAPDH forward, 5’-TGT GGG CAT CAA TGG ATT TGG-3’, reverse, 5’-ACA CCA TGT ATT CCG GGT CAA T-3’; miR-429 forward, 5’-CGC GGA TCC AGG ACC CGG AGG CCA CCC A-3’, reverse, 5’-GGA ATT CGA CCG GGC GGC TTT GCA CTG ATG AG-3’; U6 forward, 5’-TGC GGG TGC TCG CTT CGG CAG C-3’, reverse, 5’-CCA GTG CAG GGT CCG AGG T-3’.
Western blotting analysis
The total tumor cells or tissues were homogenized and the total protein was extracted by RIPA buffer. And then the extraction and detection of the proteins was performed by Western blotting. Antibodies to PDCD4 (1:1000 dilution, Abcam, Cambridge, USA) and GAPDH (1:5000 dilution, Bioscience Co., Ltd. Beijing, China) were incubated with the membranes overnight at 4°C. After three washes, the membranes were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Protein expression was assessed by Super-Signal West Pico chemiluminescent substrate (Pierce, Rockford, USA) and densitometry of the bands was quantified by using Quantity One software 4.6.2.
RNA interference
For PDCD4 gene knockdown, a set of human PDCD4 shRNA was purchased from Origene (TG302573, Rockville, MD, USA) to express short hairpin RNA (shRNA) targeting PDCD4 mRNA and shRNA containing scrambled sequence were used as control. Lenti-virion was produced in transfected 293FT packaging cells as described previously.
Plasmid construction and luciferase assay
The pmirGLO vectors containing wild-type or mutant miR-429-binding site of PDCD4 3’UTR were synthesized by Ribobio (Guangzhou, China). Luciferase activity was examined using a dual luciferase reporter assay system (Promega, USA). Briefly, 1 μg of firefly luciferase reporter plasmid, 0.5 μg of β-galactosidase expression vector (Promega, Madison, WI, USA), and equal amounts (200 pmol) of pre-miR-429 or scramble shRNA were co-transfected into cells in 24-well plates using lipofectamine 2000. The β-galactosidase vector was used as a transfection control. After 48 h, the cells were collected and luciferase activity was assessed using a Dual-Luciferase Reporter Assay kit (Promega Corporation).
Cell proliferation assays
The cells were transferred in a 96-well plate at 5×103 cells per well. MTT was added to the cells. After another 4 h, the plate was centrifuged and the precipitate was dissolved in DMSO. The absorbance at 490 nm was measured using the Quant Universal Microplate Spectrophotometer. The IC50 of each drug was calculated according to the standard curve. All experiments were performed at least three times.
In vivo tumor xenograft studies
Thirty adult male nude mice, weighing 16-18 g, were provided by Shanghai Slac Laboratory Animal Ltd (Shanghai, China). Nude mice were housed with free access to food and water under a natural day/night cycle. Rats were acclimated for 7 days before any experimental procedures. All experimental protocols were in accordance with the Institutional Animal Care and Use Committee of Anhui Medical University. SW1990/GZ cells transfected with mimics of miR-429 or with control oligonucleotides were injected into the right flank of each mouse subcutaneously, each animal was treated using 5×106 cells. The mice were also treated with twice weekly 125 mg/kg GZ by intraperitoneally (i.p.) for 28 days combined with intratumoral injection of saline (GZ plus saline group), scramble (GZ plus scramble group) and pre-miR-429 (GZ plus pre-miR-429 group) when the average tumor size reached approximately 100 mm3. And then the tumor size was monitored every 3 days by measuring the length and width via vernier calipers, and the tumor volume was calculated as V= 0.5 × L × W2, where L represents the length and W represents the width of tumor. At the end of experiments, the mice were euthanized and the tumor lesions were dissected form the animal.
Statistics
All the experiments were performed in triplicate. Quantitative values were expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed by one-way ANOVA using GraphPad Prism software (San Diego, CA); and p values less than 0.05 were considered significant.
Results
MiR-429 was suppressed and PDCD4 was down-regulated in GZ resistant SW1990 Cells
Firstly, we constructed a GZ-resistant SW1990 cell line (SW1990/GZ). As illustrated in Figure 1A, the level of PDCD4 protein Supplementary Figures 2A and 3B was markedly decreased in comparison with the parental SW1990 (P<0.01, student t-test). Meanwhile, mRNA level of PDCD4 was determined and found to be down-regulated in the SW1990/GZ (Figure 1B, P<0.01, student t-test). To further study the role of miRNAs, we also measured the mRNA expression of miR-429. As a result, the level of miR-429 was significantly down-regulated in SW1990/GZ cells compared with the parental SW1990 cells (Figure 1C, P<0.01, student t-test). Moreover, based on the result of the MTT assays, the IC50 of parental SW1990 and SW1990/GZ were 4.46 ± 0.67 μM and 218.49 ± 24.77 μM, indicating that the tolerance to GZ of SW1990/GZ cells was approximately fifty times higher than that of the parental cells (Figure 1D, P<0.01, student t-test). Thus, the results also suggest the intrinsic interaction of the role of miR-429 and its potential target PDCD4 in GZ-resistance of pancreatic cancer cells.
Figure 1.
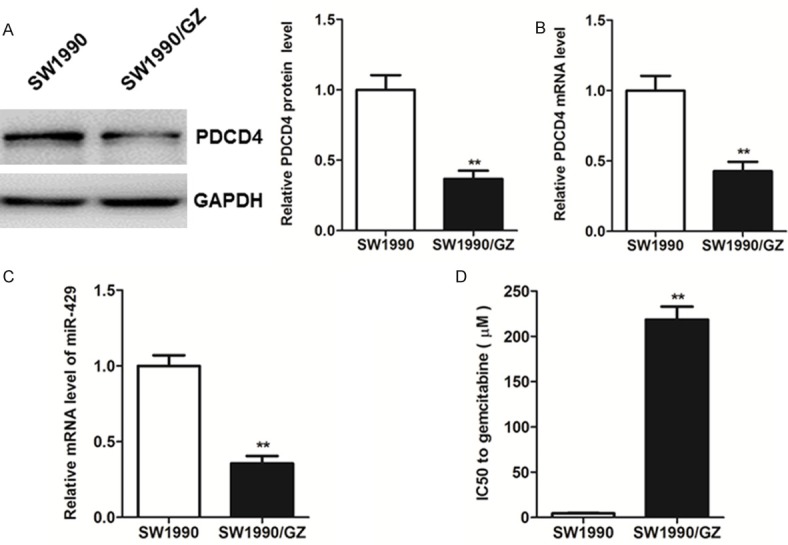
Western blot (A) and real-time PCR (B) analysis of the expression levels of PDCD4 in SW1990 and SW1990/GZ cells. (C) Real-time PCR analysis of the relative expression of miR-429 normalized to U6 RNA in SW1990 and SW1990/GZ cells. (D) The IC50 of gemcitabine-resistant pancreatic cancer cells SW1990/GZ and the parental pancreatic cancer cell line SW1990 for gemcitabine. Each bar represents the mean ± SEM. The results shown were repeated in three independent experiments. **P<0.01, student t-test.
MiR-429 sensitized gemcitabine response in GZ-resistant pancreatic cancer cell sub-line SW1990/GZ via its direct regulation of PDCD4 expression
To understand the functional role of miR-429 in the gemcitabine resistance in pancreatic cancer cells, the pre-miR-429 and scramble shRNA were transfected into SW1990/GZ cells. The miR-429 expression in SW1990/GZ transfected with pre-miR-429 and scramble shRNA was determined by qRT-PCR. As demonstrated in Figure 2A, the up-regulated expression of miR-429 in SW1990/GZ cells overexpressing pre-miR-429 transiently is associated with a remarkable increase in the protein level of PDCD4 Supplementary Figures 2B and 3A when compared with that in the scramble control cells. Interestingly, as a result, SW1990/GZ cells transfected with the pre-miR-429 decreased the IC50 for gemcitabine compared with the scramble (Figure 2C, P<0.01, student t-test).
Figure 2.
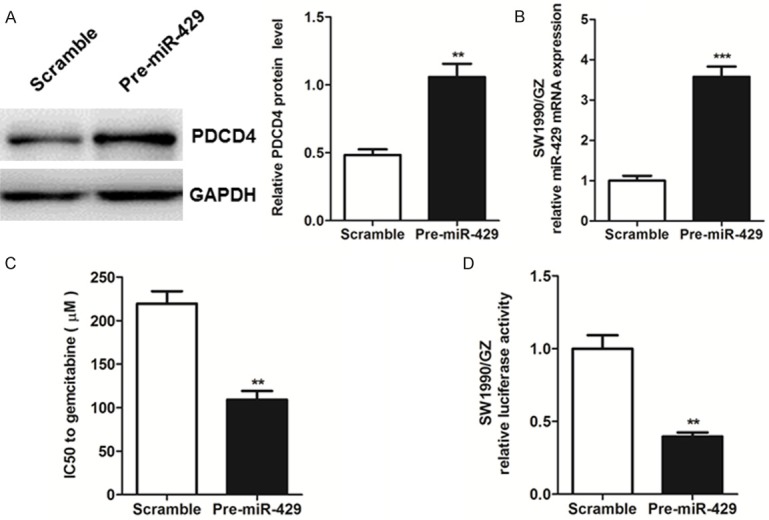
A, B. SW1990/GZ cells were transfected with the precursor oligonucleotides (pre-miR-429 and scramble). PDCD4 protein levels were determined by western blot analysis of total lysate and miR-429 levels were detected by real-time PCR d on 48 h after transfection. C. The IC50 of gemcitabine-resistant pancreatic cancer cells SW1990/GZ after transfected with the precursor oligonucleotides (pre-miR-429 and scramble) for gemcitabine. D. Effect of miR-429 expression vector on the luciferase activities of PDCD4 3’-UTR in SW1990/GZ cells. Freshly luciferase reporters containing PDCD4 3’-UTR were co-transfected into SW1990/GZ cells with the indicated precursor oligonucleotides. Dual luciferase activity was determined 48 h after transfection. Each bar represents the mean ± SEM. The results shown were repeated in three independent experiments. **P<0.01, ***P<0.001, student t-test.
Moreover, to examine the relationship between miR-429 and PDCD4, the dual luciferase assay was utilized to elucidate that transduction of pre-miR-429 in SW1990/GZ could inhibit gene promoter luciferase activities of PDCD4 3’-UTR (Figure 2D, P<0.01, student t-test). All the evidence in the present data demonstrates that miR-429 plays an important role in GZ resistance in pancreatic cancer by targeting PDCD4.
Knockdown of PDCD4 in SW1990/GZ inhibited its gemcitabine sensitivity
To further validate whether PDCD4 plays a role in the exogenous expression of miR-429 induced sensitivity to gemcitabine in SW1990/GZ, the functional evaluation of PDCD4 gene knockdown was executed. As shown in Figure 3, the expression level of PDCD4 was determined by western blotting Supplementary Figures 1A and 4A and qRT-PCR in the group transfected with PDCD4 specific shRNA, the scramble shRNA and control respectively. Interestingly, PDCD4 shRNA increased the tumor cells’ sensitivity to GZ in SW1990/GZ cells, which was proved by the result that IC50 was significantly higher than that in SW1990/GZ transduced with shScramble as well as in the parental cells (Figure 3C, P<0.01, one-way ANOVA). Those results suggested that PDCD4 effects a vital role in the development of GZ resistance in pancreatic cancer cells SW1990.
Figure 3.

The protein and mRNA expression levels of PDCD4 in SW1990/GZ cells infected with lentivirus expressing either shPDCD4 or scramble shRNA for 72 h were measured by (A) western blot and real-time PCR analysis (B) respectively. (C) The IC50 of gemcitabine-resistant pancreatic cancer cells SW1990/GZ infected with lentivirus expressing either shPDCD4 or scramble shRNA for 72 h for gemcitabine. Each bar represents the mean ± SEM. The results shown were repeated in three independent experiments. **P<0.01, one-way ANOVA.
MiR-429 suppressed SW1990 cells derived xenograft tumor growth in the presence of GZ in vivo
To confirm whether overexpression miR-429 could alleviate chemosensitivity of pancreatic cancer cells to gemcitabine resistance, a nude mouse xenograft model was used in this study. As a result, pre-miR-429 could significantly enhance the inhibition effect of gemcitabine and restrain the tumor size in the nude mice (Figure 4A, P<0.01, one-way ANOVA). To further explore its molecular mechanisms, we also performed analysis qRT-PCR and found that higher level miR-429 and PDCD4 was expressed in tumor xenografts delivered with pre-miR-429 (Figure 4B, 4C, P<0.01, one-way ANOVA). Meanwhile, the protein expression of PDCD4 (Supplementary Figures 1B and 4B) was coincided well with the mRNA level, which illustrated a significantly increased expression in the pre-miR-429 treatment group (Figure 4D, P<0.01, one-way ANOVA).
Figure 4.
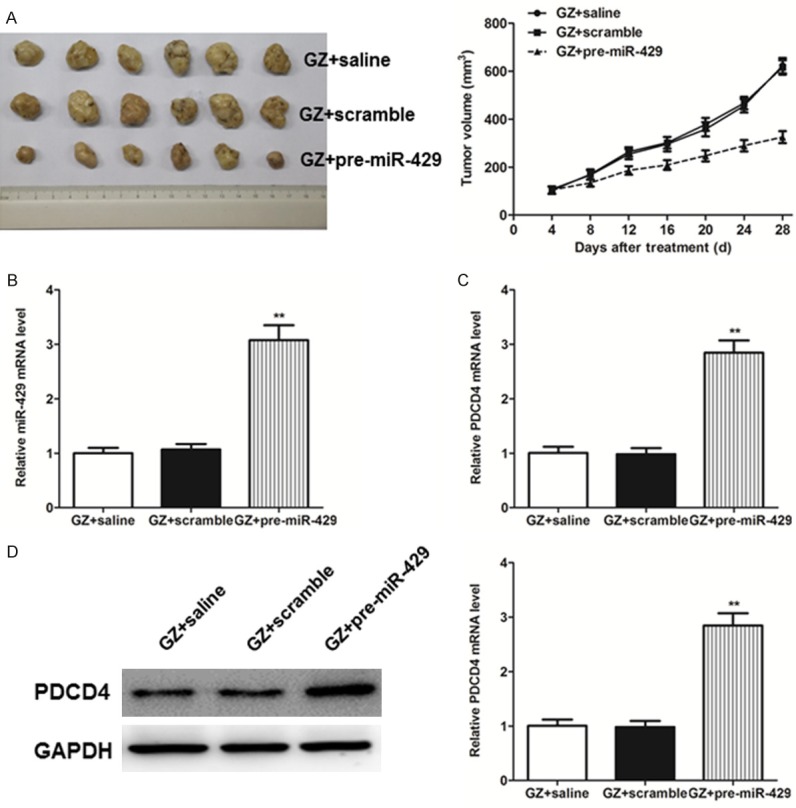
(A) Tumor growth curve in the nude mice treated with gemcitabine intraperitoneally (i.p.) (125 mg/kg, twice weekly) combined with intratumoral injection of pre-miR-429, scramble or saline for four weeks. (B) Expressions levels of miR-429 were detected by real-time PCR analysis in tumor tissues. Expression level of PDCD4 in tumor tissues were determined by real-time PCR (C) and western blot analysis (D), respectively. Each bar represents the mean ± SEM. All the results shown were repeated in three independent experiments. **P<0.01, one-way ANOVA.
Discussion
Pancreatic cancer is a highly malignant digestive system tumor, chemotherapy is the one of its main therapies [14]. As the main chemotherapy drug for pancreatic cancer, the role of gemcitabine in improving the prognosis of patients with pancreatic cancer is not obvious due to the presence of primary and acquired resistance [15-17]. Therefore, it is of great clinical significance to explore the mechanism of gemcitabine acquired resistance.
Recently, extensive studies have demonstrated that the deregulation of miRNAs has been frequently observed in cell proliferation, differentiation, apoptosis, metastasis and drug resistance [18-22]. To date, several miRNAs targeted corresponding genes display have been found to be associated with chemotherapy resistance [23]. Based on these findings, the identification of resistance relevant miRNA and its target gene may be a potential therapeutic strategy for chemoresistance. The present study identified that miR-429 was overexpressed 2.1-fold in the GZ-resistant SW1990/GZ cells compared with its parental cell line, and the subsequent qRT-PCR experiment confirmed this result. Moreover, endogenous miR-429 could reverse the resistance of human pancreatic cancer cell line to gemcitabine by MTT assays. In the vitro animal model, overexpression miR-429 could enhance the inhibition of gemcitabine on tumor growth.
As one of the target of miR-429, overexpression PDCD4 has been reported to increase the sensitivity to CDDP and paclitaxel in human prostate cancer [24]. Consistent with this finding, the present study demonstrated that PDCD4 is a target of miR-429 and that it plays a role in GZ resistance in the pancreatic cancer SW1990 cell line. Furthermore, overexpression of PDCD4 could significantly confer its gemcitabine sensitivity in pancreatic cancer cell lines such as SW1990/GZ with gemcitabine-resistance in vitro and in vivo. Thus, the experiment results provided direct evidences that the miR-429/PDCD4 axis might be a novel therapeutic target for conquering gemcitabine resistance in current pancreatic cancer treatment.
In summary, the present study demonstrated that miR-429 was a tumor suppressor in pancreatic cancer, its overexpression could sensitize pancreatic cancer cells to GZ. Meanwhile, PDCD4 could mediate miR-429-induced chemo-resistance to gemcitabine in SW1990 cells. The miR-429/PDCD4 axis may potentially be used as novel approach of the chemotherapy response and may help us develop potential therapies against pancreatic cancer.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Ohuchida K, Mizumoto K, Kayashima T, Fujita H, Moriyama T, Ohtsuka T, Ueda J, Nagai E, Hashizume M, Tanaka M. MicroRNA expression as a predictive marker for gemcitabine response after surgical resection of pancreatic cancer. Ann Surg Oncol. 2011;18:2381–2387. doi: 10.1245/s10434-011-1602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kipps E, Young K, Starling N. Liposomal irinotecan in gemcitabine-refractory metastatic pancreatic cancer: efficacy, safety and place in therapy. Ther Adv Med Oncol. 2017;9:159–170. doi: 10.1177/1758834016688816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Wu X, Liu N, Li X, Meng F, Song S. Silencing of ATF2 inhibits growth of pancreatic cancer cells and enhances sensitivity to chemotherapy. Cell Biol Int. 2017;41:599–610. doi: 10.1002/cbin.10760. [DOI] [PubMed] [Google Scholar]
- 5.Vogel A, Rommler-Zehrer J, Li JS, McGovern D, Romano A, Stahl M. Efficacy and safety profile of nab-paclitaxel plus gemcitabine in patients with metastatic pancreatic cancer treated to disease progression: a subanalysis from a phase 3 trial (MPACT) BMC Cancer. 2016;16:817. doi: 10.1186/s12885-016-2798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Xue M, Xu J, Qin X. MiR-301a is involved in adipocyte dysfunction during obesityrelated inflammation via suppression of PPARgamma. Pharmazie. 2016;71:84–88. [PubMed] [Google Scholar]
- 7.Oliveto S, Mancino M, Manfrini N, Biffo S. Role of microRNAs in translation regulation and cancer. World J Biol Chem. 2017;8:45–56. doi: 10.4331/wjbc.v8.i1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang Z, Xi Y. MicroRNAs mediate therapeutic and preventive effects of natural agents in breast cancer. Chin J Nat Med. 2016;14:881–887. doi: 10.1016/S1875-5364(17)30012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu N, Li J, Zhao Z, Han J, Jiang T, Chen Y, Hou N, Huang C. MicroRNA-302a enhances 5-fluorouracil-induced cell death in human colon cancer cells. Oncol Rep. 2017;37:631–639. doi: 10.3892/or.2016.5237. [DOI] [PubMed] [Google Scholar]
- 10.Han Y, Zhao Q, Zhou J, Shi R. miR-429 mediates tumor growth and metastasis in colorectal cancer. Am J Cancer Res. 2017;7:218–233. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Chen H, Xia B, Liu T, Lin M, Lou G. KIAA0101, a target gene of miR-429, enhances migration and chemoresistance of epithelial ovarian cancer cells. Cancer Cell Int. 2016;16:74. doi: 10.1186/s12935-016-0353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong SJ, Cai XJ, Li SJ. The clinical significance of MiR-429 as a predictive biomarker in colorectal cancer patients receiving 5-fluorouracil treatment. Med Sci Monit. 2016;22:3352–3361. doi: 10.12659/MSM.900674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Bian Y, Zhao S, Kong F, Li X. Suppression of PDCD4 mediated by the long noncoding RNA HOTAIR inhibits the proliferation and invasion of glioma cells. Oncol Lett. 2016;12:5170–5176. doi: 10.3892/ol.2016.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andren-Sandberg A. Pancreatic cancer: chemotherapy and radiotherapy. N Am J Med Sci. 2011;3:1–12. doi: 10.4297/najms.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoneyama H, Takizawa-Hashimoto A, Takeuchi O, Watanabe Y, Atsuda K, Asanuma F, Yamada Y, Suzuki Y. Acquired resistance to gemcitabine and cross-resistance in human pancreatic cancer clones. Anticancer Drugs. 2015;26:90–100. doi: 10.1097/CAD.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 16.Todaka A, Umehara R, Sasaki K, Serizawa M, Urakami K, Kusuhara M, Yamaguchi K, Yasui H. Metabolic profiling of gemcitabine- and paclitaxel-treated immortalized human pancreatic cell lines with K-RASG12D. Biomed Res. 2017;38:29–40. doi: 10.2220/biomedres.38.29. [DOI] [PubMed] [Google Scholar]
- 17.Aoki T, Matsushita H, Hoshikawa M, Hasegawa K, Kokudo N, Kakimi K. Adjuvant combination therapy with gemcitabine and autologous gammadelta T-cell transfer in patients with curatively resected pancreatic cancer. Cytotherapy. 2017;19:473–485. doi: 10.1016/j.jcyt.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Liu K, Liu S, Zhang W, Jia B, Tan L, Jin Z, Liu Y. miR-494 promotes cell proliferation, migration and invasion, and increased sorafenib resistance in hepatocellular carcinoma by targeting PTEN. Oncol Rep. 2015;34:1003–1010. doi: 10.3892/or.2015.4030. [DOI] [PubMed] [Google Scholar]
- 19.Meng W, Tai Y, Zhao H, Fu B, Zhang T, Liu W, Li H, Yang Y, Zhang Q, Feng Y, Chen G. Downregulation of miR-33a-5p in hepatocellular carcinoma: a possible mechanism for chemotherapy resistance. Med Sci Monit. 2017;23:1295–1304. doi: 10.12659/MSM.902692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin CZ, Lou XY, Lv QL, Cheng L, Wu NY, Hu L, Zhou HH. MicroRNA-184 acts as a potential diagnostic and prognostic marker in epithelial ovarian cancer and regulates cell proliferation, apoptosis and inflammation. Pharmazie. 2015;70:668–673. [PubMed] [Google Scholar]
- 21.Guo Y, Pang Y, Gao X, Zhao M, Zhang X, Zhang H, Xuan B, Wang Y. MicroRNA-137 chemosensitizes colon cancer cells to the chemotherapeutic drug oxaliplatin (OXA) by targeting YBX1. Cancer Biomark. 2017;18:1–9. doi: 10.3233/CBM-160650. [DOI] [PubMed] [Google Scholar]
- 22.Ning ZQ, Lu HL, Chen C, Wang L, Cai W, Li Y, Cao TH, Zhu J, Shu YQ, Shen H. MicroRNA-30e reduces cell growth and enhances drug sensitivity to gefitinib in lung carcinoma. Oncotarget. 2017;8:4572–4581. doi: 10.18632/oncotarget.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R, Li X, He B, Hu W. MicroRNA-410 regulates autophagy-related gene ATG16L1 expression and enhances chemosensitivity via autophagy inhibition in osteosarcoma. Mol Med Rep. 2017;15:1326–1334. doi: 10.3892/mmr.2017.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Xu H, Shen H, Li H. microRNA-106a modulates cisplatin sensitivity by targeting PDCD4 in human ovarian cancer cells. Oncol Lett. 2014;7:183–188. doi: 10.3892/ol.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


