Abstract
Mesenchymal stem cells (MSCs) provide promising applications for clinical treatments. However, patients often take medications that affect the viability of transplanted MSCs. The aim of this study was to assess the effects and underlying mechanism of action of aspirin on the proliferation of MSCs. We showed that aspirin inhibited the growth of MSCs in a concentration- and time-dependent manner. Analysis of cell-cycle distributions showed significantly increased cell populations in the G0/G1 phase and decreased cell populations in the S phase and G2/M phase with increasing concentrations of aspirin. We further analyzed the expression of cyclins and found that the level of cyclin D1 was significantly reduced after aspirin treatment, while there was no obvious effect on the levels of cyclin A2 and cyclin E1. Because we showed that the expression of miRNA145 was significantly increased after aspirin treatment, we further transfected MSCs with an miRNA145 mimic or miRNA145 inhibitor. Transfection with the miRNA145 mimic resulted in decreased expression of cyclin D1, while transfection with miRNA145 inhibitor resulted in increased expression of cyclin D1. Transfection with miRNA145 inhibitor abolished the downregulation of cyclin D induced by aspirin. The results suggested that aspirin inhibited the proliferation of MSCs and caused cell-cycle arrest in the G0/G1 phase through downregulation of cyclin D1, which could be related to the increased expression of miRNA145.
Keywords: Mesenchymal stem cell, aspirin, proliferation, cyclin
Introduction
Researchers have investigated the use of mesenchymal stem cells (MSCs) to treat many human diseases, such as bone defects, cartilage deterioration, and cardiovascular diseases [1-3]. Our previous study showed that MSCs were promising candidates for cartilage repair [2]. To achieve good restorative results, cell-based therapy must maintain the viability of transplanted MSCs at the target site; however, patients who are candidates for MSC therapy often use medications that may influence the viability of MSCs.
Aspirin, a nonsteroidal anti-inflammatory drug (NSAID), is one of the most widely used medications for treatment of pain, fever, and inflammation. Given its anti-thrombotic properties, aspirin has also been recommended for the prevention of cardiovascular diseases [4]. In recent years, studies have indicated that aspirin inhibits the growth of cancer cells [5,6]. Similarly, NSAIDs may have side effects on physiological processes by inhibiting cell growth. For example, NSAIDs have been reported to inhibit bone repair, formation, and remodeling in vivo, which may be related to the suppression of osteoblast proliferation [7]. Thus, it is important to determine whether aspirin has adverse effects on the proliferation of MSCs.
MicroRNAs (miRNAs) are a class of endogenous small noncoding RNAs that cause translational repression and negative regulation of protein expression through binding to the 3’-untranslated regions (3’-UTRs) of target mRNAs [8]. Evidence suggests that miRNAs play important roles in the regulation of cell proliferation, apoptosis, and differentiation [9,10]. miRNA145 is involved in regulating proliferation and the cell cycle. Its expression is low in proliferating cells and increases in less proliferating cells [11]. Several studies have reported that miRNA145 can inhibit the growth of cancer cells [12,13]. miRNA145 is expressed in embryonic stem cells and that it regulated the expression of OCT4 and SOX2 [14].
In the present study, we demonstrated that aspirin influenced the proliferation and cell cycle of MSCs. Our results suggest that miRNA145 is involved in inhibition of proliferation and cell-cycle arrest caused by aspirin via decreased expression of cyclin D1.
Materials and methods
Cell isolation and culture
Synovial tissue was harvested from primary knee osteoarthritis patients undergoing total knee arthroplasty. Written informed consent was obtained from patients before the use of their tissues. The research protocol was approved by the Ethics Committee at Zhongshan Hospital, Fudan University. All cell separation and identification procedures are described in our previous study [15]. The isolated cells were positive for CD29, CD44, and CD90, but negative for CD45. The osteogenic and chondrogenic differentiation potentials of isolated cells were also confirmed.
Cell proliferation and colony formation assay
For proliferation assays, cells were plated in 96-well plates at a density of 5,000 cells/well. After 12 hours, the medium was replaced with Dulbecco’s Modified Eagle’s Medium (DMEM) containing various concentrations of aspirin (Sigma-Aldrich, St. Louis, MO, USA) [5,16]. According to the manufacturer’s instructions, cell proliferation was assessed by the Cell Counting Kit-8 (CCK-8) assay (Beyotime, Jiangsu, China). For colony formation, cells were plated in 6-well plates at a density of 1,000 cells/well and incubated for 14 days. The cells were fixed with 4% paraformaldehyde for 10 min and stained with 0.5% crystal violet for 5 min prior to being photographed. Cell colonies with a diameter larger than 50 µm were counted using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Cell-cycle analysis
Cells were trypsinized, washed with phosphate-buffered saline (PBS), and fixed with -20°C precooled 70% ethanol overnight at 4°C. After the cells were washed, 100 µL RNase A was added and incubated for 30 min at 37°C, then 400 µL propidium iodide (PI) stain was added and incubated for 30 min at 4°C. The stained cells were examined using flow cytometry (BD FACSAriaTM II; BD Biosciences, San Jose, CA, USA) at an excitation wavelength of 488 nm. Data were processed using FlowJo software (FlowJo, Inc., Ashland, OR, USA).
Bioinformatics predictions of miRNA
The nucleotide sequence of the cyclin D1 3’-UTR was obtained from the NCBI database. The miRNA databases, including TargetScan, PicTar, miRanda, DIANA, miRBase, and miRTarBase were used to predict the miRNA targeting the 3’-UTR of cyclin D1.
MiRNA145 mimic or inhibitor transfection
Cells were plated in 6-well plates at a density of 1×105 cells/mL, incubated overnight, and transfected with 50 nM miRNA145 mimics, 150 nM miRNA145 inhibitors, or control miRNAs of the same concentrations (RiboBio, Guangzhou, China) according to the manufacturer’s instructions. After 24 h, the cells were harvested for the following experiments.
Western blot analysis
Cell lysates were extracted using radioimmunoprecipitation assay buffer (RIPA) (Beyotime) containing PMSF and phosphatase inhibitor (Roche, Basel, Switzerland) at 4°C. The extracts were prepared using Cytoplasmic Extraction Reagents (Pierce Biotechnology, Waltham, MA, USA). Protein samples were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% bovine serum albumin (BSA) for 2 h, washed, and then incubated with primary antibodies for cyclin D1, cyclin E1, cyclin A2, and tubulin (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. After washing, the membranes were incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and detected using enhanced chemiluminescence (Pierce). The protein expressions were quantified using densitometry with ImageJ software and normalized to the relative tubulin expression. All experiments were performed in triplicate.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells using Trizol® reagent (Invitrogen, Waltham, MA, USA). For the cyclin D1 mRNA, cDNA was synthesized using the Prime Script RT Reagent Kit (Takara, Shiga, Japan) and qRT-PCR analyses were performed by using SYBR® Premix Ex TaqTM (Clontech, Mountain View, CA, USA). The sense and antisense primers used were: cyclin D1, 5’-CTACTACCGCCTCACACGCTT-3’ and 5’-GGCTTGACTCCAGGGCT-3’; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5’-GCACCGTCAAGGCTGAGAAC-3’ and 5’-TGGTGAAGAACGCCA GTGGA-3’. For miRNA analysis, the first-strand cDNAs were synthesized using the reverse transcriptase with miRNAs-specific stem-loop primer (RiboBio). Quantitative PCR amplification was conducted with a real-time fluorescent measurement system (ABI7500; Thermo Fisher Scientific, Scotts Valley, CA, USA) using the Bulge-LoopTM miRNA qRT-PCR Starter Kit (RiboBio) according to the protocol provided. The relative expression of miRNA was normalized to that of endogenous control U6 by the 2-ΔΔCt method. All experiments were performed in triplicate.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc, La Jolla, CA, USA). All data were expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to assess group differences. P values < 0.05 were considered statistically significant.
Results
Aspirin inhibited the growth of MSCs
MSCs were treated with various concentrations of aspirin for different periods of time. The aspirin exhibited concentration- and time-dependent inhibitory effects on the proliferation of MSCs (Figure 1A). Growth inhibition was also observed with colony formation, and the number of colony forming units was significantly decreased using 1 mM and 2.5 mM aspirin (Figure 1B).
Figure 1.
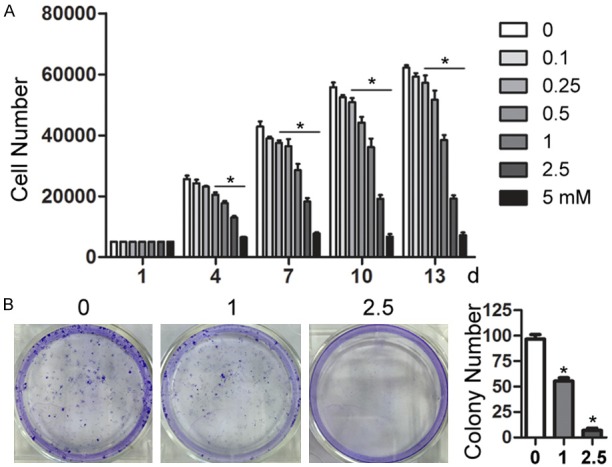
Aspirin inhibited the growth of MSCs. A. Mesenchymal stem cells (MSCs) were incubated with various concentrations of aspirin (0-5 mM) for the indicated time periods, followed by Cell Counting Kit-8 (CCK-8) assay analysis. B. MSCs were incubated with aspirin (0, 1, and 2.5 mM) for 14 days and cell colonies were stained with crystal violet. Cell colonies with a diameter larger than 50 µm were counted using ImageJ software. All experiments were performed three times. *P < 0.05 vs. control.
Aspirin-induced G0/G1 arrest
The proliferation inhibition of aspirin could be due to cell-cycle arrest; therefore, cell-cycle analysis was conducted using flow cytometry. Cell-cycle distribution analysis showed significantly increased cell populations in the G0/G1 phase and decreased cell populations in the S phase and G2/M phase, with increasing concentrations of aspirin (Figure 2). These results suggested that the growth inhibition with aspirin treatment might be associated with its ability to induce cell growth arrest in the G0/G1 phase.
Figure 2.
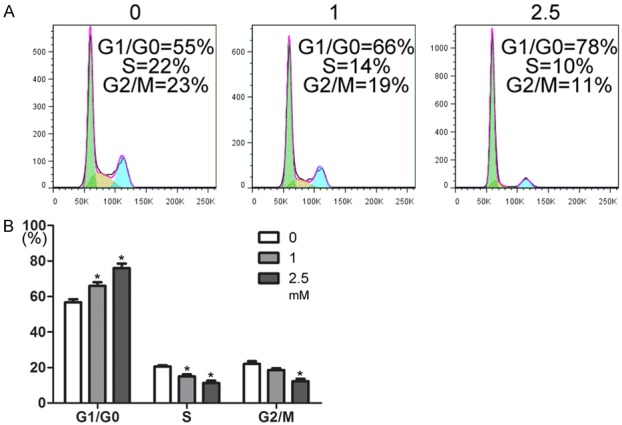
Cell-cycle analysis after aspirin treatment. A. Representative data show the distribution of propidium iodide (PI) stained cells in each stage of the cell cycle (G1/G0, green; S, yellow; and G2/M, cyan). B. The plot shows the percentage of MSCs in each phase after incubation with aspirin (0, 1, or 2.5 mM) for 2 days. *P < 0.05 vs. control.
Aspirin caused the downregulation of cyclin D1 expression
To understand the mechanism of the cell-cycle arrest, the expression levels of cell cycle regulatory proteins were analyzed by western blots. As shown in Figure 3A, aspirin treatment specifically inhibited expression of cyclin D1, while there was no obvious change observed in the expression of cyclin A2 and cyclin E1 under the same experimental conditions. Decreased expression of cyclin D1 was concentration- and time-dependent. qRT-PCR was performed to determine the cyclin D1 mRNA expression levels. Treatment with aspirin also resulted in significant (*P < 0.05) reduction of cyclin D1 mRNA (Figure 3B).
Figure 3.
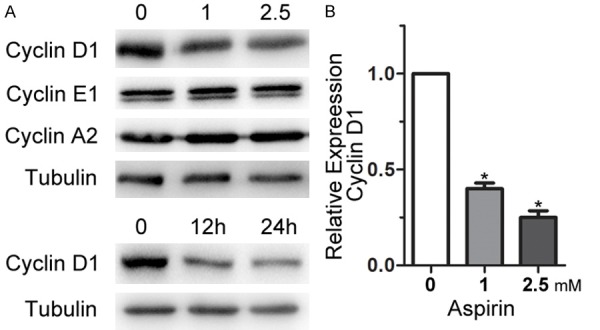
Effect of aspirin on cyclin expression levels. A. MSCs were incubated with two different doses (1 or 2.5 mM) of aspirin for 24 h, or incubated with 1 mM aspirin for two different durations (12 or 24 h). Western blot analysis show cyclin expression. B. Cyclin D1 mRNA levels were detected by qRT-PCR after MSCs were incubated with 1 mM or 2.5 mM aspirin for 12 h. The relative gene expression was normalized to GAPDH and calculated by the 2-ΔΔCt method with the control designated as a value of 1. *P < 0.05 vs. control.
MiRNA145 mediated the proliferation inhibition of aspirin
Because miRNA145 may play an important role in regulating cell proliferation and the cell cycle, we determined whether miRNA145 mediated the aspirin-induced reduction in MSC proliferation. For this purpose, six databases were queried to predict whether miRNA145 targeted the 3’-UTR of cyclin D1. The results identified three databases, specifically miRanda, TargetScan, and DIANA, that predicted the mRNA and miRNA interactions, and two databases (miRBase and miRTarBase) predicted mRNA and miRNA interactions based on reported next generation sequencing (NGS) results. A reverse analysis was also performed to verify whether cyclin D1 might be a target of miR-NA145. Then, we determined if miRNA145 mediated the aspirin-induced reduction in cell proliferation. We found that aspirin treatment significantly increased miRNA145 expression in a concentration- and time-dependent manner (Figure 4A). To further determine whether miRNA145 was involved in aspirin-induced growth inhibition, an miRNA145 mimic and inhibitor were used to transfect MSCs. As shown in Figure 4B, transfection with the miRNA145 mimic significantly increased miRNA145 expression and resulted in the decreased expression of cyclin D1. Transfection with the miRNA145 inhibitor significantly decreased miRNA145 expression and increased cyclin D1 expression. More importantly, transfection with the miRNA145 inhibitor abolished the aspirin-induced downregulation of cyclin D1 (Figure 4C). These results demonstrated that miRNA145 induced by aspirin was responsible for inhibition of cyclin D1 expression that caused cell arrest in the G0/G1 phase and inhibited the proliferation of MSCs.
Figure 4.
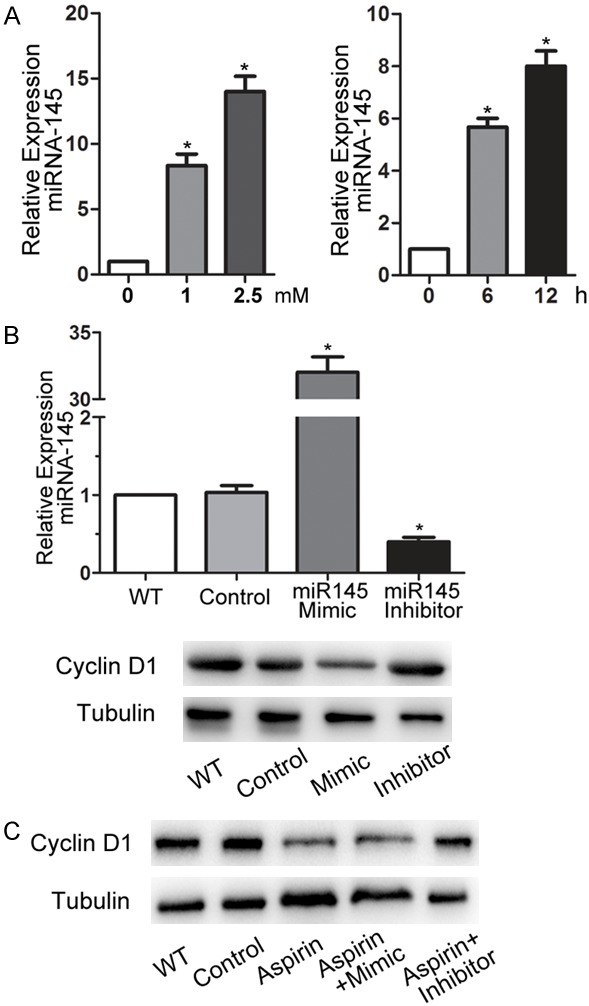
MiRNA145 mediated the downregulation of cyclin D1. A. The relative expression of miRNA145 was normalized to that of U6 and calculated by the 2-ΔΔCt method after mesenchymal stem cells (MSCs) were incubated with two different doses (1 or 2.5 mM) of aspirin for 12 h, or incubated with 1 mM aspirin for two different durations (6 or 12 h). B. miRNA145 and cyclin D1 expression were detected by qRT-PCR and western blot, respectively, after transfection with 50 nM mimics, 150 nM inhibitors, control miRNAs, or no transfection (wild type; WT). C. Cyclin D1 expression in WT, control, 1 mM aspirin treatment alone, and aspirin combined with the miRNA145 mimic- or inhibitor-treated cells.
Discussion
MSCs are a promising source of cells for a wide range of cellular therapies due to their self-renewal capacity, multi-lineage differentiation potentials, paracrine effects, and immunosuppressive properties [17]. However, the viability of transplanted MSCs is a prerequisite for therapeutic outcomes in cell-based regenerative medicine [18]. Although many factors can affect the in vivo growth of transplanted MSCs, medications taken by patients are factors that are relatively easy to control. Aspirin is widely used drug with a variety of indications. Here, we determined that aspirin inhibited the proliferation of MSCs in a concentration- and time-dependent manner in vitro at concentrations used in previous studies [5,16].
Because cell proliferation is closely related to the cell cycle, we further analyzed the cell-cycle distribution and aspirin significantly increased cell populations in the G0/G1 phase. The effects of aspirin on the cell cycle were found to vary according to the cell type. For example, Zong et al. [5] reported that aspirin induced cell-cycle arrest in the G0/G1 phase, while Gao et al. [19] reported that aspirin induced cell-cycle arrest in the G2/M phase. Our results indicated that aspirin treatment induced MSC arrest in the G0/G1 phase.
Cell cycle regulatory proteins play a crucial role in regulating the cell cycle [20]. Therefore, we analyzed the expression of cyclins, a family of proteins that control cell-cycle progression. We found significantly reduced cyclin D1 levels after aspirin treatment, but we found no obvious changes in the levels of cyclin A2 and cyclin E1. Cyclin D1 is required for progression through the G1 phase of the cell cycle [21]. This cyclin forms a complex with cyclin-dependent kinase (CDK) 4 or CDK6, and functions as a regulatory subunit of that complex. The cyclin D1-CDK complex promotes passage through the G1 phase by inhibiting the retinoblastoma tumor suppressor [22]. The downregulation of cyclin D1 after aspirin treatment is consistent with cell arrest in the G0/G1 phase. A recent study reported that aspirin downregulated cyclin A in cancer cells [23]. This may further suggest that different cell types have varied responses to aspirin.
The mechanisms by which aspirin inhibits the growth of cancer cells were widely studied [24]. However, the underlying mechanism of aspirin-induced growth inhibition in MSCs has not been fully elucidated. A previous study reported that aspirin inhibited the proliferation of MSCs through downregulation of the Wnt/β-catenin signal pathway [16]. In this study, we explored the role of miRNA in growth inhibition by aspirin in MSCs. We used bioinformatics to predict miRNAs targeting the 3’-UTR of cyclin D1 and identified miRNA145 as a candidate. miRNA145 is considered a tumor suppressor and inhibits the development of tumors by regulating cell growth; it is downregulated in many types of cancers [11]. miRNA145 has also been reported to inhibit the proliferation of other cells [25,26]. We further found that aspirin significantly increased the expression of miRNA145, and transfection with the miRNA145 inhibitor abolished the downregulation of cyclin D1 induced by aspirin.
In conclusion, we demonstrated that aspirin inhibited the proliferation of synovium-derived MSCs and caused cell arrest in the G0/G1 phase through downregulation of cyclin D1, which was related to the increased expression of miRNA145. The present findings help to advance our understanding of the molecular mechanisms of the anti-proliferative effects of aspirin on MSCs.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81672157 and 81672142).
Disclosure of conflict of interest
None.
References
- 1.Heo SC, Shin WC, Lee MJ, Kim BR, Jang IH, Choi EJ, Lee JS, Kim JH. Periostin accelerates bone healing mediated by human mesenchymal stem cell-embedded hydroxyapatite/tricalcium phosphate scaffold. PLoS One. 2015;10:e0116698. doi: 10.1371/journal.pone.0116698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan WS, Wang YM, Yuan L, Li S, Pan JF, Chen C, Yan ZQ, Guo CG. Enhancing synovial mesenchymal stem cell adhesion and selection via an avidin-biotin-CD105 binding system for cartilage tissue engineering. J Biomater Tissue Eng. 2016;6:27–34. [Google Scholar]
- 3.Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010-2015) Stem Cell Res Ther. 2016;7:82. doi: 10.1186/s13287-016-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stovitz SD, Satin DJ, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276–2277. doi: 10.1001/jama.2016.16748. [DOI] [PubMed] [Google Scholar]
- 5.Zong M, Fan DD, Lin S, Song YP, Wang ZY, Ma XL, Qiu WH, Bai YH, Li L, Li S. Anti-cancer activity and potential mechanism of a novel aspirin derivative. Eur J Pharmacol. 2016;791:137–146. doi: 10.1016/j.ejphar.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Li JH, Wang Y, Xie XY, Yin X, Zhang L, Chen RX, Ren ZG. Aspirin in combination with TACE in treatment of unresectable HCC: a matchedpairs analysis. Am J Cancer Res. 2016;6:2109–2116. [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JK, Li CJ, Wu SC, Yeh CH, Chen CH, Fu YC, Wang GJ, Ho ML. Effects of anti-inflammatory drugs on proliferation, cytotoxicity and osteogenesis in bone marrow mesenchymal stem cells. Biochem Pharmacol. 2007;74:1371–1382. doi: 10.1016/j.bcp.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 8.Ul Hussain M. Micro-RNAs (miRNAs): genomic organisation, biogenesis and mode of action. Cell Tissue Res. 2012;349:405–413. doi: 10.1007/s00441-012-1438-0. [DOI] [PubMed] [Google Scholar]
- 9.Yang B, Guo HF, Zhang YL, Chen L, Ying DJ, Dong SW. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS One. 2011;6:e21679. doi: 10.1371/journal.pone.0021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shenoy A, Blelloch RH. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol. 2014;15:565–576. doi: 10.1038/nrm3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui SY, Wang R, Chen LB. MicroRNA-145: a potent tumour suppressor that regulates multiple cellular pathways. J Cell Mol Med. 2014;18:1913–1926. doi: 10.1111/jcmm.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CY, Tao WY, Ni SB, Chen QY, Zhao ZS, Ma L, Fu YM, Jiao ZX. Tumor-suppressive microRNA-145 induces growth arrest by targeting SENP1 in human prostate cancer cells. Cancer Sci. 2015;106:375–382. doi: 10.1111/cas.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu YF, Li DF, Luo QF, Wei CK, Song HM, Hua KY, Song JL, Luo Y, Li XY, Fang L. MicroRNA-145 inhibits human papillary cancer TPC1 cell proliferation by targeting DUSP6. Int J Clin Exp Med. 2015;8:8590–8598. [PMC free article] [PubMed] [Google Scholar]
- 14.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Fan WS, Li JH, Wang YM, Pan JF, Li S, Zhu L, Guo CA, Yan ZQ. CD105 promotes chondrogenesis of synovium-derived mesenchymal stem cells through Smad2 signaling. Biochem Biophys Res Commun. 2016;474:338–344. doi: 10.1016/j.bbrc.2016.04.101. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Chen X, Zhu WQ, Zhang H, Hu SS, Cong XF. Growth inhibition of mesenchymal stem cells by aspirin: involvement of the wnt/beta-catenin signal pathway. Clin Exp Pharmacol Physiol. 2006;33:696–701. doi: 10.1111/j.1440-1681.2006.04432.x. [DOI] [PubMed] [Google Scholar]
- 17.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7:125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim I, Lee SK, Yoon JI, Kim DE, Kim M, Ha H. Fibrin glue improves the therapeutic effect of MSCs by sustaining survival and paracrine function. Tissue Eng Part A. 2013;19:2373–2381. doi: 10.1089/ten.tea.2012.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao L, Williams JL. Nitric oxide-donating aspirin induces G(2)/M phase cell cycle arrest in human cancer cells by regulating phase transition proteins. Int J Oncol. 2012;41:325–330. doi: 10.3892/ijo.2012.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang XP, Zhou WH, Zhang YF, Liu Y. High expression of PTGR1 promotes NSCLC cell growth via positive regulation of cyclin-dependent protein kinase complex. Biomed Res Int. 2016:5230642. doi: 10.1155/2016/5230642. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Li XS, Gong XH, Chen J, Zhang JH, Sun JH, Guo M. miR-340 inhibits glioblastoma cell proliferation by suppressing CDK6, cyclin-D1 and cyclin-D2. Biochem Biophys Res Commun. 2015;460:670–677. doi: 10.1016/j.bbrc.2015.03.088. [DOI] [PubMed] [Google Scholar]
- 22.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 23.Dachineni R, Ai GQ, Kumar DR, Sadhu S, Tummala H, Gunaje JB. Cyclin A2 and CDK2 as novel targets of aspirin and salicylic acid: a potential role in cancer prevention. Mol Cancer Res. 2016;14:241–252. doi: 10.1158/1541-7786.MCR-15-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Zeng XR, Xu JW, Xu DR, Li JX, Jin HL, Jiang GS, Han XS, Huang CS. Isorhapontigenin suppresses growth of patient-derived glioblastoma spheres through regulating miR-145/SOX2/cyclin D1 axis. Neuro Oncol. 2016;18:830–839. doi: 10.1093/neuonc/nov298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou DD, Wang X, Wang Y, Xiang XJ, Liang ZC, Zhou Y, Xu A, Bi CH, Zhang L. MicroRNA-145 inhibits hepatic stellate cell activation and proliferation by targeting ZEB2 through Wnt/betacatenin pathway. Mol Immunol. 2016;75:151–160. doi: 10.1016/j.molimm.2016.05.018. [DOI] [PubMed] [Google Scholar]


