Abstract
Type 1 diabetes mellitus (T1DM) is an autoimmune disease due to progressive injury of islet cells mediated by T lymphocytes (T cells). Our previous studies have shown that only cathepsin G (CatG), not other proteases, is involved in the antigen presentation of proinsulin, and if the presentation is inhibited, the activation of CD4+ T cells induced by proinsulin is alleviated in T1DM patients, and CatG-specific inhibitor reduces the activation of CD4+ cells induced by proinsulin in T1DM patients. Therefore, we hypothesize that CatG may play an important role in the activation of CD4+ T cells in T1DM. To this end, mouse studies were conducted to demonstrate that CatG impacts the activation of CD4+ T cells in non-obese diabetic (NOD) mice. CatG gene expression and the activation of CD4+ T cells were examined in NOD mice. The effect of CatG inhibitor was investigated in NOD mice on the activation of CD4+ T cells, islet β cell function, islet inflammation and β-cell apoptosis. Furthermore, NOD mice were injected with CatG siRNA in early stage to observe the effect of CatG knockdown on the activation status of CD4+ T cells and the progression of diabetes. During the pathogenesis of diabetes, the expression level of CatG in NOD mice gradually increased and the CD4+ T cells were gradually activated, resulting in more TH1 cells and less TH2 and Treg cells. Treatment with CatG-specific inhibitor reduced the blood glucose level, improved the function of islet β cells and reduced the activation of CD4+ T cells. Early application of CatG siRNA improved the function of islet β cells, reduced islet inflammation and β cell apoptosis, and lowered the activation level of CD4+ T cells, thus slowing down the progression of diabetes.
Keywords: NOD mice, cathepsin G, type 1 diabetes mellitus, CD4+ T cells
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease due to progressive injury of β cells mediated by T lymphocytes (T cells) [1,2]. So far, the molecular mechanisms of pathogenesis of T1DM are still largely unknown. Although numerous efforts have been made to develop effective treatments for T1DM, especially immune therapies, immunotherapy is not available for life-long relief of the disease [3-5]. Therefore, it is of great significance to have better understanding of the pathogenesis of T1DM and identify new and effective therapeutic targets.
Studies have shown that a variety of immune cells are involved in the pathogenesis of T1DM, such as CD4+ and CD8+ T cells and B cells. Among them, CD4+ T cells play a central role [6]. According to the secreted cytokines, CD4+ T cells can be roughly divided into helper T cells (TH), such as TH1, TH2, TH17, regulatory T cells (Treg) and other subsets. Many studies have shown that these cells and their cytokines play an important role in the damage of islet cells, the aggravation of islet inflammation and the occurrence of diabetes. TH1/TH2 cell imbalance is shown to play an important role in the pathogenesis of T1DM and islet cell injury mediated by cell cytokines secreted by TH1 is the cause of T1DM, while cytokines secreted by TH2 and Treg cells can protect β cells [7,8]. Previously, we found that only cathepsin G (CatG), not other proteases, is involved in the antigen presentation of proinsulin, and its inhibitor reduces the proinsulin-induced activation of CD4+ T cells in T1DM patients [9]. Therefore, we speculate that CatG may be closely related to the activation of CD4+ T cells in T1DM patients.
Cathepsins were found in 1920. They are lysosomal protein hydrolases activated in acidic environment. According to the amino acids in the active sites, cathepsins are divided into three categories: serine protease (CatA and G), aspartic protease (CatD and E) and cysteine protease (CatB, C, F, H, K, L, S, V, X and AEP). Studies have found that cathepsins are associated with many diseases [10]. Studies with NOD rats have shown that CatL is essential for developing autoimmune diabetes [11], CatL knockout rats are diabetes-free, CatS or CatB knockout rats had reduced incidence of diabetes [12-14]. Floyel found that CatH can regulate β cell function and disease progression in newly diagnosed T1DM patients [15] and CatK is associated with osteoporosis [16]. Our previous studies have found that patients with T1DM had increased level of CatG [9].
CatG has been studied as a proteolytic enzyme secreted by neutrophils and found to play an important regulatory role in inflammatory response. However, little is known about its involvement in the pathogenesis of autoimmune disease, for example T1DM, as an antigen-processing enzym. Previous studies found that CatG is involved in antigen presenting and presentation of tetanus toxin fragment C, hemagglutinin and myelin basic protein [17,18]. CatG-specific inhibitor blocks the antigen processing and presenting in primary mDC [17]. In addition, we have recently found that CatG is a key protease that is responsible for the processing and presentation of proinsulin and only CatG inhibitor (not other protease inhibitors) reduces the immune response mediated by diabetogenic T cells [9]. Therefore, we speculate that CatG plays an important role in immune pathogenesis of T1DM, antigen presentation is mainly mediated by CatG and CatG is the main effector that activates CD4+ T cells. This study was conducted to demonstrate the hypothesis in vivo for potential treatment of T1DM using CatG inhibitor in the future.
Materials and methods
Animals
NOD mice used in the study were purchased from Slackking experimental animal co., Human, China, and were maintained throughout the experimental period in sterile cages, with four animals each, under controlled temperature and lighting, with free access to filtered water and diet. This study received approval by the Animal Research Ethics Board at the Second Affiliated Hospital of Nanchang University.
Diabates modelling and treatments
NOD mice were fed with standard for about 10 weeks and then measured for blood glucose twice a week. The mice that had two repeated reading of blood glucose of > 16.7 mmol/L were considered diabetes. For CatG inhibitor treatment, diabetes mice were intraperitoneally injected with CatG inhibitor (0.1 mg/animal, Sigma, USA) every other day for 8 weeks. For CatG siRNA treatment, pre-DM mice was intraperitoneally injected with 5 nmol/animal of CatG siRNA twice a week for 8 weeks. The sequences of CatG siRNA and negative control were 5’-CCTGCTCCTGTTGACCTTTATTCTA, and 5’-GGACGAGAATTCATCTATGTCCTGCTCCTAGGTAGATACA, respectively, and all animals were then scarified for analysis at the end of experiments.
Cytometry
Peripheral blood mononuclear cells (PBMCs) and spleen lymphocytes were isolated as described [19] and incubated with fluorescent-labeled anti-mouse monoclonal antibodies against CD3, CD4, CD25, CD127, IFN-γ and IL-4 (BD, USA). The cells were then sorted analyzed on a flow cytometer (FACSCalibur, BD, USA) [20].
qRT-PCR
Total RNA was extracted from PBMCs using TRIzol Reagent (Life Technologies, Carlsbad, CA, USA) according to supplier’s protocols, determined for quantity and quality spectrometrically. RNA was reversely transcripted into cDNA and quantified using real-time qRT-PCR using SYBR Green qPCR SuperMix (Invitrogen, USA) on a Real time PCR instrument (Bio-Rad, USA). Mouse β-actin was used as internal control. The primers used for the PCR reactions were CatG-F: 5’-GTTAGGACGAAGTCTTCTCGC, CatG-R: 5’-CCCTACATG GCATTTCTTCTGAT; β-actin-F: 5’-ATCGTCCACCGTAAATGC; β-actin-R: 5’-TGAAGTGGTAGTCGGGTG. The PCR was carried out in a total volume of 20 μl containing 1.5 μl of diluted and pre-amplified cDNA, 12.5 μl of 2 × ULtraSYBR Mixture and 1 μl of each fluorescence TaqMan probe. The cycling conditions were 50°C for 2 min, 95°C for 10 min followed by 40 cycles, each one consisting of 10 s at 95°C and 1 min at 51.8°C. Samples were run in triplicate and the mean value was calculated for each case.
The data were managed using the Applied Biosystems software RQ Manager v1.2.1. Relative expression was calculated by using comparative Ct method and obtaining the fold change value (2-ΔΔCt) according to previously described protocol [21].
Isolation of islet
Islet was isolated as previously described [22]. Briefly, the mice were anesthetized and celiotomized to expose the pancreas and common bile duct. The bile duct was ligated into the duodenum. 2 ml pre-chilled collagenase P was slowly injected into the pancreas from the bile duct to fully inflate the pancreas. The mice were then scarified by cutting off the heart and the pancreas was isolated and digested in 3 ml pre-chilled collagenase P at 37°C for 17 min. The digests were shaken and added with 10 ml pre-chilled Hanks solution with 10% FBS to stop the digestion. After filtered through a stainless steel mesh of 600 µm, the cells were centrifuged at 1500 rpm for 2 min and the pellet was collected after washing with pre-chilled Hanks solution with 10% FBS.
Islet hematoxylin and eosin (HE) stain
Islet tissues were fixed with 4% paraformaldehyde, embedded in paraffin and sectioned. The slices were stained as described [23] and examined under light microscope.
Apoptosis assay
Islet β cells were isolated and purified from the islet and apoptotic cells were detected through double staining with Annexin V-PI (Annexin V-PI Apoptosis Detection Kit I, BD Biosciences, USA) on a flow cytometer (BD, USA). The quantitation of apoptotic cells was calculated by CellQuest software.
Statistical analysis
All data were expressed as means ± standard error of the mean (SEM) obtained from at least three independent experiments. Statistical comparisons between experimental and control groups were assessed by using the Student’s t-test. P < 0.05 was considered statistically significant.
Results
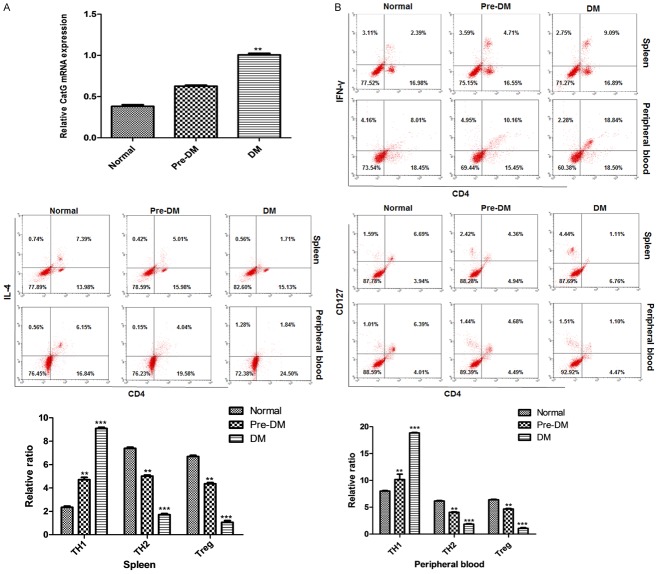
The expression of CatG and the activation of CD4+ T cells were elevated in diabetic NOD mice
Analysis showed that CatG was highly expressed in NOD diabetic mice. Compared with control mice, mice in pre-DM group and DM group had higher CatG level at mRNA (Figure 1A), and higher activation status of CD4+ T cells with significantly more TH1 cells, and less TH2 and Treg cells in both spleen and peripheral blood (Figure 1B). The results showed that the expression of CatG was different in the two groups of mice, resulting in different levels of activation of CD4+ T cells, with increased TH1 cells and reduced TH2 and Treg cells at higher CatG level.
Figure 1.
Gene expression of CatG and the activation of CD4+ T cells in NOD mice. A. mRNA level of CatG; B. Upper panel: cytometry assay of T cells, lower panel: percentage of CD4+ T subsets. ** and *** denote P < 0.01 and 0.001 vs normal.
CatG inhibitor reduced blood glucose level and improved islet β cell function
To investigate whether treatment with CatG-specific inhibitor has effect on blood glucose and islet β cell function, NOD mice were injected with the CatG inhibitor at 12 week. Analysis showed that blood glucose level was reduced and insulin content was increased (Figure 2A, 2B) after treatment with CatG inhibitor for 8 weeks. To further elucidate the protective effect, we analyzed the inflammation of the islets and apoptosis of islet β cells and found that the inflammation of the islets and apoptosis rate of β cells was significantly reduced (Figure 2C, 2D) after CatG inhibitor treatment.
Figure 2.
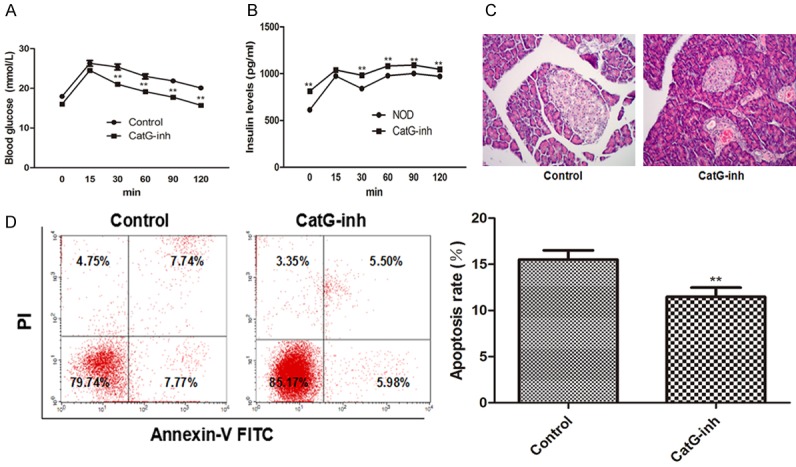
Effect of cathepsin G inhibitor on blood glucose, insulin levle and islet β cell apoptosis in NOD mice. A. Blood glucose level over time; B. Serum insulin level over time, C. Islet H&E staining; D. Flow cytometry assay of apoptotic cells and apoptotic rate. ** Denotes P < 0.01 vs control.
Treatment with CatG inhibitor reduced the activation of CD4+ T cells
We further analyzed the activation of CD4+ T cells after CatG inhibitor treatment and found that the percentage of TH1 was significantly reduced while those of Treg and TH2 cells were significantly increased (Figure 3A) as compared with control. Expression analysis showed that GatG was significantly down-regulated in the mice treated with GatG inhibitor (Figure 3B) as compared with control. These results suggest that CatG inhibitor can lower the activation of CD4+ T cells, thereby reducing the blood glucose and improving islet β cell function.
Figure 3.
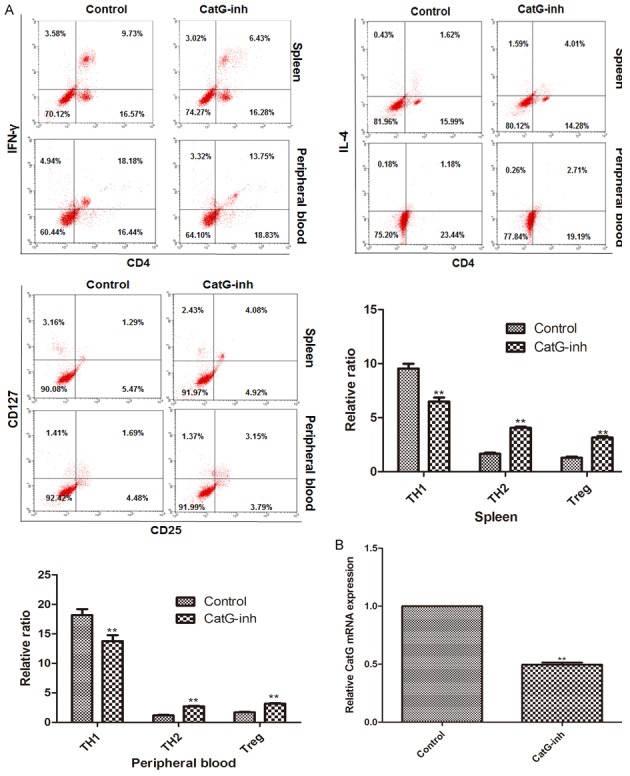
Effect of cathepsin G inhibitor on the activation of CD4+ T cells in NOD mice. A. Cytometry assay of T cells and percentage of CD4+ T subsets in spleen and peripheral blood; B. mRNA level of CatG. **P < 0.01 vs control.
Early use of CatG siRNA protected islet β cell function and delayed the progression of diabetes
To further elucidate the role of CatG in the pathogenesis of T1DM, we downregulated the expression of CatG in NOD mice using CatG siRNA in the pre-DM stage. It was found that compared with control, the expression of CatG was significantly down-regulated after CatG siRNA treatment (Figure 4A). In comparison with control, mice in the pre-DM-CatG siRNA group had significantly lower blood glucose level, higher insulin level, less inflammation and less apoptotic islet β cells (Figure 4B-E) at 16 weeks, suggesting that early application of CatG siRNA can protect islet β cell function, reduce islet β cell apoptosis, thus delaying the progression of diabetes. As a result, the mice in CatG siRNA group did not develop diabetes at 16 weeks. These results indicate that CatG plays an important role in the pathogenesis of T1DM, and the early down-regulation of GatG can delay the progression of diabetes.
Figure 4.
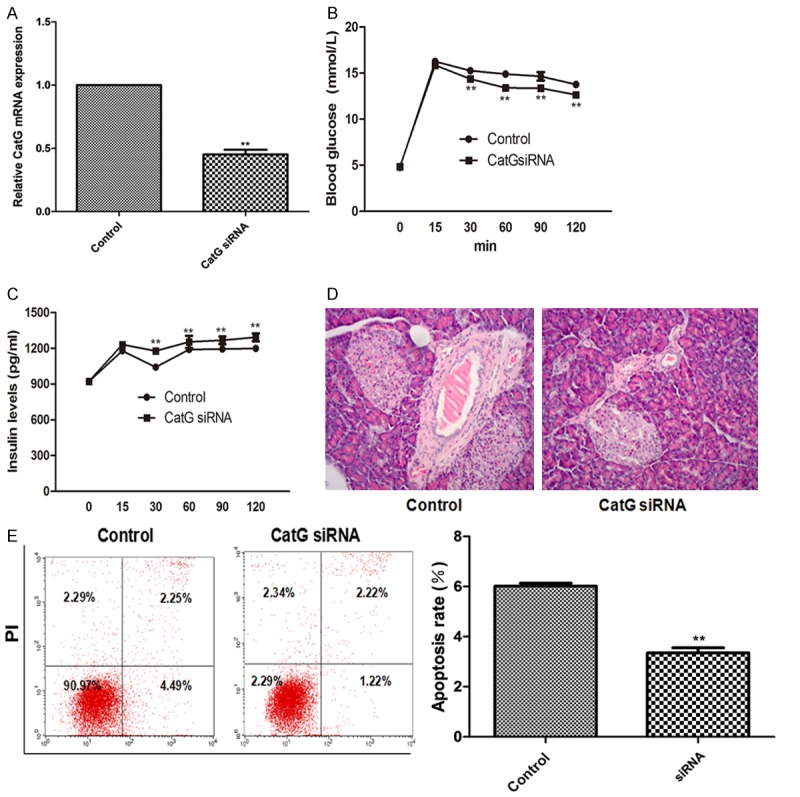
Effect of CatG siRNA on the blood glucose, insulin levels and β cell apoptosis in NOD mice. A. Relative GatG mRNA level; B and C. Blood glucose and insulin levels over time; D. Islet H&E staining; E. Flow cytometry assay and percentage of apoptosis. ** Denotes P < 0.01 vs control.
Down-regulation of CatG in pre-DM reduced the activation of CD4+ T cells
Similarly, we found that after 8 weeks of CatG siRNA injection, in addition to reduced blood glucose level, increased insulin level and reduced apoptosis in islet β cells, the percentage of TH1 cells was significantly decreased while those of TH2 and Treg cells were significantly increased as compared with control (Figure 5). These results suggest that CatG may be an important target for the treatment of T1DM.
Figure 5.
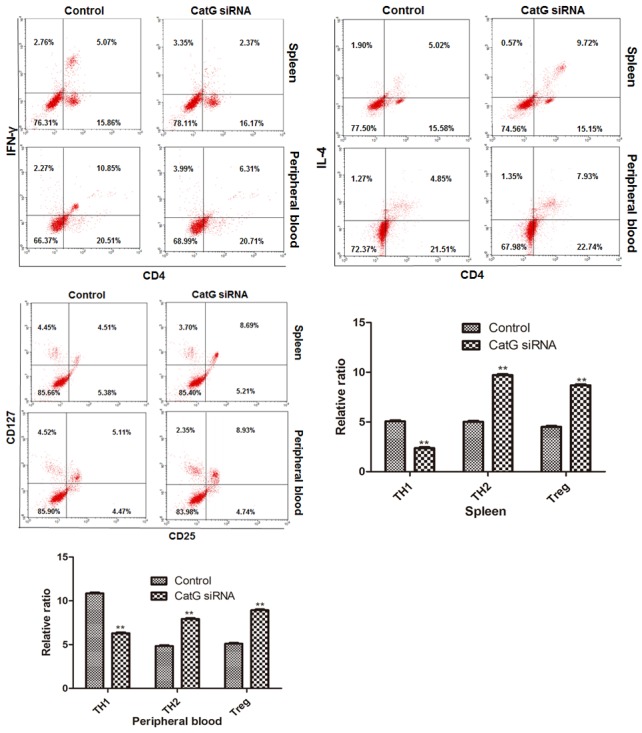
Effect of CatG siRNA treatment on the activation of CD4+ T cells in pre-DM NOD mice. Upper panel: cytometry assay of T cells, lower panel: percentage of CD4+ T subsets in spleen and peripheral blood. ** Denotes P < 0.01 vs control.
Discussion
In the pathogenesis of T1DM, autoantigens, proteolytic enzymes and antigen presenting cells (APC) are indispensable. During the process, autoantigens are degraded by cathepsins into antigen fragments, which then bind to major histocompatibility complex (MHC) molecule II in APC to be presented to T and CD4+ cells to induce their activation. This also triggers the initiation of T1DM [24]. However, it has been unclear how the autoantigens promote the evolution of T cells into diabetogenic T cells (DTLs) to mediate autoimmune response and induce cell injury. Due to the existence of multiple autoantigens in islet cell, immunosuppressive therapies targeting the autoantigens have not been able to neutralize all the antigens, while single antigen-targeted therapies are not effective enough to prevent T1DM [5,25]. Therefore, it is of great significance to deliberate how the autoantigens are degraded into antigen fragments and which cathepsins are involved in the degradation. This would result in identification of new therapeutic targets for T1DM.
In this study, we found that the expression of CatG in NOD mice was increased, and CD4+ T cells were in the state of abnormal activation. Studies have shown that CatG may be involved in the autoimmune response via the apoptotic pathway, while the deprivation of CatG reduces apoptosis [26]. When CatG was downregulated, the apoptosis of pancreatic islet β cells was significantly decreased, thus reducing the damage to pancreatic islets. Our results showed that CatG inhibitor alleviates T1DM and down-regulates the expression of CatG and reduces the apoptosis of islet β cells. Furthermore, we showed that the down-regulation of CatG has an impact on the activation of CD4+ T cells, resulting in decreased TH1 cells and increased TH2 and Treg cells. Therefore, it is likely that higher CatG may damage islet β cells.
To further demonstrate that the alleviation of T1DM by the down-regulation of CatG is achieved through impacting the activation of CD4+ T cells, we profiled CD4+ T subsets in the spleen and peripheral blood and found that once CatG was knockdown in pre-DM NOD mice, the proportion of TH1 cells decreased while those of TH2 and Treg cells increased. These results suggest that the prevention of T1DM due to the downregulation of CatG might also be associated with the activation of CD4+ T cells. Deficiency of CatL was found to increase the number of Treg cell [13], suggesting that cathepsin is involved in the activation of CD4+ T cells. Therefore, we speculate that CatG plays important role in the activation of CD4+ T cells in autoimmune diabetes of mice. This is probably due to its involvement of degradation of islet autoantigens, resulting in the conversion of T cells into DTLs that trigger the pathogenesis of diabetes.
The target of CatG siRNA may be peripheral T cells. When the atelocollagen changes, siRNA can bind it to form a nanoparticle complex of 100-300 nm in diameter. The complex is stable, resistant to nucleases and other enzymes. In addition, CatG siRNA was found to specifically reduce the mRNA level of CatG as compared with control. To further clarify the relationship between CatG and CD4+ T cell activation in NOD mice, we used CatG siRNA in pre-DM mice to knockdown CatG expression and found that the down-regulation of CatG could not only alleviate but also prevent T1DM in NOD mice and that the down-regulation reduced the activation of CD4+ T cells. These results further demonstrate that CatG is involved in the pathogenesis of TIDM and down-regulation of CatG in pre-DM stage could inhibit the actiactivation of CD4+ T cells, leading to reduced blood glucose level and protection of islet function to delay or block the pathogenesis of diabetes. TH1, TH2 and Treg cells are also important for the maintenance of immune homeostasis and prevention of T1DM [15]. Compared with the control, an increase in TH1 cells and decrease in TH2 and Treg cells were observed during the progression of T1DM in NOD mice. These data suggest that therapeutic effect of CatG on autoimmune diabetes is likely dependent on the activation of CD4+ T cells. Therefore, the use of CatG inhibitors or CatG siRNA targeting CD4+ T cells is expected to be a further therapeutic target for the prevention and treatment of T1DM, although in vivo works are needed to verify this.
Taking together, our study has demonstrated that CatG plays a very important role in the activation of CD4+ T cells in T1DM. CatG inhibitor or CatG siRNA can mitigate the activation of CD4+ T cells in autoimmune diabetic NOD mice, leading to reduced blood glucose and improved function of islet β cells. Therefore, CatG may be a new therapeutic target for autoimmune diabetes although more in vivo studies are needed for clinical use.
Acknowledgements
The work was supported by National Natural Science Foundation (grant no. 81400815) and Jiangxi Provincial Department of Science and Technology, China (grant no. 20142BAB215011).
Disclosure of conflict of interest
None.
References
- 1.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Roep BO, Tree TI. Immune modulation in humans: implications for type 1 diabetes mellitus. Nat Rev Endocrinol. 2014;10:229–242. doi: 10.1038/nrendo.2014.2. [DOI] [PubMed] [Google Scholar]
- 3.Lernmark A, Larsson HE. Immune therapy in type 1 diabetes mellitus. Nat Rev Endocrinol. 2013;9:92–103. doi: 10.1038/nrendo.2012.237. [DOI] [PubMed] [Google Scholar]
- 4.Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, Sanda S, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS Type 1 Diabetes TrialNet Canakinumab Study Group; Pickersgill L, de Koning E, Ziegler AG, Boehm B, Badenhoop K, Schloot N, Bak JF, Pozzilli P, Mauricio D, Donath MY, Castano L, Wagner A, Lervang HH, Perrild H, Mandrup-Poulsen T AIDA Study Group. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludvigsson J, Krisky D, Casas R, Battelino T, Castano L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ, Veeze HJ, Palmer J, Samuelsson U, Elding Larsson H, Aman J, Kardell G, Neiderud Helsingborg J, Lundstrom G, Albinsson E, Carlsson A, Nordvall M, Fors H, Arvidsson CG, Edvardson S, Hanas R, Larsson K, Rathsman B, Forsgren H, Desaix H, Forsander G, Nilsson NO, Akesson CG, Keskinen P, Veijola R, Talvitie T, Raile K, Kapellen T, Burger W, Neu A, Engelsberger I, Heidtmann B, Bechtold S, Leslie D, Chiarelli F, Cicognani A, Chiumello G, Cerutti F, Zuccotti GV, Gomez Gila A, Rica I, Barrio R, Clemente M, Lopez Garcia MJ, Rodriguez M, Gonzalez I, Lopez JP, Oyarzabal M, Reeser HM, Nuboer R, Stouthart P, Bratina N, Bratanic N, de Kerdanet M, Weill J, Ser N, Barat P, Bertrand AM, Carel JC, Reynaud R, Coutant R, Baron S. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 6.Sarikonda G, Pettus J, Phatak S, Sachithanantham S, Miller JF, Wesley JD, Cadag E, Chae J, Ganesan L, Mallios R, Edelman S, Peters B, von Herrath M. CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J Autoimmun. 2014;50:77–82. doi: 10.1016/j.jaut.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 8.Haskins K, Cooke A. CD4 T cells and their antigens in the pathogenesis of autoimmune diabetes. Curr Opin Immunol. 2011;23:739–745. doi: 10.1016/j.coi.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou F, Schafer N, Palesch D, Brucken R, Beck A, Sienczyk M, Kalbacher H, Sun Z, Boehm BO, Burster T. Regulation of cathepsin G reduces the activation of proinsulin-reactive T cells from type 1 diabetes patients. PLoS One. 2011;6:e22815. doi: 10.1371/journal.pone.0022815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Ma L, Yang J, Ren A, Sun Z, Yan G, Sun J, Fu H, Xu W, Hu C, Shi GP. Increased serum cathepsin S in patients with atherosclerosis and diabetes. Atherosclerosis. 2006;186:411–419. doi: 10.1016/j.atherosclerosis.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Jobs E, Ingelsson E, Riserus U, Nerpin E, Jobs M, Sundstrom J, Basu S, Larsson A, Lind L, Arnlov J. Association between serum cathepsin S and mortality in older adults. JAMA. 2011;306:1113–1121. doi: 10.1001/jama.2011.1246. [DOI] [PubMed] [Google Scholar]
- 12.Yamada A, Ishimaru N, Arakaki R, Katunuma N, Hayashi Y. Cathepsin L inhibition prevents murine autoimmune diabetes via suppression of CD8(+) T cell activity. PLoS One. 2010;5:e12894. doi: 10.1371/journal.pone.0012894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maehr R, Mintern JD, Herman AE, Lennon-Dumenil AM, Mathis D, Benoist C, Ploegh HL. Cathepsin L is essential for onset of autoimmune diabetes in NOD mice. J Clin Invest. 2005;115:2934–2943. doi: 10.1172/JCI25485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsing LC, Kirk EA, McMillen TS, Hsiao SH, Caldwell M, Houston B, Rudensky AY, LeBoeuf RC. Roles for cathepsins S, L, and B in insulitis and diabetes in the NOD mouse. J Autoimmun. 2010;34:96–104. doi: 10.1016/j.jaut.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floyel T, Brorsson C, Nielsen LB, Miani M, Bang-Berthelsen CH, Friedrichsen M, Overgaard AJ, Berchtold LA, Wiberg A, Poulsen P, Hansen L, Rosinger S, Boehm BO, Ram R, Nguyen Q, Mehta M, Morahan G, Concannon P, Bergholdt R, Nielsen JH, Reinheckel T, von Herrath M, Vaag A, Eizirik DL, Mortensen HB, Storling J, Pociot F. CTSH regulates beta-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc Natl Acad Sci U S A. 2014;111:10305–10310. doi: 10.1073/pnas.1402571111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol. 2011;7:447–456. doi: 10.1038/nrrheum.2011.77. [DOI] [PubMed] [Google Scholar]
- 17.Reich M, Lesner A, Legowska A, Sienczyk M, Oleksyszyn J, Boehm BO, Burster T. Application of specific cell permeable cathepsin G inhibitors resulted in reduced antigen processing in primary dendritic cells. Mol Immunol. 2009;46:2994–2999. doi: 10.1016/j.molimm.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Burster T, Beck A, Tolosa E, Marin-Esteban V, Rotzschke O, Falk K, Lautwein A, Reich M, Brandenburg J, Schwarz G, Wiendl H, Melms A, Lehmann R, Stevanovic S, Kalbacher H, Driessen C. Cathepsin G, and not the asparagine-specific endoprotease, controls the processing of myelin basic protein in lysosomes from human B lymphocytes. J Immunol. 2004;172:5495–5503. doi: 10.4049/jimmunol.172.9.5495. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, He Y, Niu Y. Effects of supernatant of mouse endometrial cells on proliferation and secretion activity of mouse spleen lymphocyte and goat PBMC. Journal of Northwest A & F University. 2010;23:123–127. [Google Scholar]
- 20.Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180:6411–6420. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4986. pdb prot4986. [DOI] [PubMed] [Google Scholar]
- 24.Durinovic-Bello I. Autoimmune diabetes: the role of T cells, MHC molecules and autoantigens. Autoimmunity. 1998;27:159–177. doi: 10.3109/08916939809003864. [DOI] [PubMed] [Google Scholar]
- 25.Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB, Monzavi R, Moran A, Orban T, Palmer JP, Raskin P, Rodriguez H, Schatz D, Wilson DM, Krischer JP, Skyler JS Type 1 Diabetes TrialNet GAD Study Group. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–327. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter K, Steinwede K, Aly S, Reinheckel T, Bohling J, Maus UA, Ehlers S. Cathepsin G in experimental tuberculosis: relevance for antibacterial protection and potential for immunotherapy. J Immunol. 2015;195:3325–3333. doi: 10.4049/jimmunol.1501012. [DOI] [PubMed] [Google Scholar]