Abstract
T-helper 17 (Th17) cells produce Interleukin-17 (IL-17) that plays an important role in host-defense. However, little is known whether aging affects the functions of human Th17 cells. In this study, we examine age-associated alteration in Th17-cell response; correlation between Th17-cells and endothelial cell senescence; and the occurrence of acute cerebral infarction (ACI). First, we examined Th17-frequency, phenotyping, key transcription factors, and relevant cytokines in healthy elderly, middle-aged and young-people along with elderly-patients with ACI. We detected levels of endothelial cell senescence markers in mRNA and inflammatory biomarker in serum among the groups. Correlations of Th17 frequency to levels of cytokines and endothelial cell senescence biomarkers have been analyzed. Finally, effects of IL-17 on endothelial cell senescence were explored in vitro. Our study demonstrated that healthy elderly-people have an increased Th17 frequency, RORγt expression and Th17 related cytokines (IL-17, IL-6) levels in peripheral blood compared to healthy middle-aged and young-people. Furthermore, elderly-ACI patients also have an increased Th17 expression as compared to healthy elderly-people. There was no significant difference in levels of memory Th17 frequency among the 4 groups, indicating that IL-17 is mainly produced by memory CD4+ T cells. There were no significant correlations between Th17 frequencies, levels of cytokines, inflammatory biomarkers in serum and endothelial cell senescence biomarkers in mRNA. Cell experiments about human umbilical vein endothelial cells (HUVECs) co-culture with IL-17 demonstrated that IL-17 promotes endothelial cell senescence which is closely related to ACI occurrence. Our results suggested that aging and ACI occurrence strengthen Th17-cell response. Th17/IL-17 may promote endothelial cell senescence, subsequently contributing to ACI occurrence in humans.
Keywords: Th17 cells, IL-17, endothelial cell, senescence, acute cerebral infarction, atherosclerosis
Introduction
T helper 17 (Th17) cell, an identified subset of CD4+ T cells, exerts an important effect on the pathogenesis of many experimental autoimmune diseases and human inflammatory conditions by secreting the pro-inflammatory cytokine IL-17 [1,2]. IL-17 potently induces production of IL-8 and G-CSF from epithelial cells and fibroblasts, leading to activation and recruitment of neutrophils [3].
Aging is a complex process of life that affects many aspects of human biology, including the immune system [4]. Age-related declines in immune function leave elderly individuals more susceptibility to infectious diseases or tumors resulting in an increase in morbidity and mortality.
Acute cerebral Infarction (ACI) is a leading cause of death and permanent disability in the elderly worldwide. ACI occurs as a consequence of atherosclerotic plaque erosion or rupture in which process, the Th17 cells play an important role [5]. In this study, we explore the views on age-associated alteration in Th17 cell response and the correlation between Th17 cells and endothelial cell senescence as well as ACI occurrence.
Materials and methods
Human subjects
Healthy elderly (age ≥ 65, n = 34), middle-aged (age ≥ 45 and ≤ 64, n = 33), and young subjects (age ≤ 44, n = 30) were recruited for this study (mean age ± SD, 70.4 ± 5.6, 52.8 ± 7.1 and 33.9 ± 6.9). Elderly patients with atherosclerotic cerebral infarction (ACI) (21 males and 15 females; mean age, 73.5 ± 6.9 years, n = 36). The diagnosis was based on a modification of the TOAST criteria based on the clinical, radiographic, and diagnostic information available [6].
There was no gender difference between the three groups (P > 0.05 by Chi-square test). No individuals were treated with anti-inflammatory drugs and/or immunosuppressive agents. None had any disease potentially affecting the immune system including autoimmune diseases, infectious diseases, malignancy, diabetes, asthma, other inflammatory disease, or chronic-immune-mediated disorders.
The study conformed to protocols approved by the institutional review boards of Anhui Provincial Hospital and conformed to the declaration of Helsinki. This study was cross-sectional and blinded.
Blood samples
We collected 5-10 ml of peripheral blood (PB) from all the subjects, in a fasting state, on the morning of physical examination or following admission. All samples were treated with sodium heparin and examined within 4 h. PB mononuclear cells (PBMCs) were prepared by Ficoll density gradient for analysis of flow cytometry (FCM) and reverse transcription-polymerase chain reaction (RT-PCR). Serum was obtained after centrifugation and was stored at -80°C until further use.
Cell separation and flow cytometry
For the analysis of Th17 cells, PBMCs were suspended at a density of 2.0 × 106 cells/ml in complete culture medium (Gibco BRL, USA). Cultures were stimulated with phorbolmyristate acetate (PMA, 25 ng/ml) plus ionomycin (1 μg/ml) for 4 h, in the presence of monensin (1.7 μg/ml, all from Alexis Biochemicals, San Diego, CA, USA). Cells were incubated at 37°C under a 5% CO2 environment.
Detection of Th17 cells and phenotyping
For Th17 analysis, the cells were incubated with FITC-conjugated anti-CD4 (13B8.2 clone Beckman Coulter-Immunotech, Marseille, France), APC-conjugated anti-CD45RO (UCHL1 clone, eBioscience, San Diego, CA, USA) or CD45RA (HI100 clone, eBioscience) at 4°C for 15 min and then stained with PE-conjugated anti-IL-17A (ebio64DEC17 clone, eBioscience) after fixation and permeabilization with the Fix/Perm reagent (Beckman Coulter-Immunotech) according to the manufacturer’s instructions. Stained cells were assessed by FCM using FACS Canto II flow cytometer with BD FACSDiva Software (Becton Dickinson, San Jose, CA, USA). The frequency of Th17 (CD4+IL17+) cells was expressed as a percentage of CD4+ T cells by sequential gating on lymphocytes and CD4+ T cells. The frequency of naïve (CD45RA+) and memory (CD45RO+) Th17 cells was also expressed as a percentage of CD4+IL-17+ T cells.
Measurement of blood biochemistry and inflammatory biomarker
Blood sugar, lipids and homocysteine (Hcy) were determined by the enzymatic method. High sensitive C-reactive protein (hsCRP) was measured by the immunoturbidimetric method. All of the assays were conducted on an Olympus AU2700 biochemical autoanalyzer (Olympus, Japan).
Cytokines and ox-LDL in serum
The levels of Interleukin 17 (IL-17), IL-6 and ox-LDL in serum were examined by the enzyme-linked immunosorbent assay (ELISA) and measured at 450 nm on Biocell HT1 ELISA microplate reader (IL-17 ELISA kits from Bender MedSystems; IL-6 ELISA kits from R&D system (Minneapolis, MN, USA); ox-LDL ELISA kits from Uscnlife, USA). The minimal detectable concentrations were 0.5 pg/ml for IL-17, 0.7 pg/ml for IL-6, and 4.5 μg/L for ox-LDL. Intra-assay and inter-assay coefficients of variation for all ELISA were < 5%. All samples were measured in duplicate.
Effects of IL-17 on endothelial cell senescence
HUVEC culture and IL-17 treatment
Human umbilical vein endothelial cells (HUVECs) obtained from American Tissue Culture Collection was cultured at 37°C in RPMI 1640 medium supplemented with 10% fetal bovine serum and 100 U/Ml penicillin, and 100 U/mL streptomycin in humidified 5% CO2 incubator. Cells were used at passage 5-10.
To explore the effect of IL-17A on endothelial cells senescence, HUVECs were seeded in 6-well plates at a density of 2 × 104 cells/well and cultured overnight. Then, recombinant Human IL-17A (eBioscience, USA) was added in the medium at final concentrations of 100 ng/ml. HUVECs without IL-17A incubation served as control group. The cells were incubated with IL-17A for 48 hours, then the cells were washed twice with PBS and the HUVECs were detected by Galactosidase (β-gal) staining and real-time RT-PCR analysis.
Galactosidase (β-gal) staining
Galactosidase (β-gal) staining was conducted using senescence β-galactosidase staining kit (Cell Signaling Technology, USA). HUVECs were treated with or without IL-17A. After that, the cells were washed twice with phosphate-buffered saline (PBS) and then fixed for 10-15 minutes with fixative solution at room temperature. The cells were then incubated at 37°C at least overnight with the β-galactosidase staining solution. Senescence-associated (SA)-β-gal-positive cells were observed by microscope, and over 400 cells were counted in three independent fields [7].
Expression of RORγt and endothelial cell senescence markers determined by real time-PCR
Total RNA was extracted with TRIzol (Invitrogen, USA) according to the manufacturer’s instructions. The amount and purity of the obtained RNA was determined by measurements of the optical density (OD) at 260 and 280 nm. For real-time PCR, cDNA was synthesized by using random hexamer primers and RNase H-reverse transcriptase (Invitrogen). TaqMan primers and probes for human RORγt, endothelin-1 (ET-1), vascular cell adhesion molecule-1 (VCAM-1), and endothelial nitric oxide synthase (eNOS) were purchased from Applied Biosystems. The following primer pairs were used (Table 3):
Table 3.
Primer pairs
| RORγt | F: 5’-ACGGTGGTTCCCCTCATCA-3’ | 86 bp |
| R: 5’-CCAAATAGATGTTGTATTGCTCCAC-3’ | ||
| ET-1 | F: 5’-TCAACACTCCCGAGCACGT-3’ | 96 bp |
| R: 5’-CACGGTCTGTTGCCTTTGTG-3’ | ||
| VCAM-1 | F: 5’-CGAAAGGCCCAGTTGAAGGA-3’ | 141 bp |
| R: 5’-GAGCACGAGAAGCTCAGGAGAAA-3’ | ||
| eNOS | F: 5’-ACCCTCACCGCTACAACATC-3’ | 205 bp |
| R: 5’-GCCTTCTGCTCATTCTCCAG-3’ |
All reactions were performed using the ABI Prism 7500 Sequence Detection System (Applied Biosystems). Each sample was analyzed in duplicate and mRNA expression level was normalized to the level of GAPDH housekeeping genes. GADPH was analysed using the following primers (Table 4):
Table 4.
GADPH was analysed using the primers
| GAPDH | F: 5’-CCCTTCATTGACCTCAACTACATG-3’ | 115 bp |
| R: 5’-TGGGATTTCCATTGATGACAAGC-3’ |
Statistical analysis
Values were expressed as the mean ± standard deviation (SD). Data was analysed by using statistical software (SPSS 13.0; LEAD Technologies, Inc., Chicago, IL, USA). Statistical significance for the differences in the groups was assessed by one-way analysis of variance (ANOVA). If significance was found, Bonferroni’s test was performed for post-hoc analysis to detect the differences among groups when equal variance was assumed, while Dunnett’s C test was performed when equal variance was not assumed. Spearman’s correlation was used as a test of correlation between two continuous variables. P < 0.05 was considered to be statistically significant.
Results
Patients and controls
There were no significant differences in gender, smoking rate, total triglyceride (TG) high density lipoprotein-cholesterol (HDL-C) and very low density lipoprotein-cholesterol concentrations (VLDL-C) among healthy elderly, healthy middle-aged, healthy young group and ACI patients. However, fasting blood glucose (FBG) levels in the ACI group were significantly higher than those in the 3 healthy groups (P < 0.05). Hypertension, total cholesterol (TC) and low density lipoprotein-cholesterol (LDL-C) in the ACI group were significantly higher than those in the healthy young group (P < 0.05 and P < 0.01, respectively). Furthermore, total cholesterol (TC) and low density lipoprotein-cholesterol (LDL-C) in the healthy middle-aged and elderly group were significantly higher than those in the healthy young group (Both P < 0.05). There were also no significant differences in BFS, TG, LDL-C concentrations among the 3 healthy groups (Table 1).
Table 1.
Healthy group and ACI patient characteristics
| Item | Healthy young (n = 30) | Healthy middle-aged (n = 33) | Healthy elderly (n = 34) | ACI (n = 36) |
|---|---|---|---|---|
| Age | 33.9 ± 6.9 | 52.8 ± 7.1 | 70.4 ± 5.6 | 73.5 ± 6.9 |
| Gender (male/female) | 17/13 | 19/14 | 18/16 | 21/15 |
| Hypertension, n (%) | 7 (23.3) | 12 (36.7) | 14 (41.2) | 16 (47.1)▲ |
| Smoking rate, n (%) | 9 (30) | 11 (33.3) | 13 (38.2) | 15 (41.7) |
| BFS (mmol/L) | 4.82 ± 0.54 | 5.04 ± 0.61 | 5.21 ± 0.87 | 5.85 ± 0.93* |
| TC (mmol/L) | 4.42 ± 0.81 | 5.01 ± 0.67** | 5.09 ± 0.74** | 5.26 ± 0.91** |
| TG (mmol/L) | 1.43 ± 0.29 | 1.59 ± 0.74 | 1.37 ± 0. 47 | 1.72 ± 0.53 |
| HDL-C (mmol/L) | 1.15 ± 0.30 | 1.54 ± 0.91 | 1.21 ± 0.23 | 1.28 ± 0.29 |
| LDL-C (mmol/L) | 2.39 ± 0.77 | 2.77 ± 0.65** | 2.83 ± 0.42** | 3.28 ± 0.71** |
| VLDL-C (mmol/L) | 0.52 ± 0.31 | 1.08 ± 0.63 | 0.98 ± 0.43 | 0.79 ± 0.25 |
Values are expressed as mean ± SD or number. ACI: Acute cerebral infarction; TIA: Transient ischemic attacks; NCA: Subjects with normal carotid arteries; BFS: Blood-fasting sugar; TC: Total cholesterol; TG: Total triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low density lipoprotein cholesterol.
P < 0.05 vs. healthy young, middle-aged and elderly;
P < 0.05 vs. healthy young;
P < 0.01 vs. healthy young.
The frequency and phenotyping of CD4+IL-17+ Th17 cells in the healthy groups and elderly ACI patients
The frequencies of Th17 (CD4+IL17+/CD4+ T) cells were markedly higher in elderly ACI (4.03 ± 0.67%) patients than in healthy elderly (2.31 ± 0.42%), healthy middle-aged (1.42 ± 0.37%) and healthy young group (0.94 ± 0.21%, All P < 0.01). In addition, the frequencies of Th17 cells were also markedly higher in healthy elderly group than in healthy middle-aged and young group (P < 0.05 and P < 0.01, respectively). However, there was no obvious difference in Th17 frequencies between the healthy middle-aged and young groups (P > 0.05, Figure 1).
Figure 1.
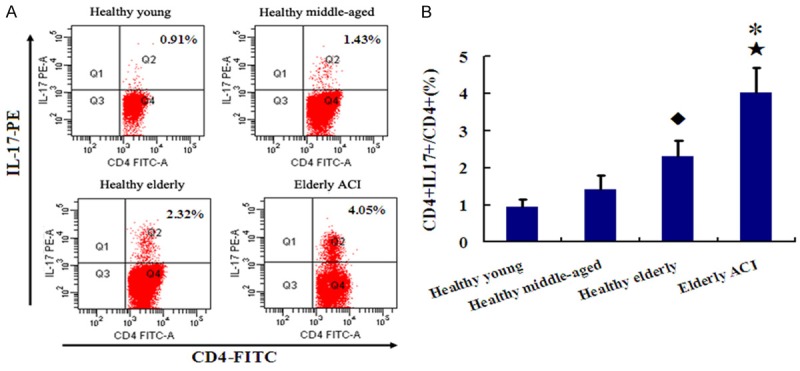
The frequencies of Th17 in Healthy groups and ACI patients. A. Representative FACS figures from a single patient in each group. The percentage of positive cells is shown in each panel. B. Comparison of Th17 expression among 4 groups. *P < 0.05 vs. Healthy elderly group; ★P < 0.01 vs. Healthy middle-aged and young groups; ◆P < 0.05 vs. Healthy middle-aged and young groups.
CD45RA is vital marker of naive T cell while CD45RO is key marker of memory T cell. There were no significant differences of memory Th17 frequencies (CD45RO+CD4+IL17+/CD4+IL17+ T cells) among elderly ACI patients (85.42 ± 7.56%), healthy elderly (83.21 ± 4.72%), healthy middle-aged (78.42 ± 5.18%) and healthy young group (81.94 ± 6.21%, All P > 0.05). Correspondingly, there were, also, no significant differences of naive Th17 frequencies (CD45RA+CD4+IL17+/CD4+IL17+ T cells) among elderly ACI patients (5.12 ± 1.65%), healthy elderly (6.71 ± 2.03%), healthy middle-aged (7.42 ± 2.38%) and healthy young group (5.94 ± 1.21%, All P > 0.05). The results indicated that IL-17 is mainly produced by memory CD4+ T cells (Figure 2).
Figure 2.
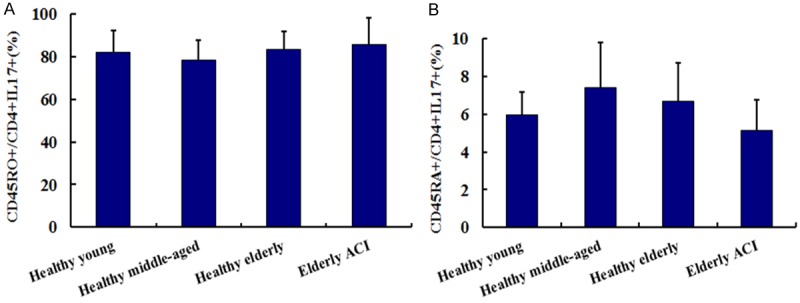
The frequencies of CD45RO (A) and CD45RA (B) in Th17 cells from Healthy groups and ACI patients.
Expression of RORγt and biomarkers of endothelial cell senescence in PBMCs from the healthy groups and elderly ACI patients
RORγt levels were markedly higher in elderly ACI patients than in the 3 healthy groups (all P < 0.01). RORγt levels were also significantly higher in healthy elderly group than in healthy middle-aged and young group (P < 0.05 and P < 0.01, respectively). There was no significant difference in RORγt level between the healthy middle-aged and young groups (P > 0.05; Figure 3).
Figure 3.
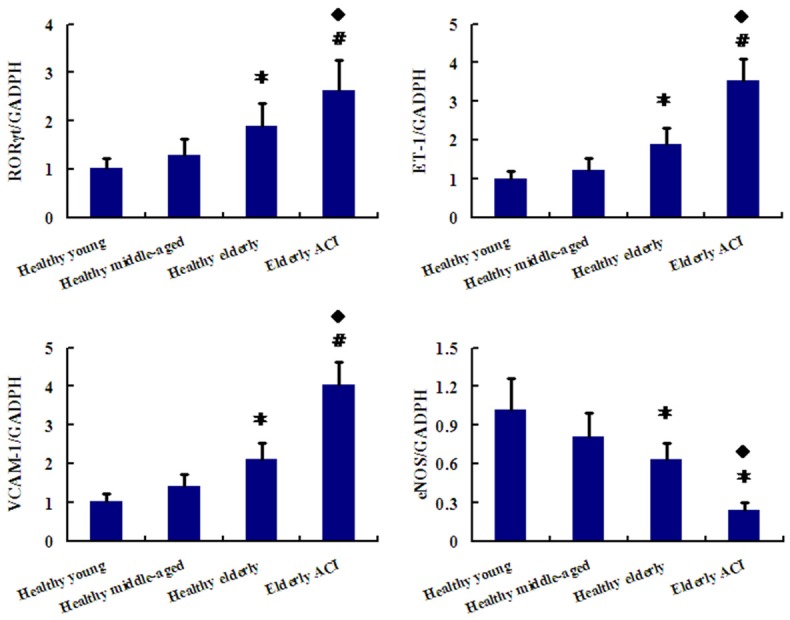
Expression of RORγt, ET-1, VCAM-1 and eNOS in PBMCs from Healthy groups along with ACI patients was determined by real time polymerase chain reaction (PCR). The ratios of RORγt/β-actin mRNA for the gray-scale value were compared in the 4 groups. *P < 0.05 vs. Healthy middle-aged and young group; #P < 0.01 vs. Healthy middle-aged and young group; ◆P < 0.05 vs. Healthy elderly group.
With respect to biomarkers of endothelial cell senescence, including endothelin-1 (ET-1), vascular cell adhesion molecule-1 (VCAM-1) and endothelial nitric oxide synthase (eNOS) levels, ET-1 as well as VCAM-1 levels were notably higher while eNOS levels were markedly lower in elderly ACI patients than in the 3 healthy groups (P < 0.01, P < 0.05). ET-1 and VCAM-1 levels were also obviously increased while eNOS levels were markedly decreased in healthy elderly group than in healthy middle-aged and young group (P < 0.05 and P < 0.01, respectively). However, there was also no apparent difference in ET-1, VCAM-1 and eNOS levels between the healthy middle-aged and young groups (P > 0.05; Figure 3).
Levels of serum cytokines and inflammatory biomarkers in the healthy groups and elderly ACI patients
The levels of IL-17 and IL-6 were markedly higher in elderly ACI patients than in the 3 healthy groups (P < 0.05 and P < 0.01, respectively). Similarly, IL-17 levels were also significantly higher in the healthy elderly group than in the healthy middle-aged and young group (P < 0.05). However, IL-6 levels in healthy elderly group were slightly higher than those in healthy middle-aged and young groups, but there was also no significant difference among the 3 healthy groups (P > 0.05). Furthermore, there was also no difference in IL-17 levels between the healthy middle-aged and young group (P > 0.05).
At the same time, the concentrations of ox-LDL, Hcy, and hsCRP were significantly increased in elderly ACI patients than in the 3 healthy groups (P < 0.01, P < 0.05 respectively). Moreover, ox-LDL and hsCRP levels were also significantly higher in healthy elderly group than in healthy middle-aged and young group, while Hcy levels were also significantly higher in healthy elderly group than in healthy young group (P < 0.05 and P < 0.01, respectively). There was also no notable difference in ox-LDL, Hcy and hsCRP levels between the healthy middle-aged and young groups (Both P < 0.05, Table 2).
Table 2.
Serum levels of cytokines, and inflammatory biomarkers in the 4 groups
| Healthy young (n = 30) | Healthy middle-aged (n = 33) | Healthy elderly (n = 34) | ACI (n = 36) | |
|---|---|---|---|---|
| IL-17 (pg/mL) | 15.98 ± 3.75 | 18.92 ± 4.93 | 27.17 ± 6.96* | 53.71 ± 10.84◆,# |
| IL-6 (pg/mL) | 4.02 ± 1.58 | 4.67 ± 1.73 | 5.84 ± 2.61 | 14.76 ± 5.83*,▲ |
| hsCRP (mg/L) | 0.87 ± 0.54 | 1.15 ± 0.81 | 2.21 ± 1.05* | 21.89 ± 16.25◆,# |
| Ox-LDL (µg/L) | 276.15 ± 30.84 | 295.04 ± 35.61 | 352.17 ± 39.49* | 447.25 ± 52.13◆,▲ |
| HCY (µmol/L) | 12.27 ± 3.26 | 14.18 ± 3.91 | 17.09 ± 4.94★ | 28.17 ± 7.12◆,▲ |
P < 0.05 vs. Healthy middle-agedand young group;
P < 0.01 vs. Healthy middle-agedand young group;
P < 0.05 vs. Healthyyoung group;
P < 0.05 vs. Healthy elderly group;
P < 0.01 vs. Healthy elderly group.
Correlation of Th17 cells to the levels of cytokines, endothelial cell senescence biomarkers, and inflammatory biomarkers
For the 4 groups, serum IL-17 and IL-6 levels were positively correlated with the frequency of Th17 cells (P < 0.01 and r = 0.849, 0.803, respectively). The endothelial cell senescence biomarkers, ET-1 and VCAM-1 mRNA levels were significant positively correlated with the frequency of Th17 cells (P < 0.01 and r = 0.784, 0.756), while eNOS mRNA levels were significant negatively correlated with the frequency of Th17 cells (P < 0.01 and r = -0.743).
The inflammatory markers ox-LDL were significant positively correlated with the frequency of Th17 cells (P < 0.01 and r = 0.791). Similar results were observed for the correlation of serum hsCRP and Hcy levels to Th17 cells (hsCRP and Th17 levels, P < 0.01 and r = 0.762; and Hcy and Th17 levels, P < 0.01 and r = 0.713).
IL-17 promotes endothelial cell senescence
We have investigated the role of IL-17A in endothelial cell senescence. IL-17A was identified as the key cytokine in the Th17-related cytokine family. Our data shows that, with 100 ng/ml IL-17A treatment, the (SA)-β-gal-positive cells were observed. At the same time, the marker of endothelial cell senescence, ET-1 and VCAM-1 levels were notably increased while eNOS levels were decreased as comparison to control group (P < 0.05 and P < 0.01, respectively). These results imply that IL-17A has the potential to induce endothelial cell senescence (Figure 4).
Figure 4.
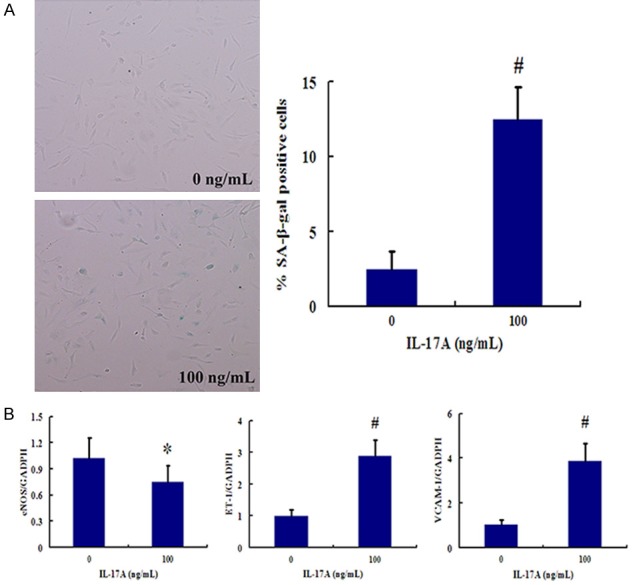
IL-17 promotes endothelial cell senescence. A. SA-β-gal activity of HUVECs treated with 100 ng/ml IL-17A. B. IL-17A promotes the expression of endothelial cell senescence markers. HUVECs were treated with 100 ng/ml IL-17A for 48 h. Then mRNA expression of VCAM-1, ET-1 and eNOS was measured by real-time RT-PCR. *P < 0.05 vs. Control group (without IL-17A treatment); #P < 0.01 vs. Control group.
Discussion
Inflammation and senescence is increasingly recognized to play an important role in atherosclerosis and stroke [8,9]. Aging is associated with alterations in T cell immunity that include thymic atrophy and changes in T cell subsets and function [10]. Recent studies have shown that age-related enhancement of Th17 cell function may contribute to significant changes in immune function [11,12]. It was demonstrated that the increase of Th17 cells is closely related to inflammation and the onset of AS [13].
Here we have found that healthy elderly people have increased Th17 frequency, RORγt expression and IL-17 and IL-6 cytokines in peripheral blood compared to healthy middle-aged and young people. Furthermore, the level of Th17 expression in elderly ACI patients is significantly increased, when compared to healthy elderly people. However, there were no marked differences between healthy middle-aged and young people. IL-17 is mainly produced by memory CD4+ T cells in all the 4 groups. Significant correlations were found between Th17 frequencies and the levels of Th17 cytokines, inflammatory biomarkers in serum as well as endothelial cell senescence biomarkers mRNA expression in PBMCs.
Incubation of HUVEC with IL-17A, IL-17A induced significant endothelial cell senescence accompanied by increased ET-1 and VCAM-1 levels as well as decreased eNOS level. These data indicate that an enhanced Th17 immune response in aged people is accompanied with endothelial cell senescence and atherosclerotic cerebral infarction in humans. Th17 cells have been implicated as a pathogenic factor of atherosclerosis and involved in the inflammatory process of plaque rupture [14]. Therefore, Th17 plays a role in the development and complications of atherosclerosis, especially ACI [15,16]. Our studies confirmed that significantly higher Th17 cells numbers, IL-17/IL-6 levels and RORγt mRNA expression were in ACI patients than in healthy groups. In addition, ACI inflammatory markers were significant positively correlated with the frequency of Th17 cells. Taken together, these results suggest that the Th17 cells are directly involved in the pathogenesis of ACI. Targeting Th17 cell development and their function may provide effective therapy for AS and ACI.
Endothelial cell senescence and injury appears to be an initial event in the formation of vasculopathy [17]. Overexpression of ET-1, VCAM-1 and low expression of eNOS occur during endothelial cell senescence, which mediated more leukocyte recruitment, adherence to senescenced endothelial cells, and exacerbated the inflammatory reaction resulting in blood vessel destruction and tissue hypoxia. Our studies have confirmed ET-1 and VCAM-1 levels were also obviously increased while eNOS levels were markedly decreased in AS and ACI.
ACI is an aging disease, and endothelial cell senescence is one of the important causes of AS or ACI occurrence. Our studies confirmed that the mRNA levels of all the 3 endothelial cell senescence biomarkers, ET-1, VCAM-1 and eNOS, were significantly correlated with the frequency of Th17 cells (P < 0.01 and r = 0.784, 0.756, -0.743).
In this study, we have investigated the role of Th17 cells in endothelial cell senescence. Our results are consistent with other studies, which suggested that Th17 cells promote the recruitment of additional T cells to vascular endothelial cells in SLE [18,19]. Our data shows that with IL-17 incubation, the (SA)-β-gal-positive cells were observed. At the same time, the marker of endothelial cell senescence, ET-1 and VCAM-1 levels were notably increased, while eNOS levels were decreased. These results imply that IL-17 has the potential to induce endothelial cell senescence. Moreover, we proved that IL-17A, the main factor secreted by Th17 cells, similarly up-regulated the expression of endothelial cell senescence markers, ET-1 and VCAM-1 and down-regulated the expression of eNOS. Taken together, this data indicates that IL-17 mediates endothelial cell senescence which is closely related to ACI occurrence.
In summary, Th17 cell response is enhanced with aging and significantly elevated in ACI. Th17/IL-17 can promote endothelial cell senescence and subsequently contribute to ACI occurrence in humans. Th17/IL-17 appears to be a novel target on the pathogenesis of endothelial cell senescence and ACI.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81472018).
Disclosure of conflict of interest
None.
References
- 1.Son HJ, Lee SH, Lee SY, Kim EK, Yang EJ, Kim JK, Seo HB, Park SH, Cho ML. Oncostatin M suppresses activation of IL-17/Th17 via SOCS3 regulation in CD4+ T Cells. J Immunol. 2017;198:1484–1491. doi: 10.4049/jimmunol.1502314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q, Liu S, Ge D, Cunningham DM, Huang F, Ma L, Burris TP, You Z. Targeting Th17-IL-17 pathway in prevention of micro-invasive prostate cancer in a mouse model. Prostate. 2017;77:888–899. doi: 10.1002/pros.23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanagisawa H, Hashimoto M, Minagawa S, Takasaka N, Ma R, Moermans C, Ito S, Araya J, Budelsky A, Goodsell A, Baron JL, Nishimura SL. Role of IL-17A in murine models of COPD airway disease. Am J Physiol Lung Cell Mol Physiol. 2017;312:L122–L130. doi: 10.1152/ajplung.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinti M, Appay V, Campisi J, Frasca D, Fulop T, Sauce D, Larbi A, Weinberger B, Cossarizza A. Aging of the immune system: focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–2301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Wang Y, Yu F, Wang YM, Zhang C, Hu C, Wu Z, Xu X, Hu S. Peripheral Th17/Treg imbalance in patients with atherosclerotic cerebral infarction. Int J Clin Exp Pathol. 2013;6:1015–1027. [PMC free article] [PubMed] [Google Scholar]
- 6.Fure B, Wyller TB, Thommessen B. TOAST criteria applied in acute ischemic stroke. Acta Neurol Scand. 2005;112:254–258. doi: 10.1111/j.1600-0404.2005.00470.x. [DOI] [PubMed] [Google Scholar]
- 7.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gencer B, Auer R, de Rekeneire N, Butler J, Kalogeropoulos A, Bauer DC, Kritchevsky SB, Miljkovic I, Vittinghoff E, Harris T, Rodondi N. Association between resistin levels and cardiovascular disease events in older adults: the health, aging and body composition study. Atherosclerosis. 2016;245:181–186. doi: 10.1016/j.atherosclerosis.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhungana H, Malm T, Denes A, Valonen P, Wojciechowski S, Magga J, Savchenko E, Humphreys N, Grencis R, Rothwell N, Koistinaho J. Aging aggravates ischemic stroke-induced brain damage in mice with chronic peripheral infection. Aging Cell. 2013;12:842–850. doi: 10.1111/acel.12106. [DOI] [PubMed] [Google Scholar]
- 10.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Bruggen N, Ouyang W. Th17 cells at the crossroads of autoimmunity, inflammation, and atherosclerosis. Immunity. 2014;40:10–12. doi: 10.1016/j.immuni.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Lee J, Zhang Y, Wang H, Liu X, Shang F, Zheng Q. Increased Th17 cells in coronary artery disease are associated with neutrophilic inflammation. Scand Cardiovasc J. 2011;45:54–61. doi: 10.3109/14017431.2010.491123. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Wang Y, Li H, Shen G, Hu S. Ox-LDL influences peripheral Th17/Treg balance by modulating Treg apoptosis and Th17 proliferation in atherosclerotic cerebral infarction. Cell Physiol Biochem. 2014;33:1849–1862. doi: 10.1159/000362963. [DOI] [PubMed] [Google Scholar]
- 14.Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol. 2009;21:414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma T, Gao Q, Zhu F, Guo C, Wang Q, Gao F, Zhang L. Th17 cells and IL-17 are involved in the disruption of vulnerable plaques triggered by short-term combination stimulation in apolipoprotein E-knockout mice. Cell Mol Immunol. 2013;10:338–348. doi: 10.1038/cmi.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon US, Choi JP, Kim YS, Ryu SH, Kim YK. The enhanced expression of IL-17-secreting T cells during the early progression of atherosclerosis in ApoE-deficient mice fed on a western-type diet. Exp Mol Med. 2015;47:e163. doi: 10.1038/emm.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valentini G, Bencivelli W, Bombardieri S, D’Angelo S, Della Rossa A, Silman AJ, Black CM, Czirjak L, Nielsen H, Vlachoyiannopoulos PG. European scleroderma study group to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann Rheum Dis. 2003;62:901–903. doi: 10.1136/ard.62.9.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, Wan L, Li M. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]


