Abstract
AIM
To analyse clinical and long-term oncologic results after laparoscopic complete mesocolic excision (CME) for colonic cancer over a 10-year period.
METHODS
Consecutive patients who received laparoscopic CME at our hospital from 2007 to 2017 were prospectively registered and retrospectively analysed. In total, 341 patients were included with tumour-nodal-metastasis (TNM) stages 0-III.
RESULTS
The mean age of the patients was 71.9 years. The median length of stay was 5 d. The mean lymph node harvest was 17.8. The mortality rate was 1.2%. Fifteen patients were reoperated on for anastomotic leaks. The local recurrence rate was 2.3%. Five-year TTR and cancer-specific survival CSS were 83.1% and 90.3%. The location of the tumour was not a significant variable for survival in unadjusted and adjusted survival analysis. TNM stage and anastomotic leaks were significant variables with respect to survival.
CONCLUSION
Laparoscopic CME results in acceptable complication rates and long-term oncologic results. It is important to avoid anastomotic leaks because of their negative effect on survival.
Keywords: Complete mesocolic excision, Central vascular ligature, Colonic cancer, Laparoscopic surgery, Time to recurrence, Cancer specific survival
Core tip: This study presents a large cohort of patients operated on with laparoscopic complete mesocolic excisions (CME) for colonic cancer. Five-year survival data are presented. For the first time in a study on laparoscopic CME, it is shown that reoperation for an anastomotic leak has a negative impact on both unadjusted and adjusted survival analysis. The location of the tumour does not impact long-term survival.
INTRODUCTION
Colonic cancer is an important challenge for specialists in gastrointestinal surgery. Complete mesocolic eccision (CME) has been put forward as a method of standardizing the surgical aspect of colon cancer with acceptable survival results. Dr. Hohenberger introduced this principle and has published impressive outcome data[1,2]. Extended lymphadenectomy or D3 excision is popular in eastern countries and is the standard for T3/T4 tumours in Japan. This technique is comparable to CME and has demonstrated excellent survival results[3]. The completeness of the specimen after CME with central vascular ligature (CVL) and D3 excision has been proven to be of the same quality as CME alone in another paper[4]. The basis of the CME technique has been published in several papers[5,6]. A recent consensus article suggests that CME with CVL should be “the gold standard” for surgery in colonic cancer[5]. The minimal invasive/laparoscopic technique for treating colonic cancer has been evolving in the last decade. CME can also be performed with the laparoscopic approach[7-9]. Laparoscopic CME for transverse colonic cancer has also been shown to be feasible with acceptable morbidity and survival results[10].
In our hospital we converted the surgery for colon cancer into CME from 2007. The goal of the present study is to analyse the last ten years with laparoscopic CME for colonic cancer and present the short-term results as well as long- term oncologic results. Five-year survival data are presented. The data are prospectively recorded and retrospectively analysed. For the first time this paper demonstrates that postoperative complications have an important role in colonic cancer survival after radical laparoscopic CME.
MATERIALS AND METHODS
Patient selection
Consecutive patients were enrolled in a prospective study from January 2007 through December 2017. Survival data were analysed and collected in April 2017. All patients had a computed tomography (CT) scan of the chest and abdomen before surgery. The Union for International Cancer Control (UICC) tumour-nodal-metastasis (TNM) system was used for staging (7th edition)[11]. Patients were excluded if they had a radiological T4 tumour on preoperative CT scan of the chest and abdomen. If some part of the CME-principles was disregarded or the surgeons were not accredited to perform CME, these procedures were excluded (n = 5). All operative reports were read in detail.
Patient eligibility
Patients were included if they had a colonic cancer detected on colonoscopy and histologically proven to be an adenocarcinoma. The patients who were operated on according to the CME principles with the laparoscopic access were included. If the surgery was converted to an open procedure within 15 min from the start of the procedure, the patient was excluded from the study. Patients were included regardless of an earlier history of malignancy. Body mass index (BMI) was not considered. Robotic surgery was not implemented in our hospital for colonic cancer.
Patient evaluation and follow-up
Patients were operated in a single hospital and they were scheduled for a follow-up visit every 6 mo after blood tests and CT scan of the chest and abdomen. The patients were followed for five years. Colonoscopy was performed after one- and four-years after surgery.
Surgery
All the patients were operated with laparoscopic CME according to the principles of CME. The specimen was extracted from a small incision in the umbilicus. In the right sided resection the anastomosis was performed extra-corporally. Between March 2009 and 2017, approximately 80% of the resections were performed by lap CME. Less than 5% of the resections were converted from laparoscopic CME to open CME in this period.
Study design
This study was a cohort study on laparoscopic CME with data extracted from 2007 through December 2017. Patients converted to open surgery within the first 15 min of the laparoscopic procedure were excluded from the study. The survival data was analysed according to the actual treatment.
Treatment outcome
Treatment outcome was extracted after 5 years according to Punt et al[12]. TTR and CSS were used as the survival endpoints. Regarding oncologic results the patients were analysed as treated, meaning that only patients that were operated on laparoscopically from start to end were included.
Statistical analysis
We used the χ2 test to compare proportions between groups. Gosset’s t-test[13] or the Wilcoxon-Mann-Whitney test[14] were used to compare means. Regarding survival the log-rank test and Kaplan-Meier[15] plots were used. Multiple prognostic factors were analysed with the Cox proportional hazards model[16]. A significance level of P ≤ 0.05 was applied. SPSS version 24 was used for statistical calculations.
RESULTS
Four hundred and seventy-five patients with TNM stages 0-IV colonic cancer operated on with laparoscopic CME were prospectively registered from May 2007 through December 2017. Patients operated on by surgeons not familiar with the CME principle were excluded (n = 5). Resections other than segmental resections were excluded, including single access procedures (n = 44). For the survival analysis only the patients with more than 12 mo of potential follow-up were included (2007-2015) (n = 375).
The aim of the study was to analyse the patients with TNM stages 0-III and this left 341 patients for inclusion. These patients had a colonic cancer in all the different locations of the colon, including the transverse colon. Rectal cancer was defined as a tumour situated less than 15 cm from the anal verge defined on rectal examination or with MRI. These patients were excluded and treated in a different hospital.
Pathology
The patients had a mean age of 71.9 years. The mean BMI was 25.8 kg/m2. One hundred and forty patients (41.1%) were operated on with a right hemicolectomy. Forty-nine patients (14.4%) were operated on with a right extended hemicolectomy for tumours in the right colonic flexure and the right transverse colon. Twenty-six patients (7.6%) were operated on with a left extended hemicolectomy for colonic tumours in the left of the mid-transverse colon, in the left flexure, and in the descending colon. One-hundred and twenty-six patients (37%) had an anterior resection. The cohort also included TNM stage 0 patients, patients with resection of a segment of the colon after R1 endoscopic resection of a malignant polyp. The resected specimen did not harbour malignancy locally or lymph nodes with metastasis. One-hundred and seventy patients were staged as TNM stage II (49.9%) and 102 patients were TNM stage III (29.9%). The patients had a mean lymph node count of 17.8 (range: 0-69). For the TNM stages 0-III patients the mean number of positive lymph nodes was 3.2 (Table 1).
Table 1.
Characteristics of 341 tumour-nodal-metastasis stages 0-III patients with colonic cancer, operated on with laparascopic complete mesocolic excision resection in a community teaching hospital in Norway in 2007-2015
| Variable category | Laparoscopic CME resection, n = 341 (%) |
| Age in years, mean, (range) | 71.9 (28-94) |
| BMI in kg/m2, mean (range) | 25.8 (15-42) |
| Surgical procedure | |
| Right hemicolectomy | 140 (41.1) |
| Extended right hemicolectomy | 49 (14.4) |
| Left extended hemicolectomy | 26 (7.6) |
| Sigmoid resection | 126 (37.0) |
| TNM stage | |
| Stage 0 | 8 (2.3) |
| Stage I | 61 (17.9) |
| Stage II | 170 (49.9) |
| Stage III | 102 (29.9) |
| No. of lymph nodes, mean (range) | 17.8 (0-69) |
| No. of positive lymph nodes, TNM st III, mean (range) | 1.2 (0-19) |
| Length of stay, d, mean (range) | 6.7 (2-57) |
TNM: Tumour-nodal-metastasis; CME: Complete mesocolic excision; BMI: Body mass index.
Morbidity and mortality
The median length of stay was 5 d. The mean length of stay was 6.7 d. Two hundred and sixty-seven patients (78.8%) had no complications or mortality. Four patients died in the first 30 d after surgery resulting in a mortality rate of 1.2%.
Fifteen patients had anastomotic leaks and were treated with surgery (4.4%). Six patients with a right-sided hemicolectomy had anastomotic leaks (4.3%). Seven patients (5.6%) with an anterior resection had anastomotic leask. Seventeen patients (5.0%) were treated for paralytic ileus (Table 2).
Table 2.
Operative morbidity and mortality in 341 patients with colonic cancer (tumour-nodal-metastasis stage 0-III) operated on with laparoscopic complete mesocolic excision in a community teaching hospital in Norway in 2007-2015 n (%)
| Variables Category | Laparoscopic CME colectomy (n = 341) |
| Morbidity | |
| No morbidity | 267 (78.8) |
| Paralytic ileus | 17 (5.0) |
| Wound infection | 4 (1.2) |
| Wound dehiscence | 8 (2.3) |
| Deep (IAA) infection | 5 (1.5) |
| Anastomotic leakage | 15 (4.4) |
| Cardiac/respiratory distress | 11 (3.2) |
| Ileus reoperation | 2 (0.6) |
| Other (bladder infection, iatrogenic perf small intestine) | 6 (1.8) |
| Mortality | 4 (1.2) |
TNM: Tumour-nodal-metastasis; CME: Complete mesocolic excision; IAA: Intra-abdominal abscess.
Oncologic outcome
The local recurrence rate was 2.3%. For right CME hemi-colectomy the local recurrence rate was 2.1% and 2.3% for anterior resection (n. s.). Twenty-two patients (6.5%) developed liver-metastasis, 5 patients (1.5%) a lung metastasis, and 19 patients (5.6%) combined metastasis. In the TNM stage III group, 14 patients (13.7%) had combined metastasis and 9 patients (8.8%) had a liver-metastasis.
Five-year time to recurrence (TTR) and cancer specific survival (CSS) was 83.1% and 90.3%. TTR was 87.3% for TNM stage II and 69.5% for TNM stage III. CSS was 94.4% for TNM stage II and 77.0% for TNM stage III (Table 3). For TTR and CSS, operative procedure (or tumour-location) did not show a significant survival difference in univariate analysis (Figures 1 and 2). Whether the patient developed an anastomotic leak or not revealed a significant difference in survival both in TTR and CSS (Figures 3-5). With respect to TTR the patients with anastomotic leaks had significantly worse survival (P = 0.037) than those without a leak. This is also shown in a Cox regression analysis according to operative procedure (Figure 3). With respect to CSS the difference was also significant (P = 0.023). In multiple Cox regression for TTR, TNM stage and an anastomotic leak were significant factors for survival (Table 4).
Table 3.
Five-year survival figures given as time to recurrence and cancer-specific survival according to tumour-nodal-metastases stages 0-III in 341 colon cancer patients that were operated on with laparoscopic complete mesocolic excision during 2007-2015 in one community teaching hospitals in Norway
| Survival type TNM | 5-yr survival (%) | P value1 |
| TTR | 83.1 | < 0.001 |
| Stage 0 | 100 | |
| Stage I | 91.9 | |
| Stage II | 87.3 | |
| Stage III | 69.5 | |
| CSS | 90.3 | < 0.001 |
| Stage 0 | 100 | |
| Stage 1 | 100 | |
| Stage 2 | 94.4 | |
| Stage 3 | 77 |
Adjusted for TNM stage with the log rank test. TNM: Tumour-nodal-metastases; CME: Complete mesocolic excision; TTR: Time to recurrence; CSS: Cancer-specific survival.
Figure 1.
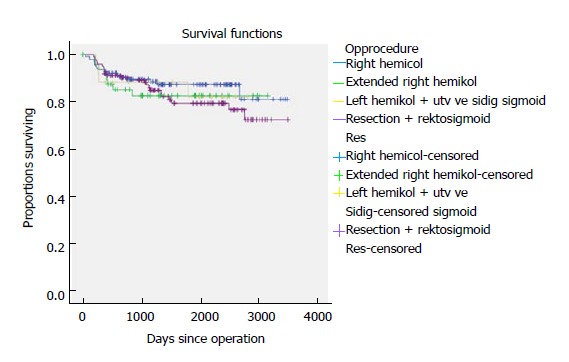
Time to recurrence shown according to resection type for 341 patients with colonic cancer operated on with laparoscopic complete mesocolic excision in a Norwegian community teaching hospital during 2007-2017 (P = NS).
Figure 2.
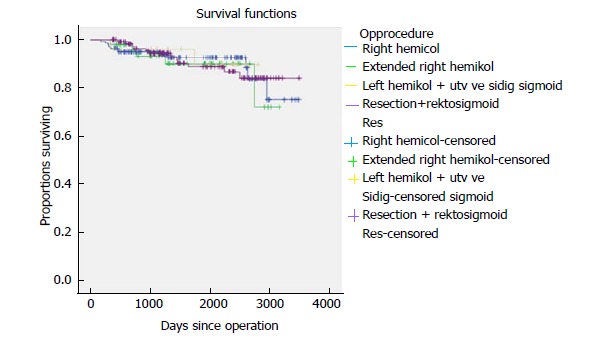
Cancer-specific survival shown according to resection type for 341 patients with colonic cancer operated on with laparoscopic complete mesocolic excision in a Norwegian community teaching hospital during 2007-2017 (P = NS).
Figure 3.
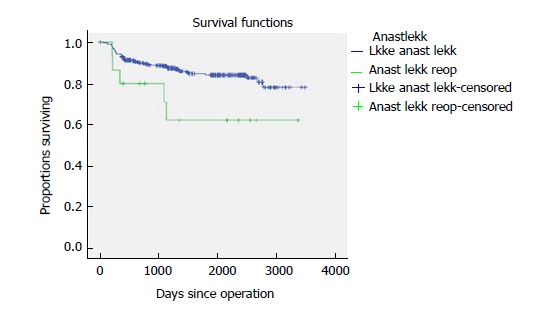
Time to recurrence shown according to reoperation for anastomotic leak for 341 patients with colonic cancer operated on with laparoscopic complete mesocolic excision in a Norwegian community teaching hospital during 2007-2017 (P = 0.037).
Figure 5.
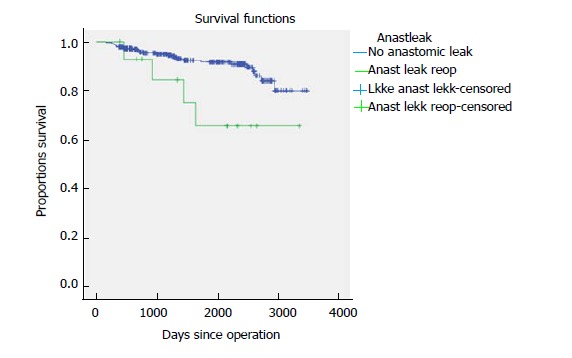
Cancer-specific survival shown according to reoperation for anastomotic leak for 341 patients with colonic cancer operated on with laparoscopic complete mesocolic excision in a Norwegian community teaching hospital during 2007-2017 (P = 0.023).
Table 4.
Uni- and multi-variate analysis of 5-year time to recurrence for 341 patients operated for tumour-nodal-metastases stage 0-III colonic cancer in a Norwegian community teaching hospital
| Unadjusted HR (95%CI) | LR test P value | Adjusted HR (95%CI) | LR test P value | |
| Age | 0.665 | 0.673 | ||
| < 70 yr | 1 (reference) | 1 (reference) | ||
| > 70 yr | 0.89 (0.51, 1.54) | 1.15 (0.60, 2.20) | ||
| BMI | 1.01 (0.94, 1.08) | 0.777 | 1.01 (0.94, 1.08) | 0.855 |
| Operative procedure | 0.696 | 0.367 | ||
| Right hemicolectomy | 1 (reference) | 1 (reference) | ||
| Extended right hemicol | 0.70 (0.37, 1.30) | 1.96 (0.76, 5.08) | ||
| Extended left hemicol | 0.97 (0.43, 2.17) | 1.63 (0.51, 5.23) | ||
| Anterior resection | 0.87 (0.30, 2.52) | 1.85 (0.88, 3.88) | ||
| Lymph nodes | 1.02 (0.98, 1.05) | 0.356 | 1.00 (0.97, 1.05) | 0.652 |
| Positive lymph nodes | 1.16 (1.09, 1.23) | < 0.001 | 1.21 (1.13, 1.29) | < 0.001 |
| Anastomotic leakage | 0.045 | 0.019 | ||
| No leak | 1 (reference) | 1 (reference) | ||
| Leak, reoperated | 2.57 (1.02, 6.47) | 3.13 (1.21, 8.10) |
BMI: Body mass index; LR: Likelihood ratio.
Figure 4.
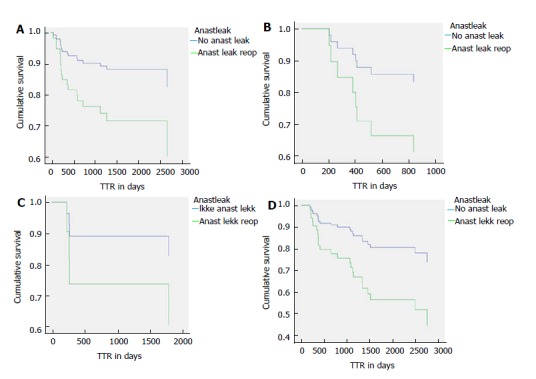
Time to recurrence and anastomotic leakage. A: Survival curves for time to recurrence (TTR) from a Cox regression model for patients with right colonic cancer operated on with laparoscopic complete mesocolic excision (CME) in a Norwegian community teaching hospital during 2007-2015 with or without an anastomotic leak (P = 0.037); B: TTR shown as Cox regression curves for patients with right flexure or right proximal transverse colonic cancer operated on with laparoscopic CME in a Norwegian community teaching hospital during 2007-2015 with or without an anastomotic leak (P = 0.037); C: TTR shown as Cox regression curves for patients with left transverse, left flexure or descending colon cancer operated on with laparoscopic CME in a Norwegian community teaching hospital during 2007-2015 with or without an anastomotic leak (P = 0.037); D: TTR shown as Cox regression curves for patients with sigmoid or recto-sigmoid colon cancer operated on with laparoscopic CME in a Norwegian community teaching hospital during 2007-2015 with or without an anastomotic leak (P = 0.037).
DISCUSSION
The interest in proper radical surgery for colonic cancer has increased in the last decade since the first publications on CME from Dr. Hohenberger. Opponents of this technique argue that this is nothing new[17].
Another interesting discussion is whether removing the apical lymph nodes in extended lymphadenectomy or in CME with CVL in patients with TNM stage III disease matters. Many argue that these patients have a systemic disease and might develop distant metastasis regardless of the CME with CVL. Many of these patients also receive chemotherapy, which influences survival[18]. Bertelsen et al[19] demonstrated better disease-free survival in patients with TNM stage I and II colonic cancer treated with CME versus conventional surgery. Our group has also demonstrated this in patients with TNM stage I and II disease[20]. Survival for the TNM stage III patients was improved in the paper by Bertelsen et al[19] but it was not significant. If the surgeon removes the apical lymph nodes in CME surgery with cancer cells, there could be an upstaging of the disease from TNM stage II to TNM stage III. This will also require treatment with chemotherapy. There is evidence indicating that this upstaging might not be the case. In a paper from our group we compared two patient cohorts in two time periods in three hospitals with increasing lymph node harvest from a mean figure of 10 lymph nodes to 16 lymph nodes. We could not show any stage migration[21].
In the recent years, there has been an increasing focus on the nature of the tumours in the right and transverse colon. Papers have focused mostly on CME for right-sided colonic cancer[8,22,23]. The complex anatomy of the right colon makes open and laparoscopic CME procedures more difficult than the conventional right hemicolectomy. The vascular anatomy can be different from patient to patient[24]. In this trial we could not show any significant difference in survival according to the operative procedure or location of the tumour. We followed 75 patients with a tumour in the transverse colon and in the flexures and 140 patients with a tumour in the right colon. Tumours located between the flexures are even more complex in anatomy and demands a technically skilled surgeon to perform laparoscopic CME. In the literature, there is very little evidence for CME surgery as a treatment for transverse colonic cancer. There is only one study from our group comparing laparoscopic CME and open CME[10]. This study shows decent survival results and no difference in survival between open and laparoscopic CME.
Morbidity is another important issue with CME surgery. With CVL of the superior mesenteric vein and artery, there is of course the possibility of vessel injury. Bertelsen et al[25] demonstrated in their study a small increase in vessel damage with CME compared to conventional surgery. The mean age of the patients in this study was 71.9 years. The mortality was 1.2%. The anastomotic leak rate was 4.4%, and these patients required reoperation. Almost 80% of the patients had no complications. Whether the surgeons should pursue even more radical surgical approaches for colonic cancer or not should be questioned.
Robotic surgery for colonic cancer and with CME has been performed. Spinoglio et al[26] demonstrated the feasibility of this procedure and also showed quite acceptable survival data. The advantage of robotic surgery is of a more technical nature. The more natural flexion and “wrist” like instruments make it easier to perform surgery and an intra-corporal anastomosis. An experienced laparoscopic surgeon might not perform better using the robot. The increased cost of the robotic platform makes it difficult to implement when the evidence of the “real” advantages does not exist. It was published in two CME-review articles in 2017. Emmanuel et al[27] concluded that there is a reasonable basis for the technique, but that there are no randomized trials from which to draw conclusions. There is no high quality evidence to recommend CME as the gold standard in colonic cancer surgery[27]. Gouvas et al[28] found evidence for a better surgical specimen after CME surgery. There are more lymph nodes and more tissue excised, which is important for a better surgical outcome. They admit, however, that there is limited evidence for an improved survival outcome after CME[28]. There might be a problem with a randomised trial of CME. What will be the other group? There is hope for a standardisation of the surgical technique in colonic cancer. This can also result in improvement of the survival of colonic cancer globally.
In this paper, we presented data on the long-term survival outcome of patients with colonic cancer operated on by laparoscopic CME. The 5-year TTR was 83.1% in TNM stage I-III patients. For TNM stage II and III, it was 87.3% and 69.5% respectively. The 5-year cancer specific survival (CSS) was 90.3%. For TNM stage II and III, it was 94.4% and 77% respectively. In both unadjusted and adjusted survival analysis, anastomotic leak was a significant variable together with TNM stage. To the best of our knowledge, this is the first paper to show an inferior survival with anastomotic leaks in laparoscopic CME surgery for colonic cancer. The limitations of this study are the sample size, the confounding factor of the selection process of patients to the laparoscopic procedure and the lack of analysis of the patients after the intention to treat principle.
It is important to perform optimal radical surgery with avoidance of anastomotic leaks in patients with colon cancer. It is also important to avoid an anastomotic leak in the patients with colonic cancer. Laparoscopic mesocolic excision (CME) is feasible and shows acceptable long-term oncological outcomes.
COMMENTS
Background
Surgery in colonic cancer has improved over the last decade. There has been an increased interest in more standardized and more extensive or radical surgery. The Japanese surgeons have standardized their resections according to the depth of tumour growth. T3 and T4 tumours are treated with D3 excision in the central area and complete mesocolic excision (CME) around the tumour and mesocolon. T1 and T2 tumours are treated less radical with a D2 excision. The CT scan is the basis for staging preoperatively and for the extent of resection. Laparoscopic surgery for colonic cancer has been proven to be not inferior to open surgery. The most important issue is the oncologic technique and the standardisation of this technique and not the open or laparoscopic approach. The benefit of laparoscopc surgery in the short term is proven in many papers and population studies. If the surgeon has the laparoscopic skills, the preferred approach for colonic cancer is by laparoscopy. The CME technique is a promising approach for colonic cancer. The evidence is building up but there are no randomised trials comparing CME + central vascular ligature (CVL) and other techniques. The problem is which procedure is less radical or more “ordinary” than CME? Is it possible to conduct a randomised trial?
Research frontiers
The authors hope for population based studies in the future comparing CME with some other technique or no standardised colonic cancer surgery. The authors have initiated a randomised trial together with Haukeland University Hospital, comparing laparoscopic CME + CVL and extended D3 excision with open surgery. The authors hope that this trail will show the extent of radical surgery needed to perform a safe and proper oncological resection for colonic cancer. What is enough? Open surgery and extended D3 excision can potentially be harmful to the patients. The authors need to evaluate this issue over the next years.
Innovations and breakthroughs
Robotic surgery is already implemented in many hospitals and more and more resections are being performed for colonic cancer. Robotic surgery is only an alternative approach in minimal invasive surgery but proponents claim that patients can have even better short term effects with the robot. There is still no evidence showing improvement in any regard with the use of the robot compared to laparoscopic surgery for colonic cancer. There is an increasing focus on mapping the vasculature around the right colon and this improves the planning before the surgery. The surgeon is delivered more knowledge about the vessels but also the tumour and lymph nodes based on improved radiological services. For the more advanced tumours of the colon (T3 and T4) there is promising studies around using neoadjuvant chemotherapy. This treatment can downstage these tumours and make surgery possible without resection of other organs. Some patients who is node positive before surgery can be node negative after treatment and then they do not need chemotherapy treatment after surgery.
Applications
This is a quite big cohort of patients operated with laparoscopic CME and CVL. The survival results show a decent 5-year survival. The short-term effect is quite good with a low mortality and few complications. These results are building up the evidence in favour of laparoscopic CME + CVL as the new “gold” standard for colonic cancer surgery.
Terminology
CME is a surgical method which focuses on doing surgery in embryological planes without tearing the planes. The standardisation of the surgery is important. CVL and D3 surgery in the central area are almost the same.
Peer-review
Well written paper on a topic of interest.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Norway
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study is part of a prospective project at Haraldsplass Deaconess Hospital. The Regional Ethical Committee for Medical Research in Western Norway approved the study. Consequently, all necessary approvals were gained from other official bodies connected with medical and biological research approval in Norway.
Clinical trial registration statement: The clinical trial is registered with clinicaltrials.gov (NCT00963352).
Informed consent statement: All study participants, or their legal guardian, provided written consent prior to study enrolment.
Conflict-of-interest statement: The authors of this manuscript have no conflict- of- interest to disclose.
Data sharing statement: There is no additional data available.
Peer-review started: June 12, 2017
First decision: July 11, 2017
Article in press: September 15, 2017
P- Reviewer: Isik A, Koda K, Rubbini M S- Editor: Ji FF L- Editor: A E- Editor: Zhao LM
Contributor Information
Kristian Eeg Storli, Department of Surgery, Haraldsplass Deaconess Hospital, Department of Clinical Medicine, University of Bergen, Bergen 5009, Norway.
Kristin Bentung Lygre, Department of Surgery, Haraldsplass Deaconess Hospital, Department of Clinical Medicine, University of Bergen, Bergen 5009, Norway.
Knut Børge Iversen, Department of Surgery, Haraldsplass Deaconess Hospital, Bergen 5009, Norway.
Maria Decap, Department of Surgery, Haraldsplass Deaconess Hospital, Bergen 5009, Norway.
Geir Egil Eide, Centre for Clinical Research, Haukeland University Hospital, Department of Global Public Health and Primary Care, University of Bergen, Bergen 5009, Norway.
References
- 1.Hohenberger W, Reingruber B, Merkel S. Surgery for colon cancer. Scand J Surg. 2003;92:45–52. doi: 10.1177/145749690309200107. [DOI] [PubMed] [Google Scholar]
- 2.Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354–364; discussion 364-365. doi: 10.1111/j.1463-1318.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y, Kitano S; Japan Clinical Oncology Group Colorectal Cancer Study Group. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg. 2014;260:23–30. doi: 10.1097/SLA.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 4.West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol. 2012;30:1763–1769. doi: 10.1200/JCO.2011.38.3992. [DOI] [PubMed] [Google Scholar]
- 5.Søndenaa K, Quirke P, Hohenberger W, Sugihara K, Kobayashi H, Kessler H, Brown G, Tudyka V, D’Hoore A, Kennedy RH, et al. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery: proceedings of a consensus conference. Int J Colorectal Dis. 2014;29:419–428. doi: 10.1007/s00384-013-1818-2. [DOI] [PubMed] [Google Scholar]
- 6.West NP, Hohenberger W, Finan PJ, Quirke P. Mesocolic plane surgery: an old but forgotten technique? Colorectal Dis. 2009;11:988–989. doi: 10.1111/j.1463-1318.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 7.Gouvas N, Pechlivanides G, Zervakis N, Kafousi M, Xynos E. Complete mesocolic excision in colon cancer surgery: a comparison between open and laparoscopic approach. Colorectal Dis. 2012;14:1357–1364. doi: 10.1111/j.1463-1318.2012.03019.x. [DOI] [PubMed] [Google Scholar]
- 8.Bae SU, Saklani AP, Lim DR, Kim DW, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol. 2014;21:2288–2294. doi: 10.1245/s10434-014-3614-9. [DOI] [PubMed] [Google Scholar]
- 9.Storli KE, Søndenaa K, Furnes B, Eide GE. Outcome after introduction of complete mesocolic excision for colon cancer is similar for open and laparoscopic surgical treatments. Dig Surg. 2013;30:317–327. doi: 10.1159/000354580. [DOI] [PubMed] [Google Scholar]
- 10.Storli KE, Eide GE. Laparoscopic Complete Mesocolic Excision versus Open Complete Mesocolic Excision for Transverse Colon Cancer: Long-Term Survival Results of a Prospective Single Centre Non-Randomized Study. Dig Surg. 2017;33:114–120. doi: 10.1159/000442716. [DOI] [PubMed] [Google Scholar]
- 11.Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. 7th edition. International Union Against Cancer. Hoboken, New York: Wiley-Blackwell; 2009. pp. 100–105. [Google Scholar]
- 12.Punt CJ, Buyse M, Köhne CH, Hohenberger P, Labianca R, Schmoll HJ, Påhlman L, Sobrero A, Douillard JY. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst. 2007;99:998–1003. doi: 10.1093/jnci/djm024. [DOI] [PubMed] [Google Scholar]
- 13.Gosset J, Bonvallet JM, Dautry P. [Data from a statistical study of a hospital (1954-5)] Mem Acad Chir (Paris) 1956;82:429–437. [PubMed] [Google Scholar]
- 14.Fay MP, Proschan MA. Wilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat Surv. 2010;4:1–39. doi: 10.1214/09-SS051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Am Stat Assoc. 1958;(53):457–481. [Google Scholar]
- 16.Cox D. Regression models and life tables. Journal R Stat Soc B. 1972;(34):187–220. [Google Scholar]
- 17.Liang J, Fazio V, Lavery I, Remzi F, Hull T, Strong S, Church J. Primacy of surgery for colorectal cancer. Br J Surg. 2015;102:847–852. doi: 10.1002/bjs.9805. [DOI] [PubMed] [Google Scholar]
- 18.Willaert W, Ceelen W. Extent of surgery in cancer of the colon: is more better? World J Gastroenterol. 2015;21:132–138. doi: 10.3748/wjg.v21.i1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16:161–168. doi: 10.1016/S1470-2045(14)71168-4. [DOI] [PubMed] [Google Scholar]
- 20.Storli KE, Søndenaa K, Furnes B, Nesvik I, Gudlaugsson E, Bukholm I, Eide GE. Short term results of complete (D3) vs. standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I-II. Tech Coloproctol. 2014;18:557–564. doi: 10.1007/s10151-013-1100-1. [DOI] [PubMed] [Google Scholar]
- 21.Storli K, Søndenaa K, Furnes B, Leh S, Nesvik I, Bru T, Gudlaugsson E, Bukholm I, Norheim-Andersen S, Eide G. Improved lymph node harvest from resected colon cancer specimens did not cause upstaging from TNM stage II to III. World J Surg. 2011;35:2796–2803. doi: 10.1007/s00268-011-1248-7. [DOI] [PubMed] [Google Scholar]
- 22.Cho MS, Baek SJ, Hur H, Soh Min B, Baik SH, Kyu Kim N. Modified complete mesocolic excision with central vascular ligation for the treatment of right-sided colon cancer: long-term outcomes and prognostic factors. Ann Surg. 2015;261:708–715. doi: 10.1097/SLA.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 23.Feng B, Sun J, Ling TL, Lu AG, Wang ML, Chen XY, Ma JJ, Li JW, Zang L, Han DP, et al. Laparoscopic complete mesocolic excision (CME) with medial access for right-hemi colon cancer: feasibility and technical strategies. Surg Endosc. 2012;26:3669–3675. doi: 10.1007/s00464-012-2435-9. [DOI] [PubMed] [Google Scholar]
- 24.Ignjatovic D, Sund S, Stimec B, Bergamaschi R. Vascular relationships in right colectomy for cancer: clinical implications. Tech Coloproctol. 2007;11:247–250. doi: 10.1007/s10151-007-0359-5. [DOI] [PubMed] [Google Scholar]
- 25.Bertelsen CA, Neuenschwander AU, Jansen JE, Kirkegaard-Klitbo A, Tenma JR, Wilhelmsen M, Rasmussen LA, Jepsen LV, Kristensen B, Gögenur I; Copenhagen Complete Mesocolic Excision Study (COMES); Danish Colorectal Cancer Group (DCCG) Short-term outcomes after complete mesocolic excision compared with ’conventional’ colonic cancer surgery. Br J Surg. 2017;103:581–589. doi: 10.1002/bjs.10083. [DOI] [PubMed] [Google Scholar]
- 26.Spinoglio G, Marano A, Bianchi PP, Priora F, Lenti LM, Ravazzoni F, Formisano G. Robotic Right Colectomy with Modified Complete Mesocolic Excision: Long-Term Oncologic Outcomes. Ann Surg Oncol. 2017;23:684–691. doi: 10.1245/s10434-016-5580-x. [DOI] [PubMed] [Google Scholar]
- 27.Emmanuel A, Haji A. Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: is it worth that extra effort? A review of the literature. Int J Colorectal Dis. 2017;31:797–804. doi: 10.1007/s00384-016-2502-0. [DOI] [PubMed] [Google Scholar]
- 28.Gouvas N, Agalianos C, Papaparaskeva K, Perrakis A, Hohenberger W, Xynos E. Surgery along the embryological planes for colon cancer: a systematic review of complete mesocolic excision. Int J Colorectal Dis. 2017;31:1577–1594. doi: 10.1007/s00384-016-2626-2. [DOI] [PubMed] [Google Scholar]


