Abstract
Enteroviruses can cause outbreaks of severe acute respiratory illness (SARI) and EV‐A, ‐B, ‐C, and ‐D species have different pathogenic profiles and circulation patterns. We aimed to characterize and determine the prevalence of enterovirus genotypes among South African patients with respiratory illness and controls during June 2012 to July 2014. Syndromic SARI and influenza‐like illness (ILI) surveillance was performed at two sentinel sites. At each site nasopharyngeal/oropharyngeal specimens were collected from SARI and ILI patients as well as controls. Specimens were tested for enterovirus by real‐time PCR. Positive specimens were further genotyped by sequencing a region of the VP1 gene. The prevalence of enterovirus was 5.8% (87/1494), 3.4% (103/3079), and 3.4% (46/1367) among SARI, ILI, and controls, respectively (SARI/controls, P = 0.002 and ILI/control, P = 0.973). Among the 101/236 (42.8%) enterovirus‐positive specimens that could be genotyped, we observed a high diversity of circulating enterovirus genotypes (a total of 33 genotypes) from all four human enterovirus species with high prevalence of Enterovirus‐B (60.4%; 61/101) and Enterovirus‐A (21.8%; 22/101) compared to Enterovirus‐C (10.9%; 11/101) and Enterovirus‐D (6.9%; 7/101) (P = 0.477). Of the enterovirus genotypes identified, Echovirus 30 (9.9%, 10/101), Coxsackie virus B5 (7.9%, 8/101) and Enterovirus‐D68 (6.9%, 7/101) were most prevalent. There was no difference in disease severity (SARI or ILI compared to controls) between the different enterovirus species (P = 0.167). We observed a high number of enterovirus genotypes in patients with respiratory illness and in controls from South Africa with no disease association of EV species with disease severity.
Keywords: enterovirus, influenza‐like illness, severe acute respiratory illness, South Africa
1. INTRODUCTION
Pneumonia is the leading cause of death in children globally and is the most common reason for hospitalization among African children.1, 2 Respiratory viruses associated with acute respiratory tract infections include influenza virus A and B, respiratory syncytial virus, parainfluenza virus types 1, 2, and 3, adenovirus and human metapneumovirus. Other respiratory viruses putatively associated with pneumonia in children also include enteroviruses, rhinovirus, human bocavirus, and human coronaviruses (229E, NL63, OC43, HKU1), although this remains controversial.3, 4 Although influenza and respiratory syncytial viruses have been well described as causes of pneumonia in South Africa, little is known about enterovirus prevalence and circulating genotypes.5, 6
Enteroviruses are members of the enterovirus genus in the family Picornaviridae.7 The capsid protein VP1, the most variable protein containing the majority of neutralization epitopes, is commonly used to characterize enteroviruses.7, 8, 9 More than 100 enterovirus genotypes are currently classified into four species, HEV‐A, HEV‐B, HEV‐C, HEV‐D, which have different pathogenic profiles and circulation patterns.7, 10
Enteroviruses (EV) can cause symptoms similar to a mild cold, but have also been associated with severe respiratory infection requiring hospitalisation and may be fatal.10 An outbreak of a re‐emerging EV‐D68 lineage causing severe acute respiratory illness (SARI) occurred in the USA and Canada in 2014, and these lineages were also detected in Europe, Asia, and South America from 2012 to 2013 samples.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Though EVs have been associated with SARI and influenza‐like illness (ILI),5 these viruses have been less well characterized in South Africa. We identified high diversity among EVs circulating in hospitalized South African patients,25 however it is unknown whether different EV genotypes are associated with mild or severe respiratory illness in South Africa.
We aimed to characterize the EV genotypes circulating among South African patients with SARI, ILI, and asymptomatic individuals from June 2012 to July 2014. In addition we assessed the association of different EV species with mild (ILI) or severe (SARI) illness compared to asymptomatic individuals.
2. METHODS
2.1. Study population and setting
2.1.1. Severe acute respiratory illness (SARI) surveillance
We conducted prospective, hospital‐based, SARI surveillance from June 2012 to July 2014 at two sites (Klerksdorp‐Tshepong Hospital Complex, Klerksdorp, North West Province, and Edendale Hospital, Pietermaritzburg, KwaZulu‐Natal Province) in South Africa.
A case of SARI was defined as a hospitalized individual with duration of symptoms ≤10 days, meeting age‐specific clinical inclusion criteria as follows: children aged 2 days through <3 months with physician‐diagnosed sepsis or lower respiratory tract infection (LRTI), children aged 3 months through <5 years with physician‐diagnosed LRTI and patients aged ≥5 years meeting a modified World Health Organization (WHO) case definition for SARI: (i) fever (>38°C) or reported fever; (ii) cough or sore throat; and (iii) shortness of breath, or difficulty breathing, with or without clinical or radiographic findings of pneumonia. Demographic and clinical characteristics were collected from all patients.
2.1.2. Influenza‐like illness (ILI) surveillance
Prospective surveillance for cases presenting with ILI were done at two outpatient clinics (Jouberton Clinic, North West Province, and Edendale Gateway Clinic, KwaZulu‐Natal Province) located in the same catchment area to the above‐mentioned hospitals from July 2012 through June 2014. An ILI case was defined as an outpatient of any age presenting with either temperature >38°C or, history of fever and cough of duration ≤10 days.5
2.1.3. Asymptomatic individuals
An asymptomatic individual or control was defined as a person presenting at the same outpatient clinics with no history of fever, respiratory, or gastrointestinal symptoms during the 14 days preceding the visit. These individuals commonly presented to the clinics for visits such as dental procedures, family planning, well baby visits, voluntary HIV counseling and testing, or acute care for non‐febrile illnesses. We aimed to enroll one HIV‐infected and one HIV‐uninfected control every week in each clinic within each of the following age categories: 0‐1, 2‐4, 5‐14, 15‐54, and ≥55 years. Individuals were not followed‐up to ensure they remained asymptomatic after enrollment.
2.1.4. Study procedures
Study staff completed case report forms for all enrolled SARI and ILI cases as well as controls. In addition, for SARI cases, hospital records were reviewed to assess disease progression and outcome (ie, discharge, transfer, or in‐hospital death). Referral to hospital was recorded for all enrolled ILI cases. ILI cases that were referred to hospital were excluded from the analysis, as these patients did not adhere to the case definition of ILI anymore.
2.1.5. Determination of HIV status
HIV results were obtained from a combination of two sources: (i) patient clinical records when available and (ii) for consenting patients, an anonymized linked dried blood spot was tested at the National Institute for Communicable Diseases (NICD). When both results were available, the NICD result was used.
2.2. Samples and laboratory procedures
Nasopharyngeal aspirates for children <5 years of age and nasopharyngeal and oropharyngeal swabs from persons ≥5 years of age were collected from all enrolled participants and placed in universal transport medium (Copan, Murruieta, CA).
All respiratory tract specimens were tested for the presence of 10 respiratory viruses (influenza virus A and B, respiratory syncytial virus, parainfluenza virus types 1, 2, and 3, adenovirus, human metapneumovirus, and rhinovirus) including EV, using a multiplex real‐time reverse transcriptase polymerase chain reaction assay.26 The real‐time reverse transpriptase polymerase chain reaction used for EV testing, is a sensitive method for the routine detection of all members of the enterovirus genus. This method has a weak cross‐reactivity to high‐titre rhinovirus stocks, but not with rhinovirus‐positive clinical samples.27
2.3. Amplification and sequencing of enterovirus VP1 gene
All specimens testing positive for enterovirus were selected for molecular characterization. A 400 base pair region of the VP1 gene was amplified and sequenced as previously described using primer set 224/222 during the first round of amplification and primer set AN89/AN88 for the nested amplification.8 This assay was originally designed to detect and identify EV, but also detects and identifies a subset of rhinoviruses.
Amplicons were purified using the ExoSAP enzyme system (Thermo Fisher Scientific, Waltham, MA) and sequenced using the Big‐Dye Terminator version 3.1 Cycle Sequencing Ready Reaction kit (Life Technologies, Carlsbad, CA) using nested primers. Sequencing reactions were purified with the BigDye® XTerminator Purification kit (Life Technologies, Carlsbad, CA) and resolved on the 3130XL Genetic Analyzer (Life Technologies, Carlsbad, CA). Sequences were analyzed and assembled using Sequencher® version 5 (Gene Codes Corporation, Ann Arbor, MI).
2.4. Phylogenetic analysis
The genetic diversity of each VP1 sequence was first determined by comparison with the reference strains in GenBank (US National Center for Biotechnology Information, NCBI, http://www.ncbi.nlm.nih.gov/, accessed 01 July 2016) using BLAST (Basic Local Alignment Search Tool) and confirmed by phylogenetic analysis of the partial VP1 sequences. Multiple sequence alignments were generated in the MAFFT (Multiple Alignment using Fast Fourier Transform) multiple sequence alignment program28 and analyzed in the BioEdit Sequence Alignment Editor Software.29 Phylogenetic trees were generated using the neighbor‐joining method and genetic distances were calculated with the Kimura‐2‐parameter model using MEGA (Molecular Evolutionary Genetics Analysis) 6 software.30
The statistical significance of the phylogenies was estimated by bootstrap analysis using 1000 pseudo replicates. Sub analysis was similarly done for the three dominant EV species in this study. Sequences of enterovirus partial VP1 genes generated in this study have been deposited in GenBank with the following accession numbers: KX940982‐KX941096.
2.5. Statistical analysis
The Chi‐squared or Fisher's exact test were used for comparison of categorical variables. Unconditional exact logistic regression was used to assess the association of EV species with disease severity among patients with mild (ILI) or severe illness (SARI) using asymptomatic individuals as control group. Exact logistic regression was used to account for the fact that no EV‐D species was detected among controls. EV‐B was used as the comparison group as it was the species most frequently detected. Significance was assessed for P < 0.05. All models were adjusted for age, HIV serostatus and underlying medical conditions. The analysis was performed using STATA 14.1 (StataCorp, College Station, TX).
2.6. Ethical approval
The SARI protocol was approved by the University of the Witwatersrand Human Research Ethics Committee (HREC) and the University of KwaZulu‐Natal Human Biomedical Research Ethics Committee (BREC), protocol numbers M081042 and BF157/08, respectively. The ILI and control protocol was approved by HREC and BREC protocol numbers M120133 and BF080/12, respectively. This surveillance was deemed non‐research by the US Centers for Disease Control and Prevention.
3. RESULTS
3.1. SARI epidemiology of the study population
During the study period we enrolled 1525 patients with SARI, 3088 with ILI, and 1401 controls, of which 1494 (97.60%), 3079 (99.7%), and 1367 (97.6%) were tested and had EV results available, respectively. The detection rate for enterovirus was 5.8% (87/1494), 3.4% (103/3079), and 3.4% (46/1367) among SARI, ILI, and controls, respectively (SARI/controls, P = 0.002 and ILI/control, P = 0.973). In children <1 year of age, the majority (61.1%, 44/72) of EV positive samples presented with SARI while in the following age groups: 1‐4 years (43.1%, 50/116), 5‐18 years (53.9%, 14/26), and >18 years (81.8%, 18/22) the majority of patients presented with ILI (P < 0.001). Among EV positive samples stratified in age groups above, HIV infection was not significant in any of the cases or controls (data not shown). We observed that among EV‐positive SARI cases with documented disease progression and outcome, 3.9% (2/51) were admitted to ICU and 2% (1/50) died however the causal role of EV infection in adverse outcomes could not be addressed in this study.
EV was detected throughout the year among SARI and ILI cases and controls (Fig. 1A‐C), with peaks above 10% from the detection rate during February‐March, August and November.
Figure 1.
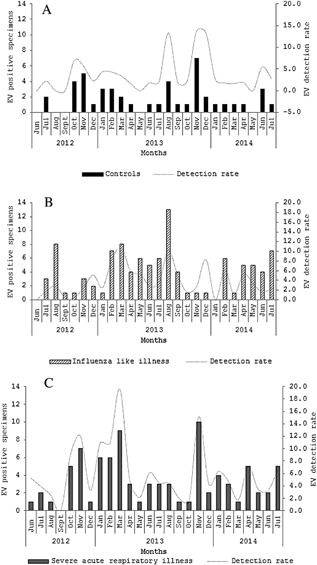
Monthly number and overall detection rate of enterovirus (EV)‐positive cases among controls (A), influenza‐like illness (B), and severe acute respiratory patients (C), Klerksdorp and Pietermaritzburg, South Africa, 2012–2014
3.2. Phylogenetic comparison of EV genotypes identified during June 2012‐July 2014
Of the 236 EV‐positive specimens detected during the study period among SARI and ILI cases and controls, 30.1% (71/236) were excluded from VP1 RT‐PCR attempts, since the EV load in these samples was considered low (Ct‐value >35) and the attempt has a higher probability of failure. An additional 16.5% (39/236) of samples were excluded from VP1 RT‐PCR and sequencing attempts, because the original specimen volume was insufficient for RNA extraction. Of the EV‐positive samples that were sequenced (53.4%, 126/236), 7.9% (10/126) could not be typed. Possible reasons for typing failure included failed sequencing reactions, mixed EV infections (co‐infections) and RV that was detected on the initial EV screen real‐time PCR assay. RV were identified in 11.9% (15/126) samples, and were excluded from the EV phylogenetic analysis. Therefore, only 101 EV‐positive samples were used for subsequent phylogenetic comparison.
Neighbor‐joining phylogenetic comparison of EVs detected in clinical samples from June 2012 to July 2014 to international reference sequences indicated that EV‐B (60.4%; 61/101) and EV‐A (21.8%; 22/101) were more commonly identified than EV‐C (10.9%; 11/101) and EV‐D (6.9%; 7/101) (Supplementary Fig. S1). The majority of EV‐A (40.9%, 9/22) and EV‐D (57.1%, 4/7) were detected among ILI cases and the majority of EV‐B (55.7%, 34/61) and EV‐C (54.6%, 6/11) were detected among SARI cases (P = 0.03).
On multivariable logistic regression analysis using EV species B as the reference group and controlling for age, HIV status and underlying illness, no association with disease severity (SARI or ILI compared to controls) was identified for any of the EV species (Supplementary Table S1).
3.3. EV group and genotypes
Enterovirus genotypes could be identified in 59% (51/87), 33% (34/103), and 35% (16/46) of enterovirus‐positive samples among SARI and ILI cases and controls, respectively. We identified a total of 33 genotypes, distributed among all four EV species. The most prevalent genotype was E30 (9.9%, 10/101), followed by CVB5 (7.9%, 8/101) and EV‐D68 (6.9%, 7/101). All other genotypes identified in the study were detected in <5% of genotyped samples (Supplementary Fig. S2).
Coxsackievirus A14 (CVA14), Echovirus 11 (E11), Echovirus 16 (E16), Echovirus 17 (E17), Echovirus 19 (E19), Echovirus 7 (E7), Echovirus 9 (E9), poliovirus 1 (Sabin vaccine strain), poliovirus 3 (Sabin vaccine strain) were detected only among SARI cases and Coxsackievirus A10 (CVA10), Coxsackievirus A16 (CVA16), Coxsackievirus B1 (CVB1), Echovirus 32 (E32), and Coxsackievirus A20 (CVA20) were detected only among ILI cases. CVB5, E30, Echovirus 6 (E6) and EV‐D68 were detected among SARI and ILI cases only (Supplementary Fig. S2).
EV‐A, EV‐B, EV‐C, and EV‐D genotypes co‐circulated in 2012‐2014, while EV‐D68 was detected sporadically in March‐May in 2013 and 2014 only (Fig. 2). The majority of EV positive samples that could be genotyped, were from children aged <5 years (89.1%, 90/101) in the EV‐B species (60.4%, 61/101) compared to the other EV species, but were not statistically significant (P = 0.08).
Figure 2.
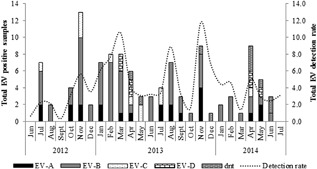
Distribution of enterovirus (EV) species (A‐D) in South Africa during June 2012 to July 2014 (dnt = did not genotype)
3.4. Molecular evolution of E30, CVB5, and EV‐D68 genotypes
3.4.1. E30
Phylogenetic analysis of E30 samples showed that all E30 samples from this study clustered with a single Australian strain from 2000 (GU232849) with 98% bootstrap support. Following the classification convention for E30 as described by Bailly et al,31 we propose to call this sub cluster genotype k. The nucleotide sequences for E30 strains were downloaded from GenBank and sequences depicted in Fig. 3 represent all known genotypes.
Figure 3.
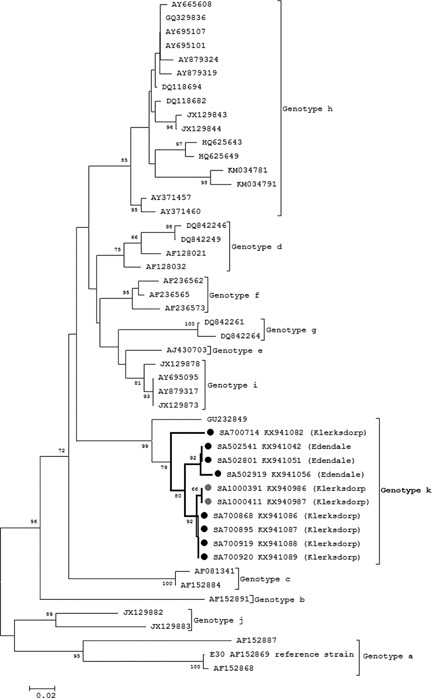
Phylogenetic analysis of E30 serotypes by neighbor‐joining phylogenetic analysis of the partial VP1 region, South Africa, 2012‐2014. Sequences with black closed circles denote serotypes identified in SARI patients and those with grey closed circles denote serotypes identified in ILI patients. Bootstrap values (1000 replicates) are shown on the branches, with values <65% omitted from the tree. The scale indicates number of base substitutions per site
All E30 strains identified in this study displayed >98% nucleotide (nt) homology and formed a distinct bi‐phyletic cluster with 79% bootstrap support (Fig. 3). In the bi‐phyletic cluster, strains from Edendale SARI cases and Klerksdorp SARI and ILI cases are distinctly located in each of the sub clusters with 92% bootstrap support, respectively.
The E30 strains in our study differ from other E30 genotypes by mean nucleotide pairwise distance of 20.2% (15.6‐27.5) and mean amino acid sequence distance of 6% (2.7‐11.6) in amino acid sequence.
3.4.2. CVB5
All CVB5 strains identified in our study (six SARI samples and two ILI samples from Edendale) were categorized as genogroup C and formed a distinct sub cluster with a bootstrap value of 91% (Fig. 4). The nucleotide sequences for CVB5 strains were downloaded from GenBank and sequences depicted in Fig. 4 represent all known genogroups.
Figure 4.
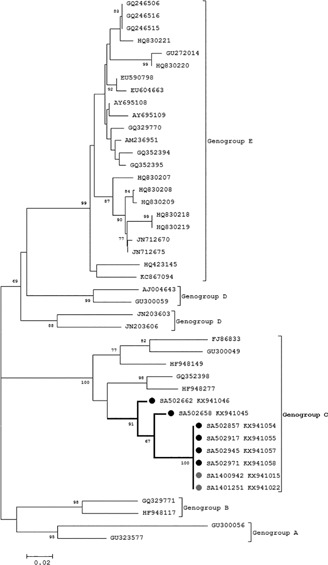
Phylogenetic analysis of CVB5 genogroups by neighbor‐joining phylogenetic analysis of the partial VP1 region, South Africa, 2012‐2014. Sequences with black closed circles denote serotypes identified in SARI patients and those with grey closed circles denote genotypes identified in ILI patients. Bootstrap values (1000 replicates) are shown on the branches, with values <65% omitted from the tree. The scale indicates number of base substitutions per site
6/8 samples (four SARI and two ILI samples) were identical (nt homology 100%). All of our CVB5 strains (identified in this study) displayed close genetic relationships with samples identified in surface water from Belarus, 2007 (GQ352398) and feces from Germany, 2009 (HF948277).
Sequences from the South African CVB5 strains differed from the other CVB5 genogroup C sequences by 13.4% in pairwise nucleotide sequence distance and 3% in pairwise amino acid sequence. Samples SA 502662 and SA 502658 which were not identical to the other South African sequences were sampled in 2012 and 2013, respectively.
3.4.3. EV‐D68
The majority (71.4%, 5/7) of EV‐D68 strains identified in 2013/2014 distinctly clustered in lineage B2 (89% bootstrap support) of which three were ILI cases and two were SARI cases, together with strains from one of the two co‐circulating EV‐D68 lineages that caused the large 2014 USA outbreak (Fig. 5). The nucleotide sequences for EVD‐68 strains were downloaded from GenBank and sequences depicted in Fig. 5 represent all known lineages.
Figure 5.
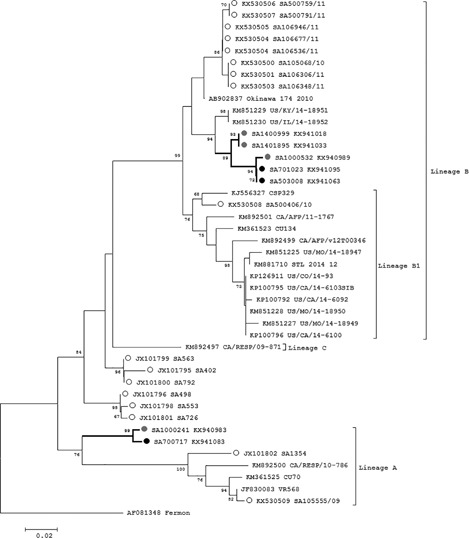
Phylogenetic analysis of EV‐D68 lineages by neighbor‐joining phylogenetic analysis of the partial VP1 region, South Africa, 2012‐2014. Sequences with black closed circles denote serotypes identified in SARI patients and those with grey closed circles denote serotypes identified in ILI patients. Sequences with open circles are EV‐D68 strains previously identified from South Africa. Bootstrap values (1000 replicates) are shown on the branches, with values <65% omitted from the tree. The scale indicates number of base substitutions per site
The lineage B EV‐D68 strains in our study differed from other lineage B EV‐D68 strains by 1.5% nucleotide and 1.4% amino acid sequence. These included two SARI cases (one from Klerksdorp and one from Edendale) and three ILI cases (one from Klerksdorp and two from Edendale).
The other South African EV‐D68 strains (28.6%, 2/7) belonged to lineage A forming a unique cluster. The two strains from 2013 are from the same geographic location and clustered with 99% bootstrap support. The lineage A EV‐D68 strains in our study differed from other lineage A EV‐D68 strains by 12.8% nucleotide and 3.0% amino acid sequence. The 2012‐2014 South African samples did not cluster with EV‐D68 samples previously identified in South African patients.25, 32
4. DISCUSSION
We describe the EV species circulating among SARI and ILI cases and asymptomatic controls in South Africa. We observed a high diversity of circulating EV genotypes (a total of 33 genotypes) from EV species A‐D with high circulation rates for EV‐B and EV‐A compared to EV‐C and EV‐D.
From our systematic surveillance across all age groups, the majority of patients testing positive for EV were <5 years of age. We did not observe any difference in disease association due to different EV species since EV disease association cannot be conclusively ascribed since the analysis did not include co‐infections with other pathogens. Epidemics of human EV disease display a seasonal pattern, with infections more common in summer and early autumn in geographical regions with a temperate climate.7, 33 Most clusters of EV‐D68 showed an atypical late seasonality compared to other EVs, with a peak in autumn, instead of summer.7 Outbreaks of EV disease in autumn, early or late, are typical. Distribution of EV‐A through ‐D in most temperate zones is from spring through autumn, with peak disease in most years in late summer‐autumn. This study showed similarities in the seasonal distribution of the EV species detected in South Africa as EV species A‐D were detected throughout the year, but EV‐D68 was sporadically detected in autumn (March‐May) during 2013‐2014.
E30, CVB‐5, and EV‐D68 were the genotypes most frequently identified in this study. Within a genotype, EVs are deemed relatively uniform, as was observed in our study for E30 and CVB5. However, some studies have suggested variation within a genotype,34 such as was observed for EV‐D68 in our study.
E30 is the most common EV strain responsible for sporadic as well as large‐scale outbreaks of aseptic meningitis in many countries of Europe and North America.31 E30 causes large outbreaks of aseptic meningitis that attracts public health attention and as a result there is a rich and large database of E30 VP1 sequences regarding aseptic meningitis, E30 has however not been associated with respiratory illness.
A common feature of E30 molecular epidemiology is the progression of circulating lineages within one prevalent genotype.9, 31 The E30 strains identified in this study clustered together (designated genotype k) although no differences were observed between viruses from SARI compared to ILI cases. There is no report of a concurrent E30 aseptic meningitis outbreak at the same time the E30 NP/OP positives were detected. It has been reported that genotypes of E30 can become dominant for 3‐4‐year periods before they disappear from circulation,34 and they are therefore known as epidemic strains. In our study, it seems that E30 genotype k became dominant and circulated in South Africa during 2012‐2014. Phylogenetic studies with VP1 sequences indicate that E30 variants are continuously emerging and replacing the circulating E30 strains, largely due to the error prone EV RNA polymerase31.
CVB5 has been detected for over 50 years and, similar to E30, sporadic cases of aseptic meningitis, as well as outbreaks, have been reported, remaining one of the most predominant reported EV genotypes in a number of countries.35 All of the CVB5 strains identified in this study formed a sub cluster in genogroup C. Phylogenetic clustering by year of study enrolment was observed for the South African strains. Different co‐circulating strains of EV are often observed in EV disease outbreaks as EV genomes vary with respect to time but may also vary by geographical distribution.36
In 2014, the largest outbreak to date of severe respiratory illness associated with EV‐D68 occurred in the USA, and almost all of the case patients were children.37 Coinciding with the 2014 USA outbreak there was an increased number of reports of children with sudden onset acute flaccid myelitis (AFM). Some recent South African EV‐D68 strains clustered with USA 2014 lineage B2 EV‐D68 strains. The USA 2014 lineage B2 co‐circulated with lineage B1 EV‐D68 during the large 2014 USA outbreak. EV‐D68 lineages B1 and B2 are both temporally associated with AFM.38 The majority of EV‐D68 strains identified in our study clustered in lineage B2 and the remaining samples clustered in lineage A, forming a unique cluster. The 2012‐2014 South African samples did not cluster with strains previously identified in South Africa during 2009‐2011.
The mechanisms for emergence of EV‐associated outbreaks are not known; however, a combination of virus‐specific, population‐level, and other external factors are likely to be involved.7
Our study has some limitations. Previous studies observed a difference in the predominant EV genotypes among neonates and older children39 but we were not powered for this analysis. Some rhinovirus sequences were detected in samples that tested positive for EV on our in‐house assay indicating that our EV detection assay is not 100% specific to detect only EVs. This study was not designed to describe the role of co‐infections with other respiratory pathogens. In addition the association with EV detection with mild and severe illness was not investigated.
In conclusion, we showed that there was a high diversity in the VP1 sequences of the EV species circulating in South Africa during 2012‐2014 and most of these genotypes are associated with meningitis worldwide. EV was detected in outpatient and hospitalized patients with SARI but was also detected in controls. We determined no disease association of EV species with disease severity; however, some genotypes (E30, CVB5, EV‐D68) were more prevalent in symptomatic cases. Further studies are needed to determine if other factors such as viral load or host interactions play a role in EV‐associated disease as well as a robust spatiotemporal phylogenetic analysis based on complete VP1 sequences.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Figure S1. Phylogenetic analysis of EV serotypes by neighbor‐joining phylogenetic analysis of the partial VP1 region, South Africa, 2012‐2014. Sequences with black closed circles denotes serotypes identified in SARI patients, those with grey closed circles denotes serotypes identified in ILI patients while those with open circles denoted serotypes identifies in asymptomatic individuals. Bootstrap values (1000 replicates) are shown on the branches, with values <65% omitted from the tree.
Figure S2. The serotypes and species (EV‐A to EV‐D) of enteroviruses identified in patients with severe acute respiratory illness (SARI), influenza‐like illness (ILI), and asymptomatic individuals (C) from June 2012 to July 2014, South Africa (CVA10 = Coxsackie virus A10, CVA14 = Coxsackie virus A14, CVA16 = Coxsackie virus A16, CVA2 = Coxsackie virus A2, CVA4 = Coxsackie virus A4, CVA6 = Coxsackie virus A6, EV‐A71 = Enterovirus A71, CVA9 = Coxsackie virus A9, CVB1 = Coxsackie virus B1, CVB2 = Coxsackie virus B2, CVB3 = Coxsackie virus B3, CVB4 = Coxsackie virus B4, CVB5 = Coxsackie virus B5, E11 = Echovirus 11, E16 = Echovirus 16, E17 = Echovirus 17, E19 = Echovirus 19, E20 = Echovirus, E25 = Echovirus 25, E3 = Echovirus 3, E30 = Echovirus 30, E32 = Echovirus 32, E6 = Echovirus 6, E7 = Echovirus 7, E9 = Echovirus 9, EV‐B75, CVA13 = Coxsackie virus A13, CVA20 = Coxsackie virus A20, EV‐C99 = Enterovirus C99, EV‐D68 = Enterovirus D68)
Table S1. Association of EV species (EV‐A to EV‐D) with influenza‐like illness (ILI) and severe acute respiratory illness (SARI) compared with asymptomatic controls, South Africa, 2009‐2010 (bold indicate factors significant at P < 0.05)
ACKNOWLEDGMENTS
We thank all members involved in SARI and ILI surveillance for the collection of specimens and data management. This work was supported by the National Health Laboratory Service, South Africa, and the United States Centers for Disease Control and Prevention, Atlanta, Georgia, USA (co‐operative agreement number: 5U51IP000155).
CONFLICTS OF INTEREST
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of their affiliated institutions or the agencies funding the study.
Hellferscee O, Tempia S, Walaza S, et al. Enterovirus genotypes among patients with severe acute respiratory illness, influenza‐like illness, and asymptomatic individuals in South Africa, 2012‐2014. J Med Virol. 2017;89: 1759–1767. 10.1002/jmv.24869
REFERENCES
- 1. Zar H J, Madhi S A. Childhood pneumonia—progress and challenges. S Afr Med J. 2006; 96:890 –900. [PubMed] [Google Scholar]
- 2.WHO. 2015. WHO Pneumonia Fact Sheet No 331.
- 3. Tiveljung‐Lindell A, Rotzén‐Östlund M, Gupta S, et al. Development and implementation of a molecular diagnostic platform for daily rapid detection of 15 respiratory viruses. J Med Virol. 2009; 81:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brittain‐Long R, Nord S, Olofsson S, Westin J, Anderson L M, Lindh M. Multiplex real‐time PCR for detection of respiratory tract infections. J Clin Virol. 2008; 41:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pretorius M A, Tempia S, Walaza S, et al. The role of influenza, RSV and other common respiratory viruses in severe acute respiratory infections and influenza‐like illness in a population with a high HIV sero‐prevalence, South Africa 2012–2015. J Clin Virol. 2016; 75:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen A L, Hellferscee O, Pretorius M, et al. Epidemiology of influenza virus types and subtypes in South Africa, 2009‐2012. Emerg Infect Dis. 2014; 20:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pons‐Salort M, Parker E P K, Grassly N C. The epidemiology of non‐polio enteroviruses. Curr Opin Infect Dis. 2015; 28:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allan Nix W, Oberste M S, Pallansch M A. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006; 44:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oberste M S, Maher K, Kilpatrick D R, Flemister M R, Brown B A, Pallansch M A. Typing of human enteroviruses by partial sequencing ofVP1. J Clin Microbiol 1999; 37:1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tapparel C, Siegrist F, Petty T J, Kaiser L. Picornavirus and enterovirus diversity with associated human diseases. Infect Genet Evol. 2013; 14:282 –293. [DOI] [PubMed] [Google Scholar]
- 11. Huang Y P, Lin T L, Lin T H, Wu H S. Molecular and epidemiological study of enterovirus D68 in Taiwan. J Microbiol Immunol Infect. 2015; 10:115. [DOI] [PubMed] [Google Scholar]
- 12. Dyrdak R, Rotzen‐Ostlund M, Samuelson A, Eriksson M, Albert J. Coexistence of two clades of enterovirus D68 in pediatric Swedish patients in the summer and fall of 2014. Infect Dis (London, England). 2015; 47:734–738. [DOI] [PubMed] [Google Scholar]
- 13. Esposito S, Zampiero A, Ruggiero L, Madini B, Niesters H, Principi N. Enterovirus D68‐associated community‐acquired pneumonia in children living in Milan, Italy. J Clin Virol. 2015; 68:94–96. [DOI] [PubMed] [Google Scholar]
- 14. Gimferrer L, Campins M, Codina M G, et al. First enterovirus D68 (EV‐D68) cases detected in hospitalised patients in a tertiary care university hospital in Spain, October 2014. Enferm Infecc Microbiol Clin. 2015; 33:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Midgley SE, Christiansen CB, Poulsen MW, Hansen CH, Fischer TK. Emergence of enterovirus D68 in Denmark, June 2014 to February 2015. Euro Surveill Bull Eur sur les Mal Transm = Eur Commun Dis Bull. 2015; 20:pii=21105. [DOI] [PubMed] [Google Scholar]
- 16. Midgley CM, Watson JT, Nix WA, et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir Med. 2015; 3:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oermann CM, Schuster JE, Conners GP, Newland JG, Selvarangan R, Jackson MA. Enterovirus D68: a focused review and clinical highlights from the 2014 U.S. outbreak. Ann Am Thorac Soc. 2015; 12:775–781. [DOI] [PubMed] [Google Scholar]
- 18. Peci A, Winter A L, Warshawsky B, et al. Epidemiology of enterovirus D68 in Ontario. PLoS ONE. 2015; 10:e0142841. 10.1371/journal.pone.0142841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poelman R, Schölvinck E H, Borger R, Niesters H G M, Van Leer‐Buter C. The emergence of enterovirus D68 in a Dutch University Medical Center and the necessity for routinely screening for respiratory viruses. J Clin Virol. 2015; 62:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poelman R, Schuffenecker I, Van Leer‐Buter C, Josset L, Niesters HGM, Lina B. European surveillance for enterovirus D68 during the emerging North‐American outbreak in 2014. J Clin Virol. 2015; 71:1–9. [DOI] [PubMed] [Google Scholar]
- 21. Reiche J, Böttcher S, Diedrich S, et al. Low‐level circulation of enterovirus D68‐associated acute respiratory infections, Germany, 2014. Emerg Infect Dis. 2015; 21:837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bragstad K, Jakobsen K, Rojahn A E, et al. High frequency of enterovirus D68 in children hospitalised with respiratory illness in Norway, autumn 2014. Influenza Other Respi Viruses. 2015; 9:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skowronski D M, Chambers C, Sabaiduc S, et al. Systematic community‐and hospital‐based surveillance for enterovirus‐D68 in three Canadian provinces, August to December 2014. Eurosurveillance. 2015; 20:pii=30047. 10.2807/1560-7917.ES.2015.20.43.30047 [DOI] [PubMed] [Google Scholar]
- 24. Torres J P, Farfan M J, Izquierdo G, Piemonte P, Henriquez J, O'Ryan M L. Enterovirus D68 infection, Chile, Spring 2014. Emerg Infect Dis United States. 2015; 21:728 –729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hellferscee O, Treurnicht F K, Tempia S, et al. Enterovirus D68 and other enterovirus serotypes identified in South African patients with severe acute respiratory illness, 2009–2011. Influenza Other Respi Viruses. 2017; 11:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pretorius M A, Madhi S A, Cohen C, et al. Respiratory viral coinfections identified by a 10‐Plex real‐time reverse‐transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness‐South Africa, 2009–2010. J Infect Dis. 2012; 206:S159 –S165. [DOI] [PubMed] [Google Scholar]
- 27. Nijhuis M, Van Maarseveen N, Schuurman R, et al. Rapid and sensitive routine detection of all members of the genus enterovirus in different clinical specimens by real‐time PCR. J Clin Microbiol. 2002; 40:3666–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katoh F K, Standley D M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013; 30:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hall T. BioEdit: a user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999; 41:95 –98. [Google Scholar]
- 30. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007; 24:1596–1599. [DOI] [PubMed] [Google Scholar]
- 31. Bailly J L, Mirand A, Henquell C, et al. Phylogeography of circulating populations of human echovirus 30 over 50 years: nucleotide polymorphism and signature of purifying selection in the VP1 capsid protein gene. Infect Genet Evol. 2009; 9:699–708. [DOI] [PubMed] [Google Scholar]
- 32. Tokarz R, Firth C, Madhi S A, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol. 2012; 93:1952–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Henquell C, Mirand A, Richter J. Phylogenetic patterns of human coxsackievirus B5 arise from population dynamics between two genogroups and reveal evolutionary factors of molecular adaptation and transmission. J Virol. 2013; 87:12249–12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yarmolskaya MS, Shumilina EY, Ivanova OE, Drexler JF, Lukashev AN. Molecular epidemiology of echoviruses 11 and 30 in Russia: different properties of genotypes within an enterovirus serotype. Infect Genet Evol. 2015; 30:244–248. [DOI] [PubMed] [Google Scholar]
- 35. Liu N, Jia L, Yin J, et al. An outbreak of aseptic meningitis caused by a distinct lineage of coxsackievirus B5 in China. Int j Infect Dis. 2014; 23:101–104. [DOI] [PubMed] [Google Scholar]
- 36. Papa A, Dumaidi K, Franzidou F, Antoniadis A. Genetic variation of coxsackie virus B5 strains associated with aseptic meningitis in Greece. Clin Microbiol Infect. 2006; 12:688–691. [DOI] [PubMed] [Google Scholar]
- 37. Midgley C M, Jackson M A, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014. Am J Transplant. 2014; 63:798 –799. [PMC free article] [PubMed] [Google Scholar]
- 38. Patel M C, Wang W, Pletneva L M. Enterovirus D‐68 infection, prophylaxis, and vaccination in a novel permissive animal model, the cotton rat (Sigmodon hispidus). PLoS ONE 2016; 11:e0166336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Janes V A, Minnaar R, Koen G, et al. Presence of human non‐polio enterovirus and parechovirus genotypes in an Amsterdam hospital in 2007 to 2011 compared to national and international published surveillance data: a comprehensive review. Eurosurveillance. 2014; 19:pii=20964. 10.2807/1560-7917.ES2014.19.46.20964 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Figure S1. Phylogenetic analysis of EV serotypes by neighbor‐joining phylogenetic analysis of the partial VP1 region, South Africa, 2012‐2014. Sequences with black closed circles denotes serotypes identified in SARI patients, those with grey closed circles denotes serotypes identified in ILI patients while those with open circles denoted serotypes identifies in asymptomatic individuals. Bootstrap values (1000 replicates) are shown on the branches, with values <65% omitted from the tree.
Figure S2. The serotypes and species (EV‐A to EV‐D) of enteroviruses identified in patients with severe acute respiratory illness (SARI), influenza‐like illness (ILI), and asymptomatic individuals (C) from June 2012 to July 2014, South Africa (CVA10 = Coxsackie virus A10, CVA14 = Coxsackie virus A14, CVA16 = Coxsackie virus A16, CVA2 = Coxsackie virus A2, CVA4 = Coxsackie virus A4, CVA6 = Coxsackie virus A6, EV‐A71 = Enterovirus A71, CVA9 = Coxsackie virus A9, CVB1 = Coxsackie virus B1, CVB2 = Coxsackie virus B2, CVB3 = Coxsackie virus B3, CVB4 = Coxsackie virus B4, CVB5 = Coxsackie virus B5, E11 = Echovirus 11, E16 = Echovirus 16, E17 = Echovirus 17, E19 = Echovirus 19, E20 = Echovirus, E25 = Echovirus 25, E3 = Echovirus 3, E30 = Echovirus 30, E32 = Echovirus 32, E6 = Echovirus 6, E7 = Echovirus 7, E9 = Echovirus 9, EV‐B75, CVA13 = Coxsackie virus A13, CVA20 = Coxsackie virus A20, EV‐C99 = Enterovirus C99, EV‐D68 = Enterovirus D68)
Table S1. Association of EV species (EV‐A to EV‐D) with influenza‐like illness (ILI) and severe acute respiratory illness (SARI) compared with asymptomatic controls, South Africa, 2009‐2010 (bold indicate factors significant at P < 0.05)


