Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD, autosomal dominant PKD or adult-onset PKD) is the most prevalent and potentially lethal kidney disease that is hereditary and lacks effective treatment. Preimplantation genetic diagnosis (PGD) of embryos in assistant reproductive technology (ART) helps to select mutation-free embryos for blocking ADPKD inheritance from the parents to their offspring. However, there are multiple pseudogenes in the PKD1 coding region, which make blocking ADPKD inheritance by PGD complicated and difficult. Therefore, this technique has not been recommended and used routinely to ADPKD family plan.
Methods and Results
Here, we report a new strategy of performing PGD in screening (target-) mutation-free embryos. We firstly used a long-range PCR amplification and next generation sequencing to identify the potential PKD1 mutant(s). After pathogenic variants were detected, multiple annealing and looping-based amplification cycles (MALBAC), a recently developed whole genome amplification method, was used to screen embryo cells. We successfully distinguished the mutated allele among pseudogenes and obtained mutation-free embryos for implantation. The first embryo transfer attempt resulted in a healthy live birth free of ADPKD condition and chromosomal anomalies which was confirmed by aminocentesis at week 18 of gestation, and by performing live birth genetic screening.
Conclusions
The first MALBAC-PGD attempt in ADPKD patient resulted in a healthy live birth free of ADPKD and chromosomal anomalies. MALBAC-PGD also enables selecting embryos without aneuploidy together and target gene mutation, thereby increasing implantation and live birth rates.
Keywords: Polycystic kidney, Autosomal dominant, Preimplantation diagnosis, MALBAC
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited condition characterized by irreversible pathological development of cysts throughout the kidneys and decline in renal function [1]. It affects about 1/400 to 1/1000 live births [2] and accounts for 7–10% of all the patients with end-stage renal disease worldwide [3].
ADPKD has always been one of the major topics in nephrology and genetics research communities. Increasing basic researches contribute to illustrate the pathogenesis of ADPKD. It is now clear that ADPKD is caused by mutations in PKD1 gene encoding polycystin-1 (85% cases) [4–7] or PKD2 encoding polycystin-2 (15% cases) [8, 9]. The mutation in either of these two genes may result in the phenotype of ADPKD. Compared to the PKD2 mutation, PKD1 mutation usually indicates more severe disease, earlier onset, and poorer prognosis. Therefore, blocking PKD1 mutation is more beneficial for the patient. While new knowledge about how mutant polycystin-1/2 result in extensive cysts growth is still needed, emerging clinical investigations focus on potential interventions minimize renal failure in ADPKD patients. While there was insufficient evidence that these treatments could improve patient outcome [10], the most promising breakthrough in this field is the proved efficiency of tolvaptan by a randomized controlled trial. Tolvaptan treatment resulted in a 50% reduction in the annual rate of kidney growth and a 33% reduction in the rate of decline in kidney function per year compared with placebo [11].
Due to the nature of ADPKD as a single-gene inherited disease, blocking the PKD1 mutant heredity with preimplantation genetic diagnosis (PGD) is the most effective way, theoretically, of reducing the incidence of ADPKD. However, technical difficulties in single-cell whole-genome amplification and sequencing of PKD1 hinder the generalization of PGD in this disease. With the development of a new whole-genome amplification method, MALBAC [12], sensitivity of detecting PKD1 mutation with single embryo cell was greatly improved. After rigorous technical tests and ethical check, we performed MALBAC-PGD for one ADPKD couple. MALBAC-PGD enables simultaneous prevention of the mutated allele, as well as chromosomal abnormalities, thereby increasing success rates of clinical pregnancy and live birth. Furthermore, the female ADPKD patient gave birth to a healthy baby on August 3, 2016 as a result of this treatment. To our knowledge, this is the first application of MALBAC-PGD in ADPKD and chromosomal disease prevention.
Methods
Ovarian stimulation, in vitro fertilization, and embryo transfer processes were performed according to the standard protocol. If more than one mutation-free embryo is retrieved, only one embryo will be transferred and others will be frozen. And after PGD process, if PKD1 mutation is still found in amniocentesis at week 18 of gestation, the female patient would decide whether to terminate the pregnancy.
PCR amplification with peripheral blood sample and library preparation
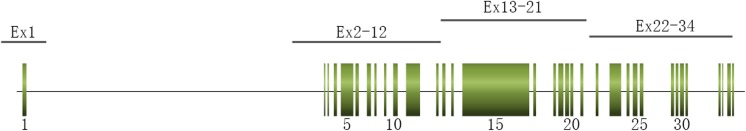
Genomic DNA was extracted from peripheral blood lymphocytes using a DNA extraction kit (QIAamp DNA Blood Mini Kit, QIAGEN, Inc., Valencia, CA, USA). The entire coding region, most of 5′ and 3′ untranslated regions, and the exon-intron boundaries of PKD1 and PKD2 were amplified in a total of six (four reactions for PKD1 gene exons 1–34 and two reactions for PKD1 gene exons 34–46 and PKD2) distinct PCR reactions using primers anchored either in the rare mismatched region with the human homologs (Fig. 1). The long-range PCR (LR-PCR) primers for PKD1 gene exons 1–34 were shown in Table 1, as published previously [13–16] and the multiplex PCR primers for PKD1 gene exons 34–46 and PKD2 were designed using software Ion AmpliSeq™ Designer. LR-PCR amplification reaction system and amplification conditions for the various LR-PCR fragments were described in Table 2 and Table 3, respectively, which were optimized based on published article previously [13]. Ampliseq multiple PCR amplification reaction system and amplification conditions were set as manufacturer’s instructions. The LR-PCR products (except for PKD1 exon 1 with an extremely high content of GC) of PKD1 gene exons 2–34 were purified with Agencourt AMPure XP Beads, then, quantified, fragmented by NEBNext Fast DNA Fragmentation Kit (New England Biolabs, Ipswich, MA, USA). Sequencing library of LR-PCR products and other Ampliseq multiplex PCR products were constructed by Ion Plus Fragment Library Kit (Thermo Fisher Scientific, Waltham, MA, USA) and then sequenced with Ion PGM™ 200 Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA). PKD1 exon 1 was amplified in another PCR reaction using specific nested primers and sequenced by Sanger method through 3730xl DNA analyzer.
Fig. 1.
Structural gene map and positional primer locations for PKD1
Table 1.
PKD1 Gene Exons 1–34 Primers for LR-PCR
| Fragment | Primers | Sequence (5′–3′) | Size(Kb) | Genomic location |
|---|---|---|---|---|
| PKD1_Ex1 [13–15] | PKD1 _1F | CGCAGCCTTACCATCCACCT | 2.3 | chr16:2185030-2187307 |
| PKD1 _1R | TCATCGCCCCTTCCTAAGCA | |||
| PKD1_Ex2-12 [13] | PKD1 _2-12F | CCAGCTCTCTGTCTACTCACCTCCGCATC | 8.7 | chr16:2163080-2171636 |
| PKD1_2-12R | CCACGGTTACGTTGTAGTTCACGGTGACG | |||
| PKD1_Ex13-21 [13] | PKD1_13-21F | TGGAGGGAGGGACGCCAATC | 7.9 | chr16:2155145-2163036 |
| PKD1_13-21R | ACACAGGACAGAACGGCTGAGGCTA | |||
| PKD1_Ex22-34 [16] | PKD1_22-34F | CCGTGTAGAGAGGAGGGCGTGTGCAAGGA | 7.5 | chr16:2147212-2154714 |
| PKD1_22-34R | TCGGCAAGGACCTGCTGGATCAGGTCTTC |
Table 2.
LR-PCR amplification reaction system
| Components | Exons 2–12 and Exons 22–34 volume (μL) | Exons 1 and Exons 13–21 Volume (μL) |
|---|---|---|
| Template DNA | ≥ 100 ng (XμL) | ≥ 100 ng (XμL) |
| Forward primer | 2 | 2 |
| Reverse primer | 2 | 2 |
| TAKARA LA Taq | 0.5 | 0.5 |
| 2*GC Buffer I | 25 | 0 |
| 2*GC Buffer II | 0 | 25 |
| dNTP mixture | 8 | 8 |
| dd Water | Up to 50 | Up to 50 |
Table 3.
LR-PCR amplification conditions
| PKD1 fragments | PCR conditions |
|---|---|
| PKD1_Ex1 | 94 °C for 5 min; followed by 35 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 2 min, with a final extension step of 72 °C for 10 min |
|
PKD1_Ex2-12 PKD1_Ex13-21 PKD1_Ex22-34 |
Touch-down protocol comprised of initial step of 94 °C for 2 min; followed by 14 cycles of 94 °C for 15 s, 66 °C for 30 s with decreasing 0.5 °C per cycle, and 68 °C for 6 min; followed by 25 cycles of 94 °C for 15 s, 59 °C for 30 s, and 68 °C for 6 min, with a final extension step of 72 °C for 10 min |
Bioinformatics analysis and mutation identification and classification
All sequencing reads were mapped to the PKD1/2 reference genome with the Torrent Server TMAP software, and then we could get bam files. The reference sequences were NM_001009944.2 for PKD1 and NM_000297.2 for PKD2. Then, bam files were analyzed using the Ion Reporter Software (version 4.4).
We adjusted ACMG (2015) classification principle [17] with PKDB classification and the actual needs of our PGD then classified variants into four different classes (Table 4). The first three classes were confirmed by long PCR and then Sanger sequencing to prevent the false positivity due to high-through put sequencing error detection or mismatch in long segment amplification.
Table 4.
Variants’ classification
| Definitely pathogenic variants | Variants which had been reported definitely pathogenic variants |
| Novel severe damage variants which include nonsense variants, frameshift variants caused by small inserts or deletions, splice site variants, and large inserts and deletions, and which were co-separation with symptoms and consistent with Mendel’s law of inheritance | |
| High suspicious pathogenic variants | Variants had been reported high suspicious variants |
| Codon variants caused by inserts or deletions with three bases | |
| Novel severe damage variants while the pedigree could not be verified or not consistent with Mendel’s law of inheritance | |
| Likely pathogenic variants | Variants had been reported likely pathogenic variants |
| Novel synonymous variants or missense variants or intron variants, of which functional structure prediction were serious damage, and which were consistent with Mendel’s law of inheritance. | |
| Other variants | Variants had been defined uncertain variants, benign variants or likely benign variants by ACMG. |
PGD procedure
Whole-genome amplification of each embryo biopsy samples was performed by MALBAC WGA kit (Yikon Genomics, Shanghai, China) following the instructions of the manufacturer. Sixty SNP markers linked to mutation alleles were selected for linkage analysis. The mutation site, as well as the SNPs, were amplified by using specific primer pairs. Then, the mixed amplification products were pooled with the MALBAC WGA products and sequenced. The chromosomal copy number as well as the mutation site and SNPs, were analyzed as published previously [18].
Results
Patient and genetic background
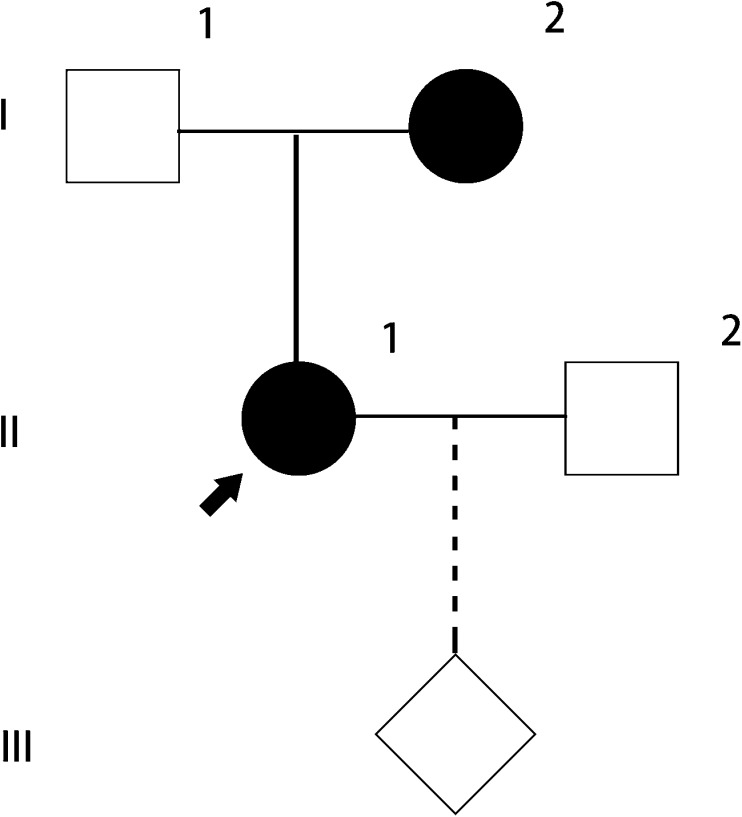
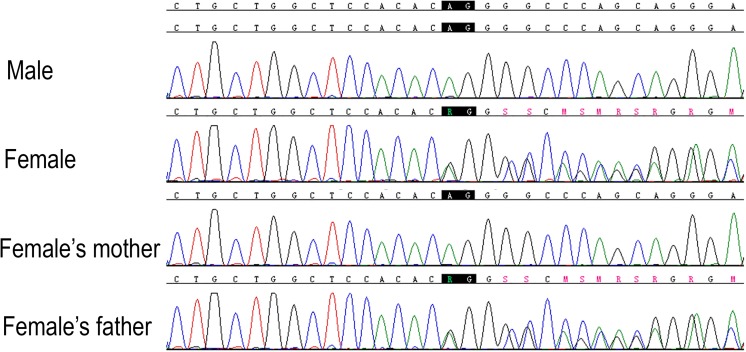
One couple (male, 35 years old, seemingly healthy; female, 31 years old, ADPKD patient, G0, P0) attended our reproductive center for artificial insemination with the husband’s semen following preimplantation genetic diagnosis. They were seen by a genetic counselor and signed a consent form approved by ethical board of our hospital before further treatment. Basic characters and routine tests results of this couple were shown in Table 5. There were no contraindications for PGD. Long-range PCR and next-generation sequencing for the couple peripheral blood sample revealed a previously reported pathogenic mutation (PKD1, IVS21-2_1del AG) in the female patient (Fig. 2). Since the female patient has clear family history of ADPKD, peripheral blood sample of her parents was collected for linkage analysis. Her mother was revealed to be the parent who transmits her PKD1 mutation to the daughter (Figs. 2 and 3).
Table 5.
Clinical background of the couple
| Male | Female | |
|---|---|---|
| Age (year) | 35 | 31 |
| BMI (kg/m2) | 25 | 23 |
| Smoking history | Non-smoker | Non-smoker |
| Genetic diseases, inborn | None | ADPKD (PKD1) |
| Total kidney volume (cm3) | NA | 408 |
| Family history of ADPKD | Negative | Positive |
| History of past illness | None | None |
| Childbearing history | NA | G0, P0 |
| WBC (×109) | 5.6 | 7.1 |
| Hb (g/L) | 175 | 140 |
| Alb (g/L) | 50 | 46 |
| TBIL (μmol/L) | 13 | 13 |
| ALT (U/L) | 13 | 13 |
| SCr (μmol/L) | 90 | 59 |
| eGFR (CKD-EPI) (mL/min × 1.73m2) | 95 | 117 |
| Karyotype analysis | No obvious anomaly | 14p12h |
| Semen examination | Asthenozoospermia and oligospermia | NA |
| Sexually transmitted diseases | None | None |
| Blood type (ABO(Rh)) | O+ | B+ |
| Anti-sperm antibody | NA | Negative |
| Antral follicle count (right/left) | NA | 4/2 |
| TORCH-related antibodies | NA | HSV1 IgG+; HSV2 IgG+; others - |
| E2 (pg/mL) | NA | 17.17 |
| P (ng/mL) | NA | 0.17 |
| T (ng/dL) | NA | 11.60 |
| PRL (ng/dL) | NA | 10.88 |
| LH (mIU/mL) | NA | 2.47 |
| FSH (mIU/mL) | NA | 7.15 |
| FSH/LH | NA | 2.89 |
NA not applicable
Fig. 2.
Pedigree of the family
Fig. 3.
Chromatogram of the peripheral blood sample from female’s family
In vitro fertilization
Ovarian stimulation was performed using protocol with GnRHa, recombinant FSH, and hCG for twice, and 17 oocytes were collected. After intracytoplasmic sperm injection procedure, totally six embryos were cultured in GIII series culture media. Embryo biopsies were carried out by mechanical method on days 5–7 for PGD.
PGD
Six biopsy samples from six embryos respectively were amplified by multiple annealing and looping-based amplification cycles [12]. Amplifications were failed in two samples. PKD1, IVS21-2_1del AG was detected in three samples by PCR and next-generation sequencing. Linkage analysis was performed to confirm the mutation detected in embryo samples by NGS. One embryo sample was found free of this mutation and then transferred on day 6. Luteal phase supports were given routinely. Serum β-hCG levels were measured at 14 days after ET. The presence of a gestational sac and fetal heartbeat by ultrasound at 5 weeks after ET evidenced clinical pregnancy. Long-range PCR and next-generation sequencing were repeated with the amniotic fluid sample collected by amniocentesis at 18 weeks of gestation. On August 3, 2016, the female patient gave birth to a child. The genotype of this child was confirmed again by long-range PCR and next-generation sequencing. No mutation of PKD1 was found.
Discussion
For the nature of ADPKD as a single-gene inherited disease, PGD should have been an ideal option for patients who have fertility desire. However, a few technical difficulties hinder the application of PGD in ADPKD. The genomic structure of the PKD1 gene included 46 exons with a ~ 2.5-kb polypyrimidine tract in intron 21. Approximately, 70% of the gene is duplicated in highly homologous loci on chromosome 16. And exon 1 is a high GC content area [19]. PCR amplification of long fragment is necessary to overcome these difficulties [20–22].
Amplification by long PCR of the entire coding region and the exon-intron boundaries of PKD1 and PKD2 is a tough task. Former researchers used eight primers and five thermal cycling conditions to PCR amplify exons 1–34 of PKD1, and all the five reactions took more than 5 h [23]. Besides time - consuming, the reported method requires inputting a large amount of DNase in the PCR template that limited the usage of the method in PGD. Although the researchers had kept optimizing the reaction conditions, there had been no significant improvement so far. The update protocol proposed by Tan required 10 PCR reaction systems to cover the entire coding region, most 5′ and 3′ untranslated regions and the exon-intron boundaries of PKD1 and PKD2, with five different reaction conditions [13]. To improve the efficiency of the experiment without increasing the cost significantly, we used Adrian Y. Tan’s four long PCR primers in the PKD1 polyclonal region. We amplified the PKD1 gene 34–46 exons and the exon-intron boundaries of PKD2 with multiplex PCR method (Ampliseq, thermofisher). Therefore, only two PCR reactions are needed in our setup. The sequencing library was then constructed with routing method of mechanical DNA fragmentation. In total, six reactions with three reaction conditions accomplished all of the amplification needed.
There are several strengths in our sequencing process. First, all sequenced reads were mapped to the PKD1/2 reference genome by the Torrent Server TMAP software to reduce false negative rate caused by true gene region alignment to pseudogene region. Second, the pathogenic mutations were confirmed by long PCR and then Sanger sequencing. In summary, we established a more effective protocol than ever to perform ADPKD-PGD in a practical manner.
Although there is only one case in this study, it is a validation of this combined PGD technology in ADPKD. To confirm efficacy and safety of this strategy, a multicenter non-randomized controlled trial (NCT02948179) using this technology is ongoing. Although in this report, ADPKD in this case was not in rapid progression, she and her family have strong desire in PGD. When PGD is generalized in clinical practice for ADPKD couples, the indication should be specified. Patients with rapid progressive ADPKD would be preferred choice. Indicators include established TKV growth rate > 5% per year, annual eGFR decline > 5 mL/min/1.73 m2, truncating PKD1 mutations and elevated plasma copeptin level [24]. However, according to a recently published survey, a proportion of patients believe that PGD should be made available to prospective parents with this disease regardless of the progression of disease [25]. To the patients with less progressive ADPKD but strong desire of performing PGD, whether the PGD should be made available is open to discussion.
Acknowledgements
This work was supported by funds from the National Natural Science Foundation of China (31371172, 81670612 to CM), Shanghai Top Priority Key Clinical Disciplines Construction Project (to CM), The National Key Research and Development Program of China (No. 2016YFC0901502), and Systemic Redesign and Demonstration for Early Detection, Evaluation and Management of Chronic Kidney Disease in Shanghai (SCREEMING Study GWIV-18 to CM).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study protocol and all subjects who participated in this study were approved by the Institutional Review Board of our institute in which informed consent was obtained from all families prior to participation in accordance with institutional and national guidelines.
Footnotes
Wen Li and Yiyi Ma contributed equally to this work.
References
- 1.Simms RJ. Autosomal dominant polycystic kidney disease. BMJ. 2016;352:i679. doi: 10.1136/bmj.i679. [DOI] [PubMed] [Google Scholar]
- 2.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329(5):332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 3.Ong AC, Devuyst O, Knebelmann B, Walz G. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet (London, England) 2015;385(9981):1993–2002. doi: 10.1016/S0140-6736(15)60907-2. [DOI] [PubMed] [Google Scholar]
- 4.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10(2):151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 5.Burn TC, Connors TD, Dackowski WR, Petry LR, Van Raay TJ, Millholland JM, et al. Analysis of the genomic sequence for the autosomal dominant polycystic kidney disease (PKD1) gene predicts the presence of a leucine-rich repeat. The American PKD1 Consortium (APKD1 Consortium) Hum Mol Genet. 1995;4(4):575–582. doi: 10.1093/hmg/4.4.575. [DOI] [PubMed] [Google Scholar]
- 6.Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. The International Polycystic Kidney Disease Consortium. Cell. 1995;81(2):289–98. [DOI] [PubMed]
- 7.Reeders ST, Breuning MH, Davies KE, Nicholls RD, Jarman AP, Higgs DR, et al. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 1985;317(6037):542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- 8.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272(5266):1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 9.Kimberling WJ, Kumar S, Gabow PA, Kenyon JB, Connolly CJ, Somlo S. Autosomal dominant polycystic kidney disease: localization of the second gene to chromosome 4q13-q23. Genomics. 1993;18(3):467–472. doi: 10.1016/S0888-7543(11)80001-7. [DOI] [PubMed] [Google Scholar]
- 10.Bolignano D, Palmer SC, Ruospo M, Zoccali C, Craig JC, Strippoli GF. Interventions for preventing the progression of autosomal dominant polycystic kidney disease. Cochrane Database Syst Rev. 2015;(7):Cd010294. doi:10.1002/14651858.CD010294.pub2. [DOI] [PMC free article] [PubMed]
- 11.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zong C, Lu S, Chapman AR, Xie XS. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science. 2012;338(6114):1622–1626. doi: 10.1126/science.1229164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan AY, Michaeel A, Liu G, Elemento O, Blumenfeld J, Donahue S, et al. Molecular diagnosis of autosomal dominant polycystic kidney disease using next-generation sequencing. J Mol Diagn: JMD. 2014;16(2):216–228. doi: 10.1016/j.jmoldx.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan YC, Michaeel A, Blumenfeld J, Donahue S, Parker T, Levine D, et al. A novel long-range PCR sequencing method for genetic analysis of the entire PKD1 gene. J Mol Diagn: JMD. 2012;14(4):305–313. doi: 10.1016/j.jmoldx.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Tan AY, Michaeel A, Blumenfeld J, Donahue S, Bobb W, et al. Development and validation of a whole genome amplification long-range PCR sequencing method for ADPKD genotyping of low-level DNA samples. Gene. 2014;550(1):131–135. doi: 10.1016/j.gene.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Rossetti S, Hopp K, Sikkink RA, Sundsbak JL, Lee YK, Kubly V, et al. Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol: JASN. 2012;23(5):915–933. doi: 10.1681/ASN.2011101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med: Off J Am Coll Me Genet. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan B, Wang S, Jia H, Liu X, Wang X. An efficient weighted tag SNP-set analytical method in genome-wide association studies. BMC Genet. 2015;16:25. doi: 10.1186/s12863-015-0182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watnick TJ, Piontek KB, Cordal TM, Weber H, Gandolph MA, Qian F, et al. An unusual pattern of mutation in the duplicated portion of PKD1 is revealed by use of a novel strategy for mutation detection. Hum Mol Genet. 1997;6(9):1473–1481. doi: 10.1093/hmg/6.9.1473. [DOI] [PubMed] [Google Scholar]
- 20.Lu S, Zong C, Fan W, Yang M, Li J, Chapman AR, et al. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Science. 2012;338(6114):1627–1630. doi: 10.1126/science.1229112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou Y, Fan W, Yan L, Li R, Lian Y, Huang J, et al. Genome analyses of single human oocytes. Cell. 2013;155(7):1492–1506. doi: 10.1016/j.cell.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, Ma F, Chapman A, Lu S, Xie XS. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu Rev Genom Hum Genet. 2015;16:79–102. doi: 10.1146/annurev-genom-090413-025352. [DOI] [PubMed] [Google Scholar]
- 23.Rossetti S, Strmecki L, Gamble V, Burton S, Sneddon V, Peral B, et al. Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am J Hum Genet. 2001;68(1):46–63. doi: 10.1086/316939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corradi V, Gastaldon F, Caprara C, Giuliani A, Martino F, Ferrari F, et al. Predictors of rapid disease progression in autosomal dominant polycystic kidney disease. Minerva Med. 2017;108(1):43–56. doi: 10.23736/S0026-4806.16.04830-8. [DOI] [PubMed] [Google Scholar]
- 25.Swift O, Vilar E, Rahman B, Side L, Gale DP. Attitudes in patients with autosomal dominant polycystic kidney disease toward prenatal diagnosis and preimplantation genetic diagnosis. Genetic testing and molecular biomarkers. 2016;20(12):741–746. doi: 10.1089/gtmb.2016.0050. [DOI] [PubMed] [Google Scholar]