Abstract
Purpose
We tested whether mitochondrial electron transport chain electron carrier coenzyme Q10 (CoQ10) increases ATP during bovine IVM and increases %M2 oocytes, mitochondrial polarization/mass, and Oct4, and decreases pAMPK and oocyte death.
Methods
Bovine oocytes were aspirated from ovaries and cultured in IVM media for 24 h with 0, 20, 40, or 60 μM CoQ10. Oocytes were assayed for ATP by luciferase-based luminescence. Oocyte micrographs were quantitated for Oct4, pAMPK (i.e., activity), polarization by JC1 staining, and mitochondrial mass by MitoTracker Green staining.
Results
CoQ10 at 40 μM was optimal. Oocytes at 40 μM enabled 1.9-fold more ATP than 0 μM CoQ10. There was 4.3-fold less oocyte death, 1.7-fold more mitochondrial charge polarization, and 3.1-fold more mitochondrial mass at 40 μM than at 0 μM CoQ10. Increased ATP was associated with 2.2-fold lower AMPK thr172P activation and 2.1-fold higher nuclear Oct4 stemness/potency protein at 40 μM than at 0 μM CoQ10. CoQ10 is hydrophobic, and at all doses, 50% was lost from media into oil by ~ 12 h. Replenishing CoQ10 at 12 h did not significantly diminish dead oocytes.
Conclusions
The data suggest that CoQ10 improves mitochondrial function in IVM where unwanted stress, higher AMPK activity, and Oct4 potency loss are induced.
Keywords: CoQ10, Oct4 potency factors, AMPK, ATP, Death, Mitochondria
Introduction
Oocytes from stressed or older females require enhanced mitochondrial function to provide sufficient energy [1]. Adaptive stress responses enable in vitro maturation (IVM) of oocytes and in embryo culture. In vitro fertilization/assisted reproductive technologies (IVF/ART) protocols such as media [2] or pipetting [3] stress oocytes and embryos, and this would hypothetically benefit if energy through ATP were created more efficiently during these protocols. In Henry Leese’s “quiet” embryo hypothesis, stress leads to increased metabolic activity which at high stress exposures overwhelms adaptive stress mechanisms and becomes maladaptive [4, 5]. Our overarching hypothesis is that the means to manage stress is to regulate the stress enzymes mediating oocyte and embryo responses, or to increase ATP to preclude the necessity of activating enzymes to levels which may have additional unwanted maladaptive effects.
Coenzyme Q10 (CoQ10) is an essential electron carrier in the mitochondrial electron transport chain (ETC) and has been added in vivo or in vitro to improve mitochondrial function in oocytes and embryos. CoQ10 has been used to improve bovine IVM after heat stress [6]. Aging in human and mouse oocytes is associated with decreased function of the CoQ10 synthetic enzyme prenyl (decaprenyl) diphosphate synthase (Pdss2) [7]. CoQ10 can improve quality of oocytes from older females [7–9]. CoQ10 decreases are associated with spontaneous miscarriage in humans [10]. Thus, CoQ10 improves oocyte and embryo function in vivo and in vitro and under stress and aging, but the mechanisms improving oocyte quality are not fully understood. In the 1990s, the best IVM media left ~ 80% of oocytes dead [11], but in two recent papers showed that 51.1% [12] and 68.6% [13] of oocytes were alive at the end of IVM culture. Although IVM culture has improved greatly, a 30–50% loss of oocytes indicates there is stress during culture that produces death.
AMP-activated protein kinase (AMPK) is the unique protein kinase that senses ATP/energy status and then regulates anabolic and catabolic molecular pathways to re-balance the ATP production and consumption equilibrium [14]. Stress induces AMPK activity in oocytes, and this adaptation mediates IVM by regulating the oocyte metabolic response while facilitating oocyte maturation [15, 16]. In these circumstances, ameliorating diabetic affects may be due to replenishing depleted AMPK activity into normal ranges. These reports do not address the possibility that AMPK activity at higher levels may be maladaptive.
But in many other circumstances, normal oocytes and embryos may be overstimulated by many types of stress and this leads to morbidity or toxicity. For example, hyperosmotic stress induces loss of caudal-related homeodomain transcription factor (Cdx)2 and inhibitor of differentiation (Id)2 potency factors in two-cell embryos, and AMPK activity is necessary and sufficient for potency loss [17]. Cdx2 and Id2 potency/stemness loss is necessary for placental trophoblast stem cells (TSCs) to differentiate in early mouse and human embryos [18, 19]. Drugs such as metformin can improve ovulation in polycystic ovarian syndrome (PCOS) patients, but metformin and aspirin cause AMPK-dependent loss of Oct4 and Rex1 potency in cultured two-cell embryos and AMPK-dependent growth rate decrease and then block of development in culture [20]. The isoform of the Oct4 protein detected by the C terminus antibody is the form that undergoes stress-induced loss in two-cell embryos which has been established as the isoform that preserves potency [21]. In an AMPK-dependent manner, hyperosmotic stress or the diet supplement BioResponse diindolylmethane (BR-DIM) also decreases potency rapidly in the two-cell embryo and then BR-DIM slows growth and kills the embryo slowly between the two-cell and blastocyst stages. AMPK activation leads to phosphorylation and decreased activity of rate-limiting anabolic enzymes such as acetyl-CoA carboxylase (ACC) in oocytes, embryos, and TSCs, which constitute 80% of cells in blastocysts [18, 22]. Thus, although AMPK can adapt oocytes and embryos to stress in vivo and in vitro, abnormally high AMPK activity may lead to potency loss and, with decreased anabolism, may lead to toxicity.
We hypothesized that IVM creates stress and that CoQ10 improves oocyte maturation development by increasing ATP production, decreasing AMPK activity, and thus enhancing potency through the pluripotency-maintaining isoform of Oct4 protein. To test this hypothesis, we cultured bovine oocytes +/− several CoQ10 doses and assayed for IVM progression by morphology and mitochondrial mass polarization/clustering. We also tested for mechanisms affected by stress and enhanced mitochondrial function: ATP and AMPK activity levels and Oct4 potency factor levels.
Materials and methods
Materials
We obtained 1168 immature bovine oocytes from slaughterhouses through Drs. George Smith and Joe Folger at Michigan State University and cultured them to the early first polar body stage to test for IVM quality. Oocytes were cultured with 0–60 μM CoQ10 and assayed by phase microscopy for fragmentation and appearance first polar body. Then, oocytes were assayed for quality of IVM assayed for pAMPK, ATP, and potency (Oct4; C10 monoclonal sc5279, Santa Cruz Technologies, Santa Cruz, CA) using immunofluorescence. The oocytes were assayed for mitochondrial mass (MitoTracker Green) and quality (polarization or clustering) using tetraethylbenzimidazolylcarbocyanine iodide dye (JC1), as well as mitochondrial inner membrane energy levels and MitoTracker Green stain (MitoTracker Green from Life Technologies, Eugene, OR).
Oocyte recovery and in vitro maturation
Briefly, ovaries obtained at a local abattoir were transported to the laboratory in sterile saline solution. Upon return to the laboratory, ovaries were washed in sterile saline and cumulus oocyte complexes (COCs) were aspirated from 2- to 7-mm visible follicles using an 18-gauge needle and 50–60 mmHg of negative pressure [23]. After sedimentation, the COCs were individually selected and washed three to four times in HEPES-buffered hamster oocyte culture medium (HECM) [114 mM NaCl, 3.2 mM KCl, 2 mM CaCl2 × 2H2O, 0.5 mM MgCl2 × 6H2O, 100 μL/mL MEM nonessential (10×) amino acids, 17 mM sodium lactate, 0.1 mM sodium pyruvate, 2 mM NaHCO3, 1 mM HEPES, 0.183 mM penicillin-G, 3 mg/mL BSA; pH 7.3–7.4; 275 mOsm/kg)] under a stereomicroscope; COCs with more than four compact layers of cumulus cells and homogeneous cytoplasm were divided into four groups according to the Coq10 concentration additive to maturation culture media (0, 20, 40, and 60 μM) and transport in a portable incubator (Hach, Loveland, CO) from MSU to WSU in Detroit, COCs were matured in TCM 199 [supplemented with 0.2 mM sodium pyruvate, 5 mg/mL gentamicin sulfate, 6.5 mM l-glutamine, 156 nM bovine luteinizing hormone (LH) (Sioux Biochemical, Sioux Center, IA), 15.6 nM bovine follicle stimulating hormone (FSH) (Sioux Biochemical), 3.67 nM 17b-estradiol, and 10% v/v defined FBS (HyClone, Logan, UT)] for 24 h in groups of 50 in four-well dishes containing 400 mL of maturation medium at 38.5 °C and 5% CO2 in air with maximum humidity. Cumulus cells were completely removed after maturation by hyaluronidase (0.1%) digestion and repeated pipetting and denuded oocytes. Metaphase II oocytes were selected based on the presence of a single polar body.
For mouth pipette preparation, mouth pipettes were prepared and used as in the previous [3, 24].
For preparation of CoQ10, coenzyme Q10 (MW 863.358) was purchased from Sigma (C9538, Sigma) and 0.00862 g dissolved in 1 mL of DMF (N,N-dimethylformamide, MW 73.1) to produce a stock concentration of CoQ10 in DMF which is 10 mM (8.6 mg in 1 mL DMF). Stock was used to make fresh CoQ10 in IVM media at 60, 40, 20, and 0 μM. The effects of DMF vehicle alone were checked for control at 0 μM and DMF concentration at the highest Coq10 concentration (60 μm). There were no significant differences found in oocyte metaphase II (MII) percentage after 24 h incubation (p < 0.05, t test).
Determination of CoQ10 concentration in bovine IVM media
A Cary 100 Bio UV-Visible spectrophotometer was used to determine CoQ10 concentration, at 25 °C, pH 7.0. Experiments were performed by placing a fixed amount of RPMI media containing increasing concentrations of CoQ10 (0, 20, 40, and 60 μm) under oil and kept in a CO2 incubator. After oil extraction, the final CoQ10 media concentrations were determined spectrophotometrically at 0, 2, 6, 12, 18, and 24 h of incubations using extinction coefficients (ε) of 76,714 l.M−1 cm−1 at 275 nm [25, 26]. The time zero dose response from 0 to 60 μM for CoQ10 was in the linear range, and thus, a standard curve was composed where concentration measured was proportional to the input dose.
Evaluation of mitochondrial characteristics in mature oocyte: death, clustering polarization, and MII development
The mitochondrial membrane potential (Δψm) of oocytes was measured in Denuded oocytes which were stained with JC1 (5,5′,6,6′-tetrachloro-1,1′, 3,3′-tetraethyl-benzimidazoyl-carbocyanine iodide (JC1), Life Technologies, Eugene, OR) as described previously [27] with slight modifications. JC1 fluorescence has two emission peaks, with red fluorescence (JC1 dimers) indicating highly polarized mitochondria (mitochondrial membranes with high potential) and green fluorescence (JC1 monomers) indicating low-polarized mitochondria (mitochondrial membranes with low potential) [27–29]. Briefly, denuded oocytes were incubated with 1 μM JC-1 and 1 μg/mL Hoechst 33342 in Dulbecco’s phosphate-buffered saline (DPBS) supplemented with 10% FCS for 20 min at 38.5 °C and oocytes were then washed three times in PBS-polyvinylpyrrolidone (PVP). Stained oocytes were transferred to a slide glass with a small amount of DPBS and pressed gently with a cover slide, and examined immediately at room temperature in a dark room to evaluate the mitochondrial activity and nuclear stage. The distributions of JC1 dimers with red fluorescence and monomers with green fluorescence were detected using the red filter and green filter of the microscope, respectively. The ratio of RITC (J-aggregate) to FITC (J-monomer) staining was determined for each oocyte, and the average ratio was calculated for each group of oocytes. Mitochondrial activity can be evaluated by the intensity of the red/green fluorescence [13, 27, 30, 31].
Mitochondrial distribution, regardless of membrane potential, was determined by staining with MitoTracker Green dye. Briefly, denuded oocytes from each group (0, 20, 40, and 60 μm) after 24 h in vitro maturation were collected and stained with 200 nM MitoTracker Green dye diluted in HEPES-TALP at 1 μg/mL Hoechst 33342 38.5 °C for 30 min. Oocytes were then washed three times in PBS with 1 mg/mL polyvinylpyrrolidone (PBS-PVP), and stained oocytes were transferred to a slide glass with a small amount of DPBS and pressed gently with a cover slide, and examined immediately at room temperature in a dark room to evaluate the mitochondrial distribution and nuclear stage. MitoTracker Green dye accumulates in the membrane lipids and mitochondrial distribution using a Hamamatsu ORCA cooled-chip digital camera and a Leica DM IRB microscope with filter sets for DAPI, FITC, and Texas Red. Oocytes were classified into four different mitochondrial distribution categories according to Stojkovic and colleagues [32]. To measure the fluorescence intensity, MitoTracker Green FM emission was assayed at 500 nm, JC1 red filter at 515 nm and green filter at 488 nm, and Hoechst 33342 at emission of 488 nm.
Dead oocytes were classified by five criteria depending on the morphological changes associated with oocytes after 24 h maturation in vitro: (1) cytoplasmic opacity vs translucency, (2) cytoplasmic shrinkage from the zona pellucida, (3) fragmentation, (4) coloration and distribution of colors, and (5) disrupted zona pellucida [33, 34].
For the evaluation of nuclear maturation after 24 h IVM, COCs were subjected to removal of the surrounding cumulus cells and oocytes were stained with 1 μg/mL Hoechst 33342 and examined under a fluorescence microscope for nuclear status. Oocytes that reached metaphase II (MII) stages were considered mature.
Immunofluorescence
Oocytes were fixed, quenched, permeabilized, and stained for Oct4 and AMPK; counterstained for DAPI by modifying previous protocols used in mouse embryonic stem cells (ESCs); and validated by immunoblot [35, 36] and used in mouse two-cell embryos [20]. Briefly, oocytes were fixed in 2% paraformaldehyde in PBS (pH 7.4) for 30 min at room temperature, quenched in 0.5 M glycine, and rinsed three times in PBS containing 0.5% bovine serum albumin (PBS/BSA). After paraformaldehyde fixation, oocytes were permeabilized for 15 min with 0.1% Triton X-100. Oct4 and AMPK thr172P antibodies were incubated overnight at 4 °C using a mouse monoclonal antibody against Oct4 diluted 1:100 from a 200-μg/mL stock (carboxy terminus, C10 monoclonal sc5279, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) [37, 38] and rabbit polyclonal to AMPK thr172P diluted to 1:200 followed by anti-mouse or anti-rabbit HRP-linked antibody diluted 1:500. Binding of primary antibody was visualized using a Texas Red-conjugated, affinity-purified, donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) [39, 40] diluted 1:500 from a 1-mg/mL stock. Oocytes were incubated for 60 min at room temperature in the secondary antibody mixture; nuclei were counterstained with 4′,6-diamidino-2-phenylindole and HCl (DAPI) diluted 1:1000 from a 5-mg/mL stock and mounted on glass slides.
Fluorescent antibody labeling was imaged using a Hamamatsu ORCA cooled-chip digital camera and a Leica DM IRB microscope with filter sets for DAPI, FITC, and Texas Red. Oocytes were imaged at an objective magnification of ×20 and an exposure time of 1.5 s. The Texas Red stain intensities were quantified using Simple PCI (Hamamatsu) imaging software and formatted using Microsoft Excel and Adobe Photoshop 7.0. Fluorescence intensities (gray levels) were determined for each antibody and non-immune IgG (background) by circumscribing the nuclei. All micrographs were exposed using the same shutter speed, and all experiments were repeated at least three times. The two proteins of interest Oct4 and AMPK thr172P antibody have previously been used in the bovine model for both immunofluorescence and immunoblot. Oct4 of the correct size (~ 35 kDa) is detected by immunoblot in bovine oocytes [37] using the N-terminus antibody from Santa Cruz. The C terminus antibody from Santa Cruz used here detects a pluripotent form of Oct4 in mouse oocytes by immunoblot and immunofluorescence, but this tends not to be nuclear until the eight-cell stage [21]. The antibody used here detects Oct4 in bovine-induced pluripotent stem cells by immunofluorescence [41]. pAMPK ser79P of the correct size (~ 63 kDa) has been detected in bovine granulosa cells by immunoblot and immunohistochemistry [39], in bovine oocytes by immunofluorescence [42], and in mouse oocytes by immunoblots [43], using the antibody used here.
Assay for bovine oocyte ATP levels
Each sample was placed in a sterile tube with 50 μL of ultrapure water and stored at − 80 °C until analysis. ATP was measured in denuded oocytes at the MII stage, after in vitro maturation. The measurement was performed using a commercial assay kit based on the luciferin-luciferase reaction (Bioluminescent Somatic Cell Assay Kit, Fl-ASC; Sigma) following the technique described by Rieger [44] and the manufacturer’s recommendations. Briefly, oocytes were thawed and kept on ice. A standard curve including 10 ATP concentrations from 0.16 to 10 pmol/25 μL in the sample buffer was generated for each series of analyses. This concentration range was linear and enabled interpolation of ATP concentrations for single oocytes isolated from IVM media containing 0 or 40 μM CoQ10. Then, 100 μL of ice-cold somatic cell reagent (FL-SAR reagent) was added to the oocyte solution and incubated for 5 min on ice. Next, 100 mL of diluted ice-cold assay mix (FL-AAM reagent; 1:25 with ATP assay mix dilution buffer, FL-AAB reagent) was added, and the tubes were kept for an additional 5 min at room temperature in the dark. The contents of the tubes were transferred to a 96-well plate. ATP content in an oocyte was quantified by measuring the luminescence using a luminometer (Synergy H1 Microplate Reader, Biotek, Winooski, VT). The ATP content was calculated using the formula derived from the linear regression of the standard curve.
Statistics
All experiments were replicated at least three times. Data were subjected to SPSS version 22.0 for analysis. Least square linear regressions were used to model the concentration loss of CoQ10 in IVM media. The half-lives of CoQ10 at 20, 40, and 60 μM were then calculated from the predicted regression equations. One-way ANOVA was used to compare the mitochondrial number, quality, and energy levels of the oocytes cultured at different CoQ10 concentrations. Percentages of dead, polarized, and clustered oocytes were transformed using the arcsine algorithm to meet the assumptions of one-way ANOVA. Data on mitochondrial number, ATP per oocyte, and AMPK and OCT4 intensities were untransformed. Following significant ANOVA, Dunnett’s post hoc tests were performed to compare CoQ10 concentrations to the control and Tukey’s post hoc tests were performed among all CoQ10 concentrations. Data are presented in the form of means ± standard error (SE). p < 0.05 indicates statistical significance.
Results
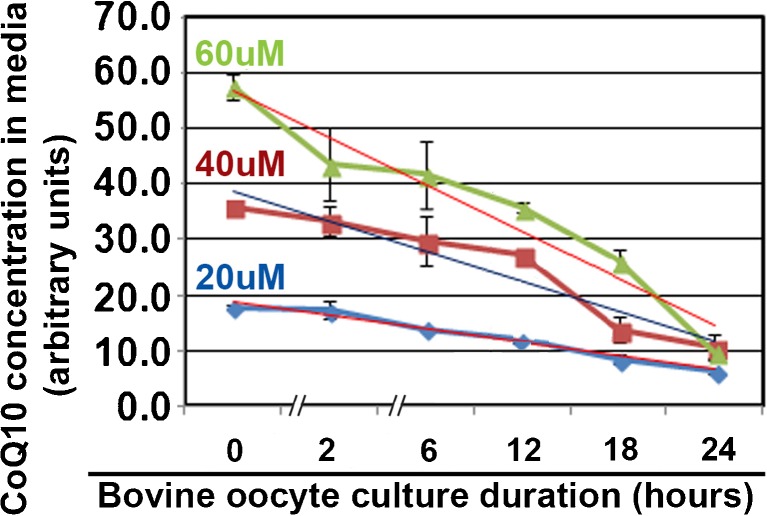
CoQ10 is known to be hydrophobic, and the half-life in IVM culture media was tested first. CoQ10 at 20, 40, and 60 μM undergoes half of the concentration loss at 11.5, 12.7, and 13.8 h, respectively (Fig. 1). This suggests that CoQ10 moves from aqueous to oil phase or from aqueous media past the zygote plasmalemma into the zygote by about the halfway point of IVM culture. Confidence in the assay is assured by input of 20, 40, and 60 μM at Tzero (time zero) creating nearly 20, 40, and 60 arbitrary units on the y-axis. Thus, the time zero dose response from 0 to 60 μM for CoQ10 was in the linear range, and thus, a standard curve was composed. Also, the variation in the triplicate experiments was generally low.
Fig. 1.
Half-life of CoQ10 in aqueous oocyte ICM media is ~ 12 h. Microdrops of oocyte IVM media were incubated under tested nonembryotoxic oil with 0, 20, 40, and 60 μM CoQ10 and tested spectrophotometrically at the indicated times for residual CoQ10.The biological experiment was repeated three times using 244 oocytes. A linear regression was done for each CoQ10 concentration and compared with starting concentration; the half concentration (shown in micromolars on the regression line) loss was calculated for time (shown in hours near the x-axis)
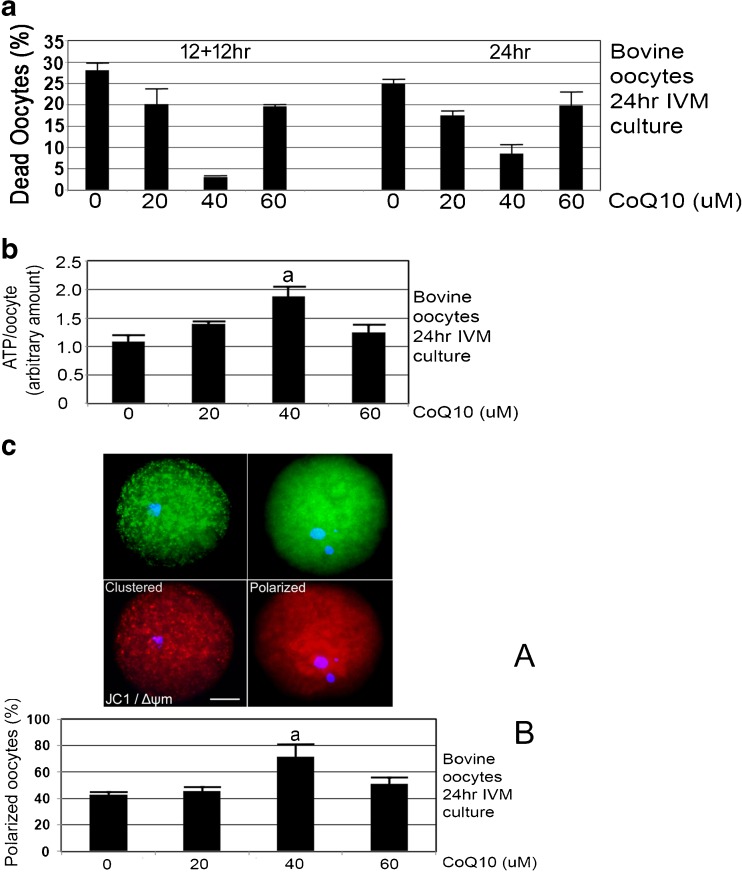
Since Fig. 1 suggested that 50% of CoQ10 remained in media at ~ 12 h, 24-h treatments were compared to replenishment at 12 h (12 h + 12 h) for decreases in oocyte death at the end of culture. CoQ10 treatment for 12 h + 12 h IVM culture produced 3.4% dead oocytes at 40 μM, 19.4% at 60 μM, 20.0% at 20 μM, and 28.2% at 0 μM. CoQ10 treatment for 24 h of IVM culture produced 7.8% dead oocytes at 40 μM, 19.5% at 60 μM, 17.3% at 20 μM, and 24.9% at 0 μM. For either 24 h or 12 h + 12 h culture, CoQ10 treatment at 40 μM significantly decreased oocyte death at the end of 24 h of IVM culture compared with 0, 20, or 60 μM CoQ10 (Fig. 2a, ANOVA and Tukey post hoc tests, p < 0.05). Thus, where oocyte death is significantly highest at 0 μM, death is significantly lowest at 40 μM. However, treatment with CoQ10 at any dose for 24 h, compared with the same dose replenished at 12 h (12 h + 12 h), produced no significant difference (Fig. 2a, ANOVA and Tukey post hoc tests, p > 0.05). Since there was no significant differences in CoQ10 replenishment, for the remaining culture we use a 24-h CoQ10 exposure without replenishment in further experiments.
Fig. 2.
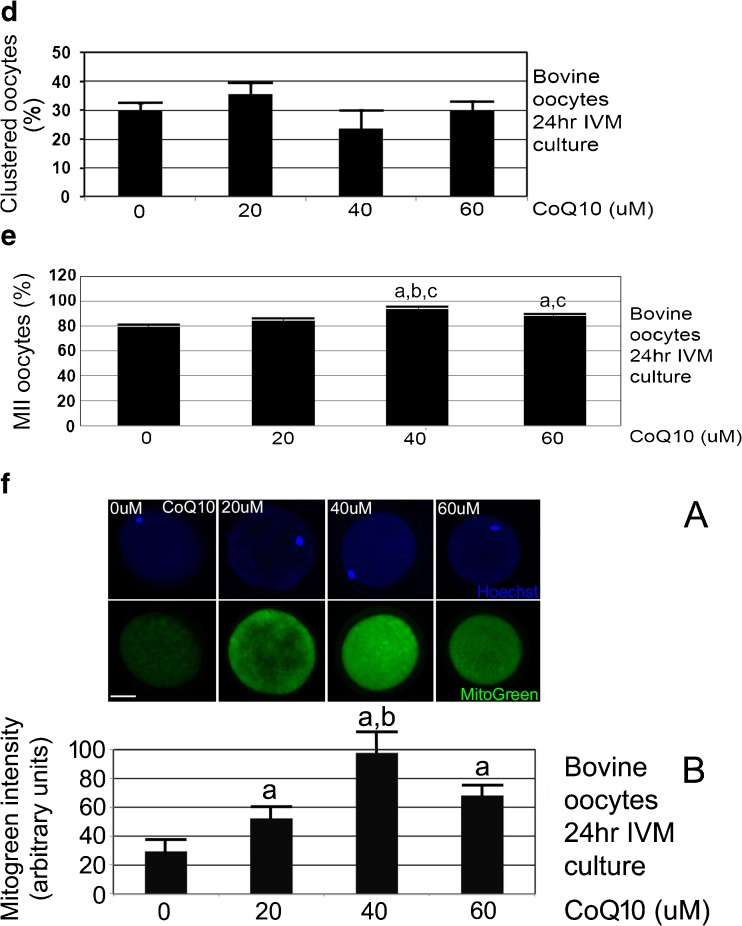
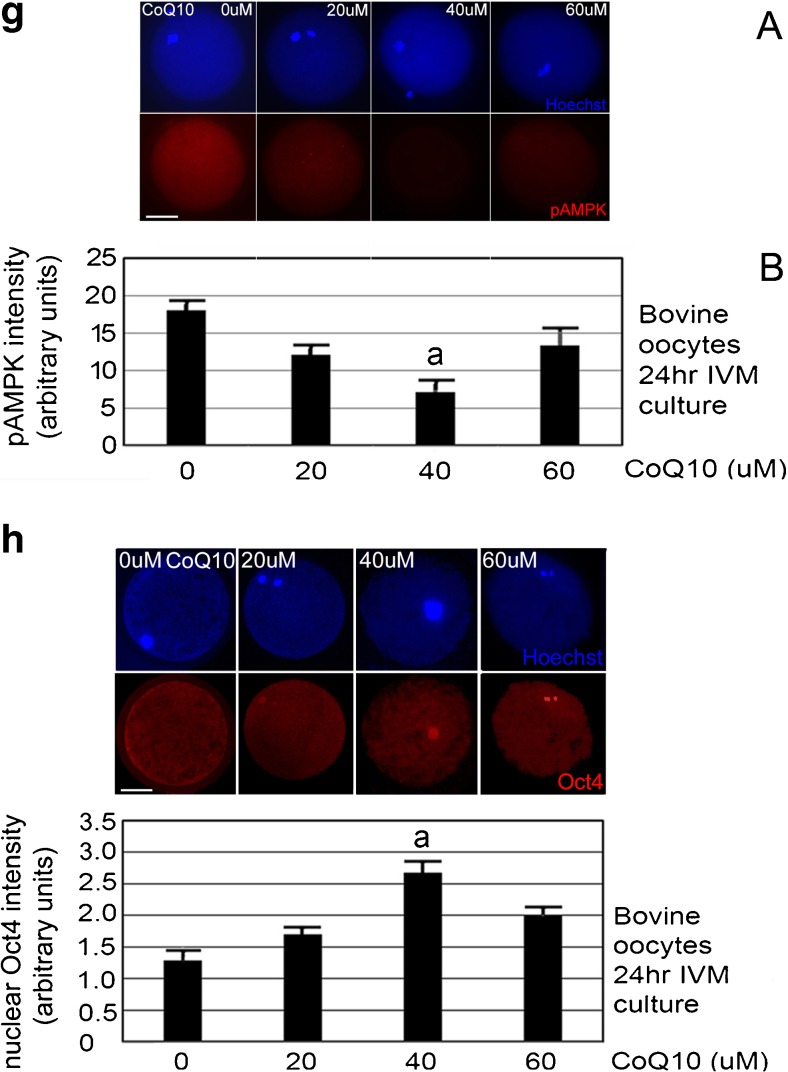
a CoQ10 treatment during 24-h IVM culture produces most ATP/oocyte at 40 μM. Oocytes were isolated by aspiration derived from slaughterhouse, incubated with indicated concentrations of CoQ10 for 24 h, and assayed by ATP luminescence assay. The biological experiment was repeated three times using 144 oocytes, error bars show s.e.m., and significance was analyzed by ANOVA followed by Tukey post hoc test. a Significant difference of 40 μM CoQ10 compared to 0 or 60 μM CoQ10 (p < 0.05). b CoQ10 treatment during 24 h IVM culture produces the smallest, 4.5% oocyte death at 40 μM with significantly higher oocyte death at 20, 60, and 0 μM CoQ10. Oocytes were isolated by aspiration derived from slaughterhouse, incubated with indicated concentrations of CoQ10 for 24 h, and micrographed and assayed for death by morphological means. The biological experiment was repeated four times using 241 oocytes, error bars show s.e.m., and significance was analyzed by ANOVA followed by Tukey post hoc test. a Significant difference of 40 μM CoQ10 compared with 0, 20, or 60 μM (p < 0.05). c CoQ10 treatment during 24 h IVM culture produces the highest fraction of oocytes with polarized mitochondria at 40 μM compared with oocytes at 20 and 0 μM CoQ10. Oocytes were isolated by aspiration derived from slaughterhouse, incubated with indicated concentrations of CoQ10 for 24 h, stained using JC1 mitochondrial charge dye, and micrographed and assayed by morphological means. a Micrographs of clustered and polarized bovine oocytes after JC1 staining for mitochondrial charge; top pair is green, low-charge stain and bottom pair is red, high-charge stain near nucleus; Δψm. b The biological experiment was repeated four times using 241 oocytes, error bars show s.e.m., and significance was analyzed by ANOVA followed by Tukey post hoc test. a Significant difference of 40 μM CoQ10 compared with 0 and 20 μM (p < 0.05). White bar shows 25 μM. d CoQ10 treatment during 24 h IVM culture produces the lowest fraction of oocytes with clustered mitochondria at 40 μM compared with oocytes at other levels. Oocytes were isolated by aspiration derived from slaughterhouse, incubated with indicated concentrations of CoQ10 for 24 h, stained using JC1 mitochondrial charge dye, and micrographed and assayed by morphological means. The biological experiment was repeated four times using 241 oocytes, error bars show s.e.m., and significance was analyzed by ANOVA followed by Tukey post hoc test. There were no significant differences at any CoQ10 levels (p > 0.05). e CoQ10 dose at 40 μM increases the fraction of oocytes reaching the MII stage. The biological experiment was repeated ten times using 503 oocytes, error bars show s.e.m., and significance was analyzed by ANOVA followed by Tukey two-sided post hoc test. a Significant difference of 40 and 60 μM CoQ10 compared with 0 μM. b Significant difference of 40 and 20 μM (p < 0.05). c No significant difference between 40 and 60 μM (p > 0.05). f Mitochondria are distributed mostly evenly through the oocyte and are at higher levels at 40 μM CoQ10-treated oocytes. Bovine oocytes were cultured with 0, 20, 40, or 60 μM CoQ10 treatment during 24 h IVM, and stained for MitoGreen or Hoechst and micrograph. a Representative micrographs for each treatment are shown above. b The biological experiment was repeated three times using 189 oocytes, error bars show s.e.m., and significance was analyzed by ANOVA followed by Tukey two-sided post hoc test. a Significant difference of 40 μM CoQ10 compared with 0, 20, or 60 μM (p < 0.05). White bar shows 25 μM. g CoQ10 treatment during 24 h IVM culture produces the smallest, 7.1% arbitrary units of oocyte AMPK thr172P at 40 μM with significantly higher oocyte levels at 20, 60, and 0 μM CoQ10. Oocytes were isolated by aspiration derived from slaughterhouse, incubated with indicated concentrations of CoQ10 for 24 h, fixed, permeabilized, developed for pAMPK thr172P and Hoechst, and micrographed. The biological experiment was repeated three times using 168 oocytes, error bars show s.e.m., and significance was analyzed by ANOVA followed by Tukey post hoc test. a Significant difference of 40 μM CoQ10 compared with 0, 20, or 60 μM (p < 0.05). White bar shows 25 μM. h CoQ10 treatment during 24 h IVM culture produces the smallest, 2.6 arbitrary units of oocyte Oct4 at 40 μM with significantly lower oocyte levels at 20, 60, and 0 μM CoQ10. Oocytes were isolated by aspiration derived from slaughterhouse, incubated with indicated concentrations of CoQ10 for 24 h, fixed, permeabilized, developed for Oct4 and Hoechst, and micrographed. The biological experiment was repeated three times using 181 oocytes, error bars show s.e.m., and significance was analyzed by ANOVA followed by Tukey post hoc test. a Significant difference of 40 μM CoQ10 compared with 0, 20, or 60 μM (p < 0.05). White bar shows 25 μM
The standard curve for ATP was linear and very sensitive, and thus, ATP levels from single oocytes could be interpolated from the curve (see “Materials and methods” section). CoQ10 treatment at 40 μM significantly increased ATP at the end of 24 h of IVM culture compared with 0 or 60 μM (Fig. 2b, ANOVA and Tukey post hoc tests, p < 0.05). At 40 μM CoQ10, there was a 1.9-fold increase in ATP compared to 0 μM, suggesting that CoQ10 enables sufficient ATP to enable simultaneous stress response to culture conditions and more oocyte maturation.
CoQ10 treatment for 24 h of IVM culture produced significantly higher fractions of oocytes with mitochondria polarized for charge Δψm detected by JC1. The stain was detected eccentrically around nuclei when cultured for 24 h at 40 μM compared with 0 and 20 μM but not significantly higher than 60 μM (Fig. 2c, ANOVA and Tukey post hoc tests, p < 0.05).
CoQ10 treatment for 24 h of IVM culture produced decreased clustering at 40 μM, but this was not significant as analyzed in Fig. 2d (ANOVA and Tukey post hoc tests, p < 0.05). This suggests that mitochondrial polarization is a clearer morphological measure of oocyte health than clustering. An interesting finding was that oocyte health was marked by high polarization at Coq10 with least death, and low polarization at highest death. But this was not evident for clustering as no dose of Coq10 produced a significantly different level of clustering. One hypothesis would be that for each dose, % clustered + % polarized oocytes = 100%. This is not the case as adding polarized and clustered subpopulations together for each CoQ10 dose gives 71, 73, 93, and 80% for the CoQ10 doses from 0 to 100%, respectively. However, if percentage of dead oocytes is added from Fig. 2a, the total oocytes in all three states is 98, 97, 97, and 100%, suggesting that clustered oocytes might produce dead oocytes at the end of the 24-h IVM, but that all oocyte states in polarized, clustered, and dead oocytes (from Fig. 2a, c, d) together constituted nearly the whole population of oocytes.
CoQ10 treatment at 40 μM for 24 h of IVM culture produced a small but significant increase, from 79.4 to 93.8% in the fraction of MII oocytes compared with 0 μM CoQ10 (Fig. 2e, ANOVA and Tukey post hoc tests, p < 0.05).
CoQ10 treatment for 24 h of IVM culture produced 3.1-fold increase in MitoTracker Green (MitoGreen) mitochondrial stain at 40 μM CoQ10 compared with 0 μM. All CoQ10 levels increased the total MitoGreen staining significantly compared with 0 μM (Fig. 2f, ANOVA and Tukey post hoc tests, p < 0.05). Compared with JC1 eccentric staining in polarized oocytes, MitoGreen staining was relatively evenly distributed. This suggests that mitochondria may be more highly charged in the proximity of polarized nuclei, but that the mitochondrial population as a whole is not polarized.
CoQ10 treatment for 24 h of IVM culture produced 7.2 arbitrary units of pAMPK thr172P in oocytes at 40 μM, 13.8 units in oocytes at 60 μM, 11.9 units at 20 μM, and 18.3 units at 0 μM. CoQ10 treatment at 40 μM significantly decreased oocyte pAMPK thr172P at the end of 24 h of IVM culture compared with 0, 20, or 60 μM CoQ10 (Fig. 2g, ANOVA and Tukey post hoc tests, p < 0.05). Thus, where ATP is significantly the highest at 40 μM, levels of activated AMPK are the lowest.
CoQ10 treatment at 40 μM significantly increases nuclear Oct4 at the end of 24 h of IVM culture compared with 0 or 60 μM (Fig. 2h, ANOVA and Tukey post hoc tests, p < 0.05). At 40 μM CoQ10, there was a 1.9-fold increase in Oct4 compared to 0 μM, suggesting that CoQ10 enables sufficient ATP to enable simultaneous low levels of pAMPK and high levels of Oct4.
Discussion
Here, we show that 40 μM CoQ10 is optimal for suppressing bovine oocyte death during IVM, and that this is associated with high levels of Oct4 pluripotency factor protein, mitochondrial Δψm charge nuclear polarization, and development to MII, which are also marks of healthy oocyte development. For the first time, we show that compared with 0 μM CoQ10, 40 μM also sustains the highest ATP levels (Fig. 3) and increases mitochondrial mass, and with this, it is the lowest activity of AMPK which is activated by low ATP and commensurate high AMP. Novel for this report is the analysis of loss of potency in oocytes during IVM stress. But potency loss occurs in an AMPK-dependent manner which occurs due to hyperosmotic stress, AMPK agonist drugs, and diet supplements in two-cell embryos, blastocysts ESCs, iPSCs, and TSCs [17, 22, 35, 36, 45–49]. Oct4 is known to regulate potency, metabolism, and stress responses and is needed to regulate transcription of a large number of genes before, and in preparation for, the stress response [18]. Significantly, the form of Oct4 regulated by IVM stress and upregulated by optimal CoQ10 here and in mouse two-cell embryos [20], blastocysts [50], and ESCs [36, 51] is the pluripotency-maintaining isoform identified by the C terminal-specific antibody to Oct4 [21]. It is known that potency loss in ESCs and TSCs enables differentiation, but defining the outcomes mediated by decreases in Oct4 in oocytes and in two-cell embryo requires further study.
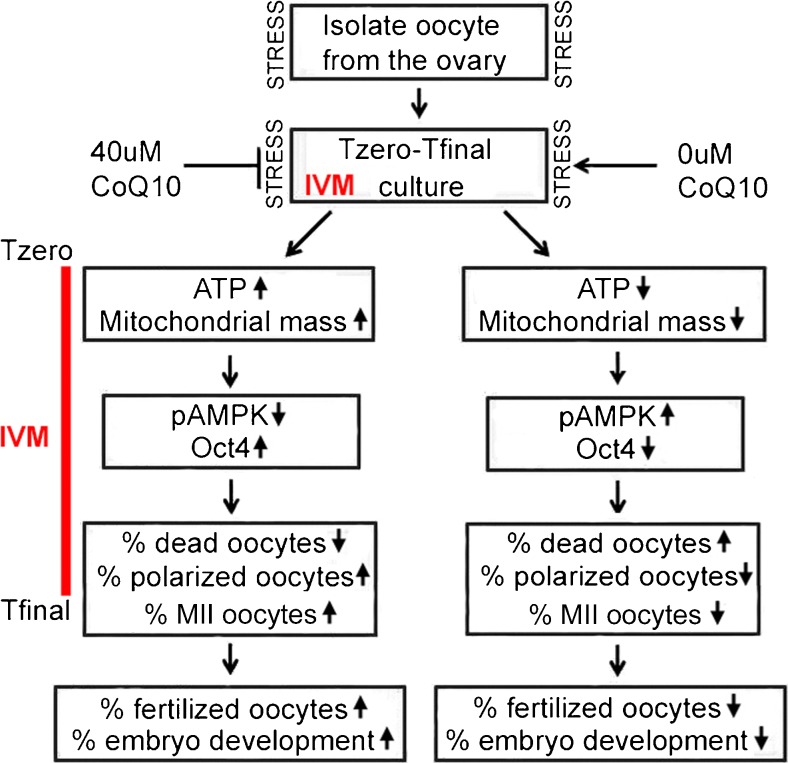
Fig. 3.
Summary diagram shows the hypothetical mechanisms induced by optimal dose of 40 μM CoQ10 compared with normal IVM without CoQ10 and the hypothetical sequelae of CoQ10 treatment after IVM. Hypothetically, isolation and culture are stressful, but the stress of IVM culture was from Tzero to Tfinal, which creates ~ 30% oocyte death at Tfinal, but can be ameliorated by an optimal CoQ10 dose that modulates three pairs of markers. These markers may represent causal mechanisms creating the best environment with the lowest oocyte death and highest potential for continuing development after fertilization
The causes of culture stress are multifactorial and include suboptimal oxygen, buffer components, nutritional components, and insufficient fluid movement to deliver nutrients and remove waste. In the study of seven media created over 30 years of research on preimplantation embryos, the most recently developed media produce about 2-fold less stress than the historical oldest media [2]. However, stage-matched, cultured blastocysts had ~ 50% higher stress levels than blastocysts ex vivo. The stress of oocyte isolation and IVM culture leads to approximately 25% oocyte death after 24 h culture without CoQ10. Although genetic and epigenetic damage is thought to be a major cause of decrease in oocyte quality [52], in studies here oocyte loss was largely reversible with increased ATP. Thus, culture stress arises from many stimuli but are higher than oocytes and embryos in vivo. What stress is the target of CoQ10? Specifically, mitochondrial efficiency and rate of ATP production is the CoQ10 target. In general, all stresses require ATP to return to cellular homeostasis. Then the oocyte (or stem cell) can return to approximate its normal developmental program and that program’s ATP requirements. If there is more nutrition and/or higher mitochondrial efficiency via CoQ10, then the normal developmental trajectory is more closely met. If not normal, development is deficient.
ATP and mitochondria mass increase during normal oocyte maturation. Although immature, mitochondria in bovine and human oocytes can produce ATP and ATP increases during oocyte maturation in vivo in abattoir-derived bovine oocytes [32, 33, 53] like those studied here. Developmental competence is marked by higher ATP and lower competence with lower ATP. Lower mitochondrial activity and ATP production are associated with poor embryogenesis, and higher activity and production are associated with enhanced progression to blastocyst stage after fertilization [32, 54–56]. Thus, the findings here suggest that CoQ10 at 40 μM improves ATP in oocytes, but should also lead to improved embryogenesis after fertilization.
Other groups have used many additives to IVM media to improve it. Spindle complex quality and MII oocyte fraction are a measure of oocyte readiness for fertilization [57]. Midkines improved MII from 78 to 86% [58], lysophosphatidic acid from 65 to 79% [59], and optimal levels of cAMP modulator from 79 to 92% [60]. Thus, the improvement here from 79 to 94% is in high baseline and improvement range compared with previous reports.
In a previous study of summer heat stress effects on bovine oocytes, a dosimetric analysis of CoQ10 effects was tested only during embryo culture [6]. Only one dose at 50 μM was used during IVM and although CoQ10 could improve some molecular/mitochondrial function in embryo culture at midrange from 0 to 100 μM tested. CoQ10 at 30–50 μM improved percentage of blastocyst from oocytes isolated in fall. But CoQ10 did not overcome heat stress during summer occurring before embryo culture (Fig. 3). Thus, in these studies heat stress in summer was more than CoQ10 could remediate. In studies here, hypothetically stress prior to IVM may have been less and improvement was seen in oocytes cultured with CoQ10 during IVM.
The following hypothesis was tested here: that IVM creates stress and that CoQ10 improves oocyte maturation development by increasing ATP production, decreasing AMPK activity, and thus enhancing potency through the pluripotency-maintaining isoform of Oct4 protein. Our study design is to use oocytes from randomized healthy ovaries that are a kind gift of a local slaughterhouse and prepared by Dr. Joe Folger using protocols developed by Dr. George Smith at Michigan State University. This randomizes oocytes over important variables of bovine age, breed, and time of year of oocyte collection (e.g., creating differences in ambient temperature and heat stress). As mentioned above, previous studies show that CoQ10 does not solve effects of certain existing variables prior to treatment such as heat stress. The strength of the study design is that it tests bovine and human IVF that uses randomized oocyte sources with many environmental and genetic variables that influence the efficacy of IVM and thus the ability of CoQ10 to increase that efficacy. The weakness of this design is that specific confounding genetic and environmental variables may lead to ineffectiveness of CoQ10 in enhancing IVM efficacy, and these confounding variables will need further studies.
One key conclusion is that in a back-to-nature approach [61], the aim of manipulations of IVF/ART is to identify, develop measurements, confirm mechanisms to be able to manage methods, and keep outcomes as close to time- or stage-matched oocytes or embryos tested immediately ex vivo. Novel measurements presented here in oocytes enable the associations of levels of ATP, AMPK activity, and potency/stemness which have known efficacy in later stages of embryos where stem cells are affected causally by these variables [18]. Interestingly in both oocytes [43, 62] and embryos [63, 64], management of AMPK by agonists such as metformin has been reported to help adapt to previous conditions such as diabetic mothers [43, 62] or of four stresses of IVM culture [15]. However, the data here and from other studies on AMPK agonism by stress, diet supplements, or drugs is that higher durations or magnitudes of AMPK activity can decrease anabolism, growth, or potency/stemness with known and unknown pathogenic consequences [17, 20, 22, 45, 47, 50, 65]. It is quite likely that the AMPK agonist does not fit the back-to-nature goals of IVF/ART, but this also needs to be tested against age- or stage-matched control oocytes and embryos from normal mothers. However, when possible, the use of CoQ10, by increasing the efficiency or rate of energy production, may preclude the need to modulate AMPK. CoQ10 may thus overcome stress prior to IVM (e.g., heat stress of bovine donors) or current stress (e.g., IVM culture, oocyte isolation, and handling) without concerns of the possible toxicity due to higher possible AMPK hyper-activity caused by agonists. CoQ10 appears to increase efficacy of IVF/ART both in vitro and in vivo, and can improve aged oocytes and decrease miscarriage, thus affecting gametes and embryos [7, 66, 67].
A limitation of the study here is the lack of studies of the consequences, and follow-up studies should test the hypothesis that CoQ10 use to overcome oocyte IVM stress produces better fertilization and blastocyst formation rates, whether or not CoQ10 use is continued during culture from zygote to blastocyst stage.
Another study optimized Coq10 doses in bovine embryo culture [68]. CoQ10 doses of 0, 10, 30, and 100 μM were tested, and 30 μm was the optimal dose with positive effects on blastocyst formation, cell proliferation, hatching, and ATP content cultured bovine embryos. The highest 100 μM CoQ10 significantly reduced the blastocyst development rate compared to 30 μM. In addition, ATP, death, and polarization were significantly optimized for 40–60 μM compared with 100 μM. Both previous studies suggest that optimal CoQ10 doses for molecular and biological outcomes in bovine embryo culture occur at midrange doses similar to those reported for bovine oocyte IVM here.
The low percentage of dead oocytes and high polarization at 40 μM in the studies here suggest that the potential mitochondria Δψm membrane charge is a key indicator of cellular survival; probably increasing ATP production at the polarized location where ATP is needed. The highest charge indicated by red polymeric JC1 stain is near the nucleus, and the highest monomeric green JC1 stain of very low charge is not near the nucleus in the 40 μM CoQ10 treatment. In this group, there is the highest total number of mitochondria indicated by MitoGreen staining. But this treatment increased mitochondrial mass 3.1-fold indicated by MitoGreen staining, and thus, the composite nuclear localization at the end of culture and accumulation of mitochondrial mass during culture amplifies the effect of localized energy production. Mitochondrial charge also reflects the pumping of hydrogen ions across the inner membrane during the process of electron transport and oxidative phosphorylation, the driving force behind ATP production [69]. But actual ATP throughput requires analysis of respiration and use of radiolabeled phosphate to test for energy production rate.
Previously, oocytes were classified for competence for fertilization and embryogenesis by morphology [34] and this was cross-referenced to ATP levels in oocytes [33]. This scoring system was used here, and these results confirm that optimal morphology, high surviving percentage of oocytes, and lowest subpopulation of dead oocytes at the highest ATP level are produced by 40 μM CoQ10.
CoQ10 is highly hydrophobic, and it was found that at all CoQ10 levels tested, ~ 50% of the mitochondrial agonist remained in the aqueous IVM media by 12 h. The amount of death was not significantly different when media were replenished at 12 h with CoQ10 or oocytes remained in initial media—despite loss of CoQ10. Lack of significant difference could be because important CoQ10 effects occur in the first 12 h of culture and/or that sufficient CoQ10 partitions into the oocyte/cumulus cells and sustains mitochondrial electron transport chain at higher activity levels during the entire 24 h. In the first half of oocyte maturation, energy is needed for increased mitochondrial mass, and in the second half or maturation, polarized, charged mitochondrial aggregates around the nucleus should provide energy for pronuclear formation [30] and calcium release induced by fertilization [70].
Mitochondria increase greatly during normal oogenesis, although about 10-fold more mitochondria are produced than the oocyte requires [71]. After fertilization, this is thought to maintain dividing cells during embryogenesis before mitochondrial biogenesis restarts. Mitochondrial biogenesis occurs mostly in the first 12 h of IVM and is reported to increase mitochondrial mass ~ 5-fold during this early part of IVM compared with an ~ 2-fold increase during maturation in vivo [72]. CoQ10 may increase mitochondrial mass by increasing biogenesis or protecting existing mitochondria during IVM stress, or both. In astrocytes, the stress of H2O2 can decrease mitochondrial mass nearly 4-fold, but coincubation with CoQ10 alleviates the decrease to 2-fold, thus protecting the surviving mitochondria [73]. CoQ10 also increases mitochondrial mass as indicated by increases in mitochondrial proteins porin, mitofilin, and mitochondrial biogenic transcription factor peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a). MitoTracker Green used here measures thiolated proteins of the inner matrix [74] and thus is also a measure of mitochondrial mass but not number. The exact effects of CoQ10 on preserving mitochondria and increasing mitochondrial biogenesis in oocytes remains to be determined.
Our focus in testing our hypothesis has been on the oocyte, and all assays were on the oocyte. However, much of the metabolism of oocytes and effects of CoQ10 are on the cumulus cells that are connected to and regulated by the oocytes during maturation in vivo and in vitro. This is reviewed elsewhere [75–79]. It is important to consider that hyaluronidase treatment is required to isolate oocytes for assays at the end of IVM. Hyaluronidase treatment activates AMPK thr172P (El Shmoury and Rappolee, unpublished); however, the low variations in the pAMPK assays and other assays suggest, as well as the low pAMPK at 40 μM, that the pAMPK activation by hyaluronidase window was avoided to obtain an activity level dependent on CoQ10 doses.
It should be noted that some AMPKα1-null embryos are lost after fertilization and before birth, but during culture there is a huge loss of null embryos in the two-to-four-cell stage [80]. This suggests that oocyte and embryo culture create stress that requires AMPK activity for an adaptive response during a period when AMPK is not needed as much in vivo. Taken together, the studies on null embryos and the studies here suggest that AMPK is needed for oocytes and embryos to resist culture stress, but increasing ATP through CoQ10 may preclude for AMPK agonists and possible toxicity.
Acknowledgements
Dr. Mohammed Abdulhasan was supported by a grant from the Iraqi Ministry of Higher Education and Research, and further support was from the Office of the Vice President of Research at Wayne State University, the Wayne State University Department of Ob/Gyn, and the Kam Moghissi Endowed Chair. Additional support was from R03HD06143101 (DAR). Oocytes were kindly provided by Dr. George Smith and Joe Folger from the Michigan State College of Animal Sciences. We thank Dr. Yu Yang, Dr. Alan Bolnick, and other lab members, and to Dr. Jose Cibelli for insightful comments on the manuscript.
References
- 1.Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28:773–783. doi: 10.1007/s10815-011-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Puscheck EE, Lewis JJ, Trostinskaia AB, Wang F, Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril. 2005;83(Suppl 1):1144–1154. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev. 2007;74:1287–1294. doi: 10.1002/mrd.20563. [DOI] [PubMed] [Google Scholar]
- 4.Leese HJ. Metabolism of the preimplantation embryo: 40 years on. Reproduction. 2012;143:417–427. doi: 10.1530/REP-11-0484. [DOI] [PubMed] [Google Scholar]
- 5.Leese HJ, Baumann CG, Brison DR, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. 2008;14:667–672. doi: 10.1093/molehr/gan065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gendelman M, Roth Z. Incorporation of coenzyme Q10 into bovine oocytes improves mitochondrial features and alleviates the effects of summer thermal stress on developmental competence. Biol Reprod. 2012;87:118. doi: 10.1093/biolreprod/87.s1.118. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–895. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentov Y, Esfandiari N, Burstein E, Casper RF. The use of mitochondrial nutrients to improve the outcome of infertility treatment in older patients. Fertil Steril. 2010;93:272–275. doi: 10.1016/j.fertnstert.2009.07.988. [DOI] [PubMed] [Google Scholar]
- 9.Bentov Y, Casper RF. The aging oocyte—can mitochondrial function be improved? Fertil Steril. 2013;99:18–22. doi: 10.1016/j.fertnstert.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Noia G, Littarru GP, De Santis M, Oradei A, Mactromarino C, Trivellini C, et al. Coenzyme Q10 in pregnancy. Fetal Diagn Ther. 1996;11:264–270. doi: 10.1159/000264313. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo PL, Illera MJ, Illera JC, Illera M. Enhancement of cumulus expansion and nuclear maturation during bovine oocyte maturation in vitro by the addition of epidermal growth factor and insulin-like growth factor I. J Reprod Fertil. 1994;101:697–701. doi: 10.1530/jrf.0.1010697. [DOI] [PubMed] [Google Scholar]
- 12.El-Raey M, Geshi M, Somfai T, Kaneda M, Hirako M, Abdel-Ghaffar AE, et al. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol Reprod Dev. 2011;78:250–262. doi: 10.1002/mrd.21295. [DOI] [PubMed] [Google Scholar]
- 13.Nabenishi H, Takagi S, Kamata H, Nishimoto T, Morita T, Ashizawa K, et al. The role of mitochondrial transition pores on bovine oocyte competence after heat stress, as determined by effects of cyclosporin a. Mol Reprod Dev. 2012;79:31–40. doi: 10.1002/mrd.21401. [DOI] [PubMed] [Google Scholar]
- 14.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006;74:585–592. doi: 10.1095/biolreprod.105.046524. [DOI] [PubMed] [Google Scholar]
- 16.LaRosa C, Downs SM. Meiotic induction by heat stress in mouse oocytes: involvement of AMP-activated protein kinase and MAPK family members. Biol Reprod. 2007;76:476–486. doi: 10.1095/biolreprod.106.057422. [DOI] [PubMed] [Google Scholar]
- 17.Xie Y, Awonuga A, Liu J, Rings E, Puscheck EE, Rappolee DA. Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells [corrected] Stem Cells Dev. 2013;22:1564–1575. doi: 10.1089/scd.2012.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puscheck EE, Awonuga AO, Yang Y, Jiang Z, Rappolee DA. Molecular biology of the stress response in the early embryo and its stem cells. Adv Exp Med Biol. 2015;843:77–128. doi: 10.1007/978-1-4939-2480-6_4. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Awonuga AO, Zhou S, Puscheck EE, Rappolee DA. Interpreting the stress response of early mammalian embryos and their stem cells. Int Rev Cell Mol Biol. 2011;287:43–95. doi: 10.1016/B978-0-12-386043-9.00002-5. [DOI] [PubMed] [Google Scholar]
- 20.Bolnick A, Abdulhasan M, Kilburn B, Xie Y, Howard M, Andresen P, et al. Commonly used fertility drugs, a diet supplement, and stress force AMPK-dependent block of stemness and development in cultured mammalian embryos. J Assist Reprod Genet. 2016;33:1027–1039. doi: 10.1007/s10815-016-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda A, Mitani A, Miyashita T, Kobayashi H, Umezawa A, Akutsu H. Spatiotemporal dynamics of OCT4 protein localization during preimplantation development in mice. Reproduction. 2016;152:417–430. doi: 10.1530/REP-16-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, et al. Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction. 2010;140:921–930. doi: 10.1530/REP-10-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettegowda A, Patel OV, Ireland JJ, Smith GW. Quantitative analysis of messenger RNA abundance for ribosomal protein L-15, cyclophilin-A, phosphoglycerokinase, beta-glucuronidase, glyceraldehyde 3-phosphate dehydrogenase, beta-actin, and histone H2A during bovine oocyte maturation and early embryogenesis in vitro. Mol Reprod Dev. 2006;73:267–278. doi: 10.1002/mrd.20333. [DOI] [PubMed] [Google Scholar]
- 24.Nagy A, Gertsenstein M, Vintersten K, Behringer RR. Manipulating the mouse embryo. A laboratory manual. 3rd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 25.Bunaciu AA, Aboul-Enein HY, Fleschin S. FT-IR spectrophotometric analysis of coenzyme Q10 (CoQ10) and its pharmaceutical formulations. Prep Biochem Biotechnol. 2007;37:59–65. doi: 10.1080/10826060601040897. [DOI] [PubMed] [Google Scholar]
- 26.Karpinska J, Mikoluc B, Piotrowska-Jastrzebska J. Application of derivative spectrophotometry for determination of coenzyme Q10 in pharmaceuticals and plasma. J Pharm Biomed Anal. 1998;17:1345–1350. doi: 10.1016/S0731-7085(98)00003-X. [DOI] [PubMed] [Google Scholar]
- 27.Tarazona AM, Rodriguez JI, Restrepo LF, Olivera-Angel M. Mitochondrial activity, distribution and segregation in bovine oocytes and in embryos produced in vitro. Reprod Domest Anim. 2006;41:5–11. doi: 10.1111/j.1439-0531.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- 28.Zampolla T, Spikings E, Zhang T, Rawson DM. Effect of methanol and Me2SO exposure on mitochondrial activity and distribution in stage III ovarian follicles of zebrafish (Danio rerio) Cryobiology. 2009;59:188–194. doi: 10.1016/j.cryobiol.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 30.Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- 31.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress. Zygote. 2012;20:249–259. doi: 10.1017/S0967199411000220. [DOI] [PubMed] [Google Scholar]
- 32.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–909. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- 33.Nagano M, Katagiri S, Takahashi Y. ATP content and maturational/developmental ability of bovine oocytes with various cytoplasmic morphologies. Zygote. 2006;14:299–304. doi: 10.1017/S0967199406003807. [DOI] [PubMed] [Google Scholar]
- 34.Nagano M, Katagiri S, Takahashi Y. Relationship between bovine oocyte morphology and in vitro developmental potential. Zygote. 2006;14:53–61. doi: 10.1017/S0967199406003510. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Gomez-Lopez N, Drewlo S, Sanchez-Rodriguez E, Dai J, Puscheck EE, et al. Development and validation of a Rex1-RFP potency activity reporter assay that quantifies stress-forced potency loss in mouse embryonic stem cells. Stem Cells Dev. 2016;25:320–328. doi: 10.1089/scd.2015.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slater JA, Zhou S, Puscheck EE, Rappolee DA. Stress-induced enzyme activation primes murine embryonic stem cells to differentiate toward the first extraembryonic lineage. Stem Cells Dev. 2014;23:3049–3064. doi: 10.1089/scd.2014.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nganvongpanit K, Muller H, Rings F, Hoelker M, Jennen D, Tholen E, et al. Selective degradation of maternal and embryonic transcripts in in vitro produced bovine oocytes and embryos using sequence specific double-stranded RNA. Reproduction. 2006;131:861–874. doi: 10.1530/rep.1.01040. [DOI] [PubMed] [Google Scholar]
- 38.Lara E, Rivera N, Rojas D, Rodriguez-Alvarez LL, Castro FO. Characterization of mesenchymal stem cells in bovine endometrium during follicular phase of oestrous cycle. Reprod Domest Anim 2017. [DOI] [PubMed]
- 39.Tosca L, Chabrolle C, Uzbekova S, Dupont J. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK) Biol Reprod. 2007;76:368–378. doi: 10.1095/biolreprod.106.055749. [DOI] [PubMed] [Google Scholar]
- 40.Bilodeau-Goeseels S, Panich PL, Kastelic JP. Activation of AMP-activated protein kinase may not be involved in AICAR- and metformin-mediated meiotic arrest in bovine denuded and cumulus-enclosed oocytes in vitro. Zygote. 2011;19:97–106. doi: 10.1017/S0967199410000195. [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi T, Tsukiyama T, Kimura K, Matsuyama S, Minami N, Yamada M, et al. Generation of naive bovine induced pluripotent stem cells using piggyBac transposition of doxycycline-inducible transcription factors. PLoS One. 2015;10:e0135403. doi: 10.1371/journal.pone.0135403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeo S, Abe T, Shirasuna K, Kuwayama T, Iwata H. Effect of 5-aminoimidazole-4-carboxamide ribonucleoside on the mitochondrial function and developmental ability of bovine oocytes. Theriogenology. 2015;84:490–497. doi: 10.1016/j.theriogenology.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Hudson E, Chi MM, Chang AS, Moley KH, Hardie DG et al. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev Biol 2006. [DOI] [PubMed]
- 44.Rieger KJ, Aljinovic G, Lazowska J, Pohl TM, Slonimski PP, Aljinovic G. A novel nuclear gene, CBT1, essential for mitochondrial cytochrome b formation: terminal processing of mRNA and intron dependence. Curr Genet. 1997;32:163–174. doi: 10.1007/s002940050262. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Jiang Z, Bolnick A, Dai J, Puscheck EE, Rappolee DA. Departure from optimal O2 level for mouse trophoblast stem cell proliferation and potency leads to most rapid AMPK activation. J Reprod Dev. 2017;63:87–94. doi: 10.1262/jrd.2016-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie Y, Zhou S, Jiang Z, Dai J, Puscheck EE, Lee I, et al. Hypoxic stress induces, but cannot sustain trophoblast stem cell differentiation to labyrinthine placenta due to mitochondrial insufficiency. Stem Cell Res. 2014;13:478–491. doi: 10.1016/j.scr.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie Y, Abdallah ME, Awonuga AO, Slater JA, Puscheck EE, Rappolee DA. Benzo(a)pyrene causes PRKAA1/2-dependent ID2 loss in trophoblast stem cells. Mol Reprod Dev. 2010;77:533–539. doi: 10.1002/mrd.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vazquez-Martin A, Vellon L, Quiros PM, Cufi S, Ruiz de Galarreta E, Oliveras-Ferraros C, et al. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle. 2012;11:974–989. doi: 10.4161/cc.11.5.19450. [DOI] [PubMed] [Google Scholar]
- 49.Chae HD, Lee MR, Broxmeyer HE. 5-Aminoimidazole-4-carboxyamide ribonucleoside induces G(1)/S arrest and Nanog downregulation via p53 and enhances erythroid differentiation. Stem Cells. 2012;30:140–149. doi: 10.1002/stem.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolnick A, Kilburn B, Abdulhasan, M, , Xie Y, Shamir A, Dai J et al. 2-cell embryos are more sensitive than blastocysts to AMPK-dependent suppression of anabolism and potency/stemness by commonly used drugs, a diet supplement and stress. J Assist Reprod Genet 2017;ReSubmittted. [DOI] [PMC free article] [PubMed]
- 51.Li Q, Louden E, Dai J, Drewlo S, Puscheck E E, Chen K et al. Stress forces first lineage differentiation of mouse ESCs, validation of a high throughput screen for toxicant stress. Development 2017;Submitted. [DOI] [PMC free article] [PubMed]
- 52.Albertini DF, Sanfins A, Combelles CM. Origins and manifestations of oocyte maturation competencies. Reprod BioMed Online. 2003;6:410–415. doi: 10.1016/S1472-6483(10)62159-1. [DOI] [PubMed] [Google Scholar]
- 53.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, et al. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev. 2011;23:424–432. doi: 10.1071/RD10133. [DOI] [PubMed] [Google Scholar]
- 55.Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2002;124:745–754. doi: 10.1530/rep.0.1240745. [DOI] [PubMed] [Google Scholar]
- 56.Ward F, Enright B, Rizos D, Boland M, Lonergan P. Optimization of in vitro bovine embryo production: effect of duration of maturation, length of gamete co-incubation, sperm concentration and sire. Theriogenology. 2002;57:2105–2117. doi: 10.1016/S0093-691X(02)00696-9. [DOI] [PubMed] [Google Scholar]
- 57.Howe K, FitzHarris G. Recent insights into spindle function in mammalian oocytes and early embryos. Biol Reprod. 2013;89:71. doi: 10.1095/biolreprod.113.112151. [DOI] [PubMed] [Google Scholar]
- 58.Ikeda S, Ichihara-Tanaka K, Azuma T, Muramatsu T, Yamada M. Effects of midkine during in vitro maturation of bovine oocytes on subsequent developmental competence. Biol Reprod. 2000;63:1067–1074. doi: 10.1095/biolreprod63.4.1067. [DOI] [PubMed] [Google Scholar]
- 59.Boruszewska D, Sinderewicz E, Kowalczyk-Zieba I, Grycmacher K, Woclawek-Potocka I. The effect of lysophosphatidic acid during in vitro maturation of bovine cumulus-oocyte complexes: cumulus expansion, glucose metabolism and expression of genes involved in the ovulatory cascade, oocyte and blastocyst competence. Reprod Biol Endocrinol. 2015;13:44. doi: 10.1186/s12958-015-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernal-Ulloa SM, Heinzmann J, Herrmann D, Hadeler KG, Aldag P, Winkler S, et al. Cyclic AMP affects oocyte maturation and embryo development in prepubertal and adult cattle. PLoS One. 2016;11:e0150264. doi: 10.1371/journal.pone.0150264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biggers JD. Thoughts on embryo culture conditions. Reprod BioMed Online. 2002;4(Suppl 1):30–38. doi: 10.1016/S1472-6483(12)60009-1. [DOI] [PubMed] [Google Scholar]
- 62.Ratchford AM, Chang AS, Chi MM, Sheridan R, Moley KH. Maternal diabetes adversely affects AMP-activated protein kinase activity and cellular metabolism in murine oocytes. Am J Physiol Endocrinol Metab. 2007;293:E1198–E1206. doi: 10.1152/ajpendo.00097.2007. [DOI] [PubMed] [Google Scholar]
- 63.Eng GS, Sheridan RA, Wyman A, Chi MM, Bibee KP, Jungheim ES, et al. AMP kinase activation increases glucose uptake, decreases apoptosis, and improves pregnancy outcome in embryos exposed to high IGF-I concentrations. Diabetes. 2007;56:2228–2234. doi: 10.2337/db07-0074. [DOI] [PubMed] [Google Scholar]
- 64.Louden ED, Luzzo KM, Jimenez PT, Chi T, Chi M, Moley KH. TallyHO obese female mice experience poor reproductive outcomes and abnormal blastocyst metabolism that is reversed by metformin. Reprod Fertil Dev. 2014;27:31–39. doi: 10.1071/RD14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Q, Yang Y, Louden E, Puscheck E, Rappolee D. High throughput screens for embryonic stem cells; stress-forced potency-stemness loss enables toxicological assays. In: Faqi A, ed. Methods In Toxicology and Pharmacology: Springer, 2016.
- 66.Bentov Y, Hannam T, Jurisicova A, Esfandiari N, Casper RF. Coenzyme Q10 supplementation and oocyte aneuploidy in women undergoing IVF-ICSI treatment. Clin Med Insights Reprod Health. 2014;8:31–36. doi: 10.4137/CMRH.S14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil Steril. 2016;105:548–559. doi: 10.1016/j.fertnstert.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Stojkovic M, Westesen K, Zakhartchenko V, Stojkovic P, Boxhammer K, Wolf E. Coenzyme Q(10) in submicron-sized dispersion improves development, hatching, cell proliferation, and adenosine triphosphate content of in vitro-produced bovine embryos. Biol Reprod. 1999;61:541–547. doi: 10.1095/biolreprod61.2.541. [DOI] [PubMed] [Google Scholar]
- 69.Nicholls DG, Ferguson SJ. Bioenergetics 3. 3. San Diego, Calif: Academic Press; 2002. [Google Scholar]
- 70.Van Blerkom J, Davis P. Mitochondrial signaling and fertilization. Mol Hum Reprod. 2007;13:759–770. doi: 10.1093/molehr/gam068. [DOI] [PubMed] [Google Scholar]
- 71.Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z, Sun Z, Chen Y, He F. A modified cryoloop vitrification protocol in the cryopreservation of mature mouse oocytes. Zygote. 2009;17:217–224. doi: 10.1017/S0967199409005309. [DOI] [PubMed] [Google Scholar]
- 73.Noh YH, Kim KY, Shim MS, Choi SH, Choi S, Ellisman MH, et al. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013;4:e820. doi: 10.1038/cddis.2013.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Presley AD, Fuller KM, Arriaga EA. MitoTracker Green labeling of mitochondrial proteins and their subsequent analysis by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;793:141–150. doi: 10.1016/S1570-0232(03)00371-4. [DOI] [PubMed] [Google Scholar]
- 75.Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. MHR: Basic Sci Reprod Med. 2010;16:715–725. doi: 10.1093/molehr/gaq031. [DOI] [PubMed] [Google Scholar]
- 76.Bertoldo MJ, Guibert E, Faure M, Rame C, Foretz M, Viollet B, et al. Specific deletion of AMP-activated protein kinase (alpha1AMPK) in murine oocytes alters junctional protein expression and mitochondrial physiology. PLoS One. 2015;10:e0119680. doi: 10.1371/journal.pone.0119680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport1. Biol Reprod. 2005;73:351–357. doi: 10.1095/biolreprod.105.041798. [DOI] [PubMed] [Google Scholar]
- 78.Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol. 2005;279:20–30. doi: 10.1016/j.ydbio.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 79.Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229:353–361. doi: 10.1002/jcp.24457. [DOI] [PubMed] [Google Scholar]
- 80.Gu L, Liu H, Gu X, Boots C, Moley KH, Wang Q. Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci: CMLS. 2015;72:251–271. doi: 10.1007/s00018-014-1739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]