Abstract
Purpose
This study tests whether metformin or diet supplement BR-DIM-induced AMP-activated protein kinase (AMPK) mediated effects on development are more pronounced in blastocysts or 2-cell mouse embryos.
Methods
Culture mouse zygotes to two-cell embryos and test effects after 0.5–1 h AMPK agonists’ (e.g., Met, BR-DIM) exposure on AMPK-dependent ACCser79P phosphorylation and/or Oct4 by immunofluorescence. Culture morulae to blastocysts and test for increased ACCser79P, decreased Oct4 and for AMPK dependence by coculture with AMPK inhibitor compound C (CC). Test whether Met or BR-DIM decrease growth rates of morulae cultured to blastocyst by counting cells.
Result(s)
Aspirin, metformin, and hyperosmotic sorbitol increased pACC ser79P ~ 20-fold, and BR-DIM caused a ~ 30-fold increase over two-cell embryos cultured for 1 h in KSOMaa but only 3- to 6-fold increase in blastocysts. We previously showed that these stimuli decreased Oct4 40–85% in two-cell embryos that was ~ 60–90% reversible by coculture with AMPK inhibitor CC. However, Oct4 decreased only 30–50% in blastocysts, although reversibility of loss by CC was similar at both embryo stages. Met and BR-DIM previously caused a near-complete cell proliferation arrest in two-cell embryos and here Met caused lower CC-reversible growth decrease and AMPK-independent BR-DIM-induced blastocyst growth decrease.
Conclusion
Inducing drug or diet supplements decreased anabolism, growth, and stemness have a greater impact on AMPK-dependent processes in two-cell embryos compared to blastocysts.
Electronic supplementary material
The online version of this article (10.1007/s10815-017-1028-x) contains supplementary material, which is available to authorized users.
Keywords: Mouse embryo, Oxygen, Blastocyst, Cell number, Apoptosis, Embryo culture, Fertility drugs, Diet supplement, Embryo development, Embryo quality
Introduction
Previous research and other studies showed that AMP-activated protein kinase (AMPK) mediates stress-driven loss of potency factor proteins in embryonic stem cells (ESCs); placental trophoblast stem cells (TSCs) and AMPK agonists block reprogramming that produces induced pluripotent stem cells (iPSCs) [1]. AMPK inhibitors compound C and AraA block stress-forced potency loss in ESCs, TSC blastocysts, and two-cell embryos suggesting that AMPK mediates potency loss. Recently, it was shown that not only stress but also other non-stressful activators of AMPK such as Rx (aspirin, metformin) and diet supplements (BR-DIM but also resveratrol) cause potency loss in two-cell embryos and high throughput screen (HTS) ESC [2, 3]. It was also shown that AMPK agonist Rx and diet supplement (DS) retard and arrest cell growth soon after initiation with cultured two-cell embryos and 2–3 days before the blastocyst stage. However, it is not known which stage of embryo, two-cell or blastocysts, is most sensitive to stress-, DS-, or Rx-caused potency loss.
This is surprising clinically where most reports of AMPK agonist use show beneficial exposures and effects. The reasons why there is a lack of a literature on AMPK agonist toxicity were discussed previously in the report that first reported these new AMPK agonist effects [3]. The Rx and DS studied here are known mostly for other mechanisms and effects other than AMPK agonism. Metformin overcomes insulin resistance to enable ovulation in infertile women by blocking glucagon-induced cAMP and inhibiting protein kinase A [4–6]. Met is used to improve ovulation in infertile women with polycystic ovarian syndrome (PCOS) [7–9] or type 2 diabetes (T2D). Aspirin is an anti-inflammatory, antipyretic, and analgesic with inhibitory effects on prostaglandin production and irreversibly inhibits cyclooxygenase (COX)1/2 activity and is used by fertile and infertile women [8, 10–20]. Met [21] and Asa [22] have therapeutic mechanisms partly through AMPK activity.
The drug DS BioResponse-3,3′-diindolylmethane (BR-DIM) can improve maternal fertility and diabetic metabolism. BR-DIM is a DS derived from yellow cruciferous vegetables [23]. DIM and BR-DIM act through mechanisms as an androgen antagonist and as a histone deacetylase inhibitor [24, 25]. However, BR-DIM diminishes growth or kills prostate cancer cells via AMPK mechanisms [26] in vivo and in vitro. Although, diminishing growth in embryos and their stem cell effects would be detrimental. Thus, diet supplements like BR-DIM, and drugs such as Asa and Met, mediate some of their activities through AMPK as well as other, sometimes better known, mechanisms.
Hyperosmotic stress was used to identify the first stress enzyme in yeast [27]. Cloning of mammalian stress enzymes used hyperosmotic stress, and this is a widely used positive control in somatic and reproductive cells [1, 28–31]. Hyperosmotic stress as a positive control supports comparison of mechanisms in embryos and somatic cells.
The AMPK heterotrimer is composed of three subunits: a catalytic α-subunit which is activated by the AMP-binding regulatory γ-subunit and inactivated by the β-subunit, and modes of activation by BR-DIM, Met, hyperosmotic stress, and aspirin were discussed previously [3]. Met and many stressors decrease ATP production increase AMP, thus activating the regulatory γ-subunit [32, 33].
This study follows up on the previous surprising finding that AMPK agonist DS and Rx arrest growth in two cell embryos as well as causing loss of potency at this stage. If AMPK causes growth arrest, one of the major putative causes is that AMPK activity leads to decreased anabolism that consumes ATP and increased catabolism that produces ATP, thus rebalancing of ATP levels during stress. This should be the mechanism activated by diet supplements and Rx that are AMPK agonists. The most commonly used marker of AMPK mediated anabolism suppression is the serine 79 phosphorylation on acetyl CoA carboxylase (ACC ser79P) which is an inactivating phosphorylation at a highly specific AMPK substrate motif.
Here we test three hypotheses: (1) that AMPK agonist Rx and DS decrease anabolism at the two cell stage and blastocyst stage and (2) decrease potency/stemness at the blastocyst stage as well as at the two-cell stage shown previously. Since AMPK is known to have effects on anabolic-to-catabolic metabolism as well as Warburg-to-OxPhos switches in stem cells, we hypothesize that the period of maximal sensitivity to AMPK agonists is the blastocyst stage when stem cells first arise and begin Warburg anaerobic glycolysis.
Materials and methods
Materials
Sorbitol, Asa (acetylsalicylic acid and metformin) were from Sigma Chemical Co. (St. Louis, MO). Mouse monoclonal anti-Oct4 (SC5279) were from Santa Cruz Biotechnology (Santa Cruz, CA) and rabbit polyclonal acetyl CoA carboxylase (ACC) ser79P were from Cell Signaling Technology (Danvers, MA)(CS3661). The AMPK inhibitor CC was from Calbiochem (San Diego, CA), and the AMPK agonist 5-amino-1-b-d-ribofuranosyl-imidazole-4-carboxamide (AICAR) was from Cell Signaling (Danvers, MA). BR-DIM was from Dr. Dou, Wayne State University School of Medicine, and was prepared as reported previously [3]. BR-DIM was purchased from BioResponse (BioResponse, Boulder, CO).
Embryo culture and treatment
Cryopreserved mouse zygotes and two-cell and morula stage embryos from superovulated female B6C3F-1 × male B6D2F-1 mice were from Embryotech Laboratories, Inc. (Haverhill, MA, USA). For experiments in Figs. 1, 2, and 3, zygotes were cultured overnight to the two-cell stage, or morula stage embryos were cultured overnight to the blastocyst state, to acclimate to media and then stimulated with sorbitol, Met, Asa, or BR-DIM for 1 h with or without compound C. For experimental groups with compound C, embryos were cultured for 2 h to inhibit AMPK prior to the addition of stimulus as done previously [34], and then CC was continued with stimulus for 1 h. Immunofluorescence for Oct4 or ACC Ser79P was done at the end of the 1 h stimulus for experiments described in Figs. 1, 2, and 3 (see the “Immunofluorescence” section). For the experiments in Fig. 4 and Supplemental Fig. 1, two-cell embryos were cultured to early blastocysts and then stimulated with sorbitol, Met, Asa, or BR-DIM for 24 h with or without compound C. For experimental groups with compound C, embryos were cultured for 2 h to inhibit AMPK prior to the addition of stimulus, and then CC was continued with stimulus for 24 h. For experiments described in Fig. 4 and Supplemental Fig. 1, embryos were fixed with 3% paraformaldehyde, Hoechst stained, and two researchers performed independent cell counts by visual inspection at × 20 magnification through the z-axis using the Leica inverted epifluorescence DM IRE microscope as done previously [31].
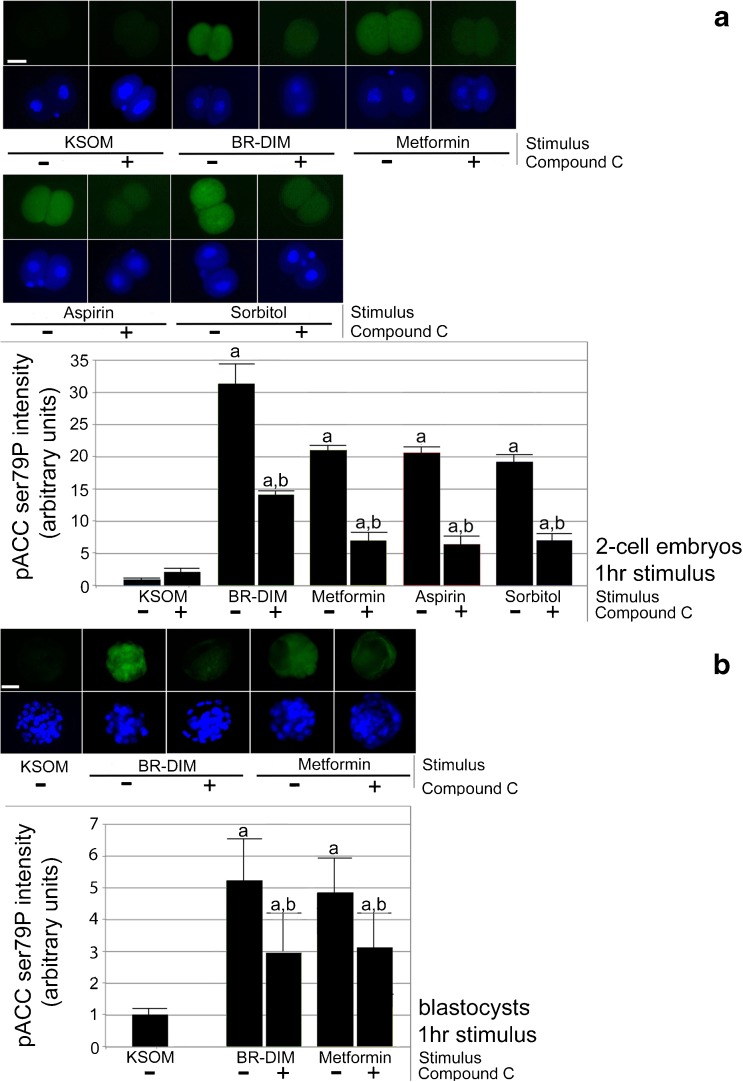
Fig. 1.
a, b Two-cell stage embryos undergo rapid diet supplement- or drug-induced 20- to 30-fold increases and blastocysts undergo rapid 5- to 6-fold increases in pACC ser79P in an AMPK-dependent (CC-sensitive) manner. Zygotes or morulae were acclimated by overnight culture and to two-cell or blastocyst stage, respectively, preloaded with CC (5 μM) for 2 h, continued +/− CC with BR-DIM (20 μM), metformin (40 μM), aspirin (10 μM), or sorbitol (200 mM) for an additional 1 h and then fixed, stained for pACC ser79P and Hoechst, micrographed quantitated and graphed. Triplicate biological experiments using 163 2-cell embryos or 267 morulae were performed, tested for normal distribution by ANOVA and significance by Tukey’s post hoc t test. Significance compared with KSOM (a) and significance for each stimulus with CC compared with the stimulus alone (b). White bar in first panel shows 25 μm
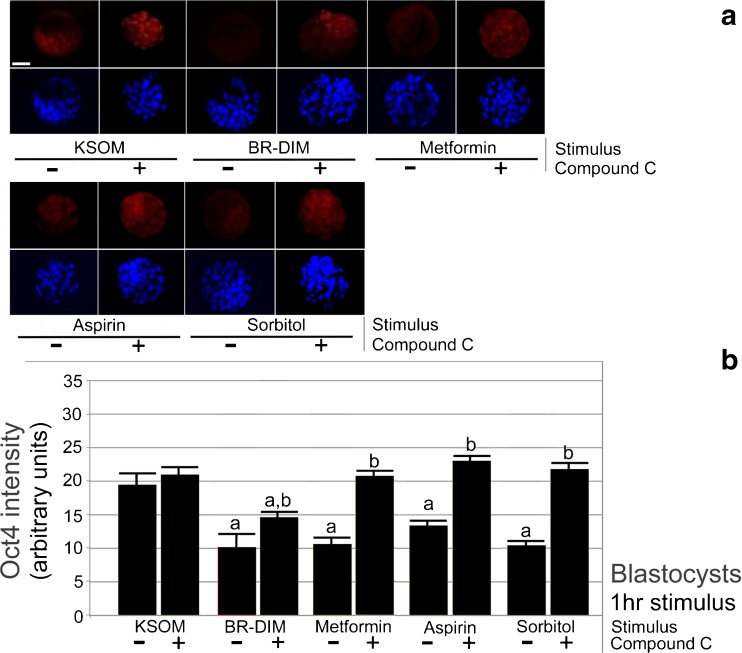
Fig. 2.
a, b. Blastocysts undergo rapid ~ 30–50% decrease in Oct4 in and AMPK-dependent (CC-sensitive) manner. Morulae were cultured overnight to acclimate to culture to blastocyst stage, preloaded with CC (5 μM) for 2 h and then continued +/− CC with stimuli BR-DIM (20 μM) or metformin (40 μM), for an additional 1 h and then fixed, stained for Oct4 and Hoechst, micrographed quantitated, and graphed. Triplicate biological experiments were performed using 190 morulae, tested for normal distribution by ANOVA and significance by Tukey’s post hoc test. Significance compared with KSOM (a) and lack of significance for each stimulus with CC compared with the stimulus alone (b). White bar in first panel shows 25 μm
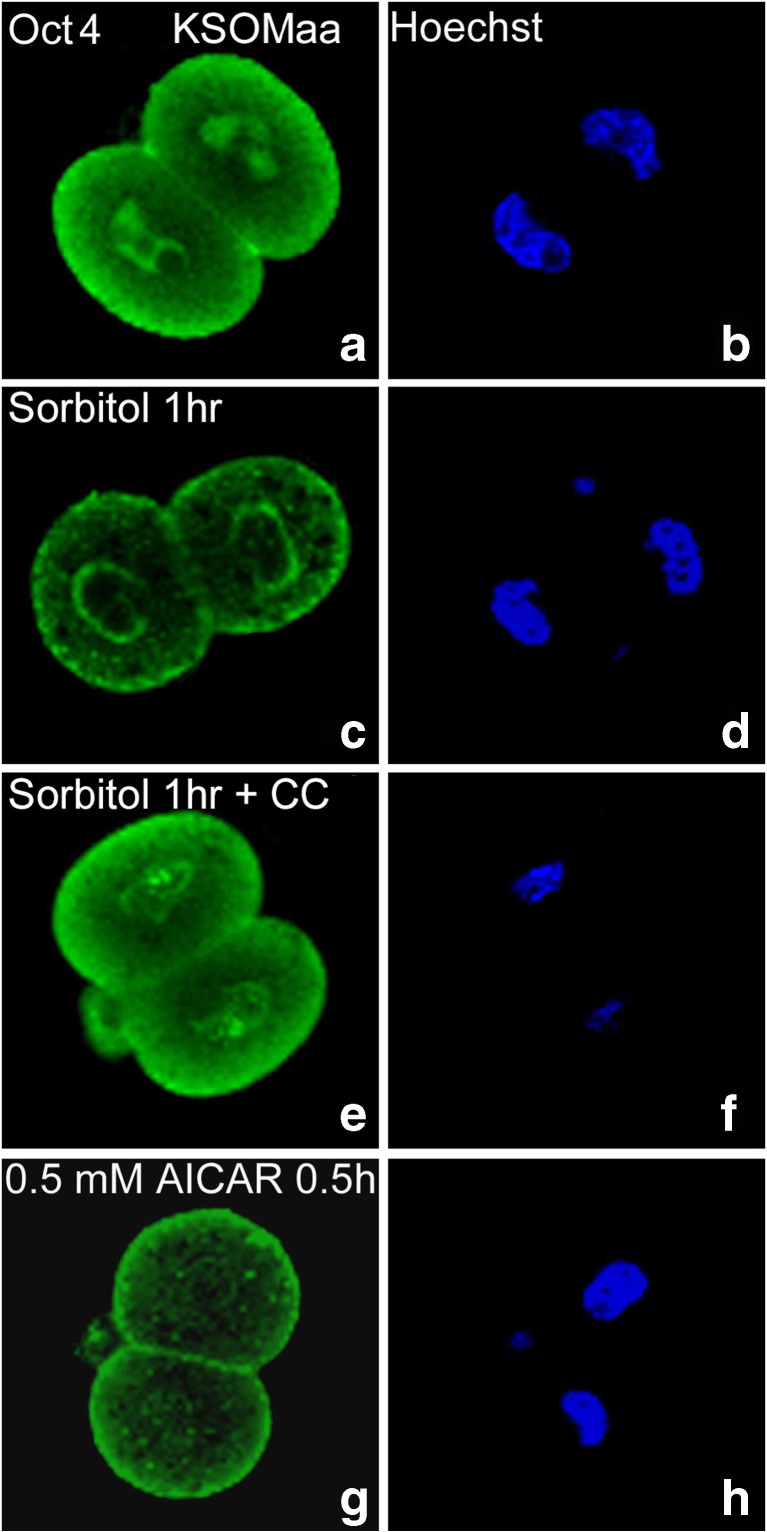
Fig. 3.
Two-cell stage embryos have AMPK agonist-induced (AICAR) and sorbitol-induced AMPK-dependent (compound C-sensitive) Oct4 losses. Compared with embryo cultured in KSOMaa (a, b), nuclear Oct4 is lost after 1 h of 200 mM (c, d) sorbitol but loss if largely reversed in the presence of 5 μM CC (e, f). AMPK agonist AICAR (0.5 mM) for 0.5 h (g, h) is sufficient to cause Oct4 loss without added stress). Eighty-three embryos were tested in triplicate experiments
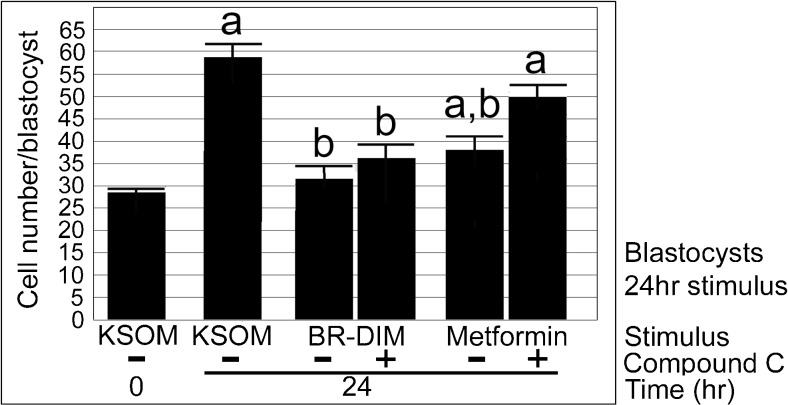
Fig. 4.
Blastocysts have significantly more cells after 24 h of culture, but BR-DIM or metformin significantly decreases cell growth compared with unstimulated embryos. Morulae were cultured overnight to acclimate to culture to blastocyst stage, one group was fixed and counted for cells and the rest were preloaded with CC (5 μM) for 2 h and then continued +/− CC with stimuli BR-DIM (20 μM) or metformin (40 μM), for an additional 24 h, and then fixed cells were counted and graphed. Triplicate biological experiments were performed using 174 embryos, tested for normal distribution by ANOVA and significance by Dunnett’s post hoc t test. Significance compared with KSOM (a) and lack of significance for each stimulus with CC compared with the stimulus alone (b)
Stimulated and control embryos were set up in triplicate biological experiment under oil and cultured in 5% CO2 at 37 °C until they were stimulated and fixed for immunofluorescence and cell counts as described previously [3]. Numbers of embryos assayed in each experiment are given in the figure legends. Standard techniques were to obtain mouse embryos [35]. Thawing was performed according to the manufacturer’s protocol. After thawing, embryos were incubated at 37 °C and 5% CO2 in KSOMAA for 18 h and examined for development. Embryos showing signs of fragmentation, delayed, or accelerated development were discarded. In all studies, embryos were equilibrated overnight in lowest stress KSOMAA [36] and stimulated with the dose and time period indicated. KSOMAA was 260–270 mOsmol, increasing 1.7-fold to 498 mOsmol by adding 200 mM sorbitol. Sorbitol (w/v) was added to produce the given molarity [30, 31]. AMPK inhibitor compound C (CC) was used at 5 μM. Embryos were treated with 200 mM sorbitol or 40 μM Met and/or 10 μM Asa or 20 μM BR-DIM in the continuing presence of 5 μM CC for 1 h at two-cell or blastocyst stage or for 1 day at early blastocyst stage.
Met was used at 40 μM because this is near peak blood levels and near the dose previously tested by the Falcone lab [37]. BR-DIM was used at 20 μM because this dose slowed growth or killed cultured prostate cancer cells [26], and a peak of ~ 7 μM BR-DIM was reported during in vivo exposure to mice [38]. Asa (salicylate) was used at 10 μM because this is a standard plasma level after enteric pill [39] and was the dose used in our previous study [3]. CC at 5 μM was validated from previous dose response testing from our lab for blocking AMPK effects in two-cell embryos, blastocysts, and TSCs without toxicity [3, 40, 41].
BR-DIM, Asa, and Met are made as stocks with 200- to 1000-fold dilution used for embryo media. These stocks are diluted in KSOM and filter sterilized just before use. These high dilutions should preclude non-specific effects on media pH or osmolality. No immediate effects on embryo morphology were noted with any treatment, suggesting that molecular and cellular outcomes were caused by drugs, DS, and compound C.
Immunofluorescence
Two-cell embryos and blastocysts were fixed, quenched, permeabilized, and stained for Oct4 and ACC ser79P and counterstained for 4′,6-diamidino-2-phenylindole (DAPI) as described previously [42, 43]. Briefly, embryos were fixed, quenched, washed, and permeabilized as described previously [3]. Oct4 (SC5279, C10) and ACC ser79 were diluted exposed to embryos as previously described [3, 44]. Fluorescein isothiocyanate (FITC)-conjugated IgG and Texas Red-conjugated IgG (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) were used to detect primary antibodies as previously described [3, 44]. Fluorescence staining was detected using a Retiga Ex cooled-chip digital camera and a Leica DM IRE microscope with filter sets for DAPI, FITC, and Texas Red. Embryos were imaged at an objective magnification of × 20 and an exposure time of 2.0 s. The FITC or Texas Red stain intensities were quantified using Simple PCI (Hamamatsu) as described previously [3, 44]. All micrographs were exposed using the same shutter speed, and all experiments were repeated at least three times.
Statistical analysis
All experiments were performed with at least three independent biological replicates. Data were analyzed with one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s t test post hoc comparisons. A general linear model was used to examine the main effects of stimulation treatments, days, and stages, and their two-way and three-way interactions. The interactions between treatments and days and between days and stages were significant, allowing the subsequent comparisons among treatments on a specific day or at a specific stage using Dunnett’s or Tukey’s post hoc tests. The cell numbers/embryo were analyzed using one-way ANOVA followed by Dunnett’s post hoc t test to examine the difference among stimulation treatments as described previously [3, 44]. Independent t test was used to compare the cell numbers per embryo between days 2 and 4 for Met + Asa or BR-DIM. All analyses were performed in the Statistical Package for the Social Sciences (SPSS) version 23.0 (SPSS Inc., Chicago, IL).
Results
We first tested for the decreased anabolism by BR-DIM (20 μM), metformin (40 μM), and aspirin (10 μM) and positive control hyperosmotic (200 mM sorbitol) after 1 h culture of two-cell embryos or blastocysts. All Rx and DS tested caused a significant ~ 20- to 30-fold increase in inactivating phosphorylation of pACC ser79P compared with two-cell embryos cultured in KSOMaa (ANOVA followed by Tukey’s post hoc test, p < 0.05; Fig. 1a). AMPK agonist compound C (CC; 5 μM) caused a 60–70% reversal of effects of stimulus alone, but reversal was still significantly different than KSOM alone. BR-DIM and Met caused a lesser, but still significant ~ 5-fold increase in pACC ser79P compared with blastocysts cultured in KSOMaa (ANOVA followed by Tukey’s post hoc test, p < 0.05; Fig. 1b). CC caused a ~ 40–50% reversal of effects of stimulus alone, but reversal was still significantly different than KSOM alone.
BR-DIM (20 μM) or Met (40 μM) caused a significant ~ 30–50% decrease in Oct4 protein in the inner cell mass (ICM) of blastocysts compared with blastocysts cultured in KSOMaa (ANOVA followed by Tukey’s post hoc test, p < 0.05). CC caused a ~ 100% reversal of effects of stimulus alone (Fig. 2a, b). Thus, the data suggest that AMPK is the major regulator of BR-DIM- and Met-induced Oct4 protein loss in blastocysts detected by immunofluorescence. This is similar to sorbitol-induced loss of Cdx2 and Id2 that was previously detected by immunofluorescence and immunoblot in blastocysts and two-cell embryos [41, 45]. Interestingly, three of four stimuli that cause significant decrease of Oct4 have the loss reversed so much by compound C that the increase in Oct4 protein makes its quantity not significantly different than unstressed levels. This is different than pACC ser79P where six of six stimuli in two-cell and blastocysts have significant phosphorylation increase and significant reversal but none of the reversals make pACC not significantly different than unstressed. There are two possible reasons for this. One is that stimulation indices for pACC are much large (as high as 30-fold in two-cell and 6-fold in blastocysts) as Oct4 (only a 2-fold decrease). A second is that AMPK actually mediates most of the Oct4 loss and AMPK antagonist CC reverses this back to background. By this logic, other enzymes work with AMPK to media phosphorylation of ACC and CC does not inhibit these other enzymes.
Here we show that Oct4 loss is rapidly AMPK-sufficient after 0.5 h as the non-metabolizable AMP mimic AICAR (0.5 mM) is sufficient to cause total apparent Oct4 loss without hyperosmotic stress which also cause nuclear Oct4 protein loss which is reversed by coaddition of compound C (Fig. 3).
There was a significant > 200% increase in cell number (28.1 to 58.4) in embryos cultured in KSOMaa for 24 h from morula to blastocyst stage (ANOVA followed by Dunnett’s post hoc t test, p < 0.05). BR-DIM (20 μM) significantly diminished cell number increase of ~ 12% over 24 h of culture to the extent that cell number was not significantly different than at the start (Dunnett’s post hoc t test, p = 0.8) and was significantly lower than cell number at Tfinal of embryo cultured with KSOMaa. Addition of CC (5 μM) did not change the effects of BR-DIM on cell number compared with Tfinal (Dunnett’s post hoc t test, p > 0.05), suggesting that AMPK did not play a role in growth after compaction. There was significant growth of ~ 40% after Tzero despite metformin (40 μM) (Dunnett’s post hoc t test, p < 0.05) but significantly fewer cells than Tfinal (Dunnett’s post hoc t test, p < 0.05). The effects of metformin on cell growth were highly AMPK-dependent as the average cell number at Tfinal for embryos treated with Met + CC were not different than unstimulated embryos in KSOMaa. An additional triplicate was done with the same design used in Fig. 4 but with no significant changes in cell number compared with Tzero for any treatment, including KSOMaa control (Supplemental Fig. 1) (for all comparisons; ANOVA followed by Tukey’s post hoc test, p > 0.05). The trends, decreased cell growth by Met and BR-DIM and reversal with CC, in this failed experiment were the same as those in Fig. 4, but without significance. This was due to an unidentified confounding variable such as incorrect cryopreservation and/or thaw or poor media. But, interestingly, there was no obvious difference in the morphology of embryos in different groups at the end of culture (data not shown). This is important when analyzing the difference in effects of drugs and diet supplements on morphological outcomes vs those on cell number outcomes. Taken together, the data show that both BR-DIM and Met slowed growth after 24 h compared with embryos cultured in KSOMaa but there were differences in the strength and AMPK-dependence of the growth effect. In addition, whether or not Met and BR-DIM slowed growth, cavitation and morphological development progressed normally.
Discussion
We tested three hypotheses here and the data supported two of them. The data support the hypothesis that Rx and DS decrease anabolism in an AMPK-dependent manner, as measured by the inactivating phosphorylation of ACC ser79P that is sensitive to CC inhibition, in two-cell embryos and blastocysts. The data also support the hypothesis that blastocysts, in addition to previously reported two-cell embryos, respond to AMPK agonist Rx and DS with CC-sensitive Oct4 loss. However, the data did not support the hypothesis that the newly arisen stem cell lineages in the blastocyst support a greater sensitivity to AMPK agonist Rx and DS than two-cell embryos. The data support the alternate hypothesis. Specifically, the suppression of anabolism by hyperosmotic stress or AMPK agonists is greater in two-cell stage embryos than blastocysts; these stimuli increase suppressive ACC ser79P ~ 20- to 30-fold in two-cell embryos, whereas equal exposures increase ACC ser79P only ~ 5- to 6-fold in blastocysts.
This alternate hypothesis is not totally unexpected. Most AMPKα1−/− mutant embryos survive to birth in vivo, but in vitro most embryos die between the zygote and four-cell stage [46]. This suggests that culture stress combined with energy needs of early development require AMPK activity to mediate adaptive stress responses.
Previously we reported a ~ 60–90% Oct4 protein decrease in two-cell embryos that was AMPK-dependent [3]. Oct4 protein loss in blastocysts was less at 30–50% although AMPK-dependence was similar for two-cell and blastocysts at 50–90% increase in Oct4 immunofluorescence after AMPK agonist coculture with compound C. Met, Asa, and BR-DIM nearly completely arrested cell proliferation in two-cell embryos 24 h after treatment and death occurred 48 h later with little cell number increase [3]. However, metformin only slightly slowed blastocyst growth after 24 h. For nearly all outcomes and stimuli, CC significantly reversed stimuli effects, although for BR-DIM, CC did not significantly reverse blastocyst growth. Metformin’s growth effects were weaker than BR-DIM, but more AMPK-dependent. The data suggest that AMPK decreases proliferation, potency, and anabolism in two-cell embryos > blastocysts.
AMPK agonists like BR-DIM and metformin also lead to decreased growth and potency in Rex1 potency reporter-RFP reporter mESCs at higher doses [2] similar to decreased growth and Oct4 in blastocysts, and Rex1 and Cdx2 potency factors decrease in two-cell embryos [3, 41]. Although AMPK is known to regulate polarity [47, 48], AMPK agonists’ biological effects at the blastocyst stage (Fig. 4; Supplemental Fig. 1) were on cell number and anabolism, but the trophectoderm and cavitation appeared normal. This is in agreement with lack of effect of LKB1 knockout, and upstream positive regulator of AMPK, on blastocyst trophectoderm [49].
Overall significance
These studies are done as part of a reductionist approach to understand the capabilities and direct homeostatic, largely cellular responses (e.g., anabolism/catabolism balance, proliferation, survival) and largely organismal responses (e.g., stemness/differentiation balance) of cells in the early embryo. However, if there is accessibility of Rx and DSs to the preimplantation embryo and the previous, first report on AMPK agonist effects suggests some accessibilities in the oviduct and uterus [discussed in [3]], and then exposure risks could be significant. A key consequence if the window of AMPK sensitivity is the same in vivo as in vitro is that two-cell embryos would likely die before implantation leaving no markers of chemical pregnancy. This would address one contributor to the surprising nature of these reports on AMPK agonist effects and the lack of epidemiological evidence in humans. Moreover, if AMPK agonists lead to greater loss at the two-cell stage > blastocyst stage in vivo in humans, then causality of loss would disappear into the many causes that lead to loss of 70% of all human fertilization embryos before birth and the approximate 35% loss that is not detected chemically (e.g., by hCG blood or urine tests) or clinically (by ultrasound) [50, 51].
Improvements in methodology compared with the initial report on AMPK agonist toxicity
Metformin dose decrease from 1000 to 40 μM in keeping with the dosimetry previously performed on cultured mouse embryos by the Falcone lab [37]. We showed here that the AMPK agonist AICAR, an AMP mimic used for only 0.5 h, was sufficient to cause Oct4 loss in two-cell embryos. The use of ACC ser79 as a more specific AMPK motif and AICAR as a specific AMPK agonist suggest more strongly that effects of some drugs and DSs on some effects are AMPK-dependent. Since Oct4 and Rex1 proteins do not have AMPK substrate motif phosphorylation sites, they thus probably undergo stress-, drug-, and DS-induced decrease that is indirectly dependent on AMPK. Compound C antagonism is not specific for AMPK [52]. However, the ACC ser79P substrate motif is highly specific for AMPK [53], and its phosphorylation and reversal with CC strongly indicate AMPK-dependent suppression of anabolism in two-cells and to a lesser degree in blastocysts.
Since ACC ser79P increase and Oct4, Rex1, and Cdx2 decrease caused by Rx and DS occur within 1 h of exposure which is well within the pharmacokinetic peaks of these stimuli in vivo [discussed previously [3]], modeling in culture replicates rapid embryonic responses in vivo.
Limitations
Exposure was longer than the in vivo peak and does not take into account pharmacokinetic clearance [discussed in [3]], and longer biological effects on growth may not be modeled accurately in the in vitro model. There are also maternal metabolic modifications and additional indirect maternal effects in vivo. Although the window of sensitivity was defined, a more precise proof of AMPK-dependence and period of reversibility and irreversibility remain to be determined.
But, there are several limitations for the studies reported here. These negative effects in blastocysts corroborate an earlier report on negative effects in cultured two-cell embryos [3] but need to be validated after gestational exposure in mice. In vitro exposure has been corroborated by follow-up in vivo tests in the AMPK-dependent prostate cancer treatments. For example, AMPK-dependent decrease by BR-DIM of activity of known AMPK substrates mTOR and ACC occurred in cultured prostate cancer cells and also after these cells formed tumors in severe combined immunodeficient mice [26].
Studies to overcome limitations and future directions
Improvements in vitro would encompass daily repeated area under the curve exposures to approximate in vivo blood magnitude at peak and clearance. In vivo studies are needed as well as higher resolution dosimetry in vitro to determine NOAEL, LOAEL, and IC50 effects on cellular, embryonic, and molecular outcomes. AMPK dependence would best be tested on knockout or knockdown embryos in vivo or in vitro where off target effects of CC are obviated. To clarify the roles and mechanisms of AMPK downstream of BR-DIM, the purified active moiety should be used to test AMPK-dependent effects on anabolism, growth, and potency of two-cell embryos and blastocysts. An expansion in the number of known AMPK-motif substrates, like ACC Ser79P, would bolster the likelihood that AMPK directly regulates a panoply of metabolic shifts in anabolism to catabolism. To test for combinatorial effects, whole DS and their active moieties should be tested by high throughput screening methods for stress-induced ESC potency loss [42] or changes in ESC G1 phase [54]. These tests could establish whether single and additive AMPK agonists from diet supplement and drug sources cause potency loss and diminish stem cell growth in vitro and then in vivo. Since no single hypothetical mechanism for AMPK-mediated pathogenicity stands out, studies of changes in the global transcriptome, epigenome, and metabolome of cultured embryos are needed to help sharpen hypotheses of mechanisms.
Electronic supplementary material
Blastocysts have significantly more cells after 24hr of culture, but BR-DIM or Metformin significantly decrease cell growth compared with unstimulated embryos at the end of culture. Morulae were cultured overnight to acclimate to culture to blastocyst stage, one group was fixed and counted for cells and the rest were preloaded with CC (5uM) for 2h and then continued +/-CC with stimuli BR-DIM (20uM) or Metformin (40uM), for an additional 24hr and then fixed cells were counted and graphed. Triplicate biological experiments using 174 embryos were performed, tested for normal distribution by ANOVA and significance by Dunnett post hoc t-test. (a) Shows significance compared with KSOM and b shows lack of significance for each stimulus with CC compared with the stimulus alone. (JPEG 224 kb)
Acknowledgements
Thanks to Dr. Erica Louden and Dr. Yu Yang for comments on the manuscript.
Funding
DAR and EEP from the Office of the Vice President for Research at Wayne State University and an R03 to DAR 1R03HD061431, from the REI fellows’ fund (AB), and from the funding of the Mary Iacobell and Kamran Moghissi Endowed Chairs.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10815-017-1028-x) contains supplementary material, which is available to authorized users.
References
- 1.Puscheck EE, Awonuga AO, Yang Y, Jiang Z, Rappolee DA. Molecular biology of the stress response in the early embryo and its stem cells. Adv Exp Med Biol. 2015;843:77–128. doi: 10.1007/978-1-4939-2480-6_4. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Yang Y, Louden E, Puscheck E, Rappolee D. High-throughput screens for embryonic stem cells; stress-forced potency-stemness loss enables toxicological assays. In: Faqi A, (eds) Developmental and reproductive toxicology. Methods in toxicology and pharmacology. 2016. Humana Press, New York, NY.
- 3.Bolnick A, Abdulhasan M, Kilburn B, Xie Y, Howard M, Andresen P, et al. Commonly used fertility drugs, a diet supplement, and stress force AMPK-dependent block of stemness and development in cultured mammalian embryos. J Assist Reprod Genet. 2016;33:1027–1039. doi: 10.1007/s10815-016-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2012. [DOI] [PubMed]
- 5.Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:951–953. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56:1898–1906. doi: 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duranteau L, Lefevre P, Jeandidier N, Simon T, Christin-Maitre S. Should physicians prescribe metformin to women with polycystic ovary syndrome PCOS? Ann Endocrinol (Paris) 2010;71:25–27. doi: 10.1016/j.ando.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Palomba S, Pasquali R, Orio F, Jr, Nestler JE. Clomiphene citrate, metformin or both as first-step approach in treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS): a systematic review of head-to-head randomized controlled studies and meta-analysis. Clin Endocrinol. 2009;70:311–321. doi: 10.1111/j.1365-2265.2008.03369.x. [DOI] [PubMed] [Google Scholar]
- 9.Sinawat S, Buppasiri P, Lumbiganon P, Pattanittum P. Long versus short course treatment with metformin and clomiphene citrate for ovulation induction in women with PCOS. Cochrane Database Syst Rev. 2008. [DOI] [PubMed]
- 10.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–258. doi: 10.1016/S0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 11.Jamal A, Milani F, Al-Yasin A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran J Reprod Med. 2012;10:265–270. [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira BC, Lanchote VL, de Jesus Antunes N, de Jesus Ponte Carvalho TM, Dantas Moises EC, Duarte G, et al. Metformin pharmacokinetics in nondiabetic pregnant women with polycystic ovary syndrome. Eur J Clin Pharmacol. 2011;67:1027–1033. doi: 10.1007/s00228-011-1053-0. [DOI] [PubMed] [Google Scholar]
- 13.Vause TD, Cheung AP, Sierra S, Claman P, Graham J, Guillemin JA, et al. Ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can. 2010;32:495–502. doi: 10.1016/S1701-2163(16)34504-2. [DOI] [PubMed] [Google Scholar]
- 14.Jungheim ES, Odibo AO. Fertility treatment in women with polycystic ovary syndrome: a decision analysis of different oral ovulation induction agents. Fertil Steril. 2010;94:2659–2664. doi: 10.1016/j.fertnstert.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genazzani AD, Ricchieri F, Lanzoni C. Use of metformin in the treatment of polycystic ovary syndrome. Women's Health (Lond Engl) 2010;6:577–593. doi: 10.2217/WHE.10.43. [DOI] [PubMed] [Google Scholar]
- 16.Palomba S, Falbo A, Russo T, Orio F, Tollino A, Zullo F. Role of metformin in patients with polycystic ovary syndrome: the state of the art. Minerva Ginecol. 2008;60:77–82. [PubMed] [Google Scholar]
- 17.Escobar-Morreale HF. Polycystic ovary syndrome: treatment strategies and management. Expert Opin Pharmacother. 2008;9:2995–3008. doi: 10.1517/14656560802559932. [DOI] [PubMed] [Google Scholar]
- 18.Moll E, van der Veen F, van Wely M. The role of metformin in polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2007;13:527–537. doi: 10.1093/humupd/dmm026. [DOI] [PubMed] [Google Scholar]
- 19.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 20.Cheang KI, Sharma ST, Nestler JE. Is metformin a primary ovulatory agent in patients with polycystic ovary syndrome? Gynecol Endocrinol. 2006;22:595–604. doi: 10.1080/09513590601005847. [DOI] [PubMed] [Google Scholar]
- 21.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3′-diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003;278:21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Li X, Guo B. Chemopreventive agent 3,3′-diindolylmethane selectively induces proteasomal degradation of class I histone deacetylases. Cancer Res. 2010;70:646–654. doi: 10.1158/0008-5472.CAN-09-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen D, Banerjee S, Cui QC, Kong D, Sarkar FH, Dou QP. Activation of AMP-activated protein kinase by 3,3′-diindolylmethane (DIM) is associated with human prostate cancer cell death in vitro and in vivo. PLoS One. 2012;7:e47186. doi: 10.1371/journal.pone.0047186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rappolee DA. Impact of transient stress and stress enzymes on development. Dev Biol. 2007;304:1–8. doi: 10.1016/j.ydbio.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 28.Mansouri L, Xie Y, Rappolee DA. Adaptive and pathogenic responses to stress by stem cells during development. Cell. 2012;1:1197–1224. doi: 10.3390/cells1041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Y, Awonuga AO, Zhou S, Puscheck EE, Rappolee DA. Interpreting the stress response of early mammalian embryos and their stem cells. Int Rev Cell Mol Biol. 2011;287:43–95. doi: 10.1016/B978-0-12-386043-9.00002-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhong W, Xie Y, Wang Y, Lewis J, Trostinskaia A, Wang F, et al. Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci. 2007;14:534–547. doi: 10.1177/1933719107307182. [DOI] [PubMed] [Google Scholar]
- 31.Xie Y, Zhong W, Wang Y, Trostinskaia A, Wang F, Puscheck EE, et al. Using hyperosmolar stress to measure biologic and stress-activated protein kinase responses in preimplantation embryos. Mol Hum Reprod. 2007;13:473–481. doi: 10.1093/molehr/gam027. [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology. 2006;131:973. doi: 10.1053/j.gastro.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 33.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 34.An Y, Sun Z, Li L, Zhang Y, Ji H. Relationship between psychological stress and reproductive outcome in women undergoing in vitro fertilization treatment: psychological and neurohormonal assessment. J Assist Reprod Genet. 2013;30:35–41. doi: 10.1007/s10815-012-9904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogan B, Beddington R, Constantini F, Lacy B. Manipulating the mouse embryo: a laboratory manual. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory; 2002. [Google Scholar]
- 36.Wang Y, Puscheck EE, Lewis JJ, Trostinskaia AB, Wang F, Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril. 2005;83(Suppl 1):1144–1154. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 37.Bedaiwy MA, Miller KF, Goldberg JM, Nelson D, Falcone T. Effect of metformin on mouse embryo development. Fertil Steril. 2001;76:1078–1079. doi: 10.1016/S0015-0282(01)02825-4. [DOI] [PubMed] [Google Scholar]
- 38.Paltsev M, Kiselev V, Muyzhnek E, Drukh V, Kuznetsov I, Pchelintseva O. Comparative preclinical pharmacokinetics study of 3,3′-diindolylmethane formulations: is personalized treatment and targeted chemoprevention in the horizon? EPMA J. 2013;4:25. doi: 10.1186/1878-5085-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross-Lee LM, Elms MJ, Cham BE, Bochner F, Bunce IH, Eadie MJ. Plasma levels of aspirin following effervescent and enteric coated tablets, and their effect on platelet function. Eur J Clin Pharmacol. 1982;23:545–551. doi: 10.1007/BF00637504. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Jiang Z, Bolnick A, Dai J, Puscheck EE, Rappolee DA. Departure from optimal O2 level for mouse trophoblast stem cell proliferation and potency leads to most rapid AMPK activation. J Reprod Dev. 2016; [DOI] [PMC free article] [PubMed]
- 41.Xie Y, Awonuga A, Liu J, Rings E, Puscheck EE, Rappolee DA. Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells [corrected] Stem Cells Dev. 2013;22:1564–1575. doi: 10.1089/scd.2012.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, Gomez-Lopez N, Drewlo S, Sanchez-Rodriquez E, Dai J, Puscheck EE, et al. Development and validation of a Rex1-RFP potency activity reporter assay that quantifies stress-forced potency loss in mouse embryonic stem cells. Stem Cells Dev. 2015; [DOI] [PMC free article] [PubMed]
- 43.Slater JA, Zhou S, Puscheck EE, Rappolee DA. Stress-induced enzyme activation primes murine embryonic stem cells to differentiate toward the first extraembryonic lineage. Stem Cells Dev. 2014;23:3049–3064. doi: 10.1089/scd.2014.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdulhasan M, Li Q, Dai J, Abdul-Soud H, Puscheck E, Rappolee D. CoQ10 increases mitochondrial mass and polarization, ATP and Oct4 potency levels, and bovine oocyte MII during IVM while decreasing AMPK activity and oocyte death. J Assist Reprod Genet. 2017; Accepted for publication.. [DOI] [PMC free article] [PubMed]
- 45.Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, et al. Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction. 2010;140:921–930. doi: 10.1530/REP-10-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertoldo MJ, Guibert E, Faure M, Rame C, Foretz M, Viollet B, et al. Specific deletion of AMP-activated protein kinase (alpha1AMPK) in murine oocytes alters junctional protein expression and mitochondrial physiology. PLoS One. 2015;10:e0119680. doi: 10.1371/journal.pone.0119680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirouse V, Billaud M. The LKB1/AMPK polarity pathway. FEBS Lett. 2011;585:981–985. doi: 10.1016/j.febslet.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci U S A. 2006;103:17272–17277. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krawchuk D, Anani S, Honma-Yamanaka N, Polito S, Shafik M, Yamanaka Y. Loss of LKB1 leads to impaired epithelial integrity and cell extrusion in the early mouse embryo. J Cell Sci. 2015;128:1011–1022. doi: 10.1242/jcs.162156. [DOI] [PubMed] [Google Scholar]
- 50.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 51.Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. 2002;8:333–343. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- 52.Secor E, Froment P, Louden E, Bolnick A, Yang Y, Abdulhasan M et al. (2017) AMPK agonists in diet supplements and Pharma mediate wide-ranging maternal and reproductive effects. eCAM. Submitted.
- 53.Tsou P, Zheng B, Hsu CH, Sasaki AT, Cantley LC. A fluorescent reporter of AMPK activity and cellular energy stress. Cell Metab. 2011;13:476–486. doi: 10.1016/j.cmet.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pauklin S, Vallier L. The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013;155:135–147. doi: 10.1016/j.cell.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blastocysts have significantly more cells after 24hr of culture, but BR-DIM or Metformin significantly decrease cell growth compared with unstimulated embryos at the end of culture. Morulae were cultured overnight to acclimate to culture to blastocyst stage, one group was fixed and counted for cells and the rest were preloaded with CC (5uM) for 2h and then continued +/-CC with stimuli BR-DIM (20uM) or Metformin (40uM), for an additional 24hr and then fixed cells were counted and graphed. Triplicate biological experiments using 174 embryos were performed, tested for normal distribution by ANOVA and significance by Dunnett post hoc t-test. (a) Shows significance compared with KSOM and b shows lack of significance for each stimulus with CC compared with the stimulus alone. (JPEG 224 kb)