Abstract
Purpose
Proteomic studies suggest an association between haptoglobin (Hp) and polycystic ovary syndrome (PCOS). Hp is a classic inflammatory marker and binds to the intravascular hemoglobin, avoiding the oxidative damages that can be caused by free hemoglobin. Inflammation and oxidative stress are important in the pathogenesis of the PCOS, one of the most frequent metabolic diseases in women.
Methods
To validate these proteomic studies, we developed a controlled cross-sectional study that aimed to evaluate the Hp levels and allelic and genotypic frequencies of Hp1-Hp2 polymorphism in Brazilian women with PCOS. We also investigated the correlation between Hp levels and several important parameters in PCOS as follows: body mass index (BMI), waist circumference (WC), fasting glucose, post-prandial glucose, homeostatic model assessment (HOMA), lipid accumulation product (LAP), C-reactive protein (CRP), and metabolization test of tetrazolium salts (MTTs—serum antioxidant capacity).
Results
Plasma Hp levels were higher in the PCOS group than in controls [8.20 (4.04) g/L; 7.98 (3.31) g/L; p = 0.018]. No significant difference was observed in the frequency of Hp1-Hp2 genotypes under additive, recessive, or dominant model of inheritance between the PCOS and the control groups. Plasma Hp levels did not differ according to the genotype. However, plasma Hp showed a negative correlation with MTT (r = − 0.383; p = 0.028), as well as a positive correlation with CRP (r = 0.361; p = 0.014) in the PCOS group.
Conclusion
Hp1-Hp2 polymorphism is not associated with PCOS but plasma Hp could be a potential biomarker for PCOS and its complications.
Keywords: Polycystic ovary syndrome, Haptoglobin, Inflammation, Oxidative stress
Introduction
Polycystic ovary syndrome (PCOS) is one of the most frequent metabolic diseases in women, which presents higher risks of insulin resistance (IR) and type 2 diabetes mellitus (DM2), dyslipidemia, metabolic syndrome, cardiovascular complications [1–3], infertility [4, 5], thrombosis [6], cancer [7], and obstetric complications [8].
Low-grade chronic inflammation is considered the link between PCOS and numerous metabolic disorders [9] and probably contributes to an increased risk of cardiovascular complications in PCOS patients [9, 10]. The visceral obesity commonly observed in this group also contributes to the increase of plasma levels of inflammatory mediators [9, 11]. Besides the chronic low-grade inflammation [11–13], PCOS women also present an imbalance of the oxidative profile, resulting in increased oxidative stress [14, 15].
Proteomic studies have suggested that haptoglobin (Hp)—an acute phase glycoprotein—may be associated to PCOS [16, 17]. The main function of Hp is to bind to the intravascular hemoglobin (Hb) released during hemolysis, avoiding the oxidative damages that can be caused by free Hb, besides the iron loss [18]. The Hp-Hb soluble complex is recognized by CD163 receptor found on the surface of phagocytic cells [19], such as monocytes, macrophages, and Kupffer cells [20, 21]. The Hp-Hb-CD163 complex induces the production of several anti-inflammatory and antioxidative mediators [22].
Serum Hp levels increase considerably in inflammatory processes [19]. Hp is synthesized primarily in the liver, whose production is influenced by cytokines, such as TNF-α [23, 24], interleukin-6 [24, 25], and interleukin-1 [24], through the bind of these cytokines in regulatory sites of the Hp promoter gene [26]. Beyond the hepatic Hp production, Hp gene expression is observed in other tissues, including the lung, spleen, kidney, and heart [27, 28]. Moreover, since this protein is also expressed in adipose tissue [27–29], the presence of obesity may further increase serum Hp levels [30].
Hp is composed by two types of peptide chains, alpha and beta [31, 32], linked by disulfide bonds [33]. Both strands are encoded by the same gene, located in 16q22.2 [31, 34] as a pre-protein, which subsequently undergoes post-translational modifications, and a proteolytic process cleaves the two subunits [35]. A common polymorphism is present in this gene, which is characterized by two alleles. The Hp1 allele has five exons and is conserved among species. Hp2 allele is human-specific and presents seven exons that likely arose from a duplication (non-homologous crossing over) involving exons 3 and 4 of the Hp1 allele [21].
The final structure of the Hp protein is genotype-dependent [34]. There are three possible genotypes for Hp gene: Hp1-1, Hp2-1, and Hp2-2 [21, 31, 32, 34]. The Hp beta chain (40 kDa) is identical for both Hp alleles [32]. Hp from individuals with the Hp1-1 genotype presents an alpha-beta dimer with 86 kDa, whereas those from the Hp2-1 genotype have compositions ranging from 86 to 300 kDa. The Hp of individuals with the Hp2-2 genotype also has several possible structures, ranging from 170 to 900 kDa. The Hp1 allele presents a single exon that encodes the multimerization domain of the protein, while Hp2 has two copies of that same exon, which explains the different Hp structures [34].
Hp1-Hp1protein products have greater ability to bind Hb and the CD163 receptor, presenting greater anti-inflammatory and antioxidant properties [36]. Contrarily, Hp2 leads to a functional impairment in Hb clearance [19] and lower antioxidant activity [34].
In an attempt to validate proteomic studies that suggest the association between Hp and PCOS [16, 17], our study aimed to evaluate the Hp levels and allelic and genotypic frequencies of Hp1-Hp2 polymorphism in Brazilian women with PCOS. Our data can help to clarify a possible role of Hp and its use as a systemic marker for PCOS.
Material and methods
Experimental design
This is a cross-sectional study, which involved 86 women with PCOS (14–42 years old) and 86 women without this syndrome—controls (20–48 years old).
The group with PCOS was selected in the Hyperandrogenism Clinic of Hospital das Clínicas, Federal University of Minas Gerais, between 2011 and 2013, according to the European Society of Human Reproduction/Embryology and the American Society for Reproductive Medicine criteria (ESHRE/ASRM) [37], which considers the presence of at least two of three criteria: (1) menstrual dysfunction and anovulation, (2) clinical or laboratory evidence of hyperandrogenism, and (3) micropolycystic ovaries evidenced by ultrasonography, defined by the presence of 12 or more follicles in the ovary each, measuring 2 to 9 mm in diameter and/or increased ovarian volume (> 10 mL). In the same period, the women of the control group were recruited among students and employees of the same university. The control group showed no signs of hyperandrogenism or reported menstrual irregularity.
We considered the following as exclusion criteria: autoimmune, adrenal, kidney, liver, thyroid, thromboembolic or renal diseases, pregnancy, diabetes mellitus, sickle-cell anemia, cancer, hyperprolactinemia, hypogonadism, inflammatory/infectious process, orthopedic implant, C-reactive protein (CRP) levels > 10 mg/dL, and use of medications such as anti-inflammatory drugs (steroidal and non-steroidal), anabolic steroids, isotretinoin, cyclosporine, antiretroviral, insulin, and hormonal contraceptives, currently or recently (past 3 months).
Venous blood samples were obtained using tubes with EDTA, sodium citrate, and tubes without anticoagulant (Vacuette®), after 12 h of fasting. The samples were centrifuged at 1500×g at 4 °C for 20 min to obtain serum or plasma samples, which were stored at − 80 °C until the analyses. An aliquot of whole EDTA blood was also stored at − 20 °C for later genomic DNA extraction.
Clinical and laboratory parameters
Insulin and testosterone levels were measured using Abbott Architect®. Serum glucose and CRP were measured in plasma citrate using Vitros kits (Johnson and Johnson®). All procedures were conducted according to the manufacturer’s instructions.
Hirsutism was assessed only in the PCOS group, according to the modified Ferriman–Gallwey scale [38], by a single observer, in order to avoid inter-examiner variation. Hyperandrogenism was considered for women that presented testosterone levels > 77 ng/dL (biochemical) and/or Ferriman–Gallwey value ≥ 8 (clinical) [38].
Body mass index (BMI) was calculated by dividing the body mass (weight, kg) by the square of the body height (m2), and waist circumference (WC) was measured midway between the lowest ribs and the iliac crest, as recommended by the World Health Organization and International Diabetes Federation [39].
Triglyceride levels were determined by using Vitros kits (Johnson and Johnson®) according to manufacturer’s instructions. The lipid accumulation product (LAP) index was calculated by using the formula [(waist circumference − 58) × (triglycerides)] [40, 41]. The homeostatic model assessment (HOMA) for IR was calculated using the formula [insulin (mU/L) × glucose (mM/L)]/22.5 [42].
The serum antioxidant capacity was determined by the metabolization test of tetrazolium salts assay (MTT) [43], according to Duarte et al. [44] and Medina et al. [45]. It is based on the ability of plasma antioxidant factors to reduce the compound 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to a colorimetric compound formed by the formazan crystals [43]. The results were expressed in arbitrary units.
Plasma Hp quantification was performed with R&D Systems® ELISA kit, using blood samples collected in citrate-coated tubes. The assay was applied according to the manufacturer’s instructions, after validation with citrate samples.
Genotyping
Genomic DNA was obtained using Biopur Mini Spin Kit (BiometrixBiotecnologia®). The Hp1-Hp2 polymorphism genotyping was performed according to Koch et al. [46]. Hp1 and Hp2 alleles diverge in a DNA segment of approximately 1700 bp, duplicated in Hp2. Two PCR reactions were used, each containing a different pair of primers: first reaction, primer A (5′-GAGGGGAGCTTGCCTTTCCATTG-3′) and primer B (5′-GAGATTTTTGAGCCCTGGCTGGT-3′); second reaction, primer C (5′-CCTGCCTCGTATTAACTGCACCAT-3′) and primer D (5′-CCGAGTGCTCCACATAGCCATGT-3′).
PCR conditions were as follows: initial denaturing at 95 °C for 2 min, followed by 35 cycles of 95 °C for 1 min, 69 °C for 2 min (1 min for reaction 2), 69 °C for 2 min (1 min for reaction 2), and final extension of 7 min at 72 °C. PCR products were visualized in 1% agarose gel electrophoresis stained with ethidium bromide solution (10 mg/mL; Sigma-Aldrich, MO, USA).
Two PCR products were visualized in the first reaction, as a 1757-bp product (Hp1 allele) and a 3481-bp product (Hp2 allele). In the second reaction, a 349-bp PCR product was generated only in the presence of Hp2, which is useful in order to confirm the results. About 60% of the samples were re-genotyped using the two reactions, and the results were confirmed.
Statistical analysis
Statistical analyses were performed with the Statistical Package of the Social Sciences (SPSS) version 17.0. The charts in this article were generated in Microsoft Office Excel 2016. We considered significant all results that presented p value < 0.05.
To evaluate the normality of each parameter, we used the Shapiro–Wilk test. Variables with normal distribution were presented as mean and standard deviation and compared with Student’s t test, whereas variables with non-normal distribution were presented as median and interquartile range (75th–25th percentiles) and compared with the Mann–Whitney test (two groups) or Kruskal–Wallis (three groups).
Correlation analyses between Hp levels and laboratorial parameters in the PCOS group were performed with Spearman’s correlation test. Regarding the strength of the correlation, we considered it weak (0 < r ≤ 0.35), moderate (0.36 ≥ r ≤ 0.67), or strong (r ≥ 0.68), as proposed by Taylor [47].
Hardy–Weinberg equilibrium (HWE) was evaluated using exact tests by GENEPOP (http://genepop.curtin.edu.au/genepop_op1.html) [48] and OEGE (http://www.oege.org/software/hardy-weinberg.html) [49]. Differences in genotypic and allelic frequencies between the groups were investigated using the chi-square (χ 2) test.
Results
Table 1 summarizes the clinical and laboratorial characteristics of the women included in the study. The two groups did not differ regarding the age, fasting glucose, and CRP levels. However, the PCOS group presented significantly increased values of BMI, WC, insulin, HOMA, LAP, testosterone, and MTT when compared to controls.
Table 1.
Clinical and laboratorial characterization of PCOS and control groups
| Parameters | PCOS | Control | p |
|---|---|---|---|
| Age (years) | 31.15 ± 4.92 | 29.0 ± 7.04 | 0.058 |
| BMI (kg/m2) | 30.17 ± 5.44 | 23.27 ± 4.23 | < 0.001* |
| WC (cm) | 98.00 (17.0) | 71.50 (16.0) | < 0.001* |
| FG (mmol/L) | 87.10 ± 7.26 | 84.75 ± 10.42 | 0.230 |
| Insulin (uUI/mL) | 15.90 (17.3) | 8.75 (5.2) | < 0.001* |
| HOMA-IR | 3.54 (4.8) | 1.68 (1.6) | < 0.001* |
| LAP | 52.95 (63.4) | 15.49 (19.1) | < 0.001* |
| Testosterone (ng/dL) | 57.00 (43.4) | 29.95 (18.5) | < 0.001* |
| Ferriman–Gallwey | 11.00 (10.0) | – | – |
| CRP (mg/dL) | 5.00 (8.6) | 3.00 (1.0) | 0.403 |
| MTTª | 0.34 (0.14) | 0.24 (0.12) | < 0.001* |
Student’s t test—for normal distribution, mean ± standard deviation; Mann-Whitney test—for non-normal distribution, median (interquartile range)
PCOS polycystic ovary syndrome, BMI body mass index, WC waist circumference, FG fasting glucose, HOMA homeostatic model assessment, LAP lipid accumulation product, CRP C-reactive protein, MTT metabolization test of tetrazolium salts, – not evaluated for control group
*p<0.05 was considered statistically significant
ªMeasured in arbitrary units of absorbance
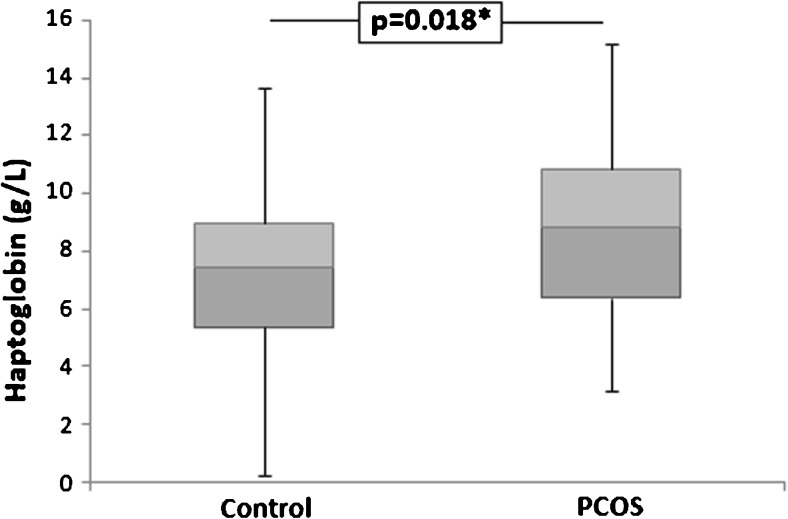
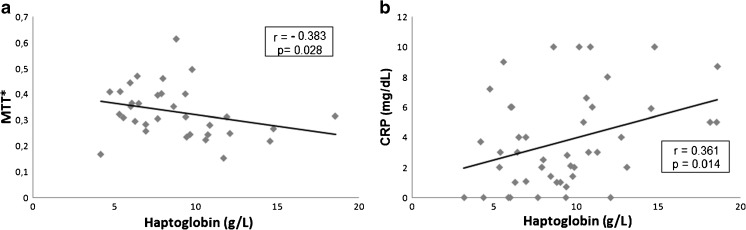
Plasma Hp levels were higher in the PCOS group [8.20 (4.04) g/L] than in controls [7.98 (3.31) g/L] (p = 0.018) (Fig. 1). Hp also showed moderate and negative correlation with MTT (r = − 0.383, p = 0.028; Fig. 2a), as well as moderate and positive correlation with CRP (r = 0.361, p = 0.014; Fig. 2b) in PCOS group. No correlation between Hp levels and BMI, WC, LAP, age, testosterone, fasting glucose, HOMA-IR, and insulin levels was observed (p > 0.05), if all individuals study were evaluated together or only patients in the PCOS group.
Fig. 1.
Plasma haptoglobin levels in PCOS and control groups. PCOS polycystic ovary syndrome. Mann–Whitney test; *p < 0.05 was considered statistically significant
Fig. 2.
Correlation coefficients between haptoglobin levels, MTT (a) and CRP levels (b) in PCOS group. PCOS polycystic ovary syndrome, CRP C-reactive protein, MTT* metabolization test of tetrazolium, expressed in arbitrary units. Spearman’s correlation test; *p < 0.05 was considered statistically significant
The polymorphism Hp1-Hp2 was in Hardy–Weinberg equilibrium (p = 0.082 for PCOS; p = 0.369 for controls) for either group. No significant difference was observed regarding the genotypic frequency between the PCOS and control groups, according to additive, recessive, or dominant model (p > 0.05) (Table 2). In both groups, the genotype Hp2-Hp1 was the most frequent [PCOS (60.5%); control (53.5%)]. The most frequent allele was Hp2 [PCOS (52.3%); control (60.5%)]; however, no significant difference in allelic frequency was observed between the groups (p = 0.128).
Table 2.
Genotypic and allelic frequencies of Hp gene in PCOS and control groups
| Genotype | PCOS (n = 86) | Control (n = 86) | OR | 95% CI | p |
|---|---|---|---|---|---|
| Additive model | |||||
| Hp1-Hp1 | 15 (17.4%) | 11 (12.8%) | 0.480 | 0.162–1.408 | 0.136 |
| Hp2-Hp1 | 52 (60.5%) | 46 (53.5%) | 0.580 | 0.270–1.237 | 0.126 |
| Hp2-Hp2 | 19 (22.1%) | 29 (33.7%) | Reference | ||
| Recessive model | |||||
| Hp2-Hp2 | 19 (22.1%) | 29 (33.7%) | 1.794 | 0.911–3.533 | 0.091 |
| Hp1-Hp1 + Hp2-Hp1 | 67 (77.9%) | 57 (66.3%) | |||
| Dominant model | |||||
| Hp2-Hp2 + Hp2-Hp1 | 71 (82.6%) | 75 (87.2%) | 1.440 | 0.620–3.347 | 0.396 |
| Hp1-Hp1 | 15 (17.4%) | 11 (12.8%) | |||
| Allele | |||||
| Hp1 | 82 (47.7%) | 68 (39.5%) | 1.393 | 0.88–2127 | 0.128 |
| Hp2 | 90 (52.3%) | 104 (60.5%) | |||
Chi-square (χ 2) test with residual analysis
PCOS Polycystic ovary syndrome
*p < 0.05 was considered statistically significant
In order to evaluate if the Hp1-Hp2 polymorphism could change the circulating levels of the protein, plasma Hp levels were compared between the three genotypes. Plasma Hp levels did not differ according to the genotype in PCOS, nor in the control group (all p > 0.05). When compared the same genotype between the groups, no difference was also observed (all p > 0.05) (Table 3).
Table 3.
Haptoglobin plasma levels (g/L) according to Hp genotypes
| Genotype | PCOS | Control | |
|---|---|---|---|
| Hp1-Hp1 | 8.61 (2.70) | 8.43 (7.68) | p = 1.000 |
| Hp2-Hp1 | 9.23 (4.24) | 7.49 (3.48) | p = 0.790 |
| Hp2-Hp2 | 7.34 (7.82) | 6.31 (4.73) | p = 0.403 |
| p = 0.492 | p = 0.349 |
Mann–Whitney and Kruskal–Wallis test
PCOS polycystic ovary syndrome
*p < 0.05 was considered statistically significant
Discussion
In this study, we evaluated the association of plasma Hp levels, Hp1-Hp2 polymorphism, and biochemical variables in PCOS women. This is the first study that observed increased plasma Hp levels in patients with PCOS compared to controls, thereby validating the preliminary findings of proteomic studies. However, we did not find evidence that the Hp1-Hp2 polymorphism is related to the presence of the syndrome or to Hp levels in these groups.
The clinical and laboratorial characteristics of the patients reinforced common findings in patients with PCOS, as hyperandrogenism [50], increased abdominal fat [51], and IR—with consequent compensatory hyperinsulinemia [50]— as well as greater risk of metabolic syndrome, evaluated by LAP index [52]. The higher levels of MTT observed in the PCOS group could be associated to a compensatory effect, since this assay measures total plasmatic antioxidant capacity [43], and PCOS is commonly associated to a pro-oxidative profile [14, 15].
A low-grade chronic inflammation is characteristic of PCOS [9, 11–13]. Accordingly, in the present study, higher CRP levels were observed in PCOS women, but the difference between the groups was not significant, which could be explained by the low-grade inflammation in this syndrome that is not enough to significantly raise CRP values. In our study, CRP levels were minimally elevated, contrasting to conditions of acute, high-grade inflammation, such as tissue injury or infection, which typically cause CRP increase [53]. It is noteworthy that women who presented CRP levels > 10 mg/dL, associated with these conditions, were excluded from our study.
The PCOS group exhibited higher Hp levels than controls. This finding supports previous proteomic studies suggesting that Hp may be a biomarker of PCOS [16, 17]. In addition, a small study in the UK (n = 30 per group) using Western blotting to quantify Hp protein subunits found that total Hp and Hp beta chain protein abundance were elevated in women with PCOS compared with controls [54].
Additionally, we observed a positive correlation between Hp and CRP levels. This result corroborates the hypothesis that increased Hp levels are associated with inflammation in PCOS group. In fact, pro-inflammatory cytokines are important signal for Hp gene expression in white adipose tissue [55]. Moreover, the negative correlation with MTT indicates that higher Hp levels are observed with the increase of the pro-oxidative profile. Taken together, our findings suggest that higher Hp level in PCOS is a condition associated with inflammation and an increase in oxidative stress status.
Even though Hp levels increase with insulin resistance [56], neither Hp levels nor the Hp1-Hp2 polymorphism were related to glucose intolerance, since no correlation between these parameters and fasting glucose, HOMA-IR, and insulin levels was observed. Consequently, Hp does not appear to be involved in the insulin resistance of PCOS women.
Adiposity is an independent factor associated with Hp levels in humans [29]. It is known that Hp is produced by adipocytes, and that Hp gene is upregulated in white adipose tissue of the obese mice [22, 55]. The higher BMI and WC observed in PCOS could explain elevated Hp levels in this group. However, we did not observe any correlation between Hp levels and these parameters, suggesting that higher Hp concentration is independent from obesity status in this group investigated.
The genotypic and allelic frequencies of Hp1-Hp2 polymorphism were similar between the two groups. Langlois and Delanghe [21] showed that polymorphisms in alpha-chain influences circulating Hp concentrations to a lesser degree compared with its effect on the functionality of the protein. Consequently, our results suggest that higher Hp concentration in PCOS is independent of Hp1-Hp2 polymorphism, and that the protein is functionally analogous to that found in women without the syndrome.
Although no significant difference was observed, the results suggest a gene dosage effect of Hp1-Hp2 polymorphism on Hp levels in the control group. Decreased concentration was observed with the Hp2 allele, which expresses the protein with weakest antioxidant activities and reduced ability to bind free Hb. Interestingly, this profile was not observed in PCOS group, probably because other factors strongest influence on serum levels of Hp when compared to polymorphism effect.
Contrary to our results, Alvarez-Blasco et al. [36] reported an association between PCOS and the Hp2 allele [PCOS 62%; control 52%; p = 0.023]. In addition, this same study did not observe different Hp concentrations between patients with PCOS and non-hyperandrogenic controls, neither correlation of Hp levels and genotypes. It is noteworthy that Alvarez-Blasco et al. [36] used the same methodology for genotyping by Koch et al. [46], but their study involved Spanish women, while our study included Brazilian individuals. It is possible that the allelic and genotypic frequencies for the Hp gene substantially differ between these populations, since the genetic characteristic of the Brazilian population is resulted of European, African, and Amerindian miscegenation.
The main limitation of our study is the need of a relatively large effect size of PCOS to reach statistical significance in the available number of subjects, but the study was sufficiently powered to demonstrate inter-group differences in plasma Hp levels. Still, further studies involving more subjects should be conducted to confirm our results and search for possible correlations between factors, which were not observable under the current sample size.
Conclusion
We observed increased plasma Hp concentration in PCOS women compared to a control group. No association of Hp1-Hp2 polymorphism frequency was associated with PCOS or Hp levels, which suggests that the protein functionality is not changed in this group. Nevertheless, Hp levels are related to inflammatory status, as well as a pro-oxidative profile in PCOS group, indicating that Hp could be a potential biomarker for PCOS complications.
Acknowledgements
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for the financial support. FMR and KBG are grateful to CNPq Research Fellowship. Simone Martins Gonçalves, Dalva Maria de Resende, for technical support. Special thanks to the patients involved in this study.
Compliance with ethical standards
This study was approved by the ethics committees of Federal University of Minas Gerais (Minas Gerais, Brazil)—CAAE 0379.0.203.000-11. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All the women who participated in this study signed a free and informed consent form.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Papadakis G, Kandaraki E, Papalou O, Vryonidou A, Diamanti-Kandarakis E. Is cardiovascular risk in women with PCOS a real risk? Current insights. Minerva Endocrinol. 2017. 10.23736/S0391-1977.17.02609-8. [DOI] [PubMed]
- 2.Palomba S, Falbo SSA, La Sala GB. Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Womens Health. 2015;7:745–763. doi: 10.2147/IJWH.S70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva FS, Sóter MO, Sales MF, Candido AL, Reis FM, Silva IF, Sousa MO, Ferreira CN, Gomes KB. Estrogen receptor alpha gene (ESR1) Pvu II and Xba I polymorphisms are associated to metabolic and proinflammatory factors in polycystic ovary syndrome. Gene. 2015;560:44–49. doi: 10.1016/j.gene.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 4.Jalilian A, Kiani F, Sayehmiri F, Sayehmiri K, Khodaee Z, Akbari M. Prevalence of polycystic ovary syndrome and its associated complications in Iranian women: a meta-analysis. Iran J Reprod Med. 2015;13(10):591–604. [PMC free article] [PubMed] [Google Scholar]
- 5.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez F, Kirwanj P, Rote NS, Minium J. Elevated circulating levels of tissue factor in polycystic ovary syndrome. Clin Appl Thromb Hemost. 2013;19(1):66–72. doi: 10.1177/1076029612436673. [DOI] [PubMed] [Google Scholar]
- 7.Shen CC, Yang AC, Hung J, Hu LY, Tsai J. A nationwide population-based retrospective cohort study of the risk of uterine, ovarian and breast cancer in women with polycystic ovary syndrome. Oncologist. 2015;20(1):45–49. doi: 10.1634/theoncologist.2014-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95(51):48–63. doi: 10.1097/MD.0000000000004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction. 2015;149(5):R219–R227. doi: 10.1530/REP-14-0435. [DOI] [PubMed] [Google Scholar]
- 10.Sóter MO, Ferreira CN, Sales MF, Candido AL, Reis FM, Milagres KS, Ronda C, Silva IO, Sousa MO, Gomes KB. Peripheral blood-derived cytokine gene polymorphisms and metabolic profile in women with polycystic ovary syndrome. Cytokine. 2015;76(2):227–235. doi: 10.1016/j.cyto.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Aytan AN, Bastu E, Demiral I, Blulut H, Dogan M, Buyry F. Relationship between hyperandrogenism, obesity, inflammation and polycystic ovary syndrome. Gynecol Endocrinol. 2016;19(24):1–5. doi: 10.3109/09513590.2016.1155208. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho LM, Ferreira CN, Sóter MO, Sales MF, Rodrigues KF, Martins SR, Candido AL, Reis FM, Silva IF, Campos FM, Gomes KB. Microparticles: inflammatory and haemostatic biomarkers in polycystic ovary syndrome. Mol Cell Endocrinol. 2017;443:155–162. doi: 10.1016/j.mce.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Mortada R, Kallail KJ, Dong F, Karakas S. HbA1c in patients with polycystic ovary syndrome: a potential marker of inflammation. J Reprod Infertil. 2015;16(4):203–206. [PMC free article] [PubMed] [Google Scholar]
- 14.Özer A, Bakacak M, Kıran H, Ercan Ö, Köstü B, Kanat-Pektaş M, Kılınç M, Aslan F. Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekol Pol. 2016;87(11):733–738. doi: 10.5603/GP.2016.0079. [DOI] [PubMed] [Google Scholar]
- 15.Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19(3):268–288. doi: 10.1093/humupd/dms059. [DOI] [PubMed] [Google Scholar]
- 16.Insenser M, Martínez-García MA, Montes R, San-Millán JL, Escobar-Morreale HF. Proteomic analysis of plasma in the polycystic ovary syndrome identifies novel markers involved in iron metabolism, acute-phase response, and inflammation. J Clin Endocrinol Metab. 2010;95(8):3863–3870. doi: 10.1210/jc.2010-0220. [DOI] [PubMed] [Google Scholar]
- 17.Matharoo-Ball B, Hughes C, Lancashire L, Tooth D, Ball G, Creaser C, Elgasim M, Rees R, Layfield R, Atiomo W. Characterization of biomarkers in polycystic ovary syndrome (PCOS) using multiple distinct proteomic platforms. J Proteome Res. 2007;6:3321–3328. doi: 10.1021/pr070124b. [DOI] [PubMed] [Google Scholar]
- 18.Ashleh R, Marsh S, Skilkrut M, Binah O, Guetta J, Lejbkowicz F, Enav B, Shehadeh N, Kanter Y, Lache O, Cohen O, Levy NS, Levy AP. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Mol Med. 2003;92:1193–1200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 19.Costacou T, Levy AP. Haptoglobin genotype and its role in diabetic cardiovascular disease. J Cardiovasc Transl Res. 2012;5(4):423–435. doi: 10.1007/s12265-012-9361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SKA, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 21.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42(10):1589–1600. [PubMed] [Google Scholar]
- 22.Shaer CA, Schoedon G, Imhof A, Kurrer MO, Schaer DJ. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ Res. 2006;99(9):943–950. doi: 10.1161/01.RES.0000247067.34173.1b. [DOI] [PubMed] [Google Scholar]
- 23.Berkova N, Gilbert C, Goupil S, Yan J, Korobko V, Naccache PH. TNF-induced haptoglobin release from human neutrophils: pivotal role of the TNF p55 receptor. J Immunol. 1999;162:6226–6232. [PubMed] [Google Scholar]
- 24.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 25.Nakata K, Saitoh R, Aamano J, Koshiyama A, Ichibangase T, Murao N, Ohta K, Aso Y, Ishigai M, Imai K. Alteration of intracellular secretory acute phase response proteins expressed in human hepatocyte induced by exposure with interleukin-6. Cytokine. 2012;59(2):317–323. doi: 10.1016/j.cyto.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Baumann H, Morella KK, Jahreis GP, Marinković S. Distinct regulation of the interleukin-1 and interleukin-6 response elements of the rat haptoglobin gene in rat and human hepatoma cells. Mol Cell Biol. 1990;10(11):5967–5976. doi: 10.1128/MCB.10.11.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedrichs WE, Navarijo-Ashbaugh AL, Bowman BH, Yang F. Expression and inflammatory regulation of haptoglobin gene in adipocytes. Biochem Biophys Res Commun. 1995;209(1):250–256. doi: 10.1006/bbrc.1995.1496. [DOI] [PubMed] [Google Scholar]
- 28.Kalmovarin N, Friedrichs WE, O’Brien OV, Linehan LA, Bowman BH, Yang F. Extrahepatic expression of plasma protein genes during inflammation. Inflammation. 1991;15(5):369–379. doi: 10.1007/BF00917353. [DOI] [PubMed] [Google Scholar]
- 29.Chiellini C, Santini F, Marsili A, Berti P, Bertacca A, Pelosini C, Scartabelli G, Pardini E, Lopez-Soriano J, Centorini R, Ciccarone AM, Benzi L, Vitti P, Del Prato S, Pinchera A, Maffei M. Serum haptoglobin: a novel marker of adiposity in humans. Journal of Endocrinology and Metabolism. 2004;89(6):2678–2683. doi: 10.1210/jc.2003-031965. [DOI] [PubMed] [Google Scholar]
- 30.Maffei M, Barone I, Scabia G, Santini F. The multifaceted haptoglobin in the context of adipose tissue and metabolism. Endocr Rev. 2016;37(4):403–416. doi: 10.1210/er.2016-1009. [DOI] [PubMed] [Google Scholar]
- 31.Farbstein D, Levy AP. The genetics of vascular complications in diabetes mellitus. Cardiol Clin. 2010;28(3):477–496. doi: 10.1016/j.ccl.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szafranek T, Marsh S, Levy AP. Haptoglobin: a major susceptibility gene for diabetic vascular complications. Exp Clin Cardiol. 2002;7(2/3):113–119. [PMC free article] [PubMed] [Google Scholar]
- 33.Gast MCW, Tinteren HV, Bontenban M, Van Hoesel RGCM, Nooji MA, Rodenhuis S, Span PN, Tjan-Heijnen VCG, Vries EGE, Harris N, Twisk WR, Schellens JHM, Beijnen JH. Haptoglobin phenotype is not a predictor of recurrence free survival in high-risk primary breast cancer patients. BMC Cancer. 2008;8:389. doi: 10.1186/1471-2407-8-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orchard TJ, Sun W, Cleary PA, Genuth SM, Lachin JM, Mcgee P, Paterson AD, Raskin P, Anbinder Y, Levy A. Haptoglobin genotype and the rate of renal function decline in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes. 2013;62(9):3218–3223. doi: 10.2337/db13-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boettger LM, Salem RM, Handsaker RE, Peloso GM, Kathiresan S, Hirschhorn JN, Mccarroll SA. Recurring exon deletions in the HP (haptoglobin) gene contribute to lower blood cholesterol levels. Nat Genet. 2016;48(4):359–366. doi: 10.1038/ng.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Blasco F, Martínez-García MA, Luque-Ramírez M, Parraza N, San Millán JL, Escobar-Morreale HF. Role of haptoglobin in polycystic ovary syndrome (PCOS), obesity and disorders of glucose tolerance in premenopausal women. PLoS One. 2009;4(5):e5606. doi: 10.1371/journal.pone.0005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 38.Api M, Badoglu B, Akca A, Api O, Gorden H, Cetin A. Interobserver variability of modiWedFerriman–Gallwey hirsutism score in a Turkish population. Arch Gynecol Obstet. 2009;279:473–479. doi: 10.1007/s00404-008-0747-8. [DOI] [PubMed] [Google Scholar]
- 39.Wen-Ya M, Chung-Yi Y, Shyang-Rong S, Hong-Jen H, Chi SH, Fu-Chun C, Mao-Shin L, Pi-Hua L, Cyue-Huei H, Yenh-Chen H, Lee-Ming C, Jou-Wei L, Jung-Nan W, Hung-Yuan L. Measurement of waist circumference: midabdominal or iliac crest? Diabetes Care. 2013;36:1660–1666. doi: 10.2337/dc12-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lwow F, Jedrzejuk D, Milewicz A, Szmigiero L. Lipid accumulation product (LAP) as a criterion for the identification of the healthy obesity phenotype in postmenopausal women. Exp Gerontol. 2016;82:81–87. doi: 10.1016/j.exger.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Godinjak A, Godinjak Z, Burekovic A, Surkovic I, Dizdarecic-Bostandizic A, Velija-asimi Z. Insulin resistance and lipid accumulation product in correlation to body mass index in women with polycystic ovary syndrome. Med Arch. 2012;66:409–411. doi: 10.5455/medarh.2012.66.409-411. [DOI] [PubMed] [Google Scholar]
- 42.Tang Q, Li X, Song P, Xu L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: developments in research and prospects for the future. Drug Discov Ther. 2015;9:380–385. doi: 10.5582/ddt.2015.01207. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Nair MG. Efficient and economical MTT assay for determining the antioxidant activity of plant natural product extracts and pure compounds. J Nat Prod. 2010;73(7):1193–1195. doi: 10.1021/np1000945. [DOI] [PubMed] [Google Scholar]
- 44.Duarte RCF, Golçalves LH, Campos FMF, Filho OAM, Alves MT, Fernandes AP, Borges KBG, Dusse LMS, Faria MC, Gonçalves GS, Bosco AA, Sandrim VC, Carvalho MG. Effect of acetylsalicylic acid on platelet activation and oxidative profile in a set of Brazilian patients with type 2 diabetes mellitus. Blood Coagul Fibrinolysis. 2014;25:01–08. doi: 10.1097/MBC.0b013e3283657795. [DOI] [PubMed] [Google Scholar]
- 45.Medina LO, Veloso CA, Abreu Borges E, Isoni CA, Calsolari MR, Nogueira-Machado JÁ. Determination of the antioxidant status of plasma from type 2 diabetic patients. Diabetes Res Clin Pract. 2007;77:193–197. doi: 10.1016/j.diabres.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Koch W, Latz W, Eichinger M, Roguin A, Levy AP, Schömig A, Kastrati A. Genotyping of the common haptoglobin Hp1/2 polymorphism based on PCR. Clin Chem. 2002;48(9):1377–1382. [PubMed] [Google Scholar]
- 47.Taylor R. Interpretation of the correlation coefficient: a basic review. J Diagn Med Sonography. 1990;6:35–39. doi: 10.1177/875647939000600106. [DOI] [Google Scholar]
- 48.GENEPOP. http://genepop.curtin.edu.au/genepop_op1.html. Accessed 5 Mar 2017.
- 49.OEGE. http://www.oege.org/software/hardy-weinberg.html. Accessed 5 Mar 2017.
- 50.Bachelot A. Polycystic ovarian syndrome: clinical and biological diagnosis. Ann Biol Clin. 2016;74(6):661–667. doi: 10.1684/abc.2016.1184. [DOI] [PubMed] [Google Scholar]
- 51.Zheng SH, Li XL. Visceral adiposity index as a predictor of clinical severity and therapeutic outcome of PCOS. Gynecol Endocrinol. 2016;32(3):177–183. doi: 10.3109/09513590.2015.1111327. [DOI] [PubMed] [Google Scholar]
- 52.Kim JJ, Choi YM. Dyslipidemia in women with polycystic ovary syndrome. Obstet Gynecol Sci. 2013;56(3):137–142. doi: 10.5468/ogs.2013.56.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kushner MAI. It’s time to redefine inflammation. J Clin Endocrinol Metab. 2010;95(8):3863–3870. doi: 10.1210/jc.2010-0220. [DOI] [PubMed] [Google Scholar]
- 54.Haoula Z, Shaw B, Daykin C, Hodgman C, Layfield R, Atiomo W. Validation of proteomic biomarkers previously found to be differentially expressed in women with polycystic ovary syndrome: a cross-sectional study. Gynecol Endocrinol. 2014;30(3):213–216. doi: 10.3109/09513590.2013.871520. [DOI] [PubMed] [Google Scholar]
- 55.Chiellini C, Bertacca A, Novelli SE, et al. Obesity modulates the expression of haptoglobin in the white adipose tissue via TNF-α. J Cell Physiol. 2002;190(2):251–258. doi: 10.1002/jcp.10061. [DOI] [PubMed] [Google Scholar]
- 56.De Pergola G, Di Roma P, Paoli G, Guida P, Pannacciulli N, et al. Haptoglobin serum levels are independently associated with insulinemia in overweight and obese women. J Endocrinol Investig. 2007;30:399–403. doi: 10.1007/BF03346317. [DOI] [PubMed] [Google Scholar]