Abstract
Damage in fish activates retina repair that restores sight. The purinergic signalling system serves multiple homeostatic functions and has been implicated in cell cycle control of progenitor cells in the developing retina. We examined whether changes in the expression of purinergic molecules were instrumental in the proliferative phase after injury of adult zebrafish retinas with ouabain. P2RY1 messenger RNA (mRNA) increased early after injury and showed maximal levels at the time of peak progenitor cell proliferation. Extracellular nucleotides, mainly ADP, regulate P2RY1 transcriptional and protein expression. The injury-induced upregulation of P2RY1 is mediated by an autoregulated mechanism. After injury, the transcriptional expression of ecto-nucleotidases and ecto-ATPases also increased and ecto-ATPase activity inhibitors decreased Müller glia-derived progenitor cell amplification. Inhibition of P2RY1 endogenous activation prevented progenitor cell proliferation at two intervals after injury: one in which progenitor Müller glia mitotically activates and the second one in which Müller glia-derived progenitor cells amplify. ADPβS induced the expression of lin28a and ascl1a genes in mature regions of uninjured retinas. The expression of these genes, which regulate multipotent Müller glia reprogramming, was significantly inhibited by blocking the endogenous activation of P2RY1 early after injury. We consistently observed that the number of glial fibrillary acidic protein-BrdU-positive Müller cells after injury was larger in the absence than in the presence of the P2RY1 antagonist. Ecto-ATPase activity inhibitors or P2RY1-specific antagonists did not modify apoptotic cell death at the time of peak progenitor cell proliferation. The results suggested that ouabain injury upregulates specific purinergic signals which stimulates multipotent progenitor cell response.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-017-9572-5) contains supplementary material, which is available to authorized users.
Keywords: Extracellular nucleotides, Retina regeneration, Progenitor cell, Purinergic receptors, E-NTPDases, Pluripotency genes
Introduction
The retina of teleost fish, such as zebrafish, is endowed with the capacity to grow as well as regenerate in larval and adult life [1, 2]. Ongoing cell genesis occurs at the periphery of the mature retina in a neuroepithelium called the ciliary marginal zone (CMZ) from which the retina grows by adding new differentiated cells originated from multipotent retina stem cells [3]. On the other hand, if the retina is damaged, intrinsic mechanisms activate retina repair which restore sight in fish [4–8]. During the regenerative response, a subset of Müller glial cells which are considered the multipotent progenitors partially dedifferentiate and re-enter the cell cycle [9–12]. Daughter proliferative cells which are generated by multipotent Müller glia mitotic division are denominated Müller glia-derived progenitor cells. These proliferative cells form different pools that can also be detected within the mature layers [13–15]. The regenerative response can be induced by light, laser, surgical, and chemical damage as well as transgenic cell ablation [16–20]. Any kind of damage which provokes either global or specific cell death in all, some, or any of the retina layers induces intrinsic mechanisms to repair the retina including the morphological as well as the functional aspects to reconstitute a physiologically working tissue.
Purinergic signalling has an important role in regulating cell cycle activity in early stages of the developing vertebrate [21–24]. Purinergic signals include nucleotides such as ATP, ADP, UTP, and UDP that can be released to the extracellular milieu throughout diverse mechanisms. ATP can be released through vesicular exocytosis, connexin, or pannexin hemi-channels; maxi-ion channels; or pore-forming purinergic receptors [25–27]. Lytic release by cell damage and death can be important sources of extracellular nucleotides [27, 28]. Extracellular ATP in the CNS is also released from reactive astrocytes following injury, which chemoattracts microglial cells, which, in turn, release more ATP originating a positive feedback loop [29]. Moreover, ATP released from Müller glia and astrocytes decreases neuronal excitability and regulates vascular tone, synaptic transmission, and plasticity [30].
A family of enzymes known as ecto-nucleoside tri-phosphate di-phosphohydrolases (E-NTPDases) also constitutes pivotal members of the purinergic system. E-NTPDases together with ecto-5′-nucleotidases can dephosphorylate extracellular ATP to adenosine [23, 31]. The purinergic signalling system is further composed by purine and pyrimidine membrane receptors called purinergic or P receptors [32]. P2RY (G protein-coupled) receptors have been involved in trophic effects on cell proliferation, differentiation, and death during development and tissue regeneration [23, 26, 33]. NTPDase2 activity and P2RY1 have been implicated in eye and retina morphogenesis in vertebrate species [24, 34–37]. Extracellular ATP can be dephosphorylated to ADP, mainly by NTPDase2 but also by NTPDase3, which binds with high affinity to P2RY1 [38]. We have previously reported the expression of plasma membrane E-NTPDases and their role in controlling daily rhythms of S phase activity in the CMZ of the zebrafish retina [39, 40]. We have also shown a nucleotide effect on cell proliferation in response to an injury with ouabain which provokes cell death in the inner retina only comprising ganglion and amacrine cells [11, 39, 41]. An ADP analogue at a relatively low concentration stimulated cell proliferation in undamaged retinas [42]. On the other hand, a severe injury caused by more elevated concentrations of ouabain [8] kills any kind of retina, pigmented epithelium, as well as choroid layer cells.
In this study, we sought to investigate whether an injury of intermediate severity with ouabain was able to induce significant changes in the expression of the purinergic system molecules. We have evaluated whether these changes correlated in time with the mitotic activation of progenitor cells. We have also analysed purinergic signal effects early after injury at the interval of Müller glia reprogramming and later on when Müller glia-derived progenitor cells mitotically amplified [7, 43, 44].
Our results indicated that P2RY1 expression was enhanced early after injury, and this increase was likely induced by its own endogenous ligand (ADP). These findings also suggest that injury-released nucleotides throughout activation of specific purinergic receptor signalling pathways can mediate the upregulation of pluripotency genes which are necessary for progenitor Müller glia reprogramming and for its derived neural progenitor cell mitotic activity.
Materials and methods
Materials
Paraformaldehyde, PBS (137 mM NaCl, 2.7 mM KCl, 5.7 mM phosphate, pH 7.4), TBS (20 mM Tris base, 140 mM NaCl, pH 7.5), Tween-20, MS-222 (tricaine), sucrose, cresyl violet, apyrase (EC-3.6.1.5), ouabain, 5-bromo-2′-deoxyuridine (BrdU), 8-sulfophenyl theophylline, sample buffer 5× (4% SDS and 10–20% β-mercaptoethanol), transfer buffer (25 mM of Tris-HCl, 200 mM glycine, 20% methanol), lysis buffer (Tris-HCl 100 mM, NaCl 100 mM, 0.5% Triton, 0.1% SDS, protease inhibitors), and ATPγS and ADPβS (A740003) were obtained from Sigma, MO, USA. Cangrelor (ARC69931MX) was a generous gift from AstraZeneca (London, UK). MRS2211, MRS2179, NF110, NF157, polyoxotungstate 1 (POM 1), and ARL67156 were obtained from Tocris Bioscience, Bristol, UK. Tissue freezing medium was from Biopack, Buenos Aires, Argentina. Vectashield and normal goat serum (NGS) were from Vector Labs, CA, USA. Random primers (dNTPs, MMLV, DTT, RNAseOUT) and specific primers were obtained from Invitrogen, CA, USA. Total RNA Extraction Kit (RBC Biosciences, Taiwan); GoTaq DNA polymerase, dNTP mix, and DNAse I (Promega, WI, USA); real-time polymerase chain reaction (PCR) mix (Biodynamics, Buenos Aires, Argentina); RNAzol; ultra-pure agarose (GenBiotech, Buenos Aires, Argentina; chemiluminescent HRP substrate (Thermo Scientific, IL, USA); In Situ Cell Death Detection Kit Fluorescein (Roche, Mannheim, Germany); rabbit polyclonal anti-β-actin antibody (1:200) in TBS (ab16039; Abcam, Cambridge, UK); goat polyclonal secondary antibodies (anti-mouse ALEXA 488 or Cy3-conjugated, anti-rabbit ALEXA 594; Jackson Immuno, PA, USA); and anti-rabbit IgG HRP-conjugated (1:3000) in TBS (Bio-Rad, CA, USA) were used in the study.
Animals
Wild-type zebrafish (Danio rerio) were maintained at 28 °C, raised in a 14/10 h light/dark cycle, and fed with Arthemia sp. and dry food. We used adult zebrafish of about 3.0 cm in body length. Animals were euthanized by immersion in ice-cold MS-222 anaesthetic solution (0.02% w/v), decapitated, and enucleated on ice. The committee on animal research at the University of Buenos Aires (CICUAL) approved the protocols for ethical animal use and care.
Ouabain treatment
Zebrafish were deeply anaesthetized and held wet under a dissecting microscope. Left eyes of zebrafish were injected with 0.6 μl of 20 μM ouabain, diluted in sterile saline solution, within the vitreous chamber. Left eyes of different zebrafish were injected with an equivalent volume of saline solution. Ouabain final concentration was estimated to be of 6 μM by considering a mean vitreous volume of 2.0 μl. The approximate volume was calculated from the volume difference between the eye posterior chamber and lens radius [11]. A bevelled 33-gauge needle (0.375 in., PT2) and a 5-μl syringe (75 RN) (Hamilton Company, Reno, NV, USA) were used to deliver appropriate volumes. The day of the single ouabain injection is referred to as day 0.
5-Bromo-2′-deoxyuridine administration
Groups of six to eight zebrafish were injected into the vitreous chamber with 0.6 μl of a solution containing 20 μg/μl BrdU. For the most part of the experiments, a single dose of BrdU was injected daily beginning on day 4 through day 7 after injury (an exposure period of 80 h). Estimated BrdU intravitreous concentration was 6 μg/μl. BrdU was injected alone (in vehicle-injected control groups) or together with purinergic analogues, receptor antagonists, ecto-ATPase inhibitors, or apyrase. For data depicted in supplementary figure 1, four groups of six zebrafish each were injected within the vitreous chamber with saline solution (uninjured control) or ouabain on day 0. Forty-one hours later, zebrafish were injected with 0.6 μl of 20 μg/μl BrdU and euthanized 65 h after injury. Other groups of zebrafish were treated with a single dose of BrdU on day 4, 6, or 19 after lesion and euthanized 24 h later (5, 7, or 20 dpl).
For data depicted in Fig. 8, BrdU was injected 4 h before zebrafish were euthanized 80 h after injury (Fig. 8i–k) or 7 dpl (as shown in Fig. 8o, q, s, u) that depict progenitor cells in the CMZ, whereas zebrafish euthanized 7 days after injury (Fig. 8l–n) were injected 20 h before.
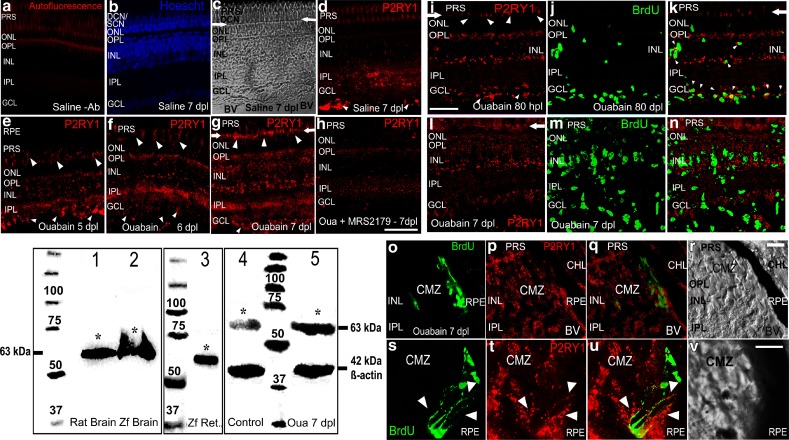
Fig. 8.
P2RY1 protein expression and localization in intact or injured retina layers. Western blot depicts P2RY1 immunodetection in the homogenates of rat and zebrafish brain which is shown in lanes 1 and 2, whereas homogenates of zebrafish neural retinas were loaded in lane 3. Lanes 4 and 5 show P2RY1 immunodetection in homogenates of saline- and ouabain-treated retinas examined 7 days after injury. Proteins from the brain (25 μg/lane) and neural retina (70 μg/lane) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) in reducing conditions, transferred to nitrocellulose membranes and incubated with antibodies. The asterisks label bands of apparent molecular weight of 63 kDa detected with the P2RY1 antibody. The bands of 42 kDa in lanes 4 and 5 were detected using an anti-β-actin antibody. Numbers 25–100 indicate molecular weights of proteins of a standard marker. Data were obtained from three to four retina pools (ten retinas each) and independent assays. Representative confocal images of retina sections from zebrafish show the expression of P2RY1 (in red) as detected by fluorescence immunocytochemistry (FICC). a A representative image of an injured retina section incubated without P2RY1 primary antibody (Ab, negative control). b–d Saline-treated uninjured retina sections stained with Hoechst to show nuclear layers (b) and two images of the same microscopic field. These pictures were taken using a transmitted irradiance channel and differential interference contrast (DIC) filters in c and the corresponding confocal image of the P2RY1 fluorescent immunoreactivity (IR) in d. Pictures in e–g depict images of mature retina sections 5, 6, and 7 days after lesion (dpl). The image in h shows P2RY1-IR in an injured-retina treated in vivo for 6 days with the antagonist MRS2179. Blurry and even red labelling in the photoreceptor segments represents autofluorescence that was also exhibited by negative control sections. Detection of 5-bromo-2′-deoxyuridine (BrdU) by FICC appears as bright green nuclei. BrdU was injected 4 h before euthanasia at the indicated intervals after lesion (i–v). Small arrowheads in d–g indicate P2RY1 strong IR in structures that likely are blood vessels. Arrows in c, d, g, i, k, l show the outer limiting membrane (OLM) location, and medium-sized arrowheads in e–g, i indicate P2RY1 labelling in inner cone and some outer segments and/or the OLM. Images of ouabain-injured mature retina sections 80 hpl and 7 dpl are depicted in i–k and l–n, respectively. Small arrowheads in k show co-localization of both markers likely in the same cell in the INL, GCL, and fibre layer regions. o–v Images of the ciliary marginal zone (CMZ) 7 dpl. The merger of red and green images of the same microscopic field is shown in k, n, q, u. Big arrowheads in s–u indicate sites of BrdU-positive nuclei surrounded by red IR. Scale bars 40 μm (a–h), 28 μm (i–n), 15 μm (o–r), and 10 μm (s–v). PRS photoreceptor segments, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, DCN double-cone nuclei, SCN single-cone nuclei, RPE retinal pigmented epithelium, CHL choroid layer, BV blood vessel
Apyrase treatments
Apyrase dephosphorylates di- and tri-phosphate nucleotides. A single dose of 0.6 μl of a saline solution containing 20 U/ml apyrase (the estimated concentration within the vitreous chamber was 6 U/ml) was injected daily after injury for 6 days (1–7 dpl). Control’s injured eyes were injected daily with heat-inactivated apyrase also for 6 days. For the data shown in Fig. 2, groups of zebrafish with uninjured retinas were injected daily with apyrase for 3 days. Control groups were injected with heat-inactivated apyrase for the same period. On the fourth day, zebrafish were euthanized and neural retinas were isolated for RNA extraction.
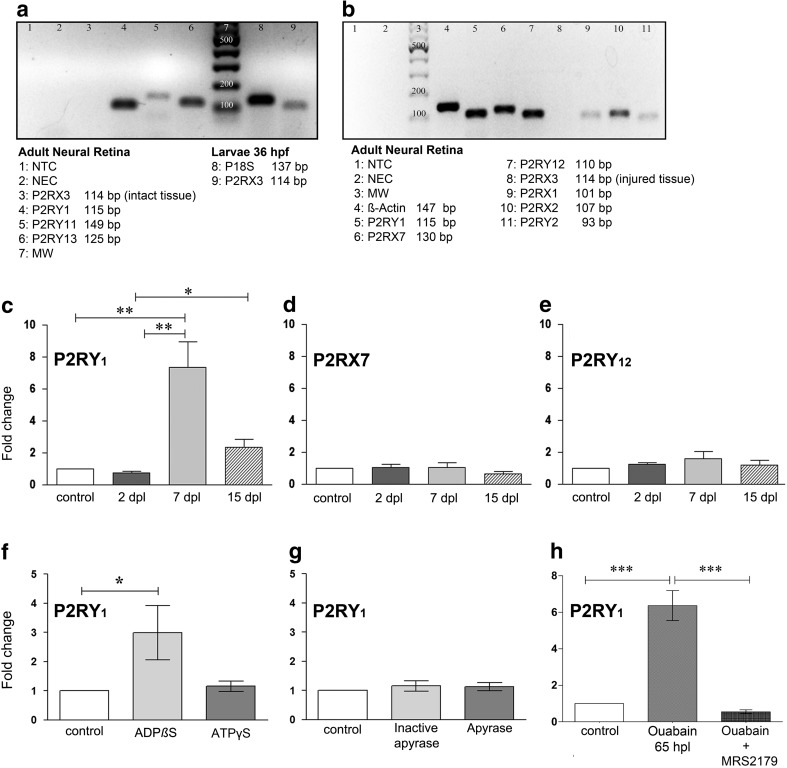
Fig. 2.
Purinergic signalling effects on P2RY1 mRNA expression in the zebrafish retina. Total RNA was purified from pools of ten retinas each obtained from intact or lesioned eyes of zebrafish at different days after lesion (dpl). Total RNA was also obtained from zebrafish larvae of 36 h post fertilization (hpf). cDNA was amplified with primers for P2 receptors: P2RY1, P2RY2, P2RY11, P2RY12, P2RY13, P2RX1, P2RX2, P2RX3a, and P2RX7. a, b Amplified cDNA products of a representative end-point RT-PCR that were separated by electrophoresis in 2% agarose gels. P2RX3a was examined in intact (a, lane 3) and injured (b, lane 8) retinas as well as zebrafish larvae (a, lane 9). NTC no template control, NEC no enzyme control, MW DNA molecular weight marker. c–e Real-time quantitative PCR performed with specific primers for P2Y1, P2X7, and P2Y12 membrane receptors. For this assay, neural retinas were excised 2, 7, or 15 dpl and considered as the samples of interest. The calibrator sample was the saline solution-treated retina pool (control group). f Total RNA was purified from pools of six retinas each obtained from uninjured eyes, which had been injected daily for 3 days with sterile saline solution, 3 μM ADPβS, or 6 μM ATPγS. g Total RNA was purified from pools of six retinas each obtained from uninjured eyes, which had been injected daily for 3 days with saline solution, 6 U/ml of apyrase, or heat-inactivated apyrase. Samples of interest: ADPβS-, ATPγS-, apyrase-, or heat-inactivated apyrase-treated retinas. Calibrator sample: saline solution-treated retinas. h Total RNA was purified from pools of ten ouabain-injured retinas treated with MRS2179 (P2RY1-specific antagonist) or saline solution (control). Ouabain-injured retinas (day 0) were treated daily with a single dose of MRS2179 (1 μM) or saline solution from 0 to 48 h after lesion (hpl), and both groups were considered samples of interest. Calibrator sample: uninjured saline solution-treated retinas. Zebrafish were euthanized, and retinas were excised 65 hpl. Real-time quantitative PCR reactions were performed with specific primers for P2RY1 as the target gene. Fold change represents mean ± SD of the relative expression ratio (rER) from three independent assays. rER is the initial fluorescence amount in the sample of interest relative to a calibrator sample (control uninjured retinas) normalized with a reference gene (P18S ribosomal protein S18 (rps18) or β-actin).*** p < 0.001, **p < 0.01, *p < 0.05, in Dunn’s multiple comparison test after the Kruskal-Wallis test
Extracellular nucleotide agonist treatments
For data shown in Figs. 2f and 7a, b, 0.6 μl of saline solution alone or containing 20 μM ATPγS or 10 μM ADPβS, with estimated intravitreous concentrations of 6 and 3 μM, respectively, was administered daily for 3 days within uninjured eyes. On the fourth day, zebrafish were euthanized and neural retinas were isolated. Peripheral tissue including the CMZ was carefully removed, and the remaining tissue (mature neural retina portion) was processed for reverse transcription quantitative PCR (RT-qPCR) assays. ATPγS and ADPβS are ATP and ADP analogues which are hydrolysed at a very low rate within the vitreous and bind to specific plasma membrane purinergic receptors with high affinity (see text below).
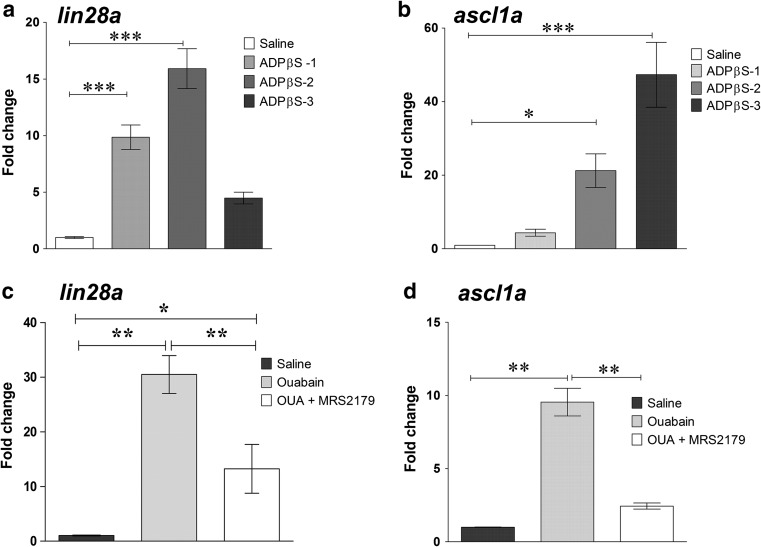
Fig. 7.
Purinergic signalling effect on lin28a and ascl1a transcriptional expression in the zebrafish retina. Total RNA was purified from pools of 10–16 neural retinas from which the peripheral tissue had been carefully removed (mature retina without the CMZ) (a, b). Each retina was obtained from uninjured eyes, which had been in vivo treated for 3 days with saline solution or 3 μM ADPβS (a slowly hydrolysable ADP analogue) solution injected within the vitreous cavity. Retina pools were obtained from three independent assays of ADPβS-treated uninjured retinas (graphical bars labelled with ADPβS-1, ADPβS-2, and ADPβS-3 tags) which were considered samples of interest. Fold change represents the relative expression ratio (rER) calculated and plotted separately for each independent assay. Calibrator samples were three independent pools of saline solution-treated uninjured retinas (fold change for each of the three control retina pools was equal to 1 and are graphically represented in the same control bar). c, d RNA was purified from pools of 10–16 retinas (mature portion only without the CMZ), each obtained from control saline-, 6 μM ouabain plus saline-, or ouabain plus 1 μM MRS2179-injected eyes. Intravitreous injections of MRS2179 were performed once daily from 0 to 48 h after lesion (hpl), and retinas were isolated 65 hpl. Fold change represents the average rER calculated from each of three independent retinal pools treated with ouabain or ouabain plus MRS2179. The calibrator sample was calculated as the average of three independent pools of saline solution-treated uninjured retinas. Data were expressed as mean ± SE. ***p < 0.001; **p < 0.01; *p < 0.05, in Tukey’s multiple comparison test after ANOVA
Extracellular nucleotide/nucleoside antagonist treatments
Retinas were treated with a single dose of ouabain on day 0. Then, 0.6 μl of saline solution either alone or containing 17 μM cangrelor, 38 μM 8-sulfophenyl theophylline (8-SPT), or 17 μM NF157 plus 17 μM NF110 was injected daily for 6 days. Estimated concentrations of these analogues within the vitreous chamber were 5, 11, or 5 plus 5 μM, respectively. Three micromolars of MRS2179 (estimated intravitreous concentration of 1 μM) was injected daily into the vitreous chamber for 6 days starting on day 1 after injury (1–7 dpl), 3 days immediately after injury (0–3 dpl), or the last 3 days beginning on day 4 after injury (4–7 dpl). One hundred micromolars of A740003 (estimated intravitreous concentration of 30 μM) was injected daily for 3 days starting immediately after injury (0–3 dpl).
Purinergic receptor agonist and antagonist potency and affinities
Cangrelor (ARC 69931MX): competitive antagonist of P2RY12 and non-competitive antagonist of P2RY13 with no activity on P2RY1 [45–48]. EC50 = 0.4 nM as an anti-ADP-induced platelet aggregation agent. pEC50: cangrelor 8.4 > ADP 7.2 (on P2RY12) and cangrelor 8.4 > ADP 7.9 > MRS2211 6.0 (on P2RY13) [49]
MRS2179: competitive P2RY1 antagonist (IC50 = 0.330 μM; K i = 100 nM), selective over P2RX1/P2RX3/P2RX2/P2RX4 and P2RY2/P2RY4/P2RY6. Agonist specificity: ADPβS > ADP >> ATP
A740003: high affinity competitive antagonist for ATP-activated P2RX7. It displays selectivity over P2RX1/P2RX3/P2RX2/P2RX4 and P2RY up to a concentration of 100 μM. IC50 = 18 and 40 nM for rat and human receptors, respectively. Agonist specificity: BzATP >>> ATPγS > ATP
NF110: high affinity antagonist for ATP-activated P2RX3/P2RX1/P2RX2 (IC50 = 0.527 μM; K i = 36/82/4144 nM, respectively) shows no activity on P2RY1/P2RY2/P2RY11 (IC50 > 10 μM)
NF157: antagonist selective for P2RY11 (IC50 = 0.463 μM) and P2RX1 over P2RY1/P2RY2 and P2RX2/P2RX3/P2RX4/P2RX7. Agonist specificity: ATPγS > ATP [50]
8-SPT: competitive adenosine antagonist on P1 receptors (K i = 1.2 μM)
Treatments with ecto-ATPase and ecto-nucleotidase activity inhibitors
ARL67156 selectively inhibits ecto-ATPase activity without activating purinergic receptors [51]. A volume of 0.6 μl saline solution containing 200 μM ARL67156 was injected within the vitreous (pIC50 = 4.62 and 5.1 in the human blood and rat vas deferens, respectively) [52, 53]. ARL67156 was administered inside the vitreous chamber during three different intervals: 1–7, 0–3, and 4–7 dpl.
POM 1 (Na6 [H2W12O40]) is a more potent inhibitor of E-NTPDase activity than ARL67156. POM 1 K i values are 2.58, 3.26, >10, and 28.8 μM for NTPDase1, NTPDase3, P2RY12, and NTPDase2, respectively [54]. A volume of 0.6 μl of a solution containing 120 μM of POM 1 was administered daily for 3 days immediately after injury (0–3 dpl).
Control groups
Different groups of zebrafish were injured with 6 μM ouabain (except for saline solution-injected uninjured control groups) and injected with the different vehicles in which the drugs had been dissolved during the same intervals after injury described for purinergic analogue-treated groups.
Terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling assay
We detected DNA breaks that occur at the early stages of apoptosis by terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling (TUNEL) by using an in situ cell death detection kit. Retina sections were treated for 25 min at 37 °C with 2 M HCl, washed with PBS plus 0.1% Tween-20 (PBST), and incubated with terminal deoxynucleotidyl transferase (TdT) and fluorescein-dUTP. In negative controls, TdT was omitted. Uninjured retina sections treated with 2 M HCl did not show TUNEL-positive labelling. TUNEL assay was performed either alone or followed by detection of BrdU on the same retina sections.
Tissue processing and fluorescence immunocytochemistry
To prepare eyecups, the cornea and lens were removed. Eyecups were fixed with 4% paraformaldehyde for 1 h at room temperature, incubated overnight with 20% sucrose, and embedded in the tissue freezing medium. Eyecups were cut in 16-μm cryosections parallel to the meridian plane (nasal to temporal). Each slide contained sections representing different portions of the eyecup. Retina sections were incubated in PBST and 5% NGS for 1 h at room temperature. For BrdU labelling, retinas were previously incubated in 2 M HCl for 25 min at 37 °C and washed with PBST. Then, sections were incubated at 4 °C overnight with a mouse monoclonal anti-BrdU antibody diluted (1:250) in 3% NGS (Roche Applied Sciences, Mannheim, Germany). Slides were washed and incubated in darkness for 2 h at room temperature with a fluorescent secondary antibody (1:400 in 3% NGS). For detecting P2RY1 IR, a similar protocol was carried out using a rabbit polyclonal anti-P2RY1 antibody (1:100; Invitrogen, CA, USA). For double-labelling assays, sections were incubated with the anti-BrdU antibody and either the rabbit polyclonal antibody which specifically detects the glial fibrillary acidic protein (GFAP) (1:500; Dako, Denmark) or the anti-P2RY1 antibody. Negative controls were performed by omitting either primary or secondary antibodies.
Counting of BrdU-positive nuclei
We counted BrdU-positive nuclei in retinal layers under direct observation with an epifluorescence microscope. We counted all BrdU-positive nuclei throughout the surface of six non-adjacent sections from each eye in a double-blind assay. Serial confocal images throughout the z-axis were also taken for several sections. Bi-dimensional reconstructions were performed, and cells were counted to confirm the number of cells we have computed under direct observation. For each experiment, the number of BrdU-positive cells per retinal section was normalized and the average value for the ouabain-treated group was equal to 1. Normalization consisted in dividing the individual values from each group by the average number of labelled cells in the ouabain-treated retina sections. Statistical significance was not modified by this procedure. Fluorescent small dots and speckles were not taken into account to compute BrdU-labelled nuclei.
Microscopy
Counting was performed with an epifluorescence microscope BX50 (Olympus, Japan). Objectives ×40 and ×60 (numerical aperture (NA) = 0.65 and 1.35). Microphotographs were captured with a FV1000 Fluoview confocal spectral microscope with objectives SAPO-60× oil and SAPO-40× oil (NA = 1.35 and 0.9, respectively; Olympus). Images from double-labelled retinas were taken with adequate laser beams and spectral filters with a maximal depth of 1.0 μm in the z-axis and in a fix xy-plane of the same microscopic field. Tri- and bi-dimensional reconstructions were performed with Fluoview software (Olympus). Images were adjusted for brightness and contrast against background fluorescence including photoreceptor segment autofluorescence, combined, and labelled with Adobe Photoshop CS5 extended, version 12.
RT and PCR
Ten to sixteen neural retinas were homogenized and considered as one sample. At least three independent samples were examined for each treatment and target gene. RNA was extracted, quantified, and treated with DNAse I. Copy DNA was reverse transcribed from RNA with random primers. Nucleic acids were quantified with a NanoDrop 3300 spectrophotometer (Thermo Fisher Scientific, MA, USA); 100–150 ng RNA was used for standard or real-time qPCR (Rotor Gene 6000; Qiagen, CA, USA, and Applied Biosystems 1500; Thermo Fisher Scientific). Specific primers for target and reference genes are shown in Table 1. Standard end-point PCR and qPCR products were checked by electrophoresis in 2% agarose gels. Controls in which MMLV reverse transcriptase was omitted (No-RT) were also amplified by qPCR. Samples were run in triplicate. Ribosomal protein S18 (rps18), β-actin, or elongation factor 1-alpha (ef1-α) was used as an internal reference gene and amplified in parallel with target genes. Reference genes showed no significant treatment-induced variations.
Table 1.
Specific primers for target and reference genes
| Gene symbol (transcript ID) | Forward | Reverse |
|---|---|---|
| rps18 (ENSDART00000017975) | ACCCTCGCCAGTACAAAATCC | CCTGATCTTCTTCAGCCTCTCC |
| β-Actin (ENSDART00000054987) | TCCCAAAGCCAACAGAGAGAAG | GTCACACCATCACCAGAGTCC |
| efl1-alpha (ENSDARG00000020850) | CAGCAGCTGAGGAGTGATCT | GTAGATCAGATGGCCGGTGG |
| ntpdase1 (ENSDART00000066257) | TCACAGACACAGCGAAACCC | GAATGTGCAACGGAGGTAAGC |
| ntpdase2-mq (ENSDART00000043259) | GAGCAGCAGCACGTAGCC | GACCTCAGCCGACTCTTTGG |
| ntpdase2-mv (ENSDART00000051434) | TGATGGAGTCTTTCAGCCCAAC | AGCAGCGTCCTCTAAATGAGC |
| ntpdase3 (ENSDART00000051167) | GGCTGGATCACTGTCAACTACC | CCCAAATCCAATGACCCAACTG |
| p2ry1 (ENSDART00000109100) | TATGGACAATGCTCCGCTTAGG | CGCTCTCATGTTCAGGTTCTTC |
| p2ry2 (ENSDART00000092965) | TCTGGTGTTTGTGTTCGGTCTG | ATGTAGATGGTGTTGGGCTTCC |
| p2ry11 (ENSDART00000008240) | CTTCCTCTTCACCAGCAACCTC | TGAACCACACCAGCACACTTG |
| p2ry12 (ENSDART00000102224) | AGCGTCTCCAACAGTTCATCC | GCCAGAGCGTTCAGGGATAATC |
| p2ry13 (ENSDART00000102221) | ACAAAGCAGCAGTGACGTAACC | GCGTCTGGCTGAGGGTGTAC |
| p2rx1 (ENSDART00000002866) | GGCAGGTGTATGAACAAAACCC | TCTGCTGTCAGTAGGATTGGAG |
| p2rx2 (ENSDART00000019461) | GCCAGAGAGAAATTCAGCGAAC | TATGTGGGAATACACGCAGACG |
| p2rx3a (ENSDART00000021417) | ATTCGCTTCCCTCTCTTTGGTG | AAAGATGGGACAGAACGGATGG |
| p2rx7 (ENSDART00000062229) | ACACCGAGAGGAAGTTTGAGG | AGGGGTTTTGTCTCCTGTAGTC |
| lin28a (ENSDART00000098970) | TTTCTGTCCATGACCCACCG | TCAGACTGCGAAAACCCTCC |
| ascl1a (ENSDART00000056005) | ACGACCCTCTGAGTCCAGAA | GACATCCTCCCAAGCGAGTG |
Primers were selected by using exonic sequences from the reported zebrafish genome (Ensembl database). RNA transcript identification number (ID) from the Ensembl database is shown in the first column. Selected primer pairs did not hybridize (at least five mismatches) with other sequences in the zebrafish genome (tested with primer BLAST)
Quantitative PCR data analysis was performed throughout the “gene expression’s CT difference” (GED) method [55], which considers efficiencies (E) of each amplification reaction. For Figs. 2 and 3, complementary DNA (cDNA) obtained from retinas 2, 7, and 15 dpl was used as samples of interest. For Figs. 2 and 7, cDNA obtained from ADPβS-, ATPγS-, heat-inactivated apyrase-, or apyrase-treated retinas was used as samples of interest; cDNA obtained from ouabain-treated mature retinas with or without MRS2179 (freed from the peripheral tissue containing the CMZ) 65 h after injury was also used as samples of interest. In every case, saline solution-treated (control) retinas were used as calibrator samples.
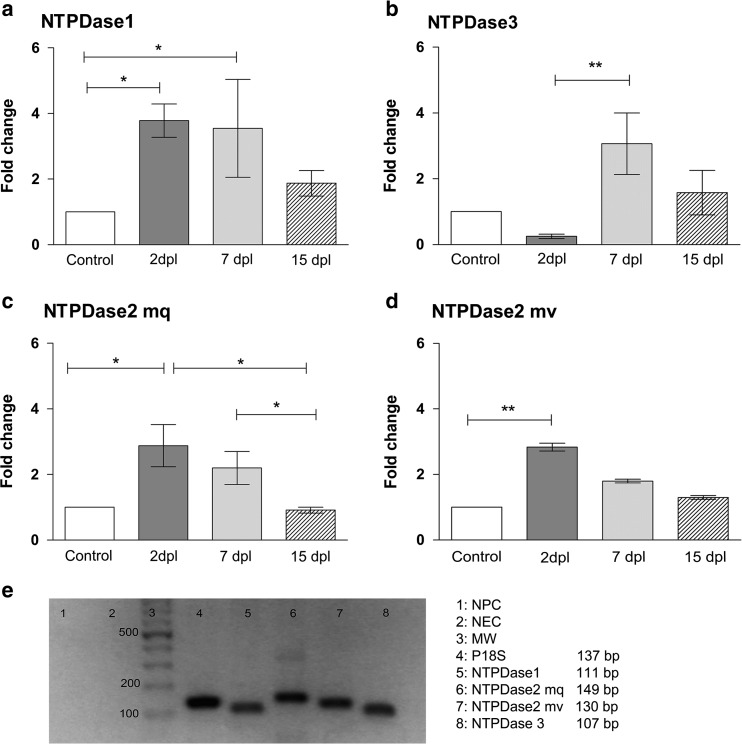
Fig. 3.
Transcriptional expression of E-NTPDases in the injured retina of zebrafish. Total RNA was purified from pools of ten retinas each obtained from saline solution-injected or ouabain-injured eyes on different days after lesion (dpl). Real-time quantitative PCR reactions were performed with primers for NTPDase1 (a), NTPDAse3 (b), NTPDAse2 mq isoform (c), and NTPDase2 mv isoform (d) as target genes. Fold change represents mean ± SD of the relative expression ratio from three independent experiments, which is the initial fluorescence amount in the sample of interest (retinas were isolated 2, 7, or 15 dpl) relative to a calibrator sample (control uninjured retinas) normalized with a reference gene (P18S ribosomal protein S18 (rps18)). **p < 0.01, *p < 0.05, in Dunn’s multiple comparison test after the Kruskal-Wallis test. e End-point (45 cycles)-amplified products of a representative quantitative PCR were separated by electrophoresis in a 2% agarose gel. NPC no primer control, NEC no enzyme control, MW DNA molecular weight marker
Western blot assay
Zebrafish brains and neural retinas as well as Sprague Dawley rat brains were isolated and homogenized on ice-chilled buffer to extract membrane proteins. Tissue homogenates were centrifuged at 14,000×g for 10 min at 4 °C. Aliquots of tissue homogenates were separated in SDS-(9%) polyacrylamide gels under reducing conditions. Protein loadings were 25 μg (rat and zebrafish brain) or 70 μg (neural retinas). Proteins were transferred onto nitrocellulose membranes. Immunoblots were performed by incubating membranes with a polyclonal antibody against P2RY1 (1:300) overnight at 4 °C. Thereafter, membranes were incubated with a secondary [HRP]-linked antibody for 60 min at room temperature and carefully washed. A mild stripping procedure was performed on some membranes for removing antibodies and re-probing with a primary antibody against β-actin as a loading control. Proteins were visualized by chemiluminescence, and images were captured in a G box (Syngene, Cambridge, UK). Data were obtained from four independent retinal pools and assays.
Results
We first performed a morphological assessment of the retinal tissue at different times after injury with 6 μM ouabain, which caused a lesion of intermediate severity between the damage caused by 2 or 10 μM ouabain injected within the vitreous cavity [11].
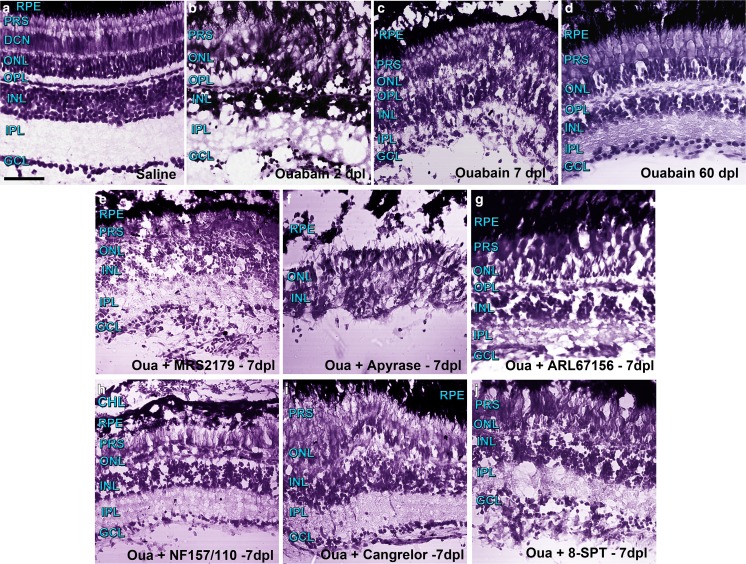
Representative images of retina sections stained for histological analysis under different treatments are depicted in Fig. 1. Figure 1a–d depicts the temporal progression of retinal damage and repair with a single dose of 6 μM ouabain 2, 7, and 60 days after lesion (dpl).
Fig. 1.
Representative photomicrographs of retina sections from zebrafish labelled with Nissl staining were captured with a confocal microscope using differential interference contrast (DIC) filters and the transmitted irradiance detector channel. Groups of five to six zebrafish underwent injections within the vitreous chamber with saline solution (a) or a solution containing 6 μM ouabain on day 0 and were kept alive for 2 (b), 7 (c), and 60 (d) days after lesion (dpl). Other groups of zebrafish were injured in the same manner and received intravitreal treatments with different compounds daily for 6 days starting on day 1 after injury. Control groups of zebrafish (from which images are not shown) underwent intravitreal injections with saline solution, other vehicles such as dimethyl sulfoxide, or inactivated apyrase also for 6 days. Zebrafish were euthanized 7 days after injury to obtain retina sections treated with the following: e 1 μM MRS2179 (antagonist of ADP-activated P2RY1), f 6 U/ml apyrase (ecto-nucleotidase), g 60 μM ARL67156 (ecto-ATPase inhibitor), h 5 μM NF157 plus 5 μM NF110 (antagonists of ATP-activated P2RX3, P2RX2, P2RX1, and P2RY11), i 5 μM cangrelor (antagonist of ADP-activated P2RY12 and P2RY13), or j 11 μM 8-SPT (antagonist of adenosine-activated P1R). Scale bar 40 μm. RPE retinal pigmented epithelium, CH choroid layer, PRS photoreceptor segments, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer
We observed more severe damage in the inner retina including the ganglion cell layer (GCL), inner plexiform layer (IPL), and inner nuclear layer (INL), but tissue injury and cell death were also observed in the outer plexiform layer (OPL), photoreceptor cell layer (including the outer nuclear layer (ONL) and photoreceptor segments (PRSs)), retinal pigmented epithelium (RPE), as well as the extra-retinal choroid layer (CHL).
Histological assessment of retinas treated with purinergic receptor antagonists, apyrase, and ATPase activity inhibitors
Images of retina sections show the morphological effects of different treatments performed daily for 6 days and analysed 7 dpl (Fig. 1e–j). Daily intravitreal treatments included one injection with pharmacological agents that have been documented as specific antagonists for P2X1, P2X2, and P2X3; P2Y1, P2Y11, P2Y12, and P2Y13 (nucleotidergic); as well as any kind of adenosine-activated (P1) plasma membrane receptors such as NF-110, NF-157, MRS2179, cangrelor, and 8-SPT. The antagonists were assessed within the eye in concentrations which have been described to be effective in several vertebrate species, but relatively low final concentrations were used to target the corresponding type of P receptor. In this way, treatments with the specific antagonist of P2RY1 (MRS2179) caused a significant decrease in the number of retinal cells and notably affected layer integrity 7 dpl. An excess of apyrase completely inhibited eye tissue recovery and likely promoted additional cell death and delamination of the retinal tissue in the injury environment. Likewise, the ecto-ATPase inhibitor (ARL67156) decreased the number of retinal cells and prevented tissue recovery, like it was normally observed on day 7 after damage with 6 μM ouabain without further pharmacological treatment. On the other hand, the other antagonists assessed, such as cangrelor (P2RY12 and P2RY13), NF-110 + NF-157 (P2RX1, P2RX2, P2RX3, and P2RY11), or 8SPT (P1R), did not apparently affect tissue recovery, nuclei number, and morphology of the retinal layers when compared with the features of the injured tissue without further treatment observed 7 days after damage.
To further characterize this injury paradigm in our conditions, we examined Müller glia-derived progenitor cell proliferation profile on different days after the ouabain injection. Supplementary figure 1 shows images of retina sections obtained from zebrafish treated with a pulse of BrdU of 24 h and euthanized 65 h or 5, 7, or 20 days after lesion. The number of BrdU-positive nuclei showed a significant increase 65 h after injury, mainly in the INL whereas the maximal increase in progenitor cell proliferative activity was observed 7 dpl as has been shown previously with different ouabain concentrations [11, 56].
The adult zebrafish retina expresses several members of the P2 receptor family
Among the plasma membrane receptors described in vertebrate neural tissues, we observed that metabotropic receptors P2RY1, P2RY2, P2RY11, P2RY12, and P2RY13 were transcriptionally expressed in the adult retina of zebrafish (Fig. 2a, b). Ionotropic P2RX1, P2RX2, and P2RX7 messenger RNAs (mRNAs) were also detected in intact neural retinas. We did not detect P2RX3a mRNA expression either in intact or injured retinas. As a positive control, we were able to detect P2RX3a mRNA expression by using the same pair of primers in 36-hpf zebrafish embryos.
P2RY1 mRNA relative levels in uninjured and damaged retinas
We quantified the mRNA expression level of P2RY1, P2RY12, and P2RX7 during the degenerative and regenerative phases after injury (Fig. 2c–e). P2RY1 (a biologically ADP-activated receptor) mRNA levels showed a sevenfold increase at 7 dpl compared with control uninjured retinas or with retinas analysed 2 days after damage. P2RY1 mRNA expression level in control zebrafish was not statistically different from the expression levels observed 15 days after damage however; the latter were significantly higher than those at 2 dpl. The mRNA levels of P2RY12 (another metabotropic P receptor activated mainly by extracellular ADP) or P2RX7 (a calcium permeant ionotropic receptor widely implicated in cell death induction when activated by high levels of extracellular ATP) did not show significant changes after injury with 6 μM ouabain.
Additionally, the injury-induced upregulation of P2RY1 mRNA was abolished by a relatively low dose of MRS2179 (this receptor’s own antagonist) administered in vivo during 3 days starting immediately after injury (Fig. 2h).
We next investigated whether extracellular treatment of the uninjured retina with ADP or ATP analogues—or whether the endogenous presence of extracellular nucleotides in the undamaged environment—was able by themselves to modify P2RY1 transcriptional expression. In this regard, P2RY1 mRNA levels were upregulated by threefold in intact retinas treated in vivo with ADPβS (3 μM) for 3 days (Fig. 2f). On the other hand, a 3-day treatment with the ATP analogue ATPγS (Fig. 2f) or the scavenging of endogenous extracellular nucleotides by apyrase (Fig. 2g) did not significantly affect the P2RY1 mRNA expression level in uninjured retinas.
Quantitative analysis of E-NTPDase transcriptional expression after injury
Cell-extrinsic nucleotide hydrolysis is principally driven by E-NTPDase activity in the plasma membrane; therefore, changes in the expression of these enzymes could modify nucleotide and nucleoside extracellular availability to bind and activate different kinds of purinergic receptors in the plasma membrane of retinal cells. So, we examined whether the mRNA expression level of NTPDases 1, 2, and 3—the plasma membrane enzymes of the kind expressed in the nervous system of many vertebrate species—underwent changes after injury. NTPDase1 mRNA relative levels showed a significant three-to fourfold increase 48 h and 7 days after injury, respectively (Fig. 3a). The mRNA expression levels of this enzyme were not significantly different from control values 15 dpl. NTPDase3 mRNA relative levels showed a threefold increase at the time of peak cell proliferation (Fig. 3b). We examined mRNA relative levels of two isoforms of NTPDase2. The mq isoform showed a threefold increment 48 h after injury and a smaller increment at the time of peak cell proliferation compared with the mRNA levels of this enzyme 15 days after damage. The mv isoform mRNA level also showed an increment of about 2.5-fold 48 h after lesion (Fig. 3c, d).
Extracellular nucleotide and ecto-ATPase activity effects on cell proliferation in different intervals after damage
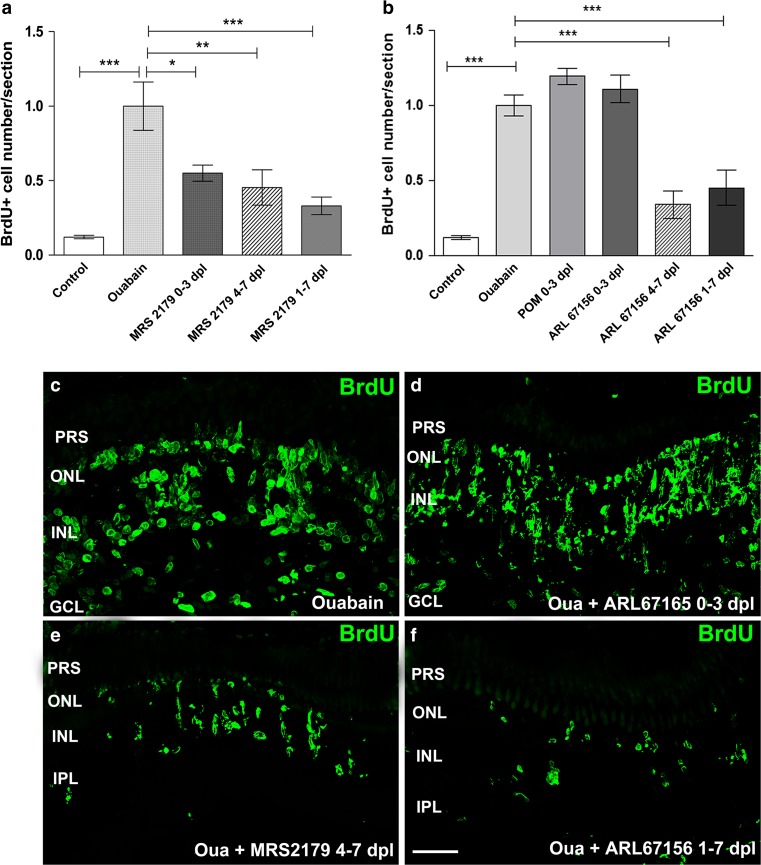
The number of BrdU-positive nuclei per retinal section varied between 400 and 1200 (mean ± SD = 731 ± 234, n = 25) among different groups of ouabain-treated zebrafish when they were assessed 7 days after lesion. Experiments shown in Fig. 4 were designed to evaluate purinergic receptor antagonist and NTPDase activity inhibitor effects on progenitor cell proliferation during different intervals after damage with ouabain. We first examined the effect of MRS2179 within the eye from 24 h to 7 days after damage. Figure 4a depicts the number of BrdU-labelled nuclei within retinal layers per section, detected in ouabain-treated retinas followed by injections of vehicle or MRS2179, the P2RY1-selective antagonist, for 6 days after lesion (1–7 dpl). This treatment inhibited the injury-induced cell progenitor amplification peak normally observed on day 7 after lesion, which is due to the mitotic division of Müller glia-derived neuronal progenitor cells.
Fig. 4.
MRS2179 and ARL67156 treatment effect on in vivo cell proliferation at different times following injury. Eyes underwent a single injection within the vitreous chamber of saline solution or 6 μM ouabain on day 0. Eyes were also injected in vivo with 1 μM MRS2179 (a specific antagonist of P2RY1), 60 μM ARL67156 (an ecto-ATPase activity inhibitor), or 36 μM POM 1 (an E-NTPDase activity inhibitor that also antagonizes P2RY12). These compounds were delivered daily for different intervals: starting on day 0 through day 3 after injury (0–3 dpl), starting on day 1 through day 7 after lesion (1–7 dpl), or starting on day 4 through day 7 after injury (4–7 dpl). All the groups of zebrafish were treated with a single intravitreous injection of 5-bromo-2′-deoxyuridine (BrdU) on day 4 through day 7 after injury. The normalized number of BrdU-labelled nuclei per retina section treated with MRS2179 or ARL67156 is depicted in a, b, respectively. c–f Representative confocal images (bi-dimensionally reconstructed from z-stacks) depicting BrdU-labelled nuclei in retina sections from ouabain-treated eyes with different treatments as indicated in each panel. Scale bar 40 μm. Normalized data were expressed as mean ± SE (n = 6–8 zebrafish per group). ***p < 0.001, **p < 0.01, *p < 0.05, in Dunnett’s multiple comparison test (vs. the ouabain treatment) after ANOVA. PRS photoreceptor segments, ONL outer nuclear layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, Oua ouabain
To examine whether the effect of extracellular nucleotides on progenitor cell mitotic activity also occurred during the injury-induced activation of multipotent progenitor Müller glia, we treated zebrafish retinas for only 3 days with MRS2179 beginning immediately after injury (0–3 dpl). We also examined this antagonist effect on Müller glia-derived progenitor cell proliferative activity with a delayed treatment that began 4 days after lesion (4–7 dpl). MRS2179 significantly decreased the number of BrdU-positive nuclei when it was assessed 7 dpl regardless of the interval of treatment (Fig. 4a). We then investigated the timing of ARL67156 treatment by using a similar protocol. Inhibition of ecto-ATPase activity (including plasma membrane-expressed E-NTPDases) by ARL67156 from 1 to 7 dpl significantly decreased the number of BrdU-positive nuclei at the time of peak progenitor cell proliferation (Fig. 4b). Additionally, the inhibitor reduced the number of BrdU-positive nuclei regularly observed on day 7 after injury when it was present within the eye at the time of Müller glia-derived progenitor cell amplification (from 4 to 7 dpl). Treatments with ARL67156 or POM 1 (a more potent inhibitor of the E-NTPDase activity) applied only at the time of multipotent Müller glia activation and early derived proliferating progenitor cells (0–3 dpl) failed to reduce the injury-induced increase in the number of Müller glia-derived neuronal progenitors normally observed at the time of the peak of cell proliferation after damage (Fig. 4b).
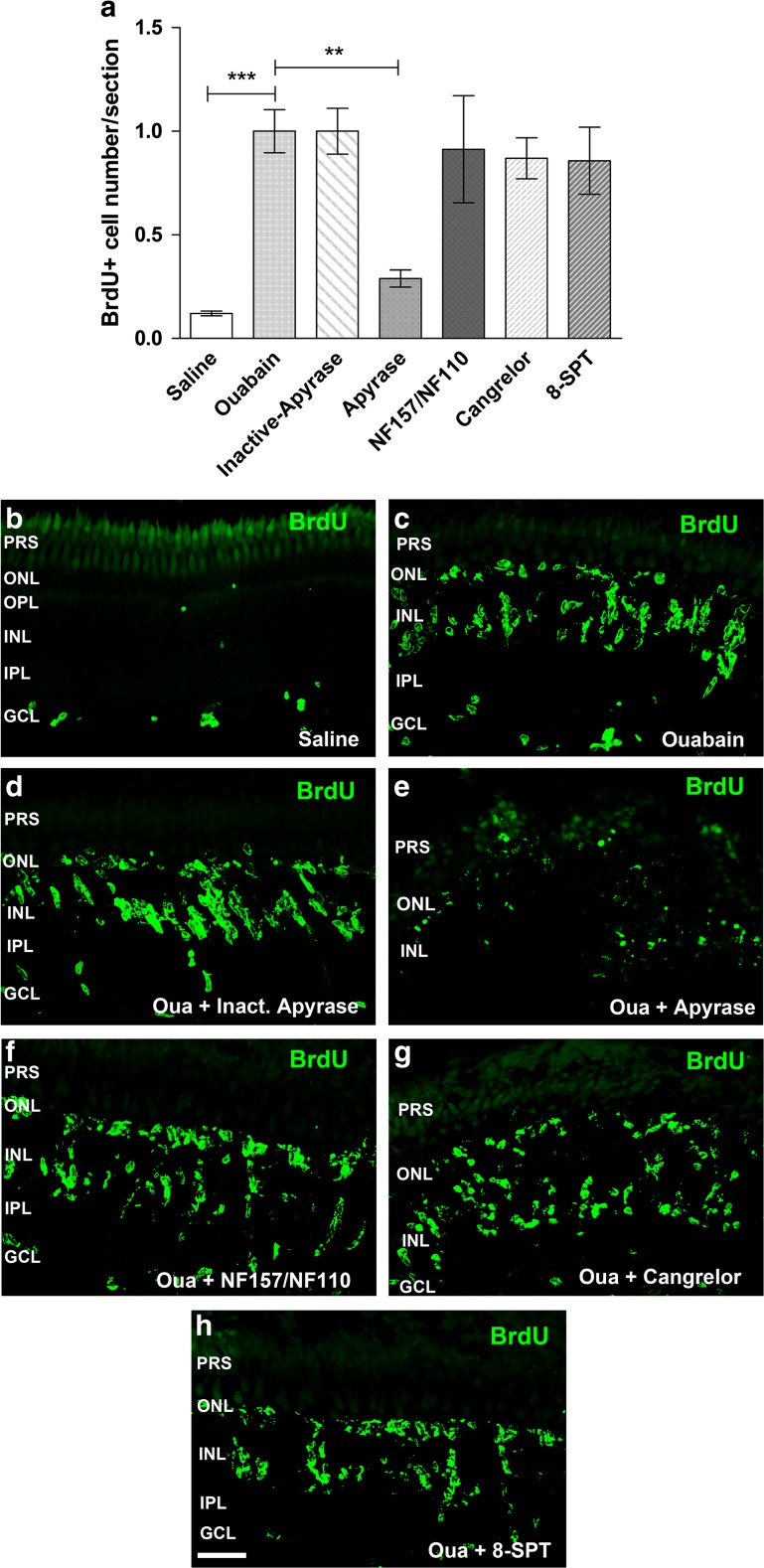
Apyrase and P2R and P1R antagonist effects on the BrdU-positive cell number after injury
To continue examining a purinergic signalling effect on proliferative activity of progenitor cells for retina repair after injury, we treated zebrafish eyes with apyrase to eliminate the presence of endogenous extracellular nucleotide in the vitreal cavity and the retinal tissue. As shown in Fig. 5e, treatments with apyrase were able to completely inhibit the injury-induced increase in the number of BrdU-positive nuclei at the time of peak cell proliferation. The control group was treated for 6 days after damage with heat-inactivated apyrase. This group did not show a significant effect on cell proliferation, indicating no toxic effects of the inactive enzyme injected within the eye.
Fig. 5.
Apyrase and purinergic receptor antagonist treatment effect on in vivo cell proliferation after injury. Eyes underwent a single intravitreal injection of saline solution or ouabain on day 0. Eyes were injected daily for 6 days after lesion (dpl) with saline solution, apyrase (di- and tri-phosphate nucleotidase), heat-inactivated apyrase, or different purinergic receptor antagonists: cangrelor (P2RY12, P2RY13), 8-SPT (adenosine P1R), or NF110 plus NF157 (P2RX1, P2RX2, P2RX3, and P2RY11). A single dose of 5-bromo-2′-deoxyuridine (BrdU) was injected for 3 days starting on day 4 after injury together with the enzyme apyrase or the antagonists. Zebrafish were euthanized 7 days after injury. a Normalized number of BrdU-positive nuclei per retinal section. b–h Representative confocal images (bi-dimensionally reconstructed from z-stacks) of BrdU-labelled nuclei in 16-μm sections obtained from ouabain-treated retinas that were in vivo exposed to the different antagonists as indicated in each panel. Normalized data were expressed as mean ± SE (n = 4–8 zebrafish per group). ***p < 0.001, **p < 0.01, Dunnett’s multiple comparison test (vs. the ouabain treatment) after ANOVA. Scale bar 40 μm. PRS photoreceptor segments, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, Oua ouabain
On the other hand, treatments for 6 days after injury with cangrelor (which was present within the eye from 24 h to 7 days after damage), a selective antagonist of both P2RY12 and P2RY13 which are primarily activated by ADP, did not significantly affect the number of BrdU-positive nuclei observed on day 7 after damage. Other antagonists (NF110 plus NF157) of P2 receptors primarily activated by ATP such as P2RY11, P2RX1, P2RX2, and P2RX3 or the antagonist (8-SPT) of adenosine-activated P1 receptors did not significantly affect BrdU-positive nuclei count, also detected at the time of peak progenitor cell mitotic amplification. Whenever these antagonists failed to affect BrdU-positive cell count, they did not obviously affect either progenitor cell nuclei morphology or cluster formation in the INL. The presence of progenitor or precursor cells in the ONL or GCL was neither significantly affected.
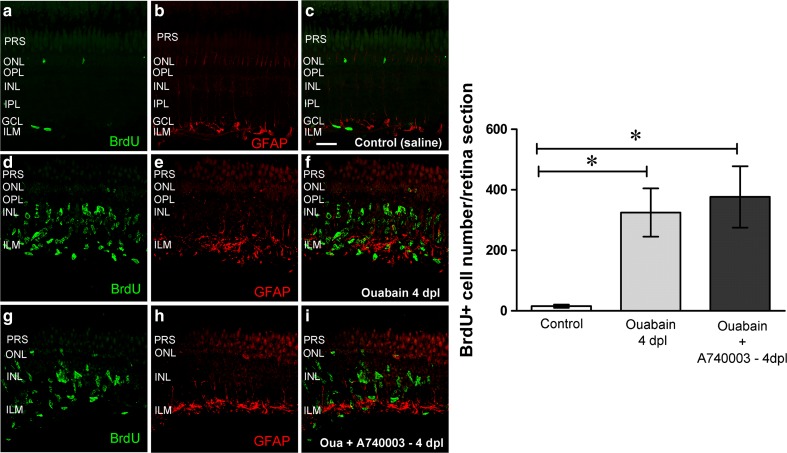
Blocking of P2RX7 effect on progenitor cell mitotic activation
We further investigated a possible participation of P2X7 receptors in progenitor cell number regulation after damage, because these receptors have been involved in cell death induction in pathological environments due to their capacity of forming calcium pores in the presence of very high extracellular concentrations of ATP that might be the case during extensive cell damage in the early injured environment. So, a single dose of the potent and specific antagonist of P2RX7, A740003, was intravitreally injected daily for 3 days starting on day 0 through day 2 after injury to assess its efficacy in modifying the proliferative activity of progenitor Müller glia and its early derived neuronal progenitor cells. Figure 6 depicts representative pictures of double-labelled images which show IR for GFAP and a 24-h pulse of BrdU which was incorporated by proliferative progenitor cells before performing animal euthanasia 4 days (96 h) after lesion. GFAP was upregulated in Müller glia evidencing reactive gliosis to injury. In the presence of the P2RX7 antagonist, GFAP expression was also increased by injury and BrdU-positive cell number was not significantly different from the number of positive cells observed in injured retinas without treatment with this antagonist.
Fig. 6.
P2X7-specific antagonist in vivo effect on progenitor cell mitotic activity early after injury. Eyes underwent a single intravitreal injection with saline solution or 6 μM ouabain on day 0. Then, a single dose of the specific P2RX7 antagonist A740003 (30 μM) or saline solution was injected daily inside the eyes starting immediately after lesion (0 hpl) and 24 and 48 h after lesion (hpl). A single dose of BrdU was injected within the vitreous chamber at 72 hpl. Retina sections were obtained from zebrafish euthanized 4 days (96 h) after lesion (dpl). Staining of 5-bromo-2′-deoxyuridine (BrdU) and glial fibrillary acidic protein (GFAP) immunoreactivity is depicted in green and red, respectively. The third column shows the merger of red and green images. Photographs depict representative confocal images of saline-treated retina sections (control; a–c), ouabain-injured and saline-treated retina sections (d–f), and ouabain plus A740003-treated retina sections (g–i). Data (BrdU-positive nuclei number per 16-μm retina section) were expressed as mean ± SE (n = 6-8 zebrafish per group). *p < 0.01, Dunnett’s multiple comparison test after ANOVA. Scale bar 25 μm. PRS photoreceptor segments, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, ILM inner limiting membrane
Quantitative analysis of lin28a and ascl1a mRNA levels in the mature retina
To investigate the purinergic signal effect on gene expression of pluripotency markers in the intact mature retina, we measured the relative expression level of the pluripotency gene lin28a, which codifies an RNA-binding protein which is only expressed by injury-activated progenitor Müller glia and Müller glia-derived progenitor cells in the mature tissue. We also determined the mRNA relative levels of the proneural transcription factor ascl1a, which has been described as an upstream regulator of lin28a in mechanically injured retinas [7]. We separated the mature portion of the neural retina from the CMZ, the germinal zone responsible for retinal growth containing multipotent progenitor cells which are different from progenitor Müller glial cells, and assessed the lin28a and ascl1a mRNA expression at 65 h after injury in the mature region exclusively. We first examined the expression of these genes in uninjured retinas of zebrafish, which had been treated in vivo for 3 days with 5 μM ADPβS. The slowly hydrolysable analogue of ADP significantly upregulated mRNA levels of lin28a by inducing 5- to 16-fold increments compared with the mRNA levels observed in saline solution-treated retinas (Fig. 7a). A similar treatment with ADPβS provoked significant increases in the relative levels of ascl1a mRNA of 6- to 27-folds also compared with the levels detected in the retinas obtained from saline solution-injected eyes (Fig. 7b).
We also examined the effect of 6 μM ouabain damage on pluripotency gene expression to then investigate the participation of P2RY1 in mediating injury-induced pluripotency gene expression in the mature neural retina. The injury significantly induced lin28a and ascl1a transcriptional expression compared with the expression levels shown by uninjured groups of retinas. In several samples, lin28a mRNA levels were undetectable in uninjured neural retinas (considering the mature portion only) whereas they were clearly detected in injured retinas at relatively low quantitative PCR cycle threshold values. However, we were able to detect a low level of lin28a mRNA in intact retinas by increasing the number of cycle threshold for all the experimental groups. Noteworthy, the specific antagonist of P2RY1 MRS2179 injected within the vitreous at 0, 24, and 48 h after injury with ouabain caused a significant inhibition of about 63 and 75% of the injury-induced expression of lin28a and ascl1a genes, respectively (Fig. 7c, d).
P2RY1 protein expression was detected in the zebrafish retina by western blot and fluorescence immunocytochemistry
To examine the protein expression level of the P2RY1 in intact and injured retinas 7 days after damage, we detected the relative expression level of the P2RY1 by western blot in control (saline solution-injected) or ouabain-injured retinas, as depicted in Fig. 8. As a positive control, we used rat brain homogenates which showed a clear band of 63 kDa (lane 1). Concordantly, the brain (lane 2) as well as the neural retina (lane 3) of zebrafish exhibited single bands of the same molecular weight. Furthermore, thicker bands of 63 kDa were observed in injured retinas compared to the ones detected in control retinas.
To further evaluate the expression level and particularly the expression pattern of P2RY1 in the intact as well as injured retina layers at different intervals after lesion, fluorescence immunocytochemistry (FICC) assays also demonstrated expression of the P2RY1 in retina sections from saline solution-injected (control) and 80 hpl, 5 dpl, 6 dpl, and 7 dpl ouabain-treated zebrafish. Specific labelling showed a granular pattern differentially distributed on retinal layers. Blurry and even red or green label on photoreceptor segments (Fig. 8d, f-h, l) represents autofluorescence that was also observed in negative control sections (Fig. 8a). In uninjured retinas (Fig. 8d), P2RY1 protein was particularly expressed in the IPL synaptic layers and GCL. A strong IR for the P2RY1 was observed in structures which likely are inner retina blood vessels located in the fibre layer area (small arrowheads). Eighty hours after injury, P2RY1-IR level was unchanged relative to control saline solution-injected retinas whereas the receptor expression pattern significantly decreased in the IPL and increased in the inner nuclear and outer plexiform layers (Fig. 8i). The staining for the receptor gradually enhanced in the INL, OPL, and photoreceptor segments 5 days (Fig. 8e), 6 days (Fig. 8f), and 7 days (Fig. 8g) after damage. Moreover, IR for the P2RY1 also progressively increased in the IPL and GCL regions from days 5 to 7 after lesion. Staining in injured retinas 7 dpl also revealed labelling in the outer limiting membrane (OLM) region formed by Müller cell processes in the outer retina (arrows). The apparent staining in the OLM region may be also due to a more elevated expression in photoreceptor cell inner segments.
To evaluate whether endogenous extracellular nucleotides regulated their own receptor protein expression, zebrafish were injected within the vitreous cavity with the antagonist of P2RY1 daily from 24 h to day 7 after damage. P2RY1-IR was strongly reduced by the antagonist below levels detected in intact retinas (Fig. 8h).
Eighty hours after lesion, double-labelling assays showed that a few BrdU-positive nuclei, detected in a short interval of 4 h, co-localized with P2RY1-IR in apparently the same cells in the INL as well as the remaining tissue with proliferating cells in the GCL and fibre layer regions (Fig. 8i–k). On day 7 after injury, many nuclei labelled with a 4-h pulse of BrdU and mainly localized in the INL, but also some of them in the GCL and ONL, were surrounded by a dense and grainy P2RY1-IR (Fig. 8l–n). Pictures of retina sections depicting the CMZ which contains stem cells as well as progenitor and differentiating cells for retina growth are shown in Fig. 8o–v. Retinas were also labelled in vivo with a 4-h pulse of BrdU. BrdU-positive nuclei, which likely represent the nuclei of slowly dividing progenitor cells because they were detected in close proximity to the RPE and blood vessels (BVs), were tightly bordered with P2YR1-IR in the CMZ (Fig. 8o–v).
Injury and MRS2179 effects on GFAP expression and BrdU incorporation
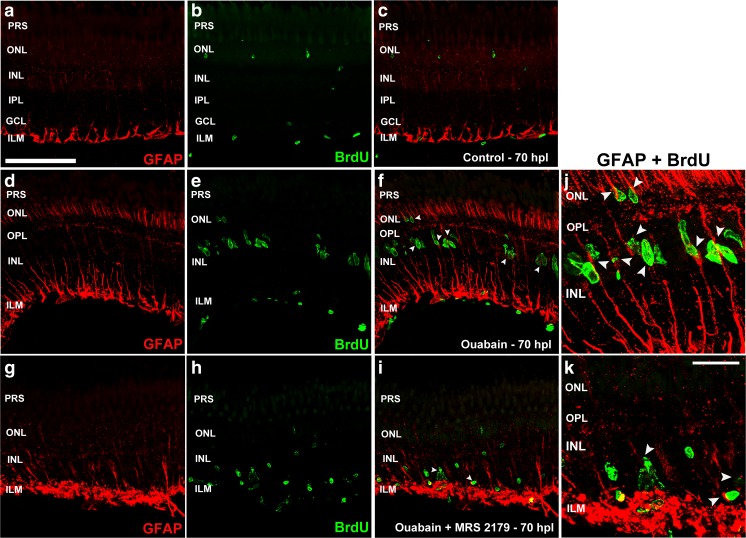
Figure 9 depicts GFAP-IR in Müller glia in uninjured, injured with ouabain, and ouabain plus MRS2179-treated retina sections which were analysed 65 h after injury. GFAP-IR was increased in Müller glia throughout retinal layers in response to injury. In the presence of the P2RY1 antagonist, GFAP-IR was also increased but it was particularly enhanced in the Müller glia feet normally forming the inner limiting membrane (ILM) whereas this staining was less conspicuous in the INL and outer retina. In injured retinas with no further treatment, nuclei labelled with a 4-h pulse of BrdU were aligned in the outer half of the INL. A few of these nuclei, which had initiated the S phase in a 4-h time window, co-localized with GFAP processes across the INL whereas the majority of the BrdU-positive nuclei formed clusters associated to the same GFAP-positive processes. This particular spatial arrangement suggests that the labelled nuclei are postmitotic nuclei of multipotent Müller glia and clustered nuclei belong to mitotically active derived neuronal progenitors. To analyse the possible participation of P2RY1 activation by extracellular nucleotides early after lesion in regulating multipotent Müller glia reprogramming and early derived progenitor cell cycle progression, retinas were treated in vivo with the specific antagonist of the P2RY1 MRS2179 at 0, 24, and 48 h after damage. BrdU incorporation in mitotically active cells was detected for a short interval of 4 h before euthanasia that occurred at 65 h after lesion. The treatment with the antagonist caused a significant decrease in the number of BrdU-labelled nuclei and a consequent decrement in the clusters formed by mitotically active derived progenitor cells. We observed isolated and dispersed BrdU-positive nuclei, which were not aligned in the outer portion of the INL like they were in damaged control retinas, with scant co-localization with the GFAP process across the INL.
Fig. 9.
BrdU-positive nuclei and GFAP expression in Müller cells. A single dose of 5-bromo-2′-deoxyuridine (BrdU) was injected at 24 and 48 h within the vitreous after ouabain or saline solution injections, and zebrafish were euthanized 70 h after lesion (hpl). a–c Control saline-treated uninjured retinas; d–f 6 μM ouabain-treated retinas; g–i 6 μM ouabain plus 1 μM MRS2179-treated retinas. A single dose of the antagonist of P2RY1 was injected three times: immediately after injury (0 hpl) and at 24 and 48 hpl. j, k Progenitor cell nuclei were detected by BrdU immunofluorescence and are depicted in green. Glial fibrillary acidic protein (GFAP) immunoreactivity (IR) appears red. The third column shows the merger of red and green images. Arrowheads indicate several green nuclei which co-localized with GFAP-IR. PRS photoreceptor segments, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, ILM inner limiting membrane. Scale bars 20 and 5 μm in a and k, respectively
ARL67156 or MRS2179 effects on the apoptotic cell number in injured retinas
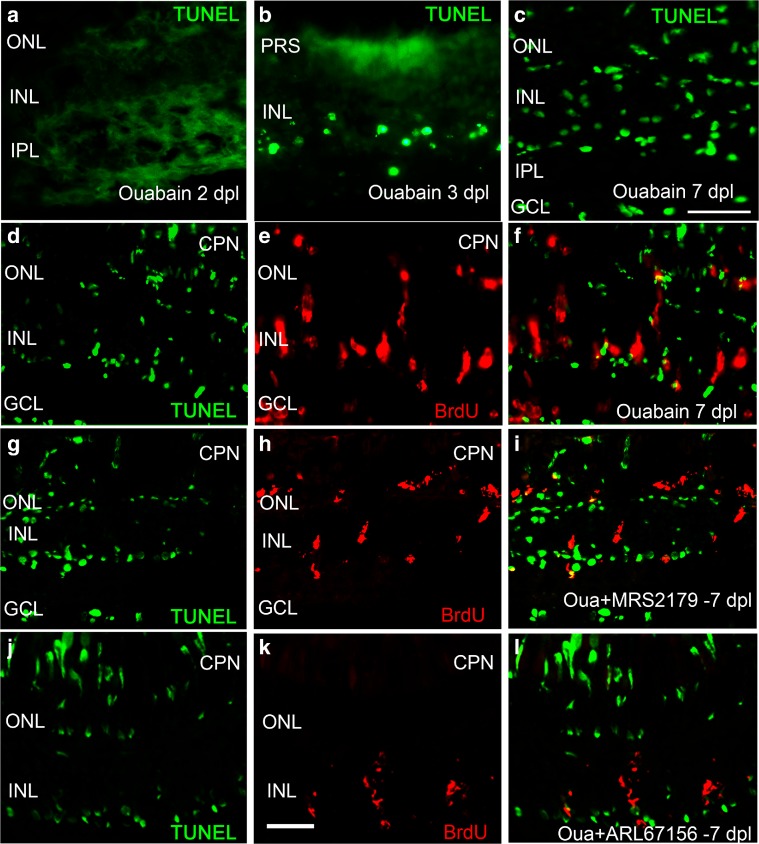
This set of assays was designed to detect apoptotic cell death at different times after injury and to examine whether purinergic antagonists or ecto-ATPase inhibitors could affect apoptotic cell death and hence tissue recovery. Figure 10 shows apoptotic cell death in retinas 2, 3, and 7 dpl (Fig. 10a–c). Apoptotic cell death was remarkably low on days 2 to 3 after injury with ouabain. Retina sections examined 7 days after injury presented an enhanced number of BrdU- and TUNEL-labelled nuclei (Fig. 10d–f) which were only partially coincident.
Fig. 10.
Apoptotic cell death and BrdU-positive nuclei double-labelling assays in injured retinas. Apoptotic fluorescein-labelled nuclei detected by the method of TUNEL (in green) 2, 3, and 7 days after lesion (dpl) with 6 μM ouabain are shown in a–c. Nuclei labelled with 5-bromo-2′-deoxyuridine (BrdU)-positive nuclei appear red in e, f, h, i, k, l. Retinas were isolated at 7 dpl (c, d, g, j) and treated with BrdU for 3 days beginning on day 4 after injury. Representative images of retina sections treated with MRS2179 (a specific antagonist of P2RY1) for 3 days starting on day 4 after injury (4–7 dpl) are shown in g–i. Representative images of retina sections treated with ARL67156 (an ecto-ATPase activity inhibitor) for 6 days starting on the first day after damage (1–7 dpl) are shown in the last row (j–l). f, i, l The merger of the green and red images. CPN cone photoreceptor nuclei, PRS photoreceptor segments, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, Oua ouabain. Scale bars in c, k 20 μm
Then, we treated retinas with the P2RY1 antagonist and the ecto-ATPase inhibitor that had caused further damage to the retinal tissue and prevented the physiological recovery observed 7 days after damage, as shown in Fig. 1. A treatment for the last 3 days (4–7 dpl) with the P2RY1 antagonist MRS2179 or for 6 days after injury (1–7 dpl) with ARL67156 did not modify the number of TUNEL-labelled nuclei identified at 7 dpl. In spite of that, the number of BrdU-positive nuclei was significantly lower when compared to control-injured retinas in the absence of the antagonist (Fig. 10g–i) or the ecto-ATPase inhibitor (Fig. 10j–l).
Discussion
The findings reported here in the zebrafish retina demonstrated that specific purinergic signals are timely induced after damage and further support a pivotal role of P2RY1 and extracellular nucleotides particularly ADP, as well as E-NTPDase activity, in regulating pluripotency gene expression in multipotent progenitor Müller glia and derived neuronal progenitor cells. The blocking of P2RY1 inhibited cell cycle progression of reprogrammed Müller glia as well as Müller glia-derived progenitor cell mitotic amplification fundamental for obtaining a sufficient number of progenitor cells that migrate and differentiate for retina repair and sight recovery.
Endogenous purinergic signals regulate tissue recovery in the injured retina
An approximate intravitreous concentration of 6 μM ouabain caused cell death in choroid, pigmented epithelium, as well as all neural retina layers; however, a more prominent damage was observed in the inner retina. Histological analysis at the time of maximal proliferation of derived progenitor cells (7 dpl) revealed apyrase and purinergic receptor antagonist effects on morphological recovery of the retinal tissue. The specific antagonist of P2RY1, the ecto-ATPase inhibitor, and apyrase treatments negatively affected tissue recovery. Blocking of other purinergic membrane receptors examined, such as P2RY12 and P2RY13 (with cangrelor or MRS2211); P2X1, P2X2, P2X3, and P2RY11 (with NF-110 + NF-157); or P1R (8-SPT), did not strongly affect the cell number and lamination of the retinal tissue when compared to control-injured retinas at the same interval as assessed by histological analysis. Nevertheless, we cannot rule out other effects of these purinergic receptors and their antagonists at different intervals after injury such as progenitor cell migration, protection, vasculogenesis, differentiation, and synaptogenesis that were not particularly evaluated in the present work and deserve further investigation.
It has been described that when multiple retinal cells die as a consequence of an injury with low (with cell death in the inner retina only) [11] or high (with an almost complete destruction of the laminated tissue) doses of ouabain [56], a peak of neuronal progenitor cell proliferation was always observed 7 days after injury. In our assay conditions with an intermediate dose of ouabain, we have observed likewise.
Several P2 receptors are expressed by cells of the neural retina of zebrafish
We have assessed in this study the transcriptional expression of different P2 receptors that have been described in the central nervous system of vertebrate from embryonic stages to adulthood [57]. We detected P2RY1, P2RY12, and P2RY13 mRNAs which are principally although not exclusively ADP-activated receptors [50]. On the other hand, mRNA of purinergic receptors which are chiefly activated by ATP (P2RX1, P2RX2, P2RX7, P2RY11, and P2RY2) was also detected. In contrast, P2RX3a mRNA was not detected in the adult retina in agreement with previous reports which indicated that P2RX3 is transiently expressed during embryonic development in the nervous system and declines in postnatal stages in rodents and zebrafish larvae [58, 59]. Injury with ouabain failed to induce P2RX3a transcriptional expression in the adult retina of zebrafish. The relative transcriptional expression of P2RY1 was unchanged at 48 hpl but showed a strong upregulation at both 65 hpl, i.e. during the phase of mitotic activity of multipotent progenitor Müller glia and early derived progenitors, and the time of peak proliferation (7 dpl) of Müller glia-derived progenitor cells. Other purinergic receptor such as P2X7 or P2Y12 transcriptional expression was not modified by the ouabain injury. P2RY13 and P2RY12 like P2RY1 are mainly activated by extracellular ADP and share intracellular signalling pathways via an inhibitory protein, G [50]. Cangrelor is an antagonist of P2RY12 and either an antagonist or a partial agonist of P2RY13 [48, 60]. Neither cangrelor in this work nor MRS2211, a potent inhibitor for P2RY13 [42 and further results not shown], modified the number of proliferative cells after injury, suggesting that P2RY12 and P2RY13 were not involved in controlling progenitor cell mitotic activity. On the other hand, P2RX7 mRNA levels were not modified by injury and a potent and specific antagonist of P2RX7, which blocked the action of the injury-induced endogenous extracellular ATP on these receptors from days 0 to 4 after damage with ouabain, did not show a significant effect on cell proliferation assessed 4 dpl. Nevertheless, we cannot rule out P2RX7 effects on either cell survival or death, including progenitor cells, depending on the extracellular ATP levels at different intervals after damage [61] or in other types of lesion that were not investigated in the present work.
The expression of P2Y1 receptors is regulated by their biological ligands
ADPβS at relatively low doses, an ADP non-hydrolysable analogue, induced p2ry 1 gene transcription. In contrast, a higher dose of ATPγS, an ATP non-hydrolysable analogue, did not change p2ry 1 gene expression. This finding suggested a mechanism by which the upregulation of P2RY1 might occur in response to damage. In fact, given that the intracellular ATP concentration overcomes in three orders the extracellular one, dying and stimulated cells release their content to the extracellular milieu and likely the concentration of extracellular ATP as well as ADP significantly increases after damage. Furthermore, extracellular ATP is converted to ADP by NTPDase2 (and in a lesser degree, by NTPDase3) activity and extracellular ADP binding to purinergic receptors may induce p2ry 1 gene transcriptional expression. Because we have further shown that the antagonist MRS2179 provoked a significant decrease of the P2RY1 mRNA and protein level 65 hpl and 7 dpl, respectively, we may conclude that endogenous extracellular ADP induces its own receptor synthesis after injury. On the other hand, apyrase did not modify the transcriptional expression of this receptor which might indicate that physiological low levels of extracellular nucleotides do not significantly modify p2ry 1 gene expression in uninjured retinas. In this regard, it has been described that treatments with apyrase did not cause damage in intact retinas [40].
P2RY1 protein was expressed and induced by injury in the adult neural zebrafish retina
The antibody used in this work detected as expected a clear band of 63 kDa in rat brain homogenates. A previous study has also described the detection of a 63-kDa band, with another polyclonal antibody, in rat and human brain membrane homogenates and in cell cultures expressing a cloned human P2RY1 [62]. We also detected a single band of 63 kDa in the brain and neural retina of zebrafish. P2RY1 protein was mainly expressed in the IPL and GCL as well as blood vessels in intact retinas whereas the expression of this receptor in the INL, OPL, and photoreceptor layer gradually increased from 80 h to 7 days after damage, in concert with the increments of this receptor mRNA relative expression levels (65 hpl and 7 dpl). Between the third and fourth day after damage (80 hpl), P2RY1 expression was diminished in the IPL which was concurrent with inner retina cell death and synaptic disorganization after injury. P2RY1 expression showed a maximal enhancement throughout all retinal layers including cone photoreceptor segments and the OLM region at the time of peak progenitor cell proliferation.
Eighty hours after lesion, a few BrdU-positive nuclei detected in the INL and remaining of the GCL and fibre layer co-localized with P2RY1 stain. The identity of the proliferative nuclei detected in the inner retina is uncertain, but they may represent mitotically active microglial and endothelial cell nuclei as well as some Müller glia-derived progenitor cells. Seven days after lesion, the enhanced expression of P2RY1 in the INL was observed in close proximity to the Müller glia-derived progenitor proliferative nuclei in the mature retina. In the CMZ at 7 dpl, we have observed co-localization of P2RY1-IR surrounding nuclei likely representing slow dividing multipotent cell progenitors due to their proximity to the RPE and BV within the proliferative ciliary margin. It was difficult to establish whether the stain for P2RY1 in plasma membranes and G2 phase or postmitotic nuclei belonged to the same cell because both markers, even if they were detected in the same cell, could be placed considerably apart. So, P2RY1 could be localized in Müller cell somatic processes away from their nuclei, plasma membrane extensions of migrating precursors, or neurites of differentiating neurons. Even so, P2RY1 was ubiquitously expressed in multiple retina cells and layers after injury and could regulate progenitor cell mitotic activity indirectly throughout other extracellular signals released by cells expressing these purinergic receptors, which were activated by ADP or ATP through paracrine mechanisms.
P2RY1-induced progenitor cell mitotic activity in the early and late intervals of the proliferative phase after injury
Treatments with the specific antagonist MRS2179 provoked inhibition of the proliferative activity after injury together with a significant decrease of P2RY1 mRNA and protein expression levels at 65 hpl and 7 dpl, respectively. MRS2179 treatment effects after injury were observed at two intervals, in which different kinds of progenitor cells become mitotically active. The specific antagonist of P2RY1 likely by inhibiting progenitor Müller glia and early derived neural progenitor’s mitotic divisions for 0–3 days after ouabain injury abolished derived progenitor cells’ amplification peak normally observed 7 dpl. Likewise, the antagonist abolished the maximal proliferative activity after injury, when it was present in the vitreous chamber for 4–7 dpl at the time progenitor Müller glia mitotic activity had likely ceased and Müller glia-derived progenitor cells amplified to reach an appropriate number for retina repair [44].
Injury-induced overexpression of P2RY1 could indicate its participation as a survival signal avoiding excessive cell death during the amplification phase (5–7 dpl). However, with an injury with ouabain at the level performed here, TUNEL assay revealed that neither MRS2179 nor ARL67156 treatment provoked a higher degree of apoptosis than that was caused by the injury per se. As aforementioned, we have described that MRS2179 provoked a strong inhibition of the injury-induced expression of P2RY1, suggesting that cell apoptosis was not significantly affected by the strong downregulation of the receptor. These evidences indicate that P2RY1 has no a crucial role in differentiating postmitotic cell survival. Nonetheless, our findings suggesting that MRS2179 caused cell cycle arrest quite early after damage might indicate that arrested progenitors went through an apoptotic process. Therefore, the relative low number of TUNEL-positive cells observed on day 7 after injury may be due to the fact that retinas treated with MRS2179 had a significantly lower number of any kind of cells. This was indeed observed in histological or FICC assessments.
We have previously demonstrated that ADPβS caused a low but significant increase in progenitor cell proliferation in the dorsal region of intact retinas, with no evidence of tissue damage, which was blocked by MRS2179 [42]. Moreover, MRS2179 caused a decrement of the proliferative activity in the CMZ of intact retinas [40]. Our previous and present results suggest that ADP, at higher than physiological levels, acting on P2RY1 increases progenitor cell proliferation in the mature retina. It has been recently described that ADP acting on P2RY1 regulates cell cycle cyclins in retinal progenitor cells of developing rats without affecting progenitor cell survival [24]. Nevertheless, it is likely that both injury-induced progenitor and differentiated cells survive further in the presence of other extracellular nucleotides and nucleosides [33, 63]. Noteworthy, a treatment with apyrase for 6 days after injury, which provokes a complete hydrolysis of extracellular nucleotides, resulted in an almost acellular and unlaminated tissue (see Fig. 1) by preventing progenitor cell proliferation and/or promoting cell death (see Fig. 5 and [42]). In this regard, it has been extensively reported that extracellular nucleotides are crucial to restore retina cell volume increase, which could be provoked by an osmotic imbalance caused by the ouabain treatment in our study. Nucleotides are essential for limiting an excessive inflammatory activation of microglia and the gliotic response of macroglial and Müller cells in the mammalian retina. So, total hydrolysis of extracellular nucleotides by apyrase might also increase the gliotic response preventing multipotent Müller glia mitotic activation in regenerating zebrafish retinas [33, 64].
Injury with ouabain and purinergic signalling regulated Müller cell gliotic reactivity and activated mitotic division
In this study, we have observed that GFAP was upregulated in Müller glia evidencing gliotic reactivity at 65 hpl. As previously shown, the gliotic response was limited, compared to the one observed in the mammalian retina, which has been described as a feature of retinas with regenerative capacity [64]. Limited gliosis of Müller glia may help to promote neuroprotective mechanisms after injury [33]. In the presence of the P2RY1 antagonist, gliosis in Müller cells in response to injury was prominent but restricted to the inner retina.
Nuclei that entered the S phase and progressed through G2 phase between 66 and 70 hpl, like it was determined with a 4-h pulse of BrdU, were detected neatly aligned in the middle of the INL and several of them closely co-localized with GFAP most likely representing postmitotic nuclei of progenitor Müller glia [10, 12, 44]. The more numerous fusiform-shaped BrdU-labelled nuclei formed clusters that were also associated with Müller glia processes across the INL; these nuclei likely represented neural progenitors derived from multipotent Müller glia first divisions. In the presence of relatively low doses of the specific antagonist of P2RY1, a lower number of scattered and spherically shaped nuclei were labelled with BrdU, suggesting an S phase entry with cell cycle arrest. Almost none of these nuclei showed an apparent co-localization with GFAP-positive processes across the INL, indicating a partial but significant inhibition of progenitor Müller glia mitotic activity. The presence of the antagonist also prevented the formation of progenitor cell clusters associated to progenitor Müller glia. Most likely, P2RY1 participates in cell cycle progression to mitotic division of both multipotent Müller glial cells and their derived neural progenitor cells.
Ecto-nucleotidase activity was necessary for Müller glia-derived progenitor cell amplification
Extracellular ATP is derived from neural, glial, or pigmented epithelium cells and the vitreous and/or retinal vessels in physiological or pathological conditions. The experiments using ARL67156 indicated that an ecto-ATPase activity, including that of the plasma membrane E-NTPDases [53], were necessary to observe a maximum in Müller glia-derived progenitor cell proliferation. Treatment with ARL67156 or POM (a more potent although less specific inhibitor of E-NTPDase activity), delivered early at the time progenitor Müller glia, was activated and did not affect the number of Müller glia-derived progenitor cells at the time of peak proliferation. Hydrolysis of extracellular nucleotides may be critical for obtaining an adequate amount of daughter progenitor cells for retina repair. The lack of effect of the ecto-ATPase inhibitor early after injury might be due to a sufficient amount of ADP and ATP to activate P2RY1 had been released by dying cells. The ecto-nucleotidase inhibitor was withdrawn at 72 h after injury, and retinas were allowed to recover for 4 days. Accumulated ATP together with an increased expression of NTPDase2 and, later on, NTPDase3 may have boosted the production of extracellular ADP which, by acting through an increased number of P2RY1, and provoked a delayed activation of progenitor Müller glia and/or Müller glia-derived progenitor cell amplification. In this regard, we have shown here that NTPDase2 and NTPDase3 transcriptional expression was induced early and late after injury, respectively. We have also described an early and lasting increment of NTPDase1 transcriptional expression after injury, which may serve as a protective mechanism for neurons from an excess of ATP, which is released by dying cells and by ATP-activated glial cells in a positive feedback loop, as it was demonstrated in tissue injuries in the mammalian CNS [65].
Purinergic signals upregulated lin28a and ascl1a expression in intact or injured retinas
Our results indicated that an ADPβS treatment for 3 days was able to induce the transcriptional expression of lin28a and ascl1a genes in the mature region of uninjured retinas. This finding suggests that injury levels of endogenous extracellular ADP may regulate the expression of lin28a, possibly by increasing ascl1a levels, which are necessary for progenitor Müller glia cell cycle re-entry. These genes are also expressed by Müller glia-derived progenitors in the zebrafish retina after injury [7, 12].
Interestingly, injury-induced lin28a and ascl1a expression that was observed 65–68 h after damage in the mature portion of the adult retina, i.e. during the interval of progenitor Müller glia reprogramming, was significantly decreased by antagonizing P2RY1. These results indicate that endogenous extracellular nucleotides, likely ADP or with less affinity ATP acting on P2RY1, induce the transcription of ascl1a and, in consequence, of lin28a genes in response to injury.
In conclusion, injured retinas exhibited the upregulation of P2RY1 and E-NTPDase expression; also, P2RY1 mRNA levels can be enhanced by an ADP analogue in intact retinas. P2RY1 mRNA and protein level increments after injury can be abolished by antagonizing the same receptors. Furthermore, progenitor cell nuclei, some of them in G2 phase, as well as other postmitotic cells in the mature layers and CMZ expressed P2RY1, which were progressively upregulated after damage with a maximum at the time of peak progenitor proliferation. Extracellular nucleotide hydrolysis by E-NTPDases was important for Müller glia-derived progenitor cell proliferation, whereas P2RY1 activation by ADP, whose extracellular concentration was probably increased after injury, was relevant for progenitor Müller glia mitotic activation as well as Müller glia-derived progenitor cell amplification. P2RY1 induction of their own synthesis and progenitor cell proliferation after damage were likely mediated by an increment in the intracellular Ca2+ concentration caused by the activation of Gq protein coupled to P2RY1. The pluripotency and proneural transcription factor genes lin28a and ascl1a, respectively, have been characterized as key regulators of progenitor Müller glial cell reprogramming for retina repair. Remarkably, ADPβS by itself was able to stimulate the transcriptional expression of the pluripotency genes whereas blocking of P2RY1 significantly but not completely prevented the injury-induced transcription of both genes.
In the injured or diseased mammalian retina, purinergic signals stimulate the gliotic response and proliferation of retinal glial cells for healing and tissue protection. However, scar formation as a consequence of an excessive gliotic response has adverse consequences for tissue function. Purinergic signalling together with growth factors induce Müller cell proliferation in proliferative retinopathies in humans. Our findings are in concert with previous reports suggesting that pharmacological blocking of P2RY1 may represent an important therapeutic strategy to limit the excessive proliferative activity of Müller glia that occurs in proliferative retinopathies [33].
Electronic supplementary material
Representative images of retina sections depicting proliferative nuclei that incorporated 5-bromo-2′-deoxyuridine (BrdU-positive nuclei, in green) in a period of 24 h. Eyes were injected with saline solution (uninjured control (a)) or ouabain (b-d) within the vitreous cavity on day 0. Then, all zebrafish received a single injection within the vitreous chamber of 20 μg/μl of BrdU 24 h before euthanasia. Different groups of zebrafish were euthanized 65 h after lesion (hpl) or 5, 6, 7 or 20 days after lesion (dpl). Bar graphs in (e) show the normalized number of BrdU-positive nuclei per retina section (i.e. the number of labelled nuclei per retina section in each interval divided by the average number of BrdU-positive nuclei at 7 dpl) expressed as mean ± SD; n: 5–6 zebrafish per group. *p < 0.05, **p < 0.01, ***p < 0.001 in Newman-Keuls Multiple Comparison Test after ANOVA. PRS: photoreceptor segments; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer. Scale bars in (b and d): 40 μm. (GIF 98.3 kb)
Acknowledgments
We thank Verónica Pastor from Dr. Bernabeu’s laboratory for providing the materials and helping with the protocols as well as the thoughtful discussions. We are very grateful to AstraZeneca for kindly providing ARC69931MX. We specially thank Dr. Nora Calcaterra for providing the 36-hpf zebrafish embryos and Dr. Pablo Schwarzbaum for providing some of the purinergic drugs and his expertise in the purinergic signalling field.
Compliance with ethical standards
Grant sponsors
The study received a grant from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 2012-2014 No. 079, UBACYT 2015-2017 No. 061), University of Buenos Aires, Buenos Aires, Argentina.
Conflict of interest
Matías P. Medrano declares that he has no conflict of interest.
Claudio A. Bejarano declares that he has no conflict of interest.
Ariadna G. Battista declares that she has no conflict of interest.
Graciela D. Venera declares that she has no conflict of interest.
Ramón O. Bernabeu declares that he has no conflict of interest.
Maria Paula Faillace declares that she has no conflict of interest.
Ethical approval
The Committee on Animal Research at the University of Buenos Aires, CICUAL, approved the protocols for ethical animal use and care.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-017-9572-5) contains supplementary material, which is available to authorized users.
References
- 1.Johns PR, Fernald RD. Genesis of rods in teleost fish retina. Nature. 1981;293:141–142. doi: 10.1038/293141a0. [DOI] [PubMed] [Google Scholar]
- 2.Reh TA, Fischer AJ. Stem cells in the vertebrate retina. Brain Behav Evol. 2001;58:296–305. doi: 10.1159/000057571. [DOI] [PubMed] [Google Scholar]
- 3.Fernald RD. Teleost vision: seeing while growing. J Exp Zool Suppl. 1990;5:167–180. doi: 10.1002/jez.1402560521. [DOI] [PubMed] [Google Scholar]
- 4.Knight JK, Raymond PA. Retinal pigmented epithelium does not transdifferentiate in adult goldfish. J Neurobiol. 1995;27:447–456. doi: 10.1002/neu.480270402. [DOI] [PubMed] [Google Scholar]
- 5.Lombardo F. Time course and localization of mitosis during the regeneration of the retina of an adult teleost. Accademia Lincei-Renicontt I Scienze Fisicali Matematiche e Naturale. 1972;53:323–327. [Google Scholar]
- 6.Mensinger AF, Powers MK. Visual function in regenerating teleost retina following surgical lesioning. Vis Neurosci. 2007;24:299–307. doi: 10.1017/S0952523807070265. [DOI] [PubMed] [Google Scholar]
- 7.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Müller glia dedifferentiation and retina regeneration via a Lin-28-dependent, let-7 miRNA signaling pathway. Nature Cell Biol. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond PA, Reifler MJ, Rivlin PK. Regeneration of goldfish retina: rod precursors are a likely source of regenerated cells. J Neurobiol. 1988;19:431–463. doi: 10.1002/neu.480190504. [DOI] [PubMed] [Google Scholar]
- 9.Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish Danio rerio retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::AID-NEU1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that functions as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman D. Müller glia reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Julian D, Ennis K, Korenbrot JI. Birth and fate of proliferative cells in the inner nuclear layer of the mature fish retina. J Comp Neurol. 1998;394:271–282. doi: 10.1002/(SICI)1096-9861(19980511)394:3<271::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Otteson DC, D’Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- 15.Faillace MP, Julian D, Korenbrot JI. Mitotic activation of proliferative cells in the inner nuclear layer of the mature fish retina: regulatory signals and molecular markers. J Comp Neurol. 2002;451:127–141. doi: 10.1002/cne.10333. [DOI] [PubMed] [Google Scholar]
- 16.Cameron DA, Gentile KL, Middleton FA, Yurco P. Gene expression profiles of intact and regenerating zebrafish retina. Mol Vis. 2005;11:775–791. [PubMed] [Google Scholar]
- 17.Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013;11:611–620. doi: 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassen SC, Ramanan V, Montgomery JE, T Burket C, Liu CG, Vithelic TS, Hyde DR. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan SA, Barthel LK, Largent BL, Raymond PA. A goldfish notch-3 homologue is expressed in neurogenic regions of embryonic, adult, and regenerating brain and retina. Dev Genet. 1997;20:208–223. doi: 10.1002/(SICI)1520-6408(1997)20:3<208::AID-DVG4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Zhao XF, Ellingsen S, Fjose A. Labelling and targeted ablation of specific bipolar cell types in the zebrafish retina. BMC Neurosci. 2009;10:107. doi: 10.1186/1471-2202-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.França GR, Freitas RC, Ventura AL. ATP-induced proliferation of developing retinal cells: regulation by factors released from postmitotic cells in culture. Int J Dev Neurosci. 2007;25:283–291. doi: 10.1016/j.ijdevneu.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Martins RAP, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 2008;1192:37–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp. 2006;276:113–128. doi: 10.1002/9780470032244.ch10. [DOI] [PubMed] [Google Scholar]
- 24.de Almeida-Pereira L, Magalhães CF, Repossi MG et al (2016) Adenine nucleotides control proliferation in vivo of rat retinal progenitors by P2Y1 receptor. Mol Neurobiol. doi:10.1007/s12035-016-0059-0 [DOI] [PubMed]
- 25.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Burnstock G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. BioEssays. 2012;34:218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- 27.Dahl G (2015) ATP release through pannexon channels. Phil Trans R Soc B Biol Sci. doi:10.1098/rstb.2014.0191 [DOI] [PMC free article] [PubMed]
- 28.Pafundo DE, Chara O, Faillace MP, Krumschnabel G, Schwarzbaum PJ. Kinetics of ATP release and cell volume regulation of hyposmotically challenged goldfish hepatocytes. Am J Physiol Regul Integr Comp Physiol. 2008;294:R220–R233. doi: 10.1152/ajpregu.00522.2007. [DOI] [PubMed] [Google Scholar]
- 29.Burnstock G, Ulrich H. Purinergic signalling in embryonic and stem cell development. Cell Mol Life Sci. 2011;68:1369–1394. doi: 10.1007/s00018-010-0614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman EA (2015) Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Phil Trans R Soc B Biol Sci. doi:10.1098/rstb.2014.0195 [DOI] [PMC free article] [PubMed]
- 31.Hunsucker SA, Mitchell BS, Spychala J. The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol Ther. 2005;107:1–30. doi: 10.1016/j.pharmthera.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? The vascular system. Gen Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 33.Reichenbach A, Bringmann A. Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology. 2016;104:194–211. doi: 10.1016/j.neuropharm.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Massé K, Bhamra S, Eason R, Dale N, Jones EA. Purine-mediated signalling triggers eye development. Nature. 2007;449:1058–1062. doi: 10.1038/nature06189. [DOI] [PubMed] [Google Scholar]
- 35.Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 36.Sanches G, de Alencar LS, Ventura AL. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Int J Dev Neurosci. 2002;20:21–27. doi: 10.1016/S0736-5748(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 37.Sugioka M, Zhou WL, Hofmann HD, Yamashita M. Involvement of P2 purinoceptors in the regulation of DNA synthesis in the neural retina of chick embryo. Int J Dev Neurosci. 1999;17:135–144. doi: 10.1016/S0736-5748(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 38.Sanderson J, Dartt DA, Trinkaus-Randall V, Pintor J, Civan MM, Delamere NA, Fletcher EL, Salt TE, Grosche A, Mitchell CH. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Müller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res. 2014;127:270–279. doi: 10.1016/j.exer.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricatti MJ, Alfie LD, Lavoie EG, Sevigny J, Schwarzbaum PJ, Faillace MP. Immunocytochemical localization of NTPDases1 and 2 in the neural retina of mouse and zebrafish. Synapse. 2009;63:291–307. doi: 10.1002/syn.20605. [DOI] [PubMed] [Google Scholar]
- 40.Ricatti MJ, Battista AG, Zorrilla Zubilete M, Faillace MP. Purinergic signals regulate daily S-phase cell activity in the ciliary marginal zone of the zebrafish retina. J Biol Rhythm. 2011;26:107–117. doi: 10.1177/0748730410395528. [DOI] [PubMed] [Google Scholar]
- 41.Maier W, Wolburg H. Regeneration of the goldfish retina after exposure to different doses of ouabain. Cell Tissue Res. 1979;202:99–118. doi: 10.1007/BF00239223. [DOI] [PubMed] [Google Scholar]
- 42.Battista AG, Ricatti MJ, Pafundo DE, Gautier MA, Faillace MP. Extracellular ADP regulates lesion-induced in vivo cell proliferation and death in the zebrafish retina. J Neurochem. 2009;111:600–613. doi: 10.1111/j.1471-4159.2009.06352.x. [DOI] [PubMed] [Google Scholar]
- 43.Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res. 2008;87:433–444. doi: 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140:4510–4521. doi: 10.1242/dev.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ingall AH, Dixon J, Bailey A, Coombs ME, Cox D, McInally JI, Hunt SF, Kindon ND, Teobald BJ, Willis PA, Humphries RG, Leff P, Clegg JA, Smith JA, Tomlinson W. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42:213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- 46.Marteau F, Le Poul E, Communi D, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- 47.Takasaki J, Kamohara M, Saito T, Matsumoto M, Matsumoto S, Ohishi T, Soga T, Matsushime H, Furuichi K. Molecular cloning of the platelet P2T(AC) ADP receptor: pharmacological comparison with another ADP receptor, the P2Y(1) receptor. Mol Pharmacol. 2001;l60:432–439. [PubMed] [Google Scholar]
- 48.Xiang B, Zhang G, Ren H, Sunkara M, Morris AJ, Gartner TK, Smyth SS, Li Z. The P2Y12 antagonists, 2MeSAMP and cangrelor, inhibit platelet activation through P2Y12/Gi-dependent mechanism. PLoS One. 2012;7(12):e51037. doi: 10.1371/journal.pone.0051037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobson KA. P2X and P2Y receptors. Tocris Biosci Sci Rev Ser. 2010;33:1–16. [Google Scholar]
- 50.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 51.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westfall TD, Kennedy C, Sneddon P. The ecto-ATPase inhibitor ARL67156 enhances parasympathetic neurotransmission in the guinea-pig urinary bladder. Eur J Pharmacol. 1997;329:169–173. doi: 10.1016/S0014-2999(97)89178-9. [DOI] [PubMed] [Google Scholar]
- 53.Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Müller CE, Iqbal J, Baqi Y, Zimmermann H, Röllich A, Stephan H. Polyoxometalates – a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg Med Chem Lett. 2006;16:5943–5947. doi: 10.1016/j.bmcl.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula. J Mol Med. 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 56.Sherpa T, Fimbel SM, Mallory DE, Maaswinkel H, Spritzer SD, Sand JA, Hyde DR, Stenkamp DL. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 2008;68:166–181. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulrich H, Abbracchio MP, Burnstock G. Extrinsic purinergic regulation of neural stem/progenitor cells: implications for CNS development and repair. Stem Cell Rev. 2012;3:755–767. doi: 10.1007/s12015-012-9372-9. [DOI] [PubMed] [Google Scholar]
- 58.Norton WH, Rohr KB, Burnstock G. Embryonic expression of a P2X(3) receptor encoding gene in zebrafish. Mech Dev. 2000;99:149–152. doi: 10.1016/S0925-4773(00)00472-X. [DOI] [PubMed] [Google Scholar]
- 59.Cheung KK, Chan WY, Burnstock G. Expression of P2X purinoceptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience. 2005;133:937–945. doi: 10.1016/j.neuroscience.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 60.Srinivasan S, Mir F, Huang JS, Khasawneh FT, Lam SC, Le Breton GC. The P2Y12 antagonists, 2-methylthioadenosine 5′-monophosphate triethylammonium salt and cangrelor (ARC69931MX) can inhibit human platelet aggregation through a Gi-independent increase in cAMP levels. J Biol Chem. 2009;284:16108–16117. doi: 10.1074/jbc.M809780200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue B, Xie Y, Xue Y, Hu N, Zhang G, Guan H, Ji M. Involvement of P2X7 receptors in retinal ganglion cell apoptosis induced by activated Müller cells. Exp Eye Res. 2016;153:42–50. doi: 10.1016/j.exer.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Moore D, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y1 purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol. 2000;421:374–384. doi: 10.1002/(SICI)1096-9861(20000605)421:3<374::AID-CNE6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 63.dos Santos-Rodrigues A, Pereira MR, Brito R, de Oliveira NA, Paes-de-Carvalho R. Adenosine transporters and receptors: key elements for retinal function and neuroprotection. In: Litwack G, editor. Vitamins & Hormones. New York: Academic; 2015. pp. 487–523. [DOI] [PubMed] [Google Scholar]
- 64.Than-Trong E, Bally-Cuif L. Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia. 2015;63:1406–1428. doi: 10.1002/glia.22856. [DOI] [PubMed] [Google Scholar]
- 65.Brisevac D, Bajic A, Bjelobaba I, et al. Expression of ecto-nucleoside triphosphate diphosphohydrolase1-3 (NTPDase1–3) by cortical astrocytes after exposure to pro-inflammatory factors in vitro. J Mol Neurosci. 2013;51:871. doi: 10.1007/s12031-013-0088-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative images of retina sections depicting proliferative nuclei that incorporated 5-bromo-2′-deoxyuridine (BrdU-positive nuclei, in green) in a period of 24 h. Eyes were injected with saline solution (uninjured control (a)) or ouabain (b-d) within the vitreous cavity on day 0. Then, all zebrafish received a single injection within the vitreous chamber of 20 μg/μl of BrdU 24 h before euthanasia. Different groups of zebrafish were euthanized 65 h after lesion (hpl) or 5, 6, 7 or 20 days after lesion (dpl). Bar graphs in (e) show the normalized number of BrdU-positive nuclei per retina section (i.e. the number of labelled nuclei per retina section in each interval divided by the average number of BrdU-positive nuclei at 7 dpl) expressed as mean ± SD; n: 5–6 zebrafish per group. *p < 0.05, **p < 0.01, ***p < 0.001 in Newman-Keuls Multiple Comparison Test after ANOVA. PRS: photoreceptor segments; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer. Scale bars in (b and d): 40 μm. (GIF 98.3 kb)