Abstract
Cell signaling mediated by P2X7 receptors (P2X7R) has been suggested to be involved in epileptogenesis, via modulation of intracellular calcium levels, excitotoxicity, activation of inflammatory cascades, and cell death, among other mechanisms. These processes have been described to be involved in pilocarpine-induced status epilepticus (SE) and contribute to hyperexcitability, resulting in spontaneous and recurrent seizures. Here, we aimed to investigate the role of P2X7R in epileptogenesis in vivo using RNA interference (RNAi) to inhibit the expression of this receptor. Small interfering RNA (siRNA) targeting P2X7R mRNA was injected into the lateral ventricles (icv) 6 h after SE. Four groups were studied: Saline-Vehicle, Saline-siRNA, Pilo-Vehicle, and Pilo-siRNA. P2X7R was quantified by western blotting and neuronal death assessed by Fluoro-Jade B histochemistry. The hippocampal volume (edema) was determined 48 h following RNAi. Behavioral parameters as latency to the appearance of spontaneous seizures and the number of seizures were determined until 60 days after the SE onset. The Saline-siRNA and Pilo-siRNA groups showed a 43 and 37% reduction, respectively, in P2X7R protein levels compared to respective vehicle groups. Neuroprotection was observed in CA1 and CA3 of the Pilo-siRNA group compared to Pilo-Vehicle. P2X7R silencing in pilocarpine group reversed the increase in the edema detected in the hilus, suprapyramidal dentate gyrus, CA1, and CA3; reduced mortality rate following SE; increased the time to onset of spontaneous seizure; and reduced the number of seizures, when compared to the Pilo-Vehicle group. Therefore, our data highlights the potential of P2X7R as a therapeutic target for the adjunct treatment of epilepsy.
Keywords: Temporal lobe epilepsy, P2X7 purinergic receptors, RNA interference, Pilocarpine, Hippocampus
Introduction
Epilepsy is a group of neurological diseases with a common phenotypic manifestation, epileptic seizures. Temporal lobe epilepsy (TLE) is the most common form of epileptic conditions in adult humans [1]. Hippocampal sclerosis, gliosis, synaptic reorganization, and granular cell dispersion are the main pathophysiological hallmarks in mesial temporal lobe epilepsy (MTLE) [2, 3]. The epilepsy model induced by intraperitoneal injection with pilocarpine reproduces the main pathophysiological findings related to human MTLE, including the appearance of drug-resistant seizures [4, 5]. The pilocarpine model of epilepsy has been widely used in research and is of major value to identify the molecular and cellular basis and players involved in epileptogenesis [4, 5].
The neuroinflammatory condition triggered by seizures has been considered an important player in hyperexcitability, a modulator of seizure threshold, and has been involved in key processes leading to cell death cascades [6]. New therapy approaches of TLE have been focused on brain inflammation, highlighting the interest of purinergic P2X7 receptors (P2X7R) as potent mediator of neuroinflammation in the epileptic brain [7–18]. P2X7R are trimeric non-selective ligand-gated ion channels activated by ATP, permeable to mono- and divalent cations (permeability: Ca2+ > Na+ > K+), resulting in the rapid depolarization of the membrane [19, 20]. P2X7R activation under pathological condition, i.e., under high level of ATP, occurring during seizures, can induce high level of intracellular calcium concentration, intensifying glutamate and GABA release, promoting pro-inflammatory cytokines release and cell death by apoptosis or necrosis [7, 9, 18, 21–23].
P2X7R have been considered an important therapeutic target in many injuries and neurological disorders, including neuropathic pain [24, 25], spinal cord injury [26], ischemia [27, 28], intracerebral hemorrhage, traumatic brain injury [29], and neurodegenerative diseases such as Alzheimer’s disease [30], Huntington’s disease [31], Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and depression [18], and also in epilepsy [19, 32, 33].
P2X7R levels are increased in the hippocampus of animal models of experimental model of epilepsy and in human patients with TLE [12, 34–37]. There are many studies aimed at elucidating the role of P2X7R in epilepsy using agonists and antagonists. The activation of P2X7R with Bz-ATP (P2X7R agonist) causes microglial activation, enhances TNF-α immunoreactivity, reduces astrocytes, and intensifies seizures expression [19, 38–40]. Conversely, P2X7R blockage promotes anticonvulsant effects and reduces electroencephalographic and behavioral seizures, IL-1β production, microglial activation, recruitment and infiltration of neutrophils into the frontoparietal cortex, and damage resulting from seizure [14, 15, 33, 38, 41–44]. Surprisingly, P2X7R antagonists have been described to exacerbate seizures and enhance cell death in the hippocampal CA3 subfield in the pilocarpine and intraamygdala kainic acid models, but do not change behavioral pattern in the intraperitoneal kainic acid and picrotoxin models of epilepsy [39, 41, 45]. Data obtained from P2X7R knockout mice (Pfizer) [46] are controversial as the severity of their seizures is reduced when compared to wild-type mice [33], but they show increased susceptibility to seizures induced by pilocarpine [45].
The disparities observed in epilepsy studies targeting the pharmacology of P2X7R can be attributed to several factors, including pharmacokinetics and pharmacodynamics of drugs, and the different animals models and experimental conditions used. RNA interference (RNAi) is a method that reduces the expression of the receptor of interest, allowing studying the direct impact in behavioral or morphological parameters besides excluding pharmacological effects [47].
Despite the evidence of a positive association between P2X7R activation, excitability, and excitotoxicity related to epilepsy, the role of purinergic signaling needs to be further clarified. In this study, we used in vivo RNAi intracerebral infusion to reduce the expression of P2X7R in pilocarpine-induced epileptic rat brain, in order to investigate the involvement of this receptor in brain alterations resulting from seizures, i.e., hippocampal damage, edema, and spontaneous and recurrent seizure expression.
Methods
Animals
Adult 2-month-old male Wistar rats weighting 200–250 g were used in this study. The animals were maintained under standard housing conditions, with free access to water and food, with light/dark cycle of 12 h (light from 7 a.m. to 19 p.m.), and with environment temperature kept constant between 21 ± 1 °C.
All experimental procedures were performed under the supervision and with the approval of our internal Ethics Committee (Federal University of São Paulo, CEP N. 0961/10). Animal protocols were conducted in accordance with national and international legislation (Guidelines of the Brazilian College of Animal Experimentation, COBEA; NIH Guide for Care and Use of Laboratory Animals), and the experiments followed the principles outlined in the Basel Declaration [48].
Bilateral cannulas implantation
For intracerebroventricular (icv) administration of small interfering RNA (siRNA) or vehicle, rats were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and fixed to a stereotaxic apparatus. Cannulas (12 mm in length and 0.55 mm in diameter) were stereotaxically implanted into the lateral ventricles (from bregma: anteroposterior, −0.08 mm; mediolateral, ±0.14 mm; dorsoventral, −0.3 mm) and fixed to the skull with dental cement [49]. After 15 days, the rats were injected i.p. with pilocarpine or with saline.
Pilocarpine model protocol
Animals were pretreated with methylscopolamine nitrate (1 mg/kg, subcutaneous (sc), Sigma) to minimize peripheral cholinergic effects of pilocarpine (diarrhea, piloerection, orofacial automatisms associated with salivation, wink, yawning, and vibrissae contractions) [50]. Thirty minutes after pretreatment, they received a systemic injection of pilocarpine hydrochloride (370 mg/kg, i.p., Merck, USA) for SE induction. Five hours after the SE onset, rats were treated with diazepam (1 mg/kg, sc, Santisa) and sodium pentobarbital (30 mg/kg, i.p., Cristália) to minimize behavioral seizures and reduce mortality rate. Six hours after the SE onset, rats received siRNA.
P2X7R siRNA:RVG-9DR preparation and administration in vivo
The siRNA targeting P2X7R (antisense, 5′ [Phos] CUUUAACGUCGGCUUGGGCUC [dT] [dT]-3′, and sense, 5′ [Phos] GCCCAAGCCGACGUUAAAGUA [dT] [dT]-3′) was planned on the basis of Thomas Tuschl protocol [51], and it was synthesized by Sigma Company.
Lyophilized single-stranded RNA oligonucleotides were re-suspended at 100 μM in sterile RNase free water (0.1%, v/v, DEPC in pure water), denatured, aligned (heated at 95 °C for 5 min), and annealed through slow decrease of T °C, obtaining double-stranded siRNA at 50 μM.
Before use, P2X7R siRNA was complexed with RVG-9DR (a peptide sequence derived from rabies virus glycoprotein with nine arginine residues in the carboxy terminal) in a 1:10 M ratio (siRNA:RVG-9DR) to transfect the siRNA, protocol developed by Kumar and colleagues in 2007 [52].
Six hours after the onset of SE, 2 μl containing 0.5 μg of siRNA:RVG-9DR were administered bilaterally icv at a flow rate of 1 μl/min, totaling 1 μg of siRNA per animal. Control rats received the same volume of vehicle instead of siRNA. The dose of siRNA was chosen based on a previous study in rats showing maximal effect in reducing the expression of P2X7R (60%) in the hippocampus, without any sign of neurotoxicity (hind-limb paralysis, vocalization, food intake, or neuroanatomical damage).
Western blotting
Western blot analysis was used for quantifying P2X7R in rat hippocampi. Following the 48-h period of siRNA or vehicle delivery, rats were decapitated and their hippocampi quickly dissected on an iced plate, washed with cold saline to remove blood, weighted, and stored at −80 °C.
Tissues were homogenized in lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 0.02% sodium azide, and 1% protease inhibitor cocktail (Sigma-Aldrich). Protein content was determined by the Bradford method [53].
Samples (40 μg) were mixed with Laemmli buffer containing 0.125 M Tris (pH 6.8), 20% glycerol, 10% beta-mercaptoethanol, 4% SDS, and 0.002% bromophenol blue, and heated at 95 °C for 5 min. Protein was loaded on a 10% SDS-PAGE gel and separated by electrophoresis using a Bio-Rad system with molecular weight standards (Rainbow-GE) at 50 V for 20 min and 90 V for 1 h. Proteins were transferred to a polyvinylidene fluoride membrane (PVDF, Amersham Pharmacia Biotech—Hybond P) at 110 V for 90 min (Mini-Protean, Bio-Rad). Membranes were washed with 0.1 M Tris–Tween 20, blocked with 0.1 M Tris containing 5% skimmed milk, and then incubated with the primary antibody rabbit anti-P2X7 receptor (1:1000, RPA-004—Alomone Labs) at 4 °C overnight. After rinsing, the membranes were incubated with the corresponding secondary antibody (goat anti-rabbit IgG, Calbiochem) at a dilution of 1:2000 in 0.1 M Tris containing 2% fetal bovine serum for 2 h at room temperature. After washing them twice with 0.1 M Tris, membranes were ready for the blocking stage to re-probing with the monoclonal anti-β-actin immunoglobulin (1:2000, A3854—Sigma-Aldrich) used as internal control of the reaction. After rinsing, bands were detected by chemiluminescence using West Pico Super Signal® kit (Thermo Scientific), revealed in photo documentation system (Uvitec, Cambridge) and band intensity was quantified using the UvitecBand software analysis. The P2X7R protein level was reported as normalized β-actin loading control.
Perfusion
Following the 48-h period after the siRNA or vehicle infusion, rats were anesthetized with 90 mg/kg of ketamine and 10 mg/kg of xylazine (i.p.), and subjected to transcardiac perfusion with buffered paraformaldehyde (PFA) to fix the brain. Using a peristaltic pump adjusted to a flow rate of 10 mL/min, saline was perfused during 1 min followed by 250 ml of 4% PFA. The brain tissue was post-fixed in the same solution overnight at 4 °C, and then cryoprotected in 30% sucrose in phosphate buffer during 3 days at 4 °C. The brains were frozen quickly in dry ice and cut into coronal slices with a cryostat (Leica). Slices were used for Fluoro-Jade B staining (40 μm) and for hippocampal volume analysis (50 μm).
Fluoro-Jade B protocol
To study neuronal degeneration, we used the anionic dye Fluoro-Jade B (FJ-B). Brain sections containing areas of interest were fixed on gelatin-coated slides and dried at room temperature. Then, the slides were immersed in absolute ethanol (5 min), 70% ethanol (2 min), and distilled water (2 min) and protected from light, in 0.06% potassium permanganate (15 min), under gentle shaking: distilled water (2 min), 0.01% FJ-B solution plus 0.1% acetic acid (30 min), and distilled water (three times for 2 min). Slides were dried at 50 °C, during 10 min, in a hot plate, dehydrated in absolute ethanol (2 min), cleared in xylene (2 min) and mounted with “Vecta Mount” (Vector), and coverslipped, based on Schmued and Hopkins, 2000 protocol [54].
Volumetric study of hippocampal formation
Volumetric study was performed in slices obtained from a segment of hippocampal formation (−1.72 to −3.84, from bregma) from rats of both groups, 48 h after infusion of siRNA or vehicle [55]. The studied subregions were: hilus, suprapyramidal dentate gyrus, infrapyramidal dentate gyrus, CA1, and CA3.
Brain slices (50 μm) obtained with 300-μm interval were selected and incubated free floating with Hoechst 33,342 (1:10,000, Life Technologies) in PBS containing 0.2% Triton for 3 h under shaking and protected from light. The sections were washed, fixed on gelatin-coated slides, mounted with Fluormont (Abcam), and coverslipped. Images were captured in an epifluorescence microscope (Axioskop 2 plus, Zeiss), with 5× lens using Axiovision software (Zeiss). The image processing and measurements of the regions of interest were made in ImageJ and Fiji-ImageJ (NIH), respectively. The volume of selected brain regions was estimated using Table Curve 2D v5.01 software.
Behavioral analysis
Two days after SE induction, rats were kept in individual acrylic boxes, and video monitored 24 h, for 60 days. The behavioral parameters analyzed were latency to the appearance of the first spontaneous seizure, the number of seizures, and the severity of seizures. The severity of seizures was determined based on behavioral changes according to the scale of Racine: (1) mouth and facial movements, (2) head nodding, (3) forelimb clonus, (4) rearing, and (5) rearing and falling [56].
Statistical analysis
Statistical analysis of the first spontaneous seizure latency and the number of seizures were performed by “t” test (unpaired). In the absence of normality, data were standardized by Z score and in the absence of homogeneity (Levene’s test) were corrected by the Welch test. The protein quantification, hippocampal volume, and severity of seizures were analyzed by two-way ANOVA, followed by Bonferroni post-test. The results with values p < 0.05 were considered significant.
Results
P2X7 protein level
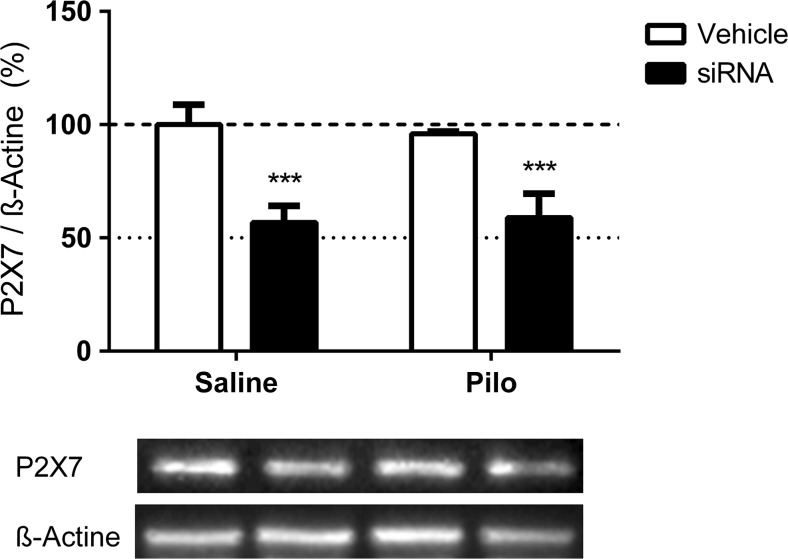
According to our western blotting analysis, the Saline-siRNA and Pilo-siRNA experimental groups showed a 43 and 37% reduction, respectively, in P2X7R protein levels in the hippocampus compared to their respective control vehicle groups (siRNA versus Vehicle, F (1, 16) = 57.71, p < 0.0001; Bonferroni: Saline-Vehicle, p = 0.0002; Pilo-Vehicle, p = 0.0008). No significant differences were observed in the levels of P2X7R when comparing the Pilo and Saline groups (F (1, 16) = 0.03151, p = 0.8613) (Fig. 1).
Fig. 1.
Effect of siRNA in decreasing P2X7R levels, as measured by western blotting. Percentages of P2X7R protein level of Saline-Vehicle, Saline-siRNA, Pilo-Vehicle, and Pilo-siRNA groups. Bars represent the mean ± standard deviation for each group (N = 5/group). Data normalized to β-actin. *** p < 0.001
Neurodegeneration
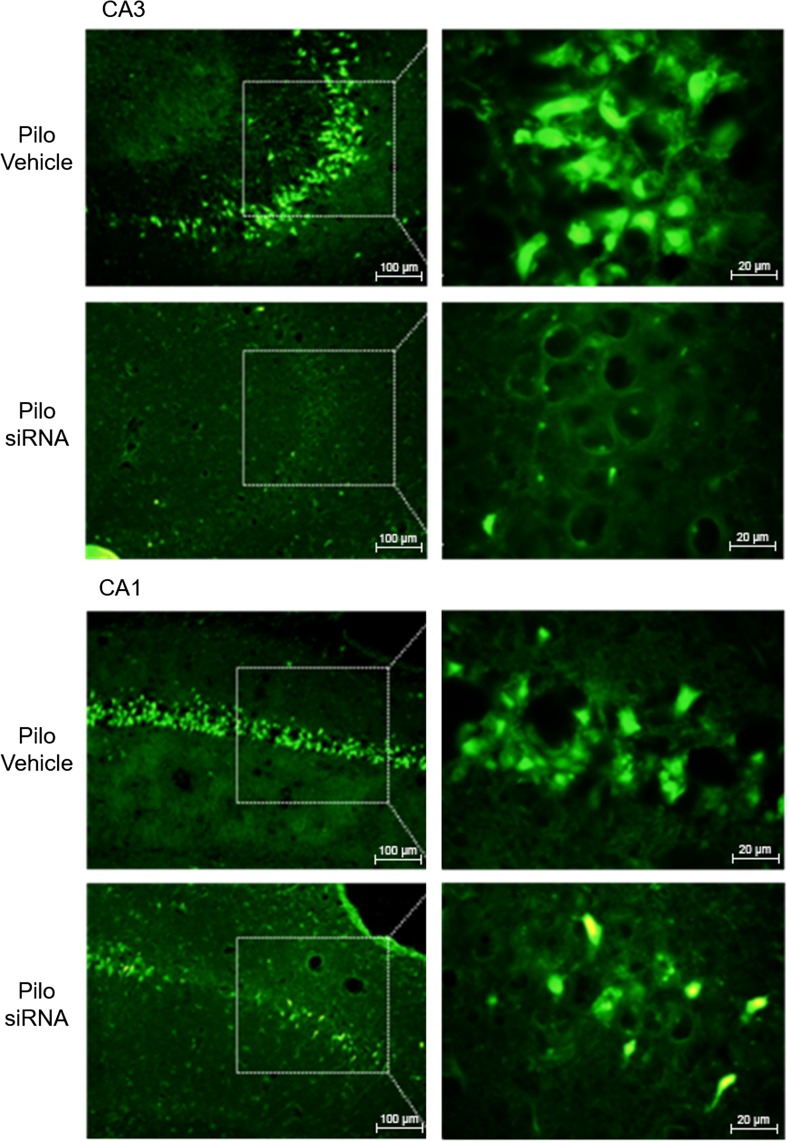
Pilo-siRNA animals showed fewer FJ-B positive cells in CA1 and CA3 pyramidal cell layers as compared with the same regions in Pilo-Vehicle treated rats, suggesting that P2X7R knockdown caused neuroprotection in these areas, especially in CA3 (Fig. 2). Interestingly, no significant differences were observed in the number of FJ-B stained cells in the amygdala, entorhinal cortex, and piriform cortex of the Pilo-siRNA group compared to Pilo-Vehicle treated rats, indicating no neuroprotection by siRNA in these regions (data not shown).
Fig. 2.
Pilocarpine-induced neuronal death in the rat hippocampus, an effect partially protected by P2X7R knockdown. FJ-B staining in CA1 and CA3 of rats injected with pilocarpine after intracerebroventricular P2X7R siRNA (Pilo-siRNA) or vehicle (Pilo-Vehicle) administration. Scale bars 100 and 20 μm
Hippocampal volumetry
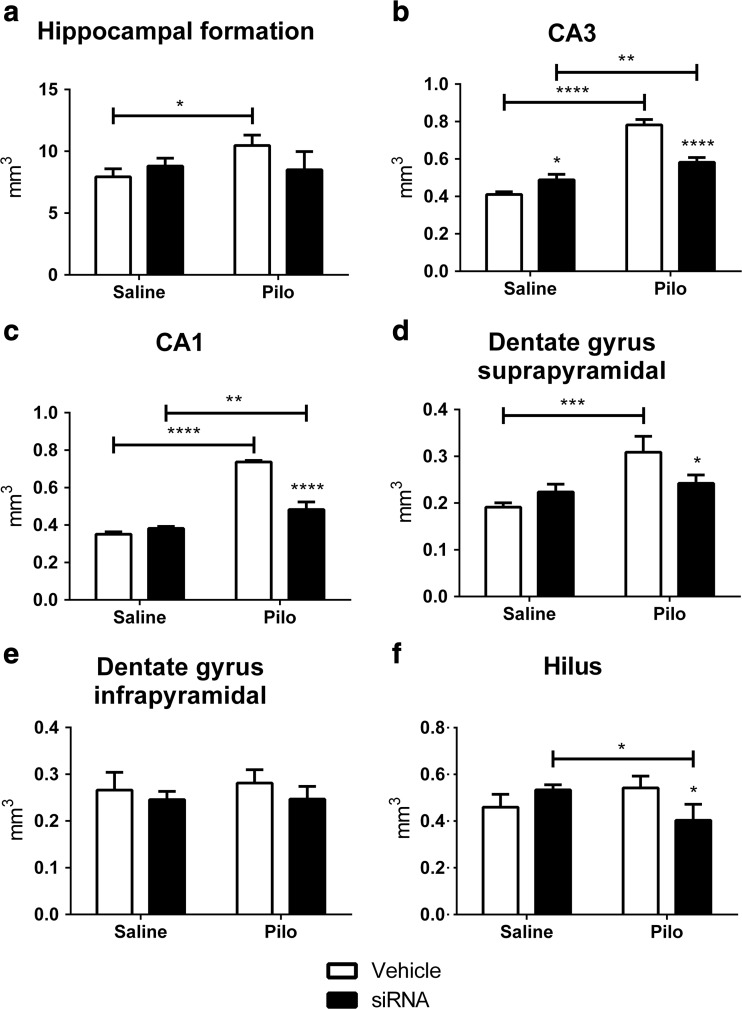
Hippocampal formation of the Pilo-Vehicle group showed an increased volume compared to the Saline-Vehicle group (Bonferroni: p = 0.0249, 32%). The P2X7R siRNA did not change the total hippocampal formation volume (F (1, 8) = 0.9792, p = 0.3514) (Fig. 3a). However, when the volume of each region was analyzed, a significant difference was observed.
Fig. 3.
Changes in hippocampal volume induced by pilocarpine and P2X7R siRNA. a Hippocampal formation, b CA3, c CA1, d dentate gyrus suprapyramidal, e dentate gyrus infrapyramidal, and f hilus of Saline-Vehicle, Saline-siRNA, Pilo-Vehicle, and Pilo-siRNA groups. Bars represent the mean ± standard deviation for each group (N = 3/group). Interactions: a* p < 0.05; b, c**** p < 0.0001; d, f** p < 0.01. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001
The volume of CA3 and CA1 regions was higher in the Pilo-Vehicle and Pilo-siRNA groups compared to their Saline groups (CA3 Bonferroni: Saline-Vehicle, p < 0.0001, 91%; Saline-siRNA, p = 0.0040, 19%; CA1 Bonferroni: Saline-Vehicle, p < 0.0001, 110%; Saline-siRNA, p = 0.0035, 26%). A lower volume in these regions was observed in the Pilo-siRNA group compared to the Pilo-Vehicle group (Bonferroni: p < 0.0001, CA3 −26%, CA1 −34%). The volume of CA3 was higher in the Saline-siRNA group compared to the Saline-Vehicle group (Bonferroni: p = 0.0331, 19%) (Fig. 3b, c).
The volume of suprapyramidal region of dentate gyrus of the Pilo-Vehicle group was higher when compared to the Saline-Vehicle group (Bonferroni: p = 0.0009, 62%) and was lower in the Pilo-siRNA group compared to the Pilo-Vehicle group (Bonferroni: p = 0.0308, −22%) (Fig. 3d). Nevertheless, no change was observed in infrapyramidal dentate gyrus comparing to all groups (F (1, 8) = 0.1792, p = 0.6832) (Fig. 3e). The volume of the hilus was lower in the Pilo-siRNA group compared to the Saline-siRNA group (Bonferroni: p = 0.0306, −24%) and to the Pilo-Vehicle group (Bonferroni: p = 0.0225, −26%) (Fig. 3f).
Behavioral data
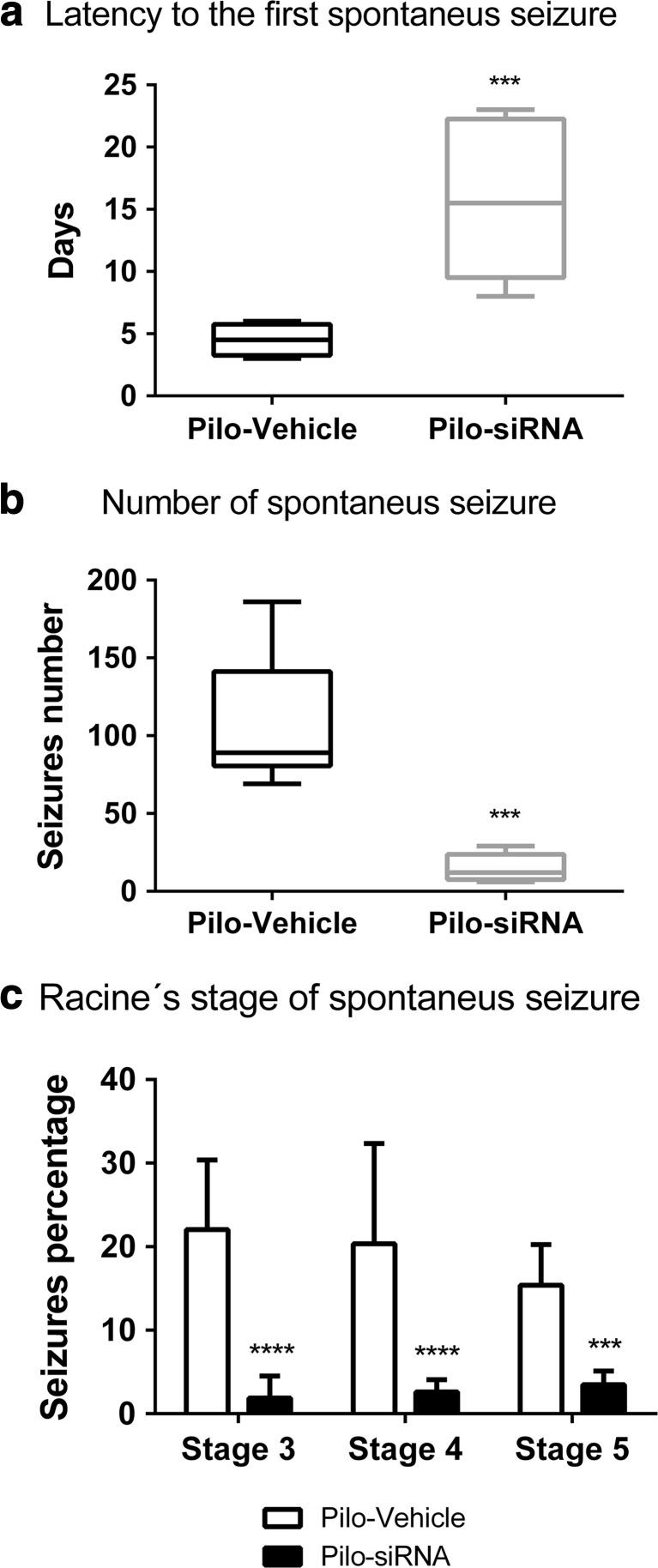
Mortality rate in the Pilo-siRNA group following SE was 63% lower than that in the Pilo-Vehicle group (38%). The latency period to the appearance of the first spontaneous seizure following pilocarpine administration was significantly increased in the Pilo-siRNA group when compared to the Pilo-Vehicle (t 5,238 = 4.027, p = 0.0009) (Fig. 4a). The number of spontaneous seizures (Racine’s scale 3–5) was significantly lower in Pilo-siRNA group than that in the Pilo-Vehicle (t 7,884 = 6.233, p = 0.0002) (Fig. 4b). Seizure severity was classified according to Racine’s scale 3–5. Seizure severity was decreased in the Pilo-siRNA group when compared to the Pilo-Vehicle (Pilo-siRNA versus Pilo-Vehicle, F (1, 36) = 59.3322, p < 0.0001; Bonferroni: stage 3, p < 0.0001; stage 4, p < 0.0001; stage 5, p = 0.0089), but no difference was observed between the stages (stages 3 versus 4 versus 5, F (2, 36) = 0.5134, p = 0.6028) (Fig. 4c).
Fig. 4.
a Latency period to the appearance of the first spontaneous seizure of Pilo-Vehicle and Pilo-siRNA groups. b Number of spontaneous seizures during 60 days following pilocarpine administration in Pilo-Vehicle and Pilo-siRNA groups. Box plot represents the median, first and third quartiles, and maximum and minimum values of each group. c Percentage of spontaneous seizure stages 3–5 during 60 days in Pilo-Vehicle and Pilo-siRNA groups. Bars represent the mean ± standard deviation for each group (Pilo-Vehicle—N = 8 and Pilo-siRNA—N = 6). *p < 0.05, ***p < 0.001, and ****p < 0.0001
Discussion
This study shows that the application of siRNA against P2X7R, 6 h after the onset of status epilepticus (SE), is able to reduce by 40% the expression of P2X7R in the rat hippocampus at 48 h later. This effect resulted in hippocampal neuroprotection, increase in the latency for the appearance of the first spontaneous seizures, and decrease in the mortality rate post SE, frequency and severity of the seizures, and protection from hippocampal volume changes, due to SE-induced edema. These data reinforce the participation of P2X7R in mechanisms underlying the increase in hyperexcitability, edema, and cell death triggered by pilocarpine-induced SE.
Previous studies performed in our group showed an increase in P2X7R expression in the hippocampus of rats submitted to pilocarpine model of SE, during acute and chronic phases of the model [12, 37]. The differential expression of P2X7R during acute (12 h) or chronic phases (90 days), indicate different roles in the progression of the epileptogenic process. During the acute phase, P2X7R were mainly located in glial cells, modulating the inflammatory process and hyperexcitability, while in chronic phase, they were mainly located in synaptic terminals modulating neurotransmitter release as glutamate and GABA [12]. Besides, a decreased level of P2X4R expression in CA1, CA2, CA3, hilus, and dentate gyrus during chronic phase of Pilo model was also observed, reflecting neuronal loss and functional alteration of reminiscent neurons following brain insult (SE) as a compensatory response to ineffective GABAergic neurotransmission [12]. Normal level of P2X7R was detected during the latent period (7 days following SE) located mainly in nerve terminals in CA3 and the dentate gyrus [12].
The pilot study performed to determine a time curve of P2X7R knockdown showed reduced expression of P2X7R 96 h following siRNA application, but the peak of the blockade was 48 h. Based on this study, the time of 48 h was chosen for the analysis following siRNA application. Considering that P2X7R increases significantly in glial cells at 12 and 24 h after SE [12], we can suppose that the knockdown induced by siRNA was sufficient to block microglial and astrocytic activation resulting in less release of cytokines in the hippocampus which consequently contributed to the neuroprotection as well as the late benefits observed in the Pilo-siRNA group.
Several studies have shown that P2X7R activation in neurons is associated with increased intracellular calcium (Ca2+) and with the facilitation of glutamate release, promoting excitotoxicity and cell death [7, 12–14, 19, 23]. In microglia, P2X7R activation is associated with the inflammatory response in the central nervous system through cytokines production and release, especially interleukin-1β (IL-1β), interleukin-18 (IL-18), tumor necrosis factor (TNF-α), signaling through nuclear factor kappa B (NF-kB), nitric oxide synthase (NOS) activation, free radical production, and proapoptotic transcription factor formation [6, 7, 9, 10, 14, 15, 17–19, 21, 57]. The P2X7R activation in astrocytes has been related to inflammatory response and facilitation of glutamate release [7, 9, 12, 13, 15, 18, 19, 21, 23, 57, 58].
Neuronal death in pilocarpine model occurs by different mechanisms [59]. The increase in intracellular Ca2+ resulting from cholinergic activation triggers a cascade of reactions involving proteases, lipases, and nucleases activations, and generation of free radicals as intermediate products, which in turn can potentiate the release of glutamate and inflammatory mediators [50, 59, 60].
Studies have shown that the P2X7R antagonists Brilliant Blue G (BBG) and A-438079 are neuroprotective against damage caused by kainic acid, by reducing neuronal death and microgliosis in the hippocampus and neocortex [41, 42]. These data corroborate other studies that also showed reduction in astrocytes loss in the molecular layer of the dentate gyrus and frontoparietal cortex following P2X7R antagonists oxidized ATP (OxATP) and BBG in pilocarpine model [40]. In contrast, some authors showed increased cell death in CA3 layer by using P2X7R antagonists OxATP, A-438079, and A-740003 following pilocarpine [39], and that inhibition of microglial activation by the P2X7R antagonists may not be sufficient to protect neurons of the excitotoxicity caused by SE [43]. We have shown that pilocarpine-induced SE caused an increase in FJ-B positive cells in accordance with previous studies [61, 62] and that the reduction in the expression of P2X7R by siRNA was neuroprotective, mainly in CA1 and CA3 hippocampal layers.
There are many reports in the literature showing that SE can cause brain edema that can by itself, contribute to the epileptogenic process [63–65], although the mechanisms underlying this process are unknown. Edema resulting from seizures in human or experimental model can be of two types, cytotoxic edema and vasogenic edema [63–69]. In the cytotoxic edema, the glutamate hyperstimulation causes intracellular Ca2+ increase and promotes cytotoxicity in neurons and glial cells [64, 70]. Conversely, in the vasogenic edema, the cellular signaling triggered by SE can induce pro-inflammatory cytokines release and increases the production of kinins [71–78]. The kinins along with the cytokines may affect the junction of blood vessel epithelial cells and reduce the integrity of the tight junctions in endothelial cells walls, leading to a dysfunction of the blood brain barrier and consequently increasing the vascular permeability and accumulation of extracellular fluid [63, 70, 79, 80]. There are a number of studies showing robust evidence that IL-1β released following seizures may be pro-convulsant in experimental models of epilepsy [6, 14, 15, 76, 81]. According to our results, P2X7R can modulate the mechanisms involved in edema since the reduction in the expression of these receptors by siRNA prevented the edema in hippocampal subareas as hilus, dentate gyrus suprapyramidal, CA1, and CA3 of rats underwent to pilocarpine-induced SE. Although the mechanisms are not elucidated, the edema processes have been associated with cell death, particularly neuronal death, and hippocampal atrophy [82, 83]. A positive relationship between cerebral edema and the occurrence of spontaneous recurrent seizures has been shown in kainic acid model, strengthening the association of edema to epileptogenesis [65]. Knowing that the P2X7R are involved in the inflammatory activation following seizures, reduction in their expression by siRNA may have caused decrease in the release of inflammatory mediators preventing edema and cell death, and decreasing seizure expression.
Previous studies have shown that P2X7R antagonists trigger anticonvulsant effect, reducing electrographic and behavioral seizures, microglia activation, decrease IL-1β production and prevent cell damage resulting from seizures [14, 15, 33, 38, 41, 42]. Studies using P2X7R (Pfizer) [46] knockout mice also show reduction in the severity of seizures compared to wild-type mice [33].
Despite the evidence that P2X7R blockade by antagonists triggers protective mechanisms following seizures, in apparent contradiction, recent studies by us in collaboration with other group demonstrate a neuroprotective effect of P2X7R antagonists BBG and AZ10606120 resulting in increased expression of spontaneous seizures in rat underwent to pilocarpine-induced SE [84, 85]. According to the authors, the blockade of P2X7R by the negative allosteric modulator AZ10606120 may have caused hilar neuroprotection and favored the survival, ectopic migration, and differentiation of neuronal progenitor cells (NPCs) expressing P2Y1 and P2X7 receptors, which in turn integrate abnormal circuits in the hippocampus, contributing with excitability [85]. Similar hypothesis that neurogenesis contributes to worsen seizures was presented previously [86]. P2X7R have been shown to modulate mechanisms involved with apoptosis/necrosis and neuronal differentiation of NPCs [87–90], while P2Y1 receptors modulate proliferation and migration of NPCs [91–93]. Klaft et al. [94] also found that P2X7R antagonists had a minor antiepileptic effect in medial entorhinal cortex on rats subjected to pilocarpine-induced epilepsy.
The heterogeneity between pharmacological studies using antagonists may occur due to a number of factors including specificity, dose, and variability of the pharmacological agents tested; the use of different experimental models of epilepsy; route of administration; and temporal window in which studies are made. However, our pharmacological data using the P2X7 antagonist AZ10606120 are in opposite with the present data using siRNA to knockdown P2X7R in pilocarpine model. The results are intriguing and require further studies to elucidate molecular mechanisms involved with P2X7R blockade in epileptic process. We also do not have data about the participation of other P2 receptors in the changes obtained with the P2X7R knockdown, being a subject to be studied in the future.
As stated in this work, there are many studies showing beneficial effect of P2X7R blockade on epilepsy, either by pharmacological antagonists or by genetic manipulation (transgenic, knockout, or RNAi) and attenuation of inflammatory cascades are among the mechanisms involved. However, there are few studies elucidating the differences between mechanisms triggered by one or other methodological condition and it is very important to increase the knowledge of the role of this receptor in epilepsy.
The main finding of this study was that knockdown of P2X7R by the administration of siRNA 6 h after SE onset reduced the mortality rate, resulted in little or no hippocampal edema, increased the latency to the onset of the first spontaneous seizure, and reduced the number and severity of spontaneous seizures in a later period. In addition, knockdown of P2X7R by siRNA induced important neuroprotection in the hippocampus of rats underwent to the pilocarpine model of SE. Further studies are required to elucidate the mechanisms associated with the knockdown of P2X7R during SE induced by pilocarpine resulting in attenuation of changes triggered by seizures.
Conclusion
Collectively, our data shows that the P2X7R may have an important role in the pathophysiology of pilocarpine-induced epilepsy, since the inhibition of the expression of these receptors in vivo, improved edema, and had neuroprotective. These data may have clinical relevance and highlight the therapeutic potential of adjunct treatment of epilepsy with P2X7R expression modulators.
Acknowledgments
This work was supported by the Brazilian funds from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, nos. 142743/2010-0 and 248728/2012-1), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). We thank Prof. Henning Ulrich who kindly provided the material for tests.
Compliance with ethical standards
All experimental procedures were performed under the supervision and with the approval of our internal Ethics Committee (Federal University of São Paulo, CEP N. 0961/10). Animal protocols were conducted in accordance with national and international legislation (Guidelines of the Brazilian College of Animal Experimentation, COBEA; NIH Guide for Care and Use of Laboratory Animals) and the experiments followed the principles outlined in the Basel Declaration [45].
Conflict of interest
Rebeca Padrão Amorim declares that she has no conflict of interest.
Michelle Gasparetti Leão Araújo declares that she has no conflict of interest.
Jorge Valero declares that he has no conflict of interest.
Iscia Lopes-Cendes declares that she has no conflict of interest.
Vinicius Davila Bitencourt Pascoal declares that he has no conflict of interest.
João Oliveira Malva declares that he has no conflict of interest.
Maria José da Silva Fernandes declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Engel J. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42(6):796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 2.Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42(2):351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- 3.Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, Bernasconi N, Bien CG, Cendes F, Coras R, Cross JH, Jacques TS, Kahane P, Mathern GW, Miyata H, Moshé SL, Oz B, Özkara Ç, Perucca E, Sisodiya S, Wiebe S, Spreafico R. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a task force report from the ILAE commission on diagnostic methods. Epilepsia. 2013;54(7):1315–1329. doi: 10.1111/epi.12220. [DOI] [PubMed] [Google Scholar]
- 4.Leite JP, Bortolotto ZA, Cavalheiro EA. Spontaneous recurrent seizures in rats: an experimental model of partial epilepsy. Neurosci Biobehav Rev. 1990;14(4):511–517. doi: 10.1016/S0149-7634(05)80076-4. [DOI] [PubMed] [Google Scholar]
- 5.Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9(3):315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- 6.Vezzani A. Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr. 2014;14(1):3–7. doi: 10.5698/1535-7511-14.s2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beamer E, Gölöncsér F, Horváth G, Bekő K, Otrokocsi L, Koványi B, Sperlágh B. Purinergic mechanisms in neuroinflammation: an update from molecules to behavior. Neuropharmacology. 2016;104:94–104. doi: 10.1016/j.neuropharm.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Bernardino L, Balosso S, Ravizza T, Marchi N, Ku G, Randle JC, Malva JO, Vezzani A. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptor-mediated IL-1β release. J Neurochem. 2008;106:271–280. doi: 10.1111/j.1471-4159.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95(2):229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Séguéla P. ADP and AMP induce interleukin-1β release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci. 2002;22(8):3061–3069. doi: 10.1523/JNEUROSCI.22-08-03061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36(3):174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Doná F, Ulrich H, Persike DS, Conceição IM, Blini JP, Cavalheiro EA, Fernandes MJ. Alteration of purinergic P2X4 and P2X7 receptor expression in rats with temporal-lobe epilepsy induced by pilocarpine. Epilepsy Res. 2009;83(2–3):157–167. doi: 10.1016/j.eplepsyres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Engel T, Alves M, Sheedy C, Henshall DC. ATPergic signalling during seizures and epilepsy. Neuropharmacology. 2016;104:140–153. doi: 10.1016/j.neuropharm.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Henshall DC, Engel T. P2X purinoceptors as a link between hyperexcitability and neuroinflammation in status epilepticus. Epilepsy Behav. 2015;49:8–12. doi: 10.1016/j.yebeh.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez-Pacheco A, Diaz-Hernandez M, Arribas-Blázquez M, Sanz-Rodriguez A, Olivos-Oré LA, Artalejo AR, Alves M, Letavic M, Miras-Portugal MT, Conroy RM, Delanty N, Farrell MA, O'Brien DF, Bhattacharya A, Engel T, Henshall DC. Transient P2X7 receptor antagonism produces lasting reductions in spontaneous seizures and gliosis in experimental temporal lobe epilepsy. J Neurosci. 2016;36(22):5920–5932. doi: 10.1523/JNEUROSCI.4009-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29(12):3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monif M, Burnstock G, Williams DA. Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol. 2010;42(11):1753–1756. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24(2):337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- 19.Engel T, Jimenez-Pacheco A, Miras-Portugal MT, Diaz-Hernandez M, Henshall DC. P2X7 receptor in epilepsy; role in pathophysiology and potential targeting for seizure control. Int J Physiol Pathophysiol Pharmacol. 2012;4(4):174–187. [PMC free article] [PubMed] [Google Scholar]
- 20.North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol. 2013;83(4):759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders BM, Wiley JS, Smith NC. The role of the P2X7 receptor in infectious diseases. PLoS Pathog. 2011;7(11):1–7. doi: 10.1371/journal.ppat.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papp L, Vizi ES, Sperlágh B. Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor−/− mice. Neuroreport. 2004;15(15):2387–2391. doi: 10.1097/00001756-200410250-00017. [DOI] [PubMed] [Google Scholar]
- 23.Sperlágh B, Köfalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, Vizi ES. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem. 2002;81(6):1196–1211. doi: 10.1046/j.1471-4159.2002.00920.x. [DOI] [PubMed] [Google Scholar]
- 24.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Sperlágh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbeloa J, Perez-Samartin A, Gottlieb M, Matute C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis. 2012;45:954–961. doi: 10.1016/j.nbd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonte C, Bernardi G, Pedata F, Sancesario G. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 2006;26:974–982. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- 29.Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One. 2012;7(7):e41229. doi: 10.1371/journal.pone.0041229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz-Hernandez JI, Gomez-Villafuertes R, Leon-Otegui M, Hontecillas-Prieto L, Del Puerto A, Trejo JL, Lucas JJ, Garrido JJ, Gualix J, Miras-Portugal MT, Diaz-Hernandez M. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3beta and secretases. Neurobiol Aging. 2012;33(8):1816–1828. doi: 10.1016/j.neurobiolaging.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Hernandez M, Diez-Zaera M, Sanchez-Nogueiro J, Gomez-Villafuertes R, Canals JM, Alberch J, Miras-Portugal MT, Lucas JJ. Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23(6):1893–1906. doi: 10.1096/fj.08-122275. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G. Purinergic mechanisms and pain—an update. Eur J Pharmacol. 2013;716(1–3):24–40. doi: 10.1016/j.ejphar.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 33.Henshall DC, Diaz-Hernandez M, Miras-Portugal MT, Engel T. P2X receptors as targets for the treatment of status epilepticus. Front Cell Neurosci. 2013;7:237. doi: 10.3389/fncel.2013.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes MS, Speciali DS, Blini J, Canzian M, Cavalheiro EA, Ulrich H, Carrete H, Jr, Centeno RS, Yacubian EM. Purinergic P2 receptors are up-regulated in the hippocampus of patients with temporal lobe epilepsy associated with hippocampal sclerosis. Epilepsia. 2009;50(10):78. [Google Scholar]
- 35.Padrão RA, Ariza CB, Canzian M, Porcionatto M, Araújo MGL, Cavalheiro EA, Ulrich H, Carrete H, Jr, Centeno RS, Yacubian EM, Fernandes MS. The P2 purinergic receptors are increased in the hippocampus of patients with temporal lobe epilepsy: what is the relevance to the epileptogenesis? Purinergic Signal. 2011;7:127. [Google Scholar]
- 36.Rappold PM, Lynd-Balta E, Joseph SA. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089:171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Vianna EP, Ferreira AT, Naffah-Mazzacoratti MG, Sanabria ER, Funke M, Cavalheiro EA, Fernandes MJ. Evidence that ATP participates in the pathophysiology of pilocarpine-induced temporal lobe epilepsy: afluorimetric, immunohistochemical, and western blot studies. Epilepsia. 2002;43(5):227–229. doi: 10.1046/j.1528-1157.43.s.5.26.x. [DOI] [PubMed] [Google Scholar]
- 38.Choi HK, Ryu HJ, Kim JE, Jo SM, Choi HC, Song HK, Kang TC. The roles of P2X7 receptor in regional-specific microglial responses in the rat brain following status epilepticus. Neurol Sci. 2012;33(3):515–525. doi: 10.1007/s10072-011-0740-z. [DOI] [PubMed] [Google Scholar]
- 39.Kim JE, Ryu HJ, Kang TC. P2X7 receptor activation ameliorates CA3 neuronal damage via a tumor necrosis factor-α-mediated pathway in the rat hippocampus following status epilepticus. J Neuroinflammation. 2011;8:62. doi: 10.1186/1742-2094-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JE, Ryu HJ, Yeo SI, Kang TC. P2X7 receptor differentially modulates astroglial apoptosis and clasmatodendrosis in the rat brain following status epilepticus. Hippocampus. 2011;21(12):1318–1333. doi: 10.1002/hipo.20850. [DOI] [PubMed] [Google Scholar]
- 41.Engel T, Gomez-Villafuertes R, Tanaka K, Mesuret G, Sanz-Rodriguez A, Garcia-Huerta P, Miras-Portugal MT, Henshall DC, Diaz-Hernandez M. Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J. 2012;26(4):1616–1628. doi: 10.1096/fj.11-196089. [DOI] [PubMed] [Google Scholar]
- 42.Jimenez-Pacheco A, Mesuret G, Sanz-Rodriguez A, Tanaka K, Mooney C, Conroy R, Miras-Portugal MT, Diaz-Hernandez M, Henshall DC, Engel T. Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia. 2013;54(9):1551–1561. doi: 10.1111/epi.12257. [DOI] [PubMed] [Google Scholar]
- 43.Kim JE, Kwak SE, Jo SM, Kang TC. Blockade of P2X receptor prevents astroglial death in the dentate gyrus following pilocarpine-induced status epilepticus. Neurol Res. 2009;31(9):982–988. doi: 10.1179/174313209X389811. [DOI] [PubMed] [Google Scholar]
- 44.Kim JE, Ryu HJ, Yeo SI, Kang TC. P2X7 receptor regulates leukocyte infiltrations in rat fronto-parietal cortex following status epilepticus. J Neuroinflammation. 2010;7:65. doi: 10.1186/1742-2094-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JE, Kang TC. The P2X7 receptor-pannexin-1 complex decreases muscarinic acetylcholine receptor-mediated seizure susceptibility in mice. J Clin Investig. 2011;121(5):2037–2047. doi: 10.1172/JCI44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 47.de França NR, Júnior DM, Lima AB, Pucci FVC, Andrade LEC, Silva NP. Interferência por RNA: uma nova alternativa para terapia nas doenças reumáticas. Rev Bras Reumatol. 2010;50(6):695–702. doi: 10.1590/S0482-50042010000600008. [DOI] [PubMed] [Google Scholar]
- 48.Basel Declaration Society (2011) The Basel Declaration. http://www.basel-declaration.org
- 49.Paxinos G, Watson C. The rat brain in stereotaxic coordenates. 6. London: Elsevier; 2007. p. 462. [Google Scholar]
- 50.Cavalheiro EA. The pilocarpine model of epilepsy. Ital J Neurol Sci. 1995;16(1–2):33–37. doi: 10.1007/BF02229072. [DOI] [PubMed] [Google Scholar]
- 51.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 52.Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 53.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 54.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874(2):123–130. doi: 10.1016/S0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 55.Snyder JS, Ferrante SC, Cameron HA. Late maturation of adult-born neurons in the temporal dentate gyrus. PLoS One. 2012;7(11):1–8. doi: 10.1371/journal.pone.0048757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 57.Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-κB through the P2Z purinoreceptor by selectively targeting NF-κB p65. J Cell Biol. 1997;139(7):1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23(4):1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scorza FA, Arida RM, Naffah-Mazzacoratti Mda G, Scerni DA, Calderazzo L, Cavalheiro EA. The pilocarpine model of epilepsy: what have we learned? An Acad Bras Cienc. 2009;81(3):345–365. doi: 10.1590/S0001-37652009000300003. [DOI] [PubMed] [Google Scholar]
- 60.Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32(6):778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- 61.Fabene PF, Andrioli A, Priel MR, Cavalheiro EA, Bentivoglio M. Fos induction and persistence, neurodegeneration, and interneuron activation in the hippocampus of epilepsy-resistant versus epilepsy-prone rats after pilocarpine-induced seizures. Hippocampus. 2004;14(7):895–907. doi: 10.1002/hipo.20003. [DOI] [PubMed] [Google Scholar]
- 62.Rosim FE, Persike DS, Nehlig A, Amorim RP, de Oliveira DM, Fernandes MJ. Differential neuroprotection by A1 receptor activation and A2A receptor inhibition following pilocarpine-induced status epilepticus. Epilepsy Behav. 2011;22(2):207–213. doi: 10.1016/j.yebeh.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Kim JE, Ryu HJ, Kang TC. Status epilepticus induces vasogenic edema via tumor necrosis factor-α/ endothelin-1-mediated two different pathways. PLoS One. 2013;8(9):1–13. doi: 10.1371/journal.pone.0074458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lassmann H, Petsche U, Kitz K, Baran H, Sperk G, Seitelberger F, Hornykiewicz O. The role of brain edema in epileptic brain damage induced by systemic kainic acid injection. Neuroscience. 1984;13(3):691–704. doi: 10.1016/0306-4522(84)90089-7. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Liu PP, Li LY, Zhang HM, Li T. Hypothermia reduces brain edema, spontaneous recurrent seizure attack, and learning memory deficits in the kainic acid treated rats. CNS Neurosci Ther. 2011;17(5):271–280. doi: 10.1111/j.1755-5949.2010.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arrigoni E, Averet N, Loiseau H, Cohadon F. Relationship between epileptic activity and edema formation in the acute phase of cryogenic lesion. Neurochem Pathol. 1987;7:207–220. doi: 10.1007/BF03160181. [DOI] [PubMed] [Google Scholar]
- 67.Choy M, Cheung KK, Thomas DL, Gadian DG, Lythgoe MF, Scott RC. Quantitative MRI predicts status epilepticus-induced hippocampal injury in the lithium–pilocarpine rat model. Epilepsy Res. 2010;88(2–3):221–230. doi: 10.1016/j.eplepsyres.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 68.Duffy BA, Chun KP, Ma D, Lythgoe MF, Scott RC. Dexamethasone exacerbates cerebral edema and brain injury following lithium-pilocarpine induced status epilepticus. Neurobiol Dis. 2014;63(100):229–236. doi: 10.1016/j.nbd.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roch C, Leroy C, Nehlig A, Namer IJ. Magnetic resonance imaging in the study of the lithium–pilocarpine model of temporal lobe epilepsy in adult rats. Epilepsia. 2002;43(4):325–335. doi: 10.1046/j.1528-1157.2002.11301.x. [DOI] [PubMed] [Google Scholar]
- 70.Emerson MR, Nelson SR, Samson FE, Pazdernik TL. Hypoxia preconditioning attenuates brain edema associated with kainic acid-induced status epilepticus in rats. Brain Res. 1999;825(1–2):189–193. doi: 10.1016/S0006-8993(99)01195-6. [DOI] [PubMed] [Google Scholar]
- 71.Argañaraz GA, Perosa SR, Lencioni EC, Bader M, Cavalheiro EA, Naffah-Mazzacoratti MG, Pesqueiro JB, Silva JA., Jr Role of kinin B1 and B2 receptors in the development of pilocarpine model of epilepsy. Brain Res. 2004;1013:30–39. doi: 10.1016/j.brainres.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 72.Argañaraz GA, Silva JA, Jr, Perosa SR, Pessoa LG, Carvalho FF, Bascands JL, Bader M, Trindade ES, Amado D, Cavalheiro EA, Pesquero JB, Naffah-Mazzacoratti MG. The synthesis and distribution of the kinin B1 and B2 receptors are modified in the hippocampus of rats submitted to pilocarpine model of epilepsy. Brain Res. 2004;1006:114–125. doi: 10.1016/j.brainres.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 73.Gouveia TL, Scorza FA, Silva MJ, Bandeira TA, Perosa SR, Argañaraz GA, Silva MP, Araujo TR, Frangiotti MI, Amado D, Cavalheiro EA, Silva JA, Jr, Naffah-Mazzacoratti MG. Lovastatin decreases the synthesis of inflammatory mediators in the hippocampus and blocks the hyperthermia of rats submitted to long-lasting status epilepticus. Epilepsy Behav. 2011;20:1–5. doi: 10.1016/j.yebeh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Perosa SR, Argañaraz GA, Goto EM, Costa LG, Konno AC, Varella PP, Santiago JF, Pesquero JB, Canzian M, Amado D, Yacubian EM, Carrete H, Jr, Centeno RS, Cavalheiro EA, Silva JA, Jr, Naffah-Mazzacoratti MG. Kinin B1 and B2 receptors are overexpressed in the hippocampus of humans with temporal lobe epilepsy. Hippocampus. 2007;17:26–33. doi: 10.1002/hipo.20239. [DOI] [PubMed] [Google Scholar]
- 75.Simões PS, Perosa SR, Arganãraz GA, Yacubian EM, Carrete H, Jr, Centeno RS, Varella PP, Santiago JF, Canzian M, Silva JA, Jr, Mortara RA, Amado D, Cavalheiro EA, Naffah-Mazzacoratti MG. Kallikrein 1 is overexpressed by astrocytes in the hippocampus of patients with refractory temporal lobe epilepsy, associated with hippocampal sclerosis. Neurochem Int. 2011;58:477–482. doi: 10.1016/j.neuint.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 76.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46(11):1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 77.Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, Perego C, De Simoni MG. Functional role of inflammatory cytokines and antiinflamatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(5):30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- 78.Voutsinos-Porche B, Koning E, Kaplan H, Ferrandon A, Guenounou M, Nehlig A, Motte J. Temporal patterns of the cerebral inflammatory response in the rat lithium-pilocarpine model of temporal lobe epilepsy. Neurobiol Dis. 2004;17:385–402. doi: 10.1016/j.nbd.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 79.Göbel K, Pankratz S, Schneider-Hohendorf T, Bittner S, Schuhmann MK, Langer HF, Stoll G, Wiendl H, Kleinschnitz C, Meuth SG. Blockade of the kinin receptor B1 protects from autoimmune CNS disease by reducing leukocyte trafficking. J Autoimmun. 2011;36(2):106–114. doi: 10.1016/j.jaut.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 80.Weissberg I, Reichert A, Heinemann U, Friedman A. Blood-brain barrier dysfunction in epileptogenesis of the temporal lobe. Epilepsy Res Treat. 2011;2011(1):1–10. doi: 10.1155/2011/143908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao Z, Peng J, Yang L, Kong H, Yin F. Interleukin-1β plays a role in the pathogenesis of mesial temporal lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in hippocampal neurons. J Neuroimmunol. 2015;282:110–117. doi: 10.1016/j.jneuroim.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 82.Coan AC, Morita ME, Campos BM, Bergo FP, Kubota BY, Cendes F. Amygdala enlargement occurs in patients with mesial temporal lobe epilepsy and hippocampal sclerosis with early epilepsy onset. Epilepsy Behav. 2013;29(2):390–394. doi: 10.1016/j.yebeh.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 83.Kumar G, Mittal S, Moudgil SS, Kupsky WJ, Shah AK. Histopathological evidence that hippocampal atrophy following status epilepticus is a result of neuronal necrosis. J Neurol Sci. 2013;334(1–2):186–191. doi: 10.1016/j.jns.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 84.Araújo MGL, Amorim RP, Buri MV, Marques-Carneiro JE, Araújo LFS, Predebon GM, Patsis VB, Paredes-Gamero EJ, Fernandes MJS. The effect of the P2X7 receptor antagonista AZ10606120 in the pilocarpine-induced epilepsy model. Purinergic Signalling. 2016;12:343–410. doi: 10.1007/s11302-016-9503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rozmer K, Gao P, Araújo MG, Khan MT, Liu J, Rong W, Tang Y, Franke H, Krügel U, Fernandes MJ, Illes P (2016) Pilocarpine-induced status epilepticus increases the sensitivity of P2X7 and P2Y1 receptors to nucleotides at neural progenitor cells of the juvenile rodent hippocampus. Cereb Cortex:1–18. doi:10.1093/cercor/bhw178 [DOI] [PubMed]
- 86.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delarasse C, Gonnord P, Galante M, Auger R, Daniel H, Motta I, Kanellopoulos JM. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J Neurochem. 2009;109:846–857. doi: 10.1111/j.1471-4159.2009.06008.x. [DOI] [PubMed] [Google Scholar]
- 88.Glaser T, de Oliveira SL, Cheffer A, Beco R, Martins P, Fornazari M, Lameu C, Junior HM, Coutinho-Silva R, Ulrich H. Modulation of mouse embryonic stem cell proliferation and neural differentiation by the P2X7 receptor. PLoS One. 2014;9:e96281. doi: 10.1371/journal.pone.0096281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Messemer N, Kunert C, Grohmann M, Sobottka H, Nieber K, Zimmermann H, Franke H, Norenberg W, Straub I, Schaefer M, Riedel T, Illes P, Rubini P. P2X7 receptors at adult neural progenitor cells of the mouse subventricular zone. Neuropharmacology. 2013;73:122–137. doi: 10.1016/j.neuropharm.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 90.Tsao HK, Chiu PH, Sun SH. PKC-dependent ERK phosphorylation is essential for P2X7 receptor-mediated neuronal differentiation of neural progenitor cells. Cell Death Dis. 2013;4:e751. doi: 10.1038/cddis.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao X, Li LP, Qin XH, Li SJ, Zhang M, Wang Q, Hu HH, Fang YY, Gao YB, Li XW, Sun LR, Xiong WC, Gao TM, Zhu XH. Astrocytic adenosine 5′-triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells. 2013;31:1633–1643. doi: 10.1002/stem.1408. [DOI] [PubMed] [Google Scholar]
- 92.Liu X, Hashimoto-Torii K, Torii M, Haydar TF, Rakic P. The role of ATP signaling in the migration of intermediate neuronal progenitors to the neocortical subventricular zone. Proc Natl Acad Sci U S A. 2008;105:11802–11807. doi: 10.1073/pnas.0805180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suyama S, Sunabori T, Kanki H, Sawamoto K, Gachet C, Koizumi S, Okano H. Purinergic signaling promotes proliferation of adult mouse subventricular zone cells. J Neurosci. 2012;32:9238–9247. doi: 10.1523/JNEUROSCI.4001-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klaft ZJ, Schulz SB, Maslarova A, Gabriel S, Heinemann U, Gerevich Z. Extracellular ATP differentially affects epileptiform activity via purinergic P2X7 and adenosine A1 receptors in naive and chronic epileptic rats. Epilepsia. 2012;53(11):1978–1986. doi: 10.1111/j.1528-1167.2012.03724.x. [DOI] [PubMed] [Google Scholar]