Abstract
We aim to investigate whether overweight/obese pregnant women have elevated plasma levels of adenosine associated with increased consumption of high-calorie food. Sixty women were included. They were divided into lean (n = 23 and n = 12) or overweight/obese (n = 7 and n = 18) non-pregnant and pregnant women, respectively. Clinical records and maternal blood samples were collected after informed consent. A self-reported dietary questionnaire was also completed. Plasma adenosine levels were determined with high-performance liquid chromatography. Biochemical parameters, including glucose, total protein, and lipid profile, were determined using standard colorimetric assays. Adenosine levels were higher in pregnant women than in non-pregnant women (18.7 ± 1.6 vs 10.8 ± 1.3 nM/μg protein, respectively, p < 0.0001). Overweight/obese pregnant women (21.9 ± 2.5 nM/μg protein) exhibited higher adenosine levels than lean pregnant (14.5 ± 1.0 nM/μg protein, p = 0.04) or non-pregnant women (11.7 ± 1.5 nM/μg protein, p = 0.0005). Also, pregnant women with elevated weight gain exhibited higher (26.2 ± 3.7 nM/μg protein) adenosine levels than those with adequate weight gain (14.9 ± 1.4 nM/μg protein, p = 0.03). These differences were not statistically significant compared with those of pregnant women with reduced weight gain (17.4 ± 2.1 nM/μg protein, p = 0.053). Body mass index and adenosine only in pregnant women were positively correlated (r = 0.39, p = 0.02). While, polyunsaturated fatty acid (PUFA) consumption was negatively correlated with plasma adenosine levels only in non-pregnant women (r = −0.33, p = 0.03). Pregnancy is associated with high plasma adenosine levels, which are further elevated in pregnant women who are overweight/obese. High PUFA intake might reduce plasma adenosine levels in non-pregnant women.
Keywords: Adenosine, Pregnancy, Obesity, Food intake
Introduction
Pregnancy is one of the most nutritionally vulnerable stages in a woman’s life [1] that in some cases may result in the consumption of excess nutrients, leading to elevated weight gain and obesity. The causes of the increasing prevalence of obesity during pregnancy are commonly described as multifactorial, with one of the main factors being food intake [2]. A rapid shift to the increased consumption of high-calorie foods, caloric beverages, animal-based foods, and caloric sweeteners is associated with obesity [3, 4]. Nutrient ingestion during pregnancy has been extensively studied [5, 6], and the Institute of Medicine (IOM) has established clinical guidelines for healthy ranges of weight gain in pregnancy [7], concluding in general that the higher the body mass index (BMI), the lower the weight gain.
Adenosine is a nucleotide derived from the breakdown of adenosine tri-phosphate (ATP), adenosine di-phosphate (ADP), adenosine monophosphate (AMP), and S-adenosine-homocysteine that has pleiotropic functions, including the regulation of nutrient ingestion [8–10]. For instance, Levine and Morley [8] described that direct injection of adenosine into the cerebral cavity leads to the suppression of food intake in rats. Other studies have also determined that adenosine stimulates the consumption of high-calorie foods in rats [10] and mice [11]. Despite these findings, the potential relationship between nutrient intake and adenosine levels in humans is unknown.
Extracellular levels of adenosine increase throughout normal pregnancy until term [12, 13]. However, causes and consequences of elevated levels of adenosine in normal pregnancy are unclear, but it has been related with immunological and cardiovascular adaptation. In addition, further elevation of adenosine extracellular levels has been reported in pathological pregnancies, including those with pre-eclampsia [14] or diabetes [15]. Nevertheless, Iriyama et al. [16] described a mice model of increased placental adenosine levels, which results in a pre-eclampsia-like syndrome, suggesting that high extracellular adenosine levels may instead have detrimental effect on normal pregnancy development. Since obesity is a well-described risk factor for pregnancy pathologies such as diabetes or pre-eclampsia, it is intriguing to ask whether adenosine plasma levels are elevated in women with overweight or obesity. In this regard, no reports concerning plasma adenosine levels in overweight/obese pregnant women have been published. But, high concentrations of this molecule were found in the homogenates of adipose tissue obtained from obese adults [17] or in plasma from obese children [18].
Therefore, we hypothesized that overweight/obese pregnant women in their third trimester would have an elevated plasma level of adenosine associated with the increased consumption of a high-calorie diet. To test this hypothesis, we quantified plasma adenosine in lean and overweight/obese non-pregnant and pregnant women. Additionally, we determined whether pregnant women who exhibited elevated weight gain also exhibited high levels of adenosine compared to those with adequate weight gain during pregnancy. Finally, we analyzed whether the anthropometric and biochemical parameters investigated in this study were correlated with plasma adenosine levels.
Methods
Patients
This cross-sectional study was approved by the Ethical Committee from the Universidad del Bío-Bío (Fondecyt 1140586, October 8th, 2013) in accordance with protocols defined by the Declaration of Helsinki Ethics Committee. Women were recruited in this study after signing an informed written consent.
Pregnant women who visited the Department of Obstetrics and Gynecology of the Herminda Martin Clinical Hospital, Chillan, Chile for their delivery were included in this study. Exclusion criteria were chronic hypertension, altered renal function, diabetes, chronic disease, twin pregnancies, recurrent miscarriages, and placental abruption. The gestational age was defined as the period of time from the first day of the mother’s last menstrual period and the delivery date; this age was confirmed with a first trimester ultrasound. Body mass index (BMI = kg/m2) was determined as described elsewhere [18]. Women were classified as lean (18.5–24.9 kg/m2) and overweight/obese (>25 kg/m2) based on BMI. Pre-gestational weight was obtained from the clinical chart. In addition, weight gain during pregnancy was classified as reduced, adequate, or elevated based on the ranges recommended by the IOM [5].
Non-pregnant women were matched to pregnant women based on their demographic characteristics, including age, marital status, multiparity, location, and BMI. The exclusion criteria for this group included a previous diagnosis of hypertension, diabetes, or chronic disease. Additionally, neither the pregnant nor the non-pregnant women had consumed any drugs, including anti-inflammatory drugs or antibiotics, in the 2 weeks prior to the study.
Clinical and nutritional interview
Participating pregnant women in their third trimester were invited to a clinical appointment with a general physician to record past medical history and undergo a physical examination. Systolic and diastolic blood pressures were recorded after 10 min of supine rest.
A nutritionist further interviewed all participants to obtain information regarding food consumption using a multiple-pass method [19]. Briefly, dietary intake was assessed with a four-step personal interview that included multiple passes through the 24 h of the previous day, during which respondents received cues to help them remember and describe the foods they consumed [19].
Data from the 24-h recalls were used to estimate nutrient and food group intake using the table of chemical composition of Chilean food [20]. Thus, nutrient intake was estimated from the participants’ daily energy intake data (i.e., fat, carbohydrate, protein, SFA = saturated fatty acids, MUFA = monounsaturated fatty acids, and PUFA = polyunsaturated fatty acids). In addition, the estimated consumption of sodium, potassium, calcium, and vitamin C was recorded from food components. The use of a prenatal supplement and medication intake during the recall period was also recorded for pregnant women. Because prenatal supplement use was not the primary objective of the present study, prenatal supplement use was excluded from the current analysis.
Sample collection
After an overnight fasting period, blood samples were taken from a vein in the antecubital fossa and divided into two tubes, with or without heparin. For adenosine analysis, heparinized plasma was mixed with stop solution containing 20 mM dipyridamole and 5 mM sodium ethylenediaminetetraacetic acid (EDTA) (Sigma Aldrich, St Louis, MO, USA) in normal saline (0.9% NaCl) as previously published [21, 22]. In some samples, the adenosine deaminase (ADA, enzyme that converts adenosine to inosine) inhibitor erythro-9-(2-hydroxyl-3-nonyl) adenine hydrochloride (EHNA, 120 mM) was included to estimate the relative contribution of ADA to plasma adenosine levels. In addition, serum and plasma samples were centrifuged at 600 ×g for 10 min, and the supernatant was immediately frozen using liquid nitrogen. Then, samples were stored at −80 °C until analysis.
Total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, and glucose levels were measured in serum samples according to the instructions provided by the manufacturer (DiaSys Diagnostic Systems, Holzheim, Germany).
HPLC for adenosine quantification
Plasma adenosine levels were measured using a high-performance liquid chromatography (HPLC) system as described previously by our group [14, 23]. Briefly, 200 μl aliquots of plasma were collected and deproteinated with 20 μl of 50% trichloroacetic acid. Samples were centrifuged (7800 g × 5 min), and 150 μl of clear supernatant was neutralized with 20 μl of 3.3 N potassium hydroxide. Under standard assay conditions, 100 μl of sample or internal standard (adenosine) was mixed with 50% aqueous chloric acetaldehyde as described previously [14, 23]. Samples were incubated (80 °C, 45 min and 4 °C, 5 min) and centrifuged (15,000 g × 4 min). Aliquots (20 μl) were injected into an Agilent 1200 Series HPLC system (Chromolith® reverse-phase column, 100 × 4.6 mm C18, 2 μm particle size, Merck Millipore). The mobile phase consisted of 0.2 M sodium phosphate buffer, pH 6.0, with 5 and 25% methanol and was run isocratically at 1 ml/min. Fluorescence was detected at an excitation wavelength of 233 nm and an emission wavelength of 415 nm using a fluorescence detector (FLD G1321A, Agilent, CA, USA). The ratio of the area under the adenosine peaks to the area under the internal standard peak was compared with a standard curve. The inter- and intra-assay variations were 9.5 ± 0.3 and 4.4 ± 0.1%, respectively. Additionally, values were standardized by total protein, which was determined with a Bradford colorimetric assay (Thermo Scientific, MA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad, San Diego, CA, USA). Values are expressed as the mean ± S.E.M. Weight gain was calculated as the arithmetic difference between weight in the third trimester and pre-pregnancy weight. Quantitative variables were analyzed using Kruskal–Wallis non-parametric analysis (ANOVA) followed by the Mann-Whitney U test. In addition, correlations between all quantitative variables were determined using a Spearman test with a confidence interval of 95%. Statistical significance was set at 0.05.
Results
A total of 60 women were included in this study, 30 of whom were not pregnant (i.e., chosen as controls) and 30 of whom had normal pregnancies. The sub-classification based on BMI, as well as the respective clinical characteristics of the patients, is shown in Table 1. As expected, BMI was significantly different among the groups studied. BMI in the third trimester (range 32 to 40 weeks of gestation) was significantly higher in the overweight/obese group than in lean pregnant women (p < 0.0001).
Table 1.
Characteristics of included patients
| Control | Normal pregnancy | ANOVA | |||
|---|---|---|---|---|---|
| Lean (n = 23) |
Overweight (n = 7) |
Lean (n = 12) |
Overweight (n = 18) |
||
| Weight (kg) | 55.3 ± 1.3 | 67.1 ± 1.6* | 65.3 ± 1.4* | 83.9 ± 3.3*† | <0.0001 |
| Height (cm) | 160.9 ± 9 | 158.6 ± 1.4 | 160.1 ± 0.02 | 159.4 ± 1.5 | NS |
| BMI1 (kg/m2) | – | – | 26.1 ± 0.4 | 33.1 ± 1.2† | – |
| BMI2 (kg/m2) | 21.5 ± 0.4 | 27.2 ± 0.8* | 22.3 ± 0.7 | 28.8 ± 1.5*† | <0.0001 |
| SBP (mm Hg) | 103.7 ± 2.3 | 110.9 ± 3.7 | 107.6 ± 2.6 | 113.7 ± 3.5 | NS |
| DBP (mm Hg) | 65.7 ± 2.8 | 66.6 ± 4.5 | 69.3 ± 4.3 | 72.4 ± 3.2 | NS |
| Menarche (years) | 12.9 ± 0.3 | 12.7 ± 0.3 | 13.1 ± 0.3 | 13.1 ± 0.4 | NS |
| Gestational age (weeks) | – | – | 35 ± 0.7 | 35.4 ± 0.4 | – |
| Total cholesterol (mg/dl) | 163 ± 6.3 | 179 ± 5.8* | 253 ± 13* | 280 ± 15* | <0.0001 |
| Cholesterol LDL (mg/dl) | 91.3 ± 5.8 | 115 ± 6.1* | 142 ± 11* | 166 ± 16* | <0.0001 |
| Cholesterol HDL (mg/dl) | 58.2 ± 3.1 | 45.4 ± 4.8 | 64.7 ± 4.3 | 57.0 ± 2.5 | 0.03 |
| Triglycerides (mg/dl) | 68.1 ± 5.2 | 93.4 ± 12.3 | 226 ± 38* | 257 ± 13*† | <0.0001 |
| Glucose (mg/dl) | 78.8 ± 1.9 | 82.5 ± 1.5 | 72.3 ± 2.1 | 82.5 ± 5.5 | NS |
| Total protein (μg/ml) | 51.9 ± 3.2 | 61.1 ± 5.8 | 63.1 ± 5.5 | 65.2 ± 5.5 | NS |
ANOVA analysis of the anthropometric, clinical evaluation, and nutritional and biochemical parameters of the studied women. Values are expressed as the mean ± S.E.M.
BMI body mass index, BMI 1 body mass index in the third trimester of pregnancy, BMI 2 non-pregnancy body mass index, SBP and DBP systolic and diastolic blood pressure, respectively
*p < 0.05 vs lean non-pregnant women
† p < 0.05 vs lean pregnant women
Regarding lipid profile, lean and obese pregnant women exhibited significantly higher levels of total cholesterol, LDL cholesterol, and triglycerides compared to lean non-pregnant women (see Table 1). Among these differences, triglyceride levels were higher in overweight pregnant women than in lean pregnant women. Higher total cholesterol and LDL cholesterol levels were found in overweight/obese non-pregnant women compared to lean non-pregnant women. Non-significant differences in glycemia or total circulating protein levels were observed among the studied groups.
With the exception of self-estimated saturated fatty acid intake, self-reported nutrient consumption (see Table 2) was similar among the studied groups. Thus, paradoxically, overweight/obese women, both pregnant and non-pregnant, reported significantly lower consumption than lean women.
Table 2.
Estimated nutrient consumption in studied patients
| Control | Normal pregnancy | ANOVA | |||
|---|---|---|---|---|---|
| Lean (n = 23) |
Overweight (n = 7) |
Lean (n = 12) |
Overweight (n = 18) |
||
| Calories (cal) | 1950 ± 128 | 1699 ± 224 | 1931 ± 115 | 1997 ± 175 | NS |
| Proteins (g/day) | 76.7 ± 6.6 | 60.7 ± 9.7 | 64.9 ± 5.8 | 67.9 ± 10.8 | NS |
| Lipids (g/day) | 70.2 ± 0.4 | 44.6 ± 6.5 | 66.9 ± 7.4 | 59.6 ± 8.2 | NS |
| Cholesterol (mg/day) | 171 ± 19 | 102 ± 31 | 237 ± 64 | 132 ± 21 | NS |
| Saturated FA (mg/day) | 19.8 ± 1.8 | 10.5 ± 1.0*† | 22.0 ± 3.0 | 16.5 ± 3.8*† | 0.006 |
| Monounsaturated FA (mg/day) | 24.5 ± 3.0 | 16.8 ± 4.7 | 22.7 ± 2.7 | 17.7 ± 2.6 | NS |
| Polyunsaturated FA (mg/day) | 11.4 ± 1.5 | 9.2 ± 2.1 | 13.4 ± 2.6 | 13.6 ± 3.8 | NS |
| Carbohydrates (g/day) | 253 ± 17 | 257 ± 36 | 274 ± 17 | 305 ± 26 | NS |
| Sodium (mg/day) | 3051 ± 324 | 2291 ± 369 | 2844 ± 257 | 2964 ± 242 | NS |
| Potassium (mg/day) | 2087 ± 230 | 1629 ± 229 | 2068 ± 325 | 1983 ± 437 | NS |
| Calcium (mg/day) | 517 ± 56 | 431 ± 46 | 592 ± 114 | 479 ± 95 | NS |
| Vitamin C (mg/day) | 70 ± 13 | 49 ± 11 | 91 ± 30 | 66 ± 10 | NS |
ANOVA analysis of self-reported nutrient consumption, as determined with the multiple-pass method. Values are expressed as the mean ± S.E.M.
FA fatty acids
*p < 0.05 vs lean non-pregnant women
† p < 0.05 vs lean pregnant women
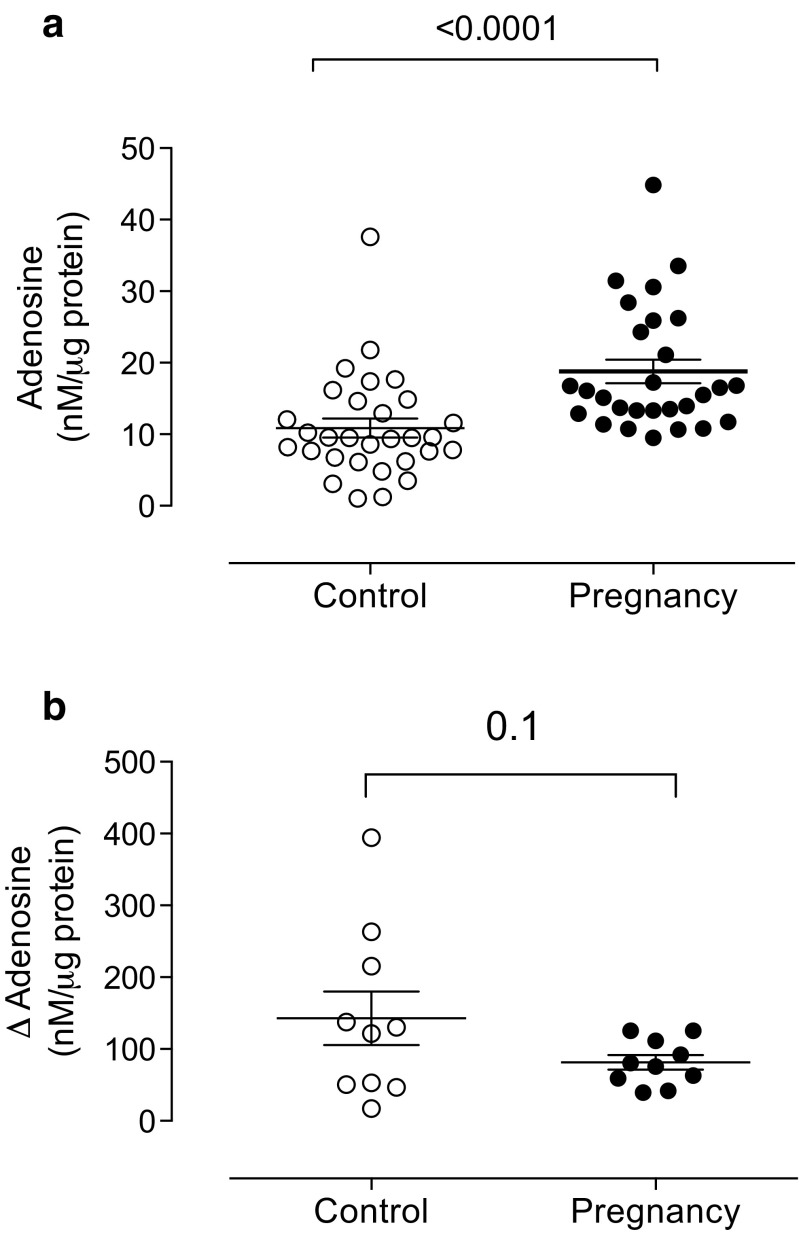
Plasma adenosine levels were 1.8-fold higher in pregnant women compared to non-pregnant women (18.7 ± 1.6 vs 10.8 ± 1.3 nM/μg protein, respectively, p < 0.0001) (Fig. 1a). Accordingly, the difference between plasma adenosine levels with or without the ADA inhibitor (i.e., relative estimation of ADA activity) was reduced (43%) in pregnant women compared to non-pregnant women (Fig. 1b), but this difference was not statistically significant (p = 0.1).
Fig. 1.
Plasma adenosine levels in pregnant women. (a) HPLC was used to measure adenosine levels in plasma samples from non-pregnant (control, n = 30) and pregnant women in their third trimester (pregnancy, n = 30). (b) The change in plasma adenosine levels in the presence of an adenosine deaminase (ADA) inhibitor (EHNA, see “Methods” section) is presented as indirect evidence of ADA activity. Each point represents an individual woman. Plasma adenosine levels are expressed relative to 1 μg of total plasma protein. Values are expressed as the mean ± S.E.M. p value is provided for each comparison
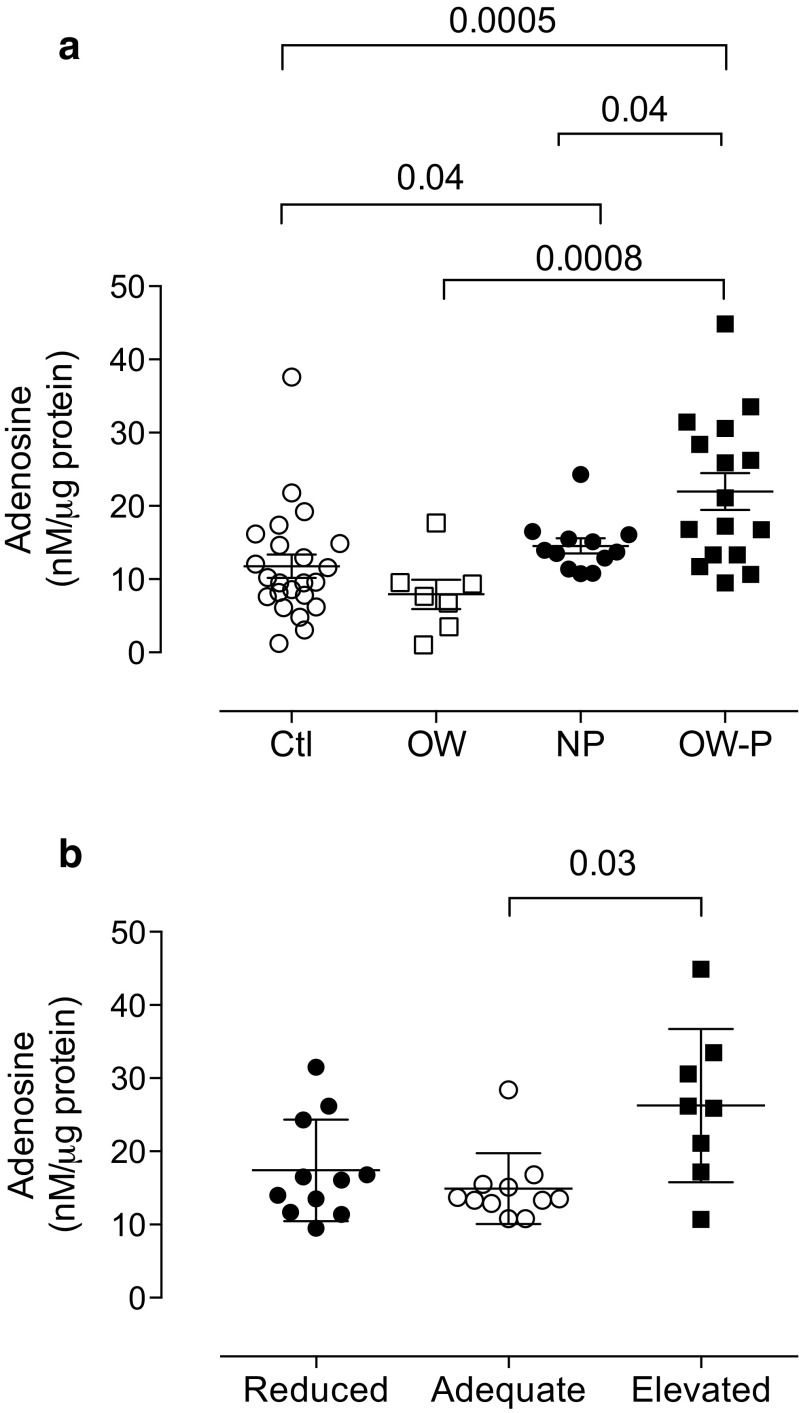
Post-hoc analysis considering BMI showed that overweight/obese pregnant women (21.9 ± 2.5 nM/μg protein) exhibited higher adenosine levels than lean pregnant (14.5 ± 1.0 nM/μg protein, p = 0.04) or non-pregnant women (11.7 ± 1.5 nM/μg protein, p = 0.0005). No differences in pregnant and non-pregnant women with overweight/obesity were observed (Fig. 2a).
Fig. 2.
Plasma adenosine levels in overweight/obese pregnant women. (a) HPLC was used to measure adenosine levels in plasma samples from lean non-pregnant (control, Ctl, n = 23), overweight/obese non-pregnant (OW, n = 7), lean pregnant (NP, n = 12), and overweight/obese pregnant women in their third trimester (OW-P, n = 18). (b) Plasma adenosine levels considering reduced (n = 11), adequate (n = 11), or elevated (n = 8) weight gain in pregnant women in their third trimester. Each point represents an individual woman. Values are expressed as the mean ± S.E.M. p value is provided for each comparison
Clinical characteristics such as gestational age and systolic and diastolic blood pressures were not statistically different among the pregnant women with adequate, reduced, or elevated weight gain (Table 3). Pregnant women with elevated weight gain exhibited significantly higher (1.7-fold, 26.2 ± 3.7 nM/μg protein) adenosine levels than those with adequate weight gain during pregnancy (14.9 ± 1.4 nM/μg protein, p = 0.03). Meanwhile, there were no differences with pregnant women with reduced weight gain (17.4 ± 2.1 nM/μg protein, p = 0.053) (Fig. 2b).
Table 3.
Characteristics of included women according to weight gain at third trimester of pregnancy
| Reduced (n = 11) |
Adequate (n = 11) |
Elevated (n = 8) |
ANOVA | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Gestational age (weeks) | 34.7 ± 0.7 | 35.8 ± 0.7 | 35.3 ± 0.6 | NS |
| BMI1 (kg/m2) | 30.5 ± 2.3 | 28.4 ± 0.9 | 32.7 ± 0.9* | 0.03 |
| BMI2 (kg/m2) | 28.3 ± 2.4 | 23.8 ± 1.2 | 26.6 ± 1.6 | NS |
| Weight gain (kg) | 5.5 ± 1.0* | 11.8 ± 0.8 | 15.4 ± 1.9*† | 0.0001 |
| SBP (mm Hg) | 110.9 ± 4.3 | 110.3 ± 3.0 | 113.1 ± 5.9 | NS |
| DBP (mm Hg) | 70.0 ± 4.3 | 71.7 ± 4.1 | 72.0 ± 5.6 | NS |
| Food consumption | ||||
| Calories (cal) | 1849 ± 163 | 2257 ± 211 | 1746 ± 180 | NS |
| Proteins (g/day) | 61.6 ± 6.5 | 76.2 ± 16.4 | 60.7 ± 9.1 | NS |
| Lipids (g/day) | 53.3 ± 7.5* | 75.5 ± 6.8 | 57.5 ± 15.8* | 0.05 |
| Cholesterol (mg/day) | 150.3 ± 31.1 | 227 ± 69.7 | 134.0 ± 34.8 | NS |
| Saturated fatty acids (mg/day) | 15.9 ± 3.3 | 21.1 ± 2.7 | 19.5 ± 8.2 | NS |
| Monounsaturated fatty acids (mg/day) | 17.1 ± 3.0 | 23.6 ± 2.9 | 18.1 ± 4.5 | NS |
| Polyunsaturated fatty acids (mg/day) | 15.2 ± 6.2 | 14.9 ± 2.8 | 9.3 ± 1.8 | NS |
| Carbohydrates (g/day) | 282.6 ± 21.9 | 313 ± 33.2 | 278.8 ± 36.1 | NS |
| Laboratory measurements | ||||
| Total cholesterol (mg/dl) | 249.5 ± 16.7 | 298.5 ± 18.0 | 258.1 ± 15.1 | NS |
| Cholesterol LDL (mg/dl) | 137.0 ± 19.0 | 189.2 ± 17.3 | 146.7 ± 14.1 | NS |
| Cholesterol HDL (mg/dl) | 64.6 ± 4.3 | 56.0 ± 3.7 | 59.5 ± 3.8 | NS |
| Triglycerides (mg/dl) | 193.9 ± 17.0* | 284.9 ± 34.4 | 259.3 ± 25.6 | 0.03 |
| Glucose (mg/dl) | 82.3 ± 8.2 | 73.4 ± 1.9 | 92.5 ± 13.6 | NS |
| Delta adenosine (nM/μg protein) | 80.7 ± 14.1 (n = 5) | 75.6 ± 20.7 (n = 3) | 92.5 ± 33.2 (n = 2) | – |
ANOVA analysis of the anthropometric, clinical evaluation, and nutritional and biochemical parameters in the studied pregnant women considering weight gain during gestation. Reduced and elevated groups include pregnant women with reduced or elevated weight gain, respectively [5]. Values are expressed as the mean ± S.E.M.
BMI body mass index, BMI 1 body mass index in the third trimester of pregnancy, BMI 2 non-pregnancy body mass index, SBP and DBP systolic and diastolic blood pressure, respectively
*p < 0.05 vs adequate weight gain
† p < 0.05 vs reduced weight gain
Pregnant women with reduced and elevated weight gain self-reported reduced lipid intake compared to those women with adequate weight gain (ANOVA, p = 0.05). No other differences were observed in the self-reported food intake in these groups of pregnant women.
Regarding lipid levels, only those women with reduced weight gain exhibited low triglycerides compared to women with adequate weight gain (Table 3). Nevertheless, the relative estimation of ADA activity and the other biochemical parameters analyzed were similar in the three groups of pregnant women.
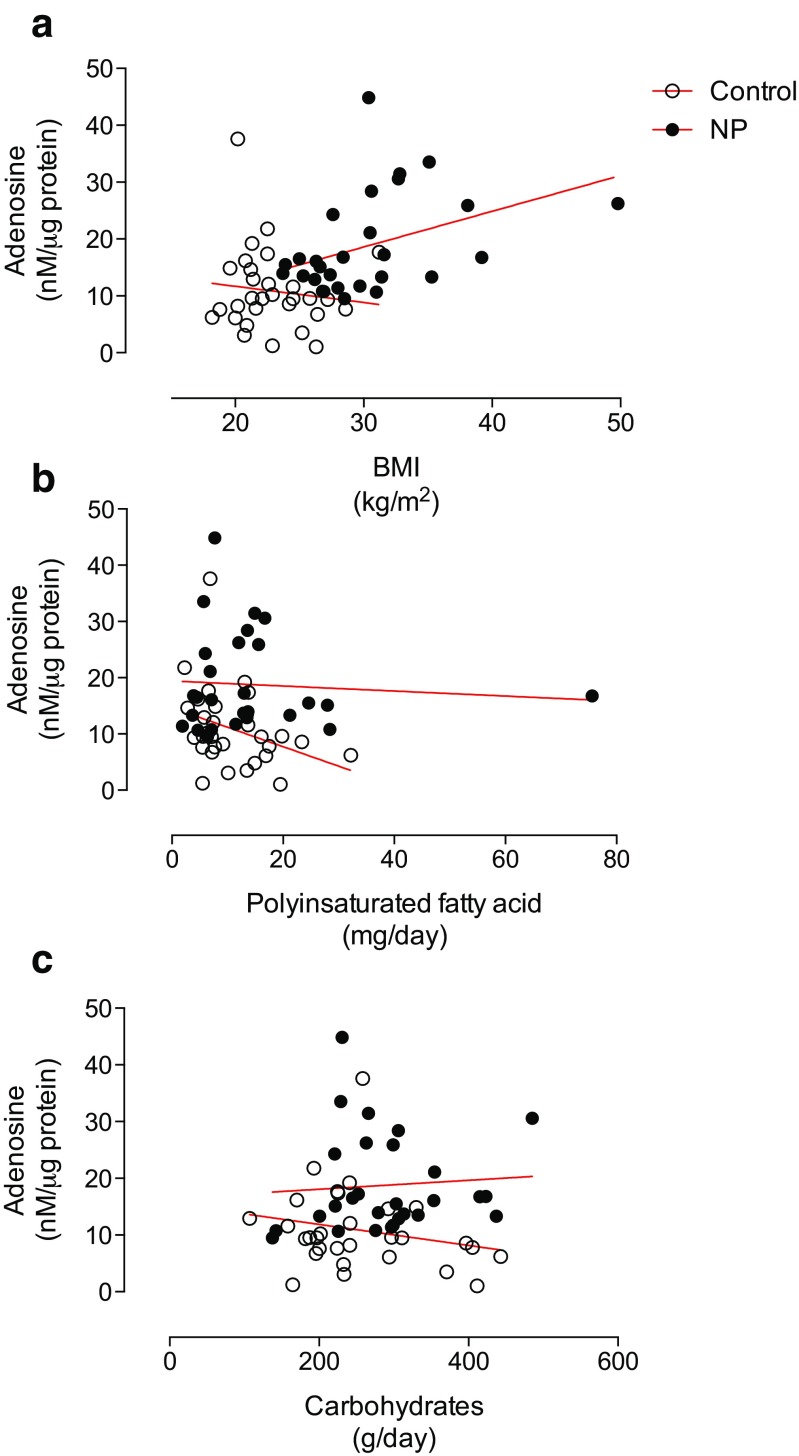
Correlative analysis showed a significant association between BMI at third trimester and plasma adenosine levels in pregnant women (r = 0.39, 95% IC 0.03 to 0.67, p = 0.02) but not in non-pregnant women (r = −0.11, 95% IC −0.45 to 0.25, p = NS) (Fig. 3a).
Fig. 3.
Correlative analysis of plasma adenosine levels in pregnant and non-pregnant women. HPLC was used to measure adenosine levels in plasma samples from non-pregnant (control, n = 30) and pregnant women (NP, n = 30). The adenosine level was correlated with (a) body mass index (BMI) or estimated consumption of (b) polyunsaturated fatty acids (PUFA) or (c) carbohydrates. Significant correlations were only observed with BMI in normal pregnancy (r = 0.39, 95% IC 0.03 to 0.67, p = 0.02) and PUFA consumption in non-pregnant women (r = −0.33, 95% IC −0.61 to 0.03, p = 0.03)
On the other hand, estimated PUFA intake was negatively correlated with plasma adenosine levels only in non-pregnant women (r = −0.33, 95% IC −0.61 to 0.03, p = 0.03) (Fig. 3b). No other significant correlations were observed with carbohydrate consumption (Fig. 3c) or the other clinical and nutritional and laboratory variables recorded in this study (Table 4).
Table 4.
Correlative analysis between adenosine plasma levels and clinical and nutritional variables
| Non-pregnant | Pregnant | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | −0.12 | 0.27 | 0.11 | 0.29 |
| Gestational age | – | – | −0.03 | 0.44 |
| SBP | −0.27 | 0.08 | 0.04 | 0.42 |
| DBP | −0.08 | 0.34 | −0.01 | 0.47 |
| Age at menarche | 0.13 | 0.25 | 0.21 | 0.13 |
| Weight before pregnancy | – | – | 0.20 | 0.14 |
| BMI before pregnancy | – | – | 0.17 | 0.19 |
| Weight gain | – | – | 0.18 | 0.17 |
| Calories intake | −0.15 | 0.21 | 0.19 | 0.15 |
| Protein intake | 0.09 | 0.33 | −0.03 | 0.43 |
| Lipids intake | −0.10 | 0.30 | 0.09 | 0.32 |
| Saturated fatty acid intake | −0.15 | 0.21 | 0.00 | 0.50 |
| Mono saturated fatty acid intake | −0.02 | 0.45 | −0.07 | 0.36 |
| Cholesterol intake | −0.08 | 0.34 | −0.02 | 0.46 |
| Sodium intake | −0.09 | 0.31 | 0.04 | 0.42 |
| Potassium intake | 0.02 | 0.45 | −0.20 | 0.14 |
| Calcium intake | 0.03 | 0.44 | −0.13 | 0.25 |
| Vitamin C intake | −0.09 | 0.32 | −0.18 | 0.17 |
| Circulating total cholesterol | −0.06 | 0.39 | 0.15 | 0.22 |
| Circulating LDL | −0.13 | 0.25 | 0.19 | 0.16 |
| Circulating HDL | 0.10 | 0.30 | −0.15 | 0.21 |
| Circulating triglycerides | 0.03 | 0.44 | 0.00 | 0.49 |
| Glycaemia | 0.06 | 0.37 | 0.18 | 0.17 |
| Insulin plasma levels | −0.24 | 0.11 | 0.03 | 0.44 |
Pearson correlation (r). p value. SBP and DBP systolic and diastolic blood pressure, respectively
Discussion
This study confirms the elevation of plasma adenosine levels in pregnant women, with these levels being even higher in overweight/obese pregnant women. Interestingly, pregnant women who exhibited elevated weight gain also exhibited high levels of adenosine compared to those with adequate weight gain during pregnancy. In addition, among the anthropometric and biochemical variables investigated in this study, only BMI was positively correlated with plasma adenosine levels in pregnant women. Elevated levels of adenosine in overweight/obese pregnant women may play a major role in overall physiological adaptation during pregnancy, including cardiovascular, neurological, and immunological adaptations.
Adenosine in pregnancy
Compared with non-pregnant women, normal pregnant women exhibit increased synthesis but reduced catabolism of adenosine [13, 24]. Yoneyama and colleagues [12, 13] showed that normal pregnant women in their third trimester had higher plasma adenosine concentrations (~0.5 μM) than non-pregnant women (~0.2 μM); this finding was also confirmed in the current study. However, the levels reported by Yoneyama’s group are higher than those reported in this manuscript by at least one order of magnitude. These differences can be attributed to the units used for expressing circulating adenosine levels. Because increased levels of adenosine in pregnancy may constitute only a matter of volume (blood volume increases by approximately 1.5 l in normal gestation), we decided to standardize adenosine concentration to protein level.
The causes and consequences of elevated plasma adenosine levels in normal pregnancy are not well understood. However, some reports have linked high plasma adenosine levels to platelet activation during normal pregnancy [12, 13]. Thus, platelet activation may release ATP, one of the main substrates for adenosine synthesis. Nevertheless, adenosine has a powerful anti-aggregation effect on platelets [25]. Thus, the elevation of adenosine levels may indicate a regulatory loop for controlling the overstimulation of platelet aggregation observed during normal pregnancy.
Because plasma adenosine levels increase throughout gestation, it is thought that adenosine might also be related to placental growth and vascular function [26, 27]. However, it is unclear whether maternal adenosine has any role in the regulation of placental circulation. Indirect evidence has indicated a negative correlation between maternal plasma adenosine levels and uterine artery pulsatility index (PI), suggesting that adenosine might lead to reduced vessel resistance in the maternal side of the placenta [28]. More recently, Iriyama and colleagues [16] found that elevated placental adenosine levels in mice were linked with abnormal placental vessel formation, maternal hypertension and proteinuria, as well as elevation of the circulating levels of anti-angiogenic proteins, all of which are hallmarks of human pre-eclampsia. Unfortunately, this last study did not measure plasma adenosine levels in the maternal circulation. However, it has been suggested that the supraphysiological elevation of plasma adenosine levels may have deleterious effects on both the vascular system and maternal and feto-placental circulation [16].
Other reports have suggested that high plasma adenosine levels in the maternal circulation might be an immunological adaptation, in particular in women with pre-eclampsia [29]; however, there is no evidence regarding how adenosine might control this phenomenon in normal pregnancies.
Elevated plasma adenosine levels in pregnancy have been linked to reduced total ADA activity [12]. Despite some discrepant results [30], this reduction in ADA activity seems to depend mainly on the soluble isoform of ADA (ADA2) rather than membrane-linked ADA (ADA1) [31]. Additionally, total ADA activity is high during the first and second trimesters but not during the third trimester [24, 32]. Our results are in agreement with this last analysis, as a trend toward reduced ADA activity was only observed in pregnant women in their third trimester compared to control women.
Adenosine in obesity
As far as we know, this study is the first to describe high plasma adenosine levels in overweight/obese with normal pregnancy during their third trimester of gestation. These results agree with previous reports in obese adults showing high adenosine levels in adipose tissue homogenates [17]. We also found high plasma adenosine levels in obese children [18]. Because adipocytes [17, 33] and the placenta [34] have all the metabolic machinery necessary for synthesizing adenosine, it is not surprising that overweight/obese pregnant women exhibit high adenosine levels.
The biological significance of high adenosine levels in overweight/obese pregnant women is unknown. In this regard, despite the fact that we could not find significant correlations between lipid and glucose levels in any of the studied groups, a potential role for adenosine in metabolic adaptations during pregnancy cannot be ruled out. More studies are required.
Adenosine might also reach other tissues such as the lungs, kidneys, and the brain. In this last regard, an intriguing idea partially addressed in this manuscript is the association between plasma adenosine levels and nutrient intake. In this regard, Levine and Morley [8] described that the intraperitoneal or intracerebroventricular administration of adenosine selectively suppressed food and water intake compared to sham-injected animals. It has also been noted that adenosine, via activation of the adenosine A2A receptor, reduces the intake of high-palatability foods in female rats [9] but may increase the intake of high-fat foods in male rats [10].
The results in the current study showed a significant negative correlation between self-reported PUFA consumption and plasma adenosine levels in non-pregnant women. Explanation for this relationship is unclear. But, an early report from Ammouche and colleagues [35] showed that the specific activity of nucleoside triphosphatase (NTP-ase, enzyme involved in degradation of ATP to ADP) was positively correlated with dietary PUFA n−6 but negatively correlated with dietary PUFA n−3 in rats. Despite we did not establish specific source of PUFA, it is feasible that PUFA consumption might regulate enzymes involved in the synthesis of adenosine. Further studies are needed to elucidate the relationship between adenosine plasma levels and PUFA consumption.
In conclusion, normal pregnancy is associated with high plasma adenosine levels, which are further elevated in pregnant women who are overweight/obese. Thus, our results suggest that increased weight gain during pregnancy results in higher plasma adenosine levels. There seems to be a relationship between high polyunsaturated fatty acid intake and plasma adenosine levels in non-pregnant women. These findings represent the first evidence presented in medical literature, and more direct studies are required to gain a better understanding of the physiological implications of these findings.
Acknowledgements
We are deeply grateful to the midwives and medical staff of the Department of Obstetrics and Gynecology at the Hospital Clinico Herminda Martin in Chillán, Chile. We would like to thank all the research staff at the Vascular Physiology Laboratory and the Group of Investigation of Tumor Angiogenesis (GIANT) of the Universidad del Bío-Bío for their technical support. We also thank the researchers in the GRIVAS Health group for an outstanding discussion of the results presented in this manuscript. We thank Dr. Padma Murthi from the Department of Medicine, School of Clinical Sciences, Monash University for editing this manuscript. This study was financially supported by Fondecyt Regular 1140586, Fondequip EQM140104, and DIUBB GI153109/EF and GI 152920/EF.
Abbreviations
- ADA
adenosine deaminase
- ADP
adenosine di-phosphate
- AMP
adenosine monophosphate
- ATP
adenosine tri-phosphate
- BMI
body mass index
- EHNA
erythro-9-(2-hydroxyl-3-nonyl) adenine hydrochloride (ADA inhibitor)
- HPLC
high performance liquid chromatography
- IOM
Institute of Medicine
- MUFA
monounsaturated fatty acids
- PUFA
polyunsaturated fatty acids
- SBP and DBP
systolic and diastolic blood pressure
- SFA
saturated fatty acids
Compliance with ethical standards
Conflicts of interest
Priscila Badillo declares that she has no conflict of interest.
Paola Salgado declares that she has no conflict of interest.
Patricia Bravo declares that she has no conflict of interest.
Katherine Guevara declares that she has no conflict of interest.
Jesenia Acurio declares that she has no conflict of interest.
Maria Angelica Gonzalez declares that she has no conflict of interest.
Carlos Oyarzun declares that he has no conflict of interest.
Rody San Martin declares that he has no conflict of interest.
Carlos Escudero declares that he has no conflict of interest.
Ethical approval
This cross-sectional study was approved by the Ethical Committee from the Universidad del Bío-Bío (Fondecyt 1140586, October 8th, 2013) in accordance with protocols defined by the Declaration of Helsinki Ethics Committee. Women were recruited in this study after signing an informed written consent.
Financial disclosure
None.
References
- 1.Darnton-Hill I, Mkparu UC. Micronutrients in pregnancy in low- and middle-income countries. Nutrients. 2015;7(3):1744–1768. doi: 10.3390/nu7031744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92(5):1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 3.Albala C, Vio F, Kain J, Uauy R. Nutrition transition in Chile: determinants and consequences. Public Health Nutr. 2002;5(1A):123–128. doi: 10.1079/PHN2001283. [DOI] [PubMed] [Google Scholar]
- 4.Bambs C, Cerda J, Escalona A. Morbid obesity in a developing country: the Chilean experience. Bull World Health Organ. 2008;86(10):813–814. doi: 10.2471/BLT.07.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Health TNACRfbNIo (2009). In: Rasmussen KM, Yaktine AL (eds) Weight gain during pregnancy: reexamining the guidelines. The national academies collection: reports funded by National Institutes of Health. Washington (DC) [PubMed]
- 6.Walsh JM, McAuliffe FM. Impact of maternal nutrition on pregnancy outcome—does it matter what pregnant women eat? Best Pract Res Clin Obstet Gynaecol. 2015;29(1):63–78. doi: 10.1016/j.bpobgyn.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1(4):170–178. [PMC free article] [PubMed] [Google Scholar]
- 8.Levine AS, Morley JE. Effect of intraventricular adenosine on food intake in rats. Pharmacol Biochem Behav. 1983;19(1):23–26. doi: 10.1016/0091-3057(83)90305-2. [DOI] [PubMed] [Google Scholar]
- 9.Micioni Di Bonaventura MV, Cifani C, Lambertucci C, Volpini R, Cristalli G, Massi M. A2A adenosine receptor agonists reduce both high-palatability and low-palatability food intake in female rats. Behav Pharmacol. 2012;23(5–6):567–574. doi: 10.1097/FBP.0b013e3283566a60. [DOI] [PubMed] [Google Scholar]
- 10.Pritchett CE, Pardee AL, McGuirk SR, Will MJ. The role of nucleus accumbens adenosine-opioid interaction in mediating palatable food intake. Brain Res. 2010;1306:85–92. doi: 10.1016/j.brainres.2009.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa M, Pardo M, Bayarri P, Lopez-Cruz L, San Miguel N, Valverde O, Ledent C, Salamone JD. Choosing voluntary exercise over sucrose consumption depends upon dopamine transmission: effects of haloperidol in wild type and adenosine A(2)AKO mice. Psychopharmacology. 2016;233(3):393–404. doi: 10.1007/s00213-015-4127-3. [DOI] [PubMed] [Google Scholar]
- 12.Yoneyama Y, Suzuki S, Sawa R, Otsubo Y, Power GG, Araki T. Plasma adenosine levels increase in women with normal pregnancies. Am J Obstet Gynecol. 2000;182(5):1200–1203. doi: 10.1067/mob.2000.104832. [DOI] [PubMed] [Google Scholar]
- 13.Yoneyama Y, Suzuki S, Sawa R, Takeuchi T, Kobayashi H, Takei R, Kiyokawa Y, Otsubo Y, Hayashi Z, Araki T. Changes in plasma adenosine concentrations during normal pregnancy. Gynecol Obstet Investig. 2000;50(3):145–148. doi: 10.1159/000010313. [DOI] [PubMed] [Google Scholar]
- 14.Escudero C, Casanello P, Sobrevia L. Human equilibrative nucleoside transporters 1 and 2 may be differentially modulated by A2B adenosine receptors in placenta microvascular endothelial cells from pre-eclampsia. Placenta. 2008;29(9):816–825. doi: 10.1016/j.placenta.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Westermeier F, Salomon C, Gonzalez M, Puebla C, Guzman-Gutierrez E, Cifuentes F, Leiva A, Casanello P, Sobrevia L. Insulin restores gestational diabetes mellitus-reduced adenosine transport involving differential expression of insulin receptor isoforms in human umbilical vein endothelium. Diabetes. 2011;60(6):1677–1687. doi: 10.2337/db11-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iriyama T, Sun K, Parchim NF, Li J, Zhao C, Song A, Hart LA, Blackwell SC, Sibai BM, Chan LL, Chan TS, Hicks MJ, Blackburn MR, Kellems RE, Xia Y (2014) Elevated placental adenosine signaling contributes to the pathogenesis of preeclampsia. Circulation. doi:10.1161/CIRCULATIONAHA.114.013740 [DOI] [PMC free article] [PubMed]
- 17.Ranta S, Kiviluoto T, Newby AC, Ohisalo JJ. Assay of adenosine in human adipose tissue. Acta Endocrinol. 1985;110(3):429–432. doi: 10.1530/acta.0.1100429. [DOI] [PubMed] [Google Scholar]
- 18.Escudero A, Carreno B, Retamal N, Celis C, Castro L, Aguayo C, Acurio J, Escudero C. Elevated concentrations of plasma adenosine in obese children. Biofactors. 2012;38(6):422–428. doi: 10.1002/biof.1039. [DOI] [PubMed] [Google Scholar]
- 19.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, Staples RC, Cleveland LE. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt-Hebbel H, Pennacchiotti I, Masson L, Mella MA. Tabla de composicion quimica de alimentos chilenos. Chile: Universidad de Chile; 1992. [Google Scholar]
- 21.Yoneyama Y, Sawa R, Suzuki S, Shin S, Power GG, Araki T. The relationship between uterine artery Doppler velocimetry and umbilical venous adenosine levels in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 1996;174(1 Pt 1):267–271. doi: 10.1016/S0002-9378(96)70406-4. [DOI] [PubMed] [Google Scholar]
- 22.Maguire MH, Szabo I, Valko IE, Finley BE, Bennett TL. Simultaneous measurement of adenosine and hypoxanthine in human umbilical cord plasma using reversed-phase high-performance liquid chromatography with photodiode-array detection and on-line validation of peak purity. J Chromatogr B Biomed Sci Appl. 1998;707(1–2):33–41. doi: 10.1016/S0378-4347(97)00581-1. [DOI] [PubMed] [Google Scholar]
- 23.Roa H, Gajardo C, Troncoso E, Fuentealba V, Escudero C, Yanez A, Sobrevia L, Pastor-Anglada M, Quezada C, San Martin R. Adenosine mediates transforming growth factor-beta 1 release in kidney glomeruli of diabetic rats. FEBS Lett. 2009;583(19):3192–3198. doi: 10.1016/j.febslet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Hwang HS, Kim BN, Kim MA, Lee JW, Park YW, Kim YH. Changes in serum adenosine deaminase activity during normal pregnancy. J Korean Med Sci. 2007;22(4):718–721. doi: 10.3346/jkms.2007.22.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuentes E, Pereira J, Mezzano D, Alarcon M, Caballero J, Palomo I. Inhibition of platelet activation and thrombus formation by adenosine and inosine: studies on their relative contribution and molecular modeling. PLoS One. 2014;9(11):e112741. doi: 10.1371/journal.pone.0112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Read MA, Boura AL, Walters WA. Vascular actions of purines in the foetal circulation of the human placenta. Br J Pharmacol. 1993;110(1):454–460. doi: 10.1111/j.1476-5381.1993.tb13832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donoso MV, Lopez R, Miranda R, Briones R, Huidobro-Toro JP. A2B adenosine receptor mediates human chorionic vasoconstriction and signals through arachidonic acid cascade. Am J Physiol Heart Circ Physiol. 2005;288(5):H2439–H2449. doi: 10.1152/ajpheart.00548.2004. [DOI] [PubMed] [Google Scholar]
- 28.Grunewald C, Kublickas M, Nisell H, Nylund L, Westgren M. The interpretation of uterine artery pulsatility index in normal and hypertensive pregnancy. Ultrasound Obstet Gynecol. 1994;4(6):476–479. doi: 10.1046/j.1469-0705.1994.04060476.x. [DOI] [PubMed] [Google Scholar]
- 29.Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Relation between adenosine and T-helper 1/T-helper 2 imbalance in women with preeclampsia. Obstet Gynecol. 2002;99(4):641–646. doi: 10.1016/s0029-7844(02)01657-5. [DOI] [PubMed] [Google Scholar]
- 30.Mokhtari M, Hashemi M, Yaghmaei M, Molashahi F, Shikhzadeh A, Niazi A, Ghavami S. Serum adenosine deaminase activity in gestational diabetes mellitus and normal pregnancy. Arch Gynecol Obstet. 2010;281(4):623–626. doi: 10.1007/s00404-009-1148-3. [DOI] [PubMed] [Google Scholar]
- 31.Yoneyama Y, Suzuki S, Sawa R, Otsubo Y, Miura A, Kuwabara Y, Ishino H, Kiyokawa Y, Doi D, Yoneyama K, Araki T. Serum adenosine deaminase activity and its isoenzyme pattern in women with normal pregnancies. Arch Gynecol Obstet. 2003;267(4):205–207. doi: 10.1007/s00404-002-0312-9. [DOI] [PubMed] [Google Scholar]
- 32.Jaqueti J, Martinez-Hernandez D, Hernandez-Garcia R, Navarro-Gallar F, Arenas-Barbero J. Adenosine deaminase in pregnancy serum. Clin Chem. 1990;36(12):2144. [PubMed] [Google Scholar]
- 33.Strouch MB, Jackson EK, Mi Z, Metes NA, Carey GB. Extracellular cyclic AMP-adenosine pathway in isolated adipocytes and adipose tissue. Obes Res. 2005;13(6):974–981. doi: 10.1038/oby.2005.114. [DOI] [PubMed] [Google Scholar]
- 34.Escudero C, Sobrevia, L (2009) Understanding physiological significance of high extracellular adenosine levels in feto-placental circulation in preeclamptic pregnancies. In: Sobrevia L, Casanello, P (ed) Membrane transporters and receptors in disease, vol 1. Research signpost, Kerala, India, pp 27–51
- 35.Ammouche A, Youyou Y, Durand G, Bourre JM. Effects of dietary fats on nucleoside triphosphatase activity and nuclear membrane fatty acid composition of rats during development. Ann Nutr Metab. 1994;38(3):132–140. doi: 10.1159/000177803. [DOI] [PubMed] [Google Scholar]