Abstract
Adenosine A2B receptors (A2BR) regulate several enteric functions. However, their implication in the pathophysiology of intestinal dysmotility associated with high-fat diet (HFD)-induced obesity has not been elucidated. We investigated the expression of A2BR in mouse colon and their role in the mechanisms underlying the development of enteric dysmotility associated with obesity. Wild-type C57BL/6J mice were fed with HFD (60% kcal from fat) or normocaloric diet (NCD; 18% kcal from fat) for 8 weeks. Colonic A2BR localization was examined by immunofluorescence. The role of A2BR in the control of colonic motility was examined in functional experiments on longitudinal muscle preparations (LMPs). In NCD mice, A2BR were predominantly located in myenteric neurons; in HFD animals, their expression increased throughout the neuromuscular layer. Functionally, the A2BR antagonist MRS1754 enhanced electrically induced NK1-mediated tachykininergic contractions in LMPs from HFD mice, while it was less effective in tissues from NCD mice. The A2B receptor agonist BAY 60-6583 decreased colonic tachykininergic contractions in LMPs, with higher efficacy in preparations from obese mice. Both A2BR ligands did not affect contractions elicited by exogenous substance P. Obesity is related with a condition of colonic inflammation, leading to an increase of A2BR expression. A2BR, modulating the activity of excitatory tachykininergic nerves, participate to the enteric dysmotility associated with obesity.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-017-9577-0) contains supplementary material, which is available to authorized users.
Keywords: A2B receptors, Colonic motor dysfunctions, Substance P, Obesity
Introduction
In the last few decades, the prevalence of obesity has reached epidemic proportions, becoming a serious challenge for global public health [1]. Indeed, beyond an excessive fat accumulation, the chronic nature of obesity gives rise to a low-grade systemic inflammation that seems to be a common root to the onset and progression of other severe disorders, such as hypertension, cardiovascular disease, type 2 diabetes, fatty liver disease, and cancer [2].
Increasing epidemiologic data indicate that obesity is tightly related also with chronic gastrointestinal complaints [3], many of which overlap with common functional digestive disorders, with particular regard for constipation [4–6]. Despite more specific investigations are needed to better characterize this correlation in the clinical setting, interesting findings, obtained in the murine model of high-fat diet (HFD)-induced obesity, have revealed the occurrence of neuroplastic changes in the enteric nervous system (ENS) of the stomach and small intestine [7, 8]. Of note, increased mucosal permeability and signs of inflammation have been observed also in bowel tissues from HFD mice or rats [9–11], leading to hypothesize that a rearrangement of ENS, along with other tissue alterations occurring under obesity, might be the consequence of a low-grade enteric inflammation. In this regard, it has been reported that intrinsic enteric tachykininergic nerves, pivotally involved in the physiological regulation of digestive motility, undergo significant rearrangements under pathological conditions, including bowel motor dysfunctions associated with inflammatory disorders [12, 13]. However, limited information are available about the cellular and molecular mechanisms underlying intestinal motor dysfunctions in the presence of obesity, and further investigations are needed.
Over the years, a critical role has been shown for the adenosine system in orchestrating interplays among ENS, enteric smooth muscle, and mucosal/immune functions to maintain homeostatic conditions in the digestive tract as well as to ensure adequate adaptive responses in the presence of adverse conditions [14]. Indeed, adenosine participates actively to the regulation of complex interactions between enteric neurons and smooth muscle cells, operating a fine tuning on gastrointestinal neuromuscular functions [14]. In this context, current data indicate that the A2B receptor is a critical player in mediating the actions of adenosine on colonic motility under physiological conditions in guinea pigs [15], rats [16], and mice [17, 18]. Moreover, a recent study showed an involvement of A2B receptors in colonic motor dysfunctions associated with experimental colitis. In particular, a marked upregulation of A2B receptor expression was observed in the colonic neuromuscular compartment upon induction of bowel inflammation [19].
Based on the above background, the significance of the present research work stems from the interest of shedding light on the possible role played by A2B adenosine receptors in the mechanisms underlying the development of enteric dysmotility associated with obesity, with the purpose of identifying a novel target to develop promising pharmacological therapeutic interventions.
Materials and methods
Animal model of diet-induced obesity
Six-week-old male C57BL/6 mice (20–25 g body weight) were purchased from ENVIGO Srl (San Pietro al Natisone UD, Italy) and employed throughout the study. Mice were fed with a high fat diet (HFD, 60% calories from fat, TD.06414) or normocaloric diet (NCD, 18% calories from fat; TD.2018) (see Table 1) for 8 weeks (eight mice per group). HFD and NCD were purchased from ENVIGO. The animals were housed, three in a cage, in temperature-controlled rooms on a 12-h light cycle at 22–24 °C and 50–60% humidity. Their care and handling were in accordance with the provisions of the European Community Council Directive 210/63/UE, recognized and adopted by the Italian Government. The experiments were approved by the Ethical Committee for Animal Experimentation of the University of Pisa and by the Italian Ministry of Health (authorization no. 744/2015-PR).
Table 1.
Effects of high-fat diet (HFD), on systematic metabolic parameters, as compared with normocaloric diet (NCD)
| NCD | HFD | |
|---|---|---|
| Glucose (mg/dl) | 134 ± 8 | 167 ± 5* |
| Cholesterol (mg/dl) | 165 ± 7 | 179 ± 4* |
| Triglycerides (mg/dl) | 125 ± 8 | 149.3 ± 6* |
*P < 0.05 versus NCD mice
Measurement of body mass index and metabolic parameters
Body mass index (BMI) was calculated by dividing body weight (g) by body length (mm) squared (BMI = body weight/body length2) as described by Smemo et al. [20].
Blood samples were taken from the tail after overnight starvation. Cholesterol, triglycerides, and glucose levels were measured using Multicare Insensor (BSI Srl, Arezzo, Italy), in accordance with the manufacturer’s instructions.
Tissue IL-6 levels
IL-6 levels in colonic neuromuscular tissues were measured with enzyme-linked immune-sorbent assay (ELISA) kits (Abcam, Cambridge, UK), following manufacturers’ protocols. For this purpose, colonic tissue samples, stored previously at −80 °C, were weighed, thawed, and homogenized in 0.4 mL of PBS, pH 7.2/20 mg of tissue at 4 °C, and centrifuged at 10,000g for 5 min. Aliquots (100 μL) of the supernatants were then used for the assay. IL-6 levels were expressed as picogram per milligram of colonic tissue.
Tissue malondialdehyde levels
Malondialdehyde (MDA) concentration in specimens of colonic neuromuscular tissues was assessed to obtain a quantitative estimation of membrane lipid peroxidation. The assay was performed as previously described [21]. Colonic tissues were weighed, minced by forceps, homogenized in 2 mL of cold buffer (20 mM RIPA buffer, pH 7.4) by a polytron homogenizer (QIAGEN, Milan, Italy), and spun by centrifugation at 1600g for 10 min at 4 °C. Colonic MDA concentrations were determined with a kit for colorimetric assay (Calbiochem, San Diego, CA, USA), and the results were expressed as nanomole of MDA per milligram of colonic tissue.
Histological analysis
Formalin-fixed and paraffin-embedded specimens of colonic tissues were cross sectioned and processed for routine hematoxylin/eosin staining, aimed at evaluating the morphology of colonic wall architecture. The severity and extent of inflammatory infiltrations were estimated as percentage of leucocytes per representative microscopic high-power field (hpf; 0.289 mm2), as well as their extension through the colonic wall layers, respectively, as proposed by Erben et al. [22]. Tissue sections were examined by a Leica DMRB light microscope, and representative photomicrographs were taken by a DFC480 digital camera (Leica Microsystems, Cambridge, UK).
Immunofluorescence imaging
Colonic tissue samples were frozen on dry ice in optimal cutting temperature mounting medium, sectioned (7 μm thick) with a cryostat microtome (Leica CM 1850 UV, Milan, Italy), and then mounted onto Superfrost Plus slides. From each colonic specimen, 100 sequential 7-μm cross sections were cut on a cryostat and 6–8 sections were subjected to immunohistochemistry as previously described [23, 24]. Colonic cryosections were then incubated overnight at room temperature with the following antibodies: rabbit polyclonal anti-human A2B receptor (A2BR23; 1∶100; Alpha Diagnostic International Inc., San Antonio, TX, USA), guinea pig polyclonal anti-mouse substance P (1∶100; Abcam, Cambridge, UK), and mouse biotin-conjugated anti-human-HuC/D (1∶100; Thermo Fisher Scientific, Milan, Italy).
Cryosections were washed and incubated with the following secondary antibodies: goat anti-rabbit IgG Dylight 649 (1:500, Jackson ImmunoResearch laboratories, West Grove, PA, USA), goat anti-guinea pig IgG Alexa Fluor 488 conjugate (1:1000; Thermo Fisher Scientific Milan, Italy), and streptavidin labeled with Alexa Fluor 555 (1:1000, Thermo Fisher Scientific, Milan, Italy) for 1 h at RT. Nuclei were stained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) (1:1000; Thermo Fisher Scientific, Milan, Italy). After three washes, colonic cryosections were mounted on glass slides using a Mowiol Mounting Medium (100 mM Tris-HCl (pH 8.5), 9% Mowiol 4–88, 25% glycerol, and 0.1% DABCO).
Negative controls were obtained by incubating sections with isotype-matched control antibodies at the same concentration as the primary antibody and/or pre-incubating each antibody with the corresponding control peptide (final concentration as indicated by manufacturer’s instructions). Confocal microscopy was performed with a Yokogawa CSU-X1 spinning disk confocal on a Nikon Ti-E inverted microscope equipped with a Plan Apo 60× NA 1.4 objective and were acquired with an Andor Technology iXon3 DU-897-BV EMCCD camera (Nikon Instruments S.p.A., Firenze, Italy). All microscope settings were set to collect images below saturation and were maintained constant for all the images. The immunoreactivity of substance P+ and A2B + cells in colonic frozen sections was determined as previously described (http://www.nature.com/protocolexchange/protocols/3213). Briefly, for each experimental group, 20 images per mouse (n = 5 mice/group) were taken blindly from different stained colonic sections and the colonic neuromuscular compartment was delimited using the Polygon selection tool and was defined as region of interest (ROI) using the open-source platform for biological-image ImageJ software (version 1.48a). Fluorescence values, indicating the fluorescence intensity of each protein of interest (i.e., A2B receptor or substance P), were normalized to the fluorescence intensity of its own DAPI and were reported as mean ± SEM, as previously described [25].
Western blot analysis
Colonic specimens were weighed and homogenized in lysis buffer containing: HEPES 10 mmol/L, NaCl 30 mmol/L, EDTA 0.2 mmol/L, phenylmethylsulfonyl fluoride 2 mmol/L, leupeptin 10 μg/mL, aprotinin 10 μg/mL, sodium fluoride 1 mmol/L, sodium orthovanadate 1 mmol/L, glycerol 2%, MgCl2 0.3 mmol/L, and Triton-X 100 1%, using a polytron homogenizer. Homogenates were spun by centrifugation at 15,000 rpm for 15 min at 4 °C, and the resulting supernatants were then separated from pellets and stored at −80 °C. Protein concentration in each sample was determined by the Bradford method [26] (Protein Assay Kit, Bio-Rad, Hercules, CA, USA). Equivalent amounts of protein lysates (50 μg) were then separated by electrophoresis on SDS-PAGE (4–15%) and transferred onto a PVDF membrane. The blots were incubated overnight at 4 °C with rabbit anti-A2BR (ALPHA DIAGNOSTIC INTERNATIONAL A2BR23-S, 1:1000 dilution), in BSA 1% TBS-T. After repeated washings with 0.1% Tween-2 in Tris-buffered saline, a peroxidase-conjugated donkey anti-rabbit antibody (dilution 1:10,000) was added for 1 h at room temperature. After repeated washings with 0.1% Tween-20 in Tris-buffered saline, immunoreactive bands were visualized by incubation with chemiluminescent reagents and exposed to Kodak Image Station 440 for signal detection and densitometric image analysis.
Recording of contractile activity
The contractile activity of distal colonic longitudinal smooth muscle was recorded as previously described by Antonioli et al. [27], with minor modifications. A midline laparotomy was performed to collect the colon, which was then quickly transferred into a dissecting dish filled with cold pre-oxygenated Krebs solution. Segments of colon were opened along the mesenteric insertion and subjected to removal of the mucosal/submucosal layer. Specimens were then cut along the longitudinal axis into strips of approximately 4-mm width and 10-mm length.
Colonic preparations were set up in organ baths containing Krebs solution at 37 °C, bubbled with 95% O2 + 5% CO2, and connected to isometric transducers (initial load = 0.5 g). Krebs solution had the following composition (mM): NaCl 113, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, and glucose 11.5 (pH 7.4 ± 0.1). Each preparation was allowed to equilibrate for at least 30 min, with intervening washings at 10-min intervals. Mechanical activity was recorded by BIOPAC MP150 (2Biological Instruments, Besozzo, VA, Italy). A pair of coaxial platinum electrodes was positioned at a distance of 10 mm from longitudinal axis of each preparation to deliver electrical stimuli by a BM-ST6 stimulator (Biomedica Mangoni, Pisa, Italy). At the end of equilibration period, each preparation was repeatedly challenged with electrical stimuli, and experiments started when reproducible responses were obtained (usually after two or three stimulations).
Preliminary experiments were performed to select the appropriate electrical stimulation frequency or substance P concentration eliciting submaximal contractions, suitable to better appreciate the effects of test drugs. For this purpose, colonic preparations, obtained from either NCD or HFD animals, were challenged with single (sES) or repeated (rES) electrical stimuli at increasing frequencies, ranging from 1 to 20 Hz (10-s single trains, 0.5 ms, 30 mA). Frequency–response curves were constructed under the following in vitro experimental conditions adopted in the present study: (1) standard Krebs solution; (2) Krebs solution added with guanethidine (noradrenergic blocker, 10 μM); (3) Krebs solution added with guanethidine and Nω-nitro-L-arginine methylester (L-NAME, 100 μM); (4) Krebs solution containing guanethidine, L-732138 (NK1 receptor antagonist, 10 μM), GR159897 (NK2 receptor antagonist, 1 μM) and SB218795 (NK3 receptor antagonist, 1 μM), and L-NAME; and (5) Krebs solution added with guanethidine, atropine (1 μM), and L-NAME.
Concentration–response curves to exogenous substance P were constructed at concentrations ranging from 0.01 to 100 μM in the presence of tetrodotoxin (1 μM). These preliminary experiments allowed to select the frequency of 10 Hz and the concentration of 1 μM of substance P, since all these settings elicited submaximal contractions suitable for evaluating the effects exerted by A2B receptor ligands.
Design of experiments
In the first set of experiments, the effects of N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl) phenoxy]-acetamide (MRS1754, A2B receptor antagonist, 0.01 μM) (see Table 2) were assayed on sES-induced motor responses of colonic preparations maintained in standard Krebs solution. The concentration of MRS1754 was selected on the basis of preliminary experiments (see Supplemental Fig. 1). To verify that MRS1754 acted specifically on A2B receptors, its effects were evaluated also under blockade of A1, A2A, and A3 receptors by incubation of colonic preparations with selective antagonists (DPCPX, 0.01 μM; ZM241385, 0.01 μM; and MRS1220, 0.1 μM, respectively) [28, 29] (see Supplemental Fig. 1).
Table 2.
Summary of the main ligands employed in the present study
| Ligand | Molecular target | Selectivity |
|---|---|---|
| DPCPX | A1 adenosine receptor | Potent and selective receptor antagonist (k i values are 3.9, 130, 50, and 4000 nM for human A1, A2A, A2B, and A3 receptors, respectively) |
| ZM 241385 | A2A adenosine receptor | Potent and highly selective adenosine antagonist (pA2 of 9.02 for A2A receptors in guinea pig cardiac vasculature and selectivities of 1000, 91, and 500,000 over A1, A2B, and A3 sites, respectively) |
| MRS 1754 | A2B adenosine receptor | Potent receptor antagonist (k i values 1.97, 16.8, 403, 503, 570, and 612 nM for hA2B, rA1, hA1, hA2A, hA3, and rA2A receptors, respectively) |
| MRS 1220 | A3 adenosine receptor | Potent and highly selective antagonist (k i values are 0.65, 305, and 52 nM at hA3, rA1, and rA2A, respectively) |
| BAY 60-6583 | A2B adenosine receptor | Potent receptor agonist (k i value 0.33 μM in mouse) |
| L-732138 | NK1 receptor antagonist | Potent and highly selective competitive receptor antagonist (IC50 = 2.3 nM) |
The second series of experiments was performed to evaluate the effects of MRS1754 (0.01 μM) on sES-evoked contractions in colonic preparations maintained in Krebs solution containing guanethidine (10 μM), in order to prevent the recruitment of noradrenergic pathways.
The third series of experiments was aimed at investigating the effects of MRS1754 on sES-evoked contractions in colonic preparations maintained in Krebs solution containing guanethidine and L-NAME (100 μM), in order to prevent the recruitment of nitrergic and noradrenergic pathways.
In the fourth series of experiments, the effects of MRS1754 were tested on contractile responses elicited by sES directed mainly to excitatory cholinergic nerves. Therefore, to prevent non-cholinergic motor responses, the colonic preparations were maintained in Krebs solution added with guanethidine, L-NAME, L-732138 (NK1 receptor antagonist, 10 μM), GR159897 (NK2 receptor antagonist 1 μM), and SB218795 (NK3 receptor antagonist, 1 μM) (see Table 2).
The fifth set of experiments was designed to assay the effects of MRS1754 on sES-evoked contractions in colonic preparations maintained in Krebs solution containing guanethidine, L-NAME, and atropine, in order to prevent the recruitment of noradrenergic, nitrergic, and cholinergic pathways. The NK receptor antagonists L-732138, GR159897, and SB218795 were then added one by one, in order to evaluate which tachykinergic receptor subtype was involved in the effect elicited by A2B receptor blockade.
In the sixth series of experiments, MRS1754 was tested on tachykininergic contractions elicited by direct pharmacological activation of NK1 receptors located on smooth muscle cells. For this purpose, colonic preparations were maintained in Krebs solution containing tetrodotoxin (1 μM) and stimulated twice with exogenous substance P (1 μM), respectively. The first stimulation was applied in the absence of other test drugs, while the second one was applied after 10-min incubation with MRS1754.
In the last series, the effects of 2-[[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy) phenyl]-2-pyridinyl] thio]-acetamide (BAY 60-6583, A2B receptor agonist, 0.001–100 μM) (see Table 2) were tested on repeated electrical stimuli (rES)-induced tachykinergic contractions as well as on NK1-mediated tachykinergic contractions elicited by exogenous substance P (1 μM) in the presence of tetrodotoxin (1 μM). Of note, the effects of BAY 60-6583 were assessed either in the absence or in the presence of MRS1754.
The effects of A2B receptor ligands were expressed as percent changes of control contractions elicited by sES or exogenous substance P.
Drugs and reagents
Atropine sulfate, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), and guanethidine monosulfate were obtained from Sigma Chemicals Co. (St. Louis, Mo, USA). Tetrodotoxin, N-[9-Chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide (MRS 1220), 4-(2-[7-amino-2- triazolo{2,3-a}{1,3,5}triazin-5-yl amino]ethyl) phenol (ZM241385), 8-(4-[{(4- cyanophenyl)carbamoylmethyl} oxy]phenyl)-1,3-di(n-propyl)xanthine (MRS1754), 2-[[6-Amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]-2-pyridinyl]thio]-acetamide (BAY 60-6583, N-acetyl-L-tryptophan 3,5-bis(trifluoromethyl)benzyl ester (L-732,138), 5-Fluoro-3-[2-[4-methoxy-4-[[(R)-phenylsulphinyl]methyl]-1-piperidinyl]ethyl]-1H–indole (GR159897), (R)-[[2-phenyl-4-quinolinyl)carbonyl]amino]-methyl ester benzeneacetic acid (SB218795), and Nω nitro-L-arginine methylester (L-NAME) were obtained from Tocris (Bristol, UK). The drugs were dissolved in dimethyl sulfoxide, and further dilutions were made with saline solution. Dimethyl sulfoxide concentration in organ bath never exceeded 0.5%.
Statistical analysis
Data are expressed as mean ± SEM. The significance of differences was evaluated for raw data, before percentage normalization, by Student’s unpaired t test or by one-way ANOVA followed by the appropriate post hoc test. P < 0.05 was considered significant. The colonic preparations included in each test group were obtained from different animals, and therefore, the number of trials was always the same as the number of animals allocated to the group. Calculations and analyses were performed using GraphPad Prism 3.0 (GraphPad Software, San Diego, CA, USA).
Results
BMI, epididymal fat weight, and metabolic parameters
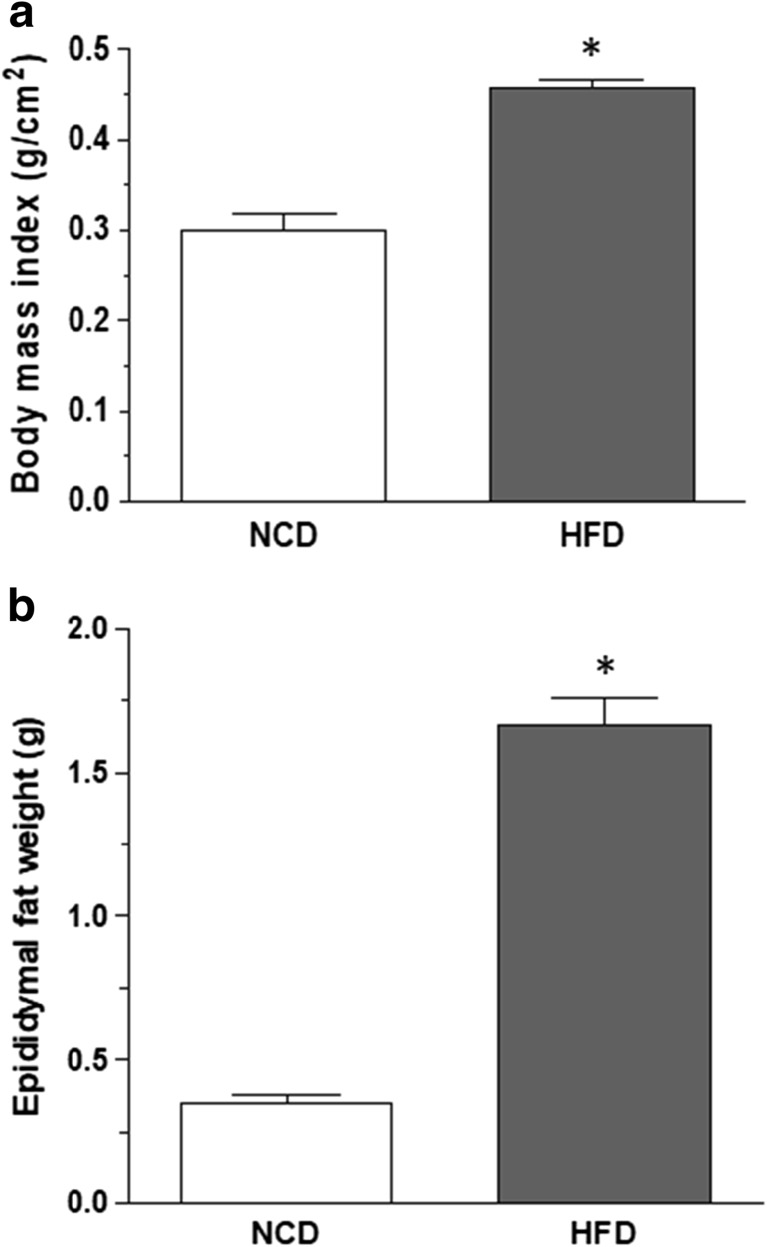
After 8 weeks, mice fed with HFD displayed a significant increase in BMI and epididymal fat weight, as compared with animals under NCD (Fig. 1a, b). Obese, but not NCD, mice displayed altered blood metabolic indices (i.e., glucose, cholesterol, and triglyceride levels) (Table 1).
Fig. 1.
Effects of normocaloric diet (NCD) or high-fat diet (HFD) on body mass index (a) and epididymal fat weight (b). Data are means ± SEM (n = 8). *P ˂ 0.05 versus NCD
Inflammatory infiltrates, IL-6 and MDA levels in colonic neuromuscular tissues
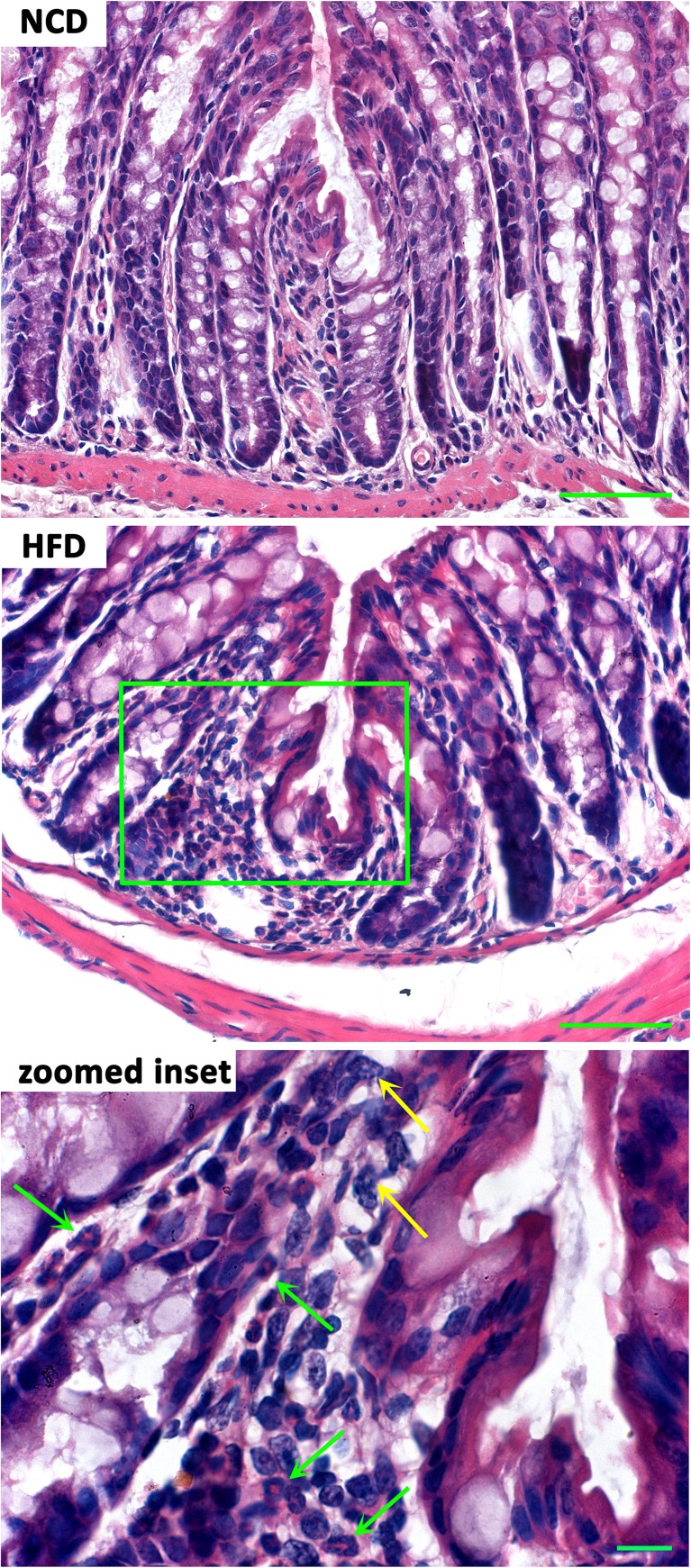
Minimal (<10%) inflammatory infiltrates of mixed leucocytes, rich in eosinophils, were observed in the tunica mucosa of colonic specimens from HFD mice, as compared to NCD mice (Fig. 2).
Fig. 2.
Representative images of the colonic architecture in hematoxylin/eosin-stained full-thickness cross sections. Interstitial inflammatory infiltrates are present in the colonic mucosa of high-fat diet (HFD) mice, at variance with normocaloric diet (NCD) mice. The magnification of the boxed area shows mixed inflammatory cells with eosinophils (green arrows) and neutrophils (yellow arrows). Scale bars 50 μm; inset 10 μm
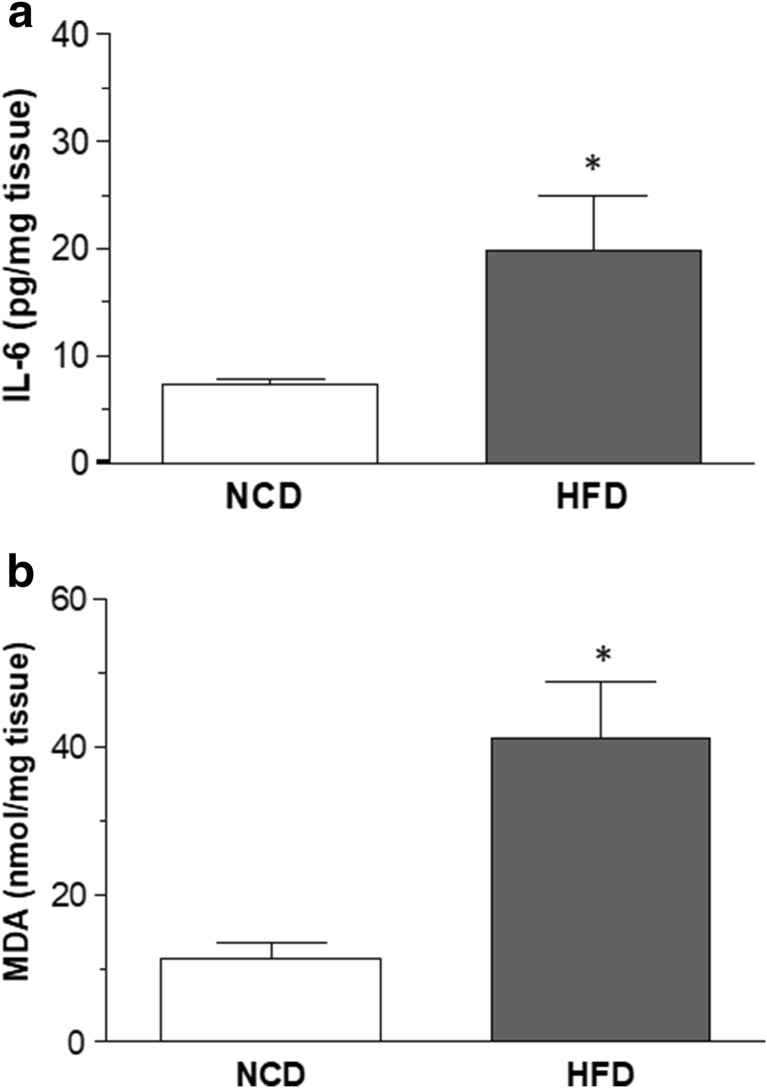
In colonic tissues from NCD animals, IL-6 levels accounted for 7.4 ± 0.4-pg/mg tissue (Fig. 3a). In colonic specimens from obese mice, IL-6 levels were significantly increased as compared with NCD mice (Fig. 3a). In colonic specimens from NCD mice, MDA levels accounted for 11.4 ± 2.1-nmol/mg tissue (Fig. 3b). HFD-induced obesity was associated with a significant increase in the oxidative stress of colonic tissues (Fig. 3b).
Fig. 3.
Effects of normocaloric diet (NCD) or high-fat diet (HFD) on colonic tissue levels of IL-6 (a) and malondialdehyde (b). Data are means ± SEM (n = 8). *P ˂ 0.05 versus NCD
Immunofluorescence analysis
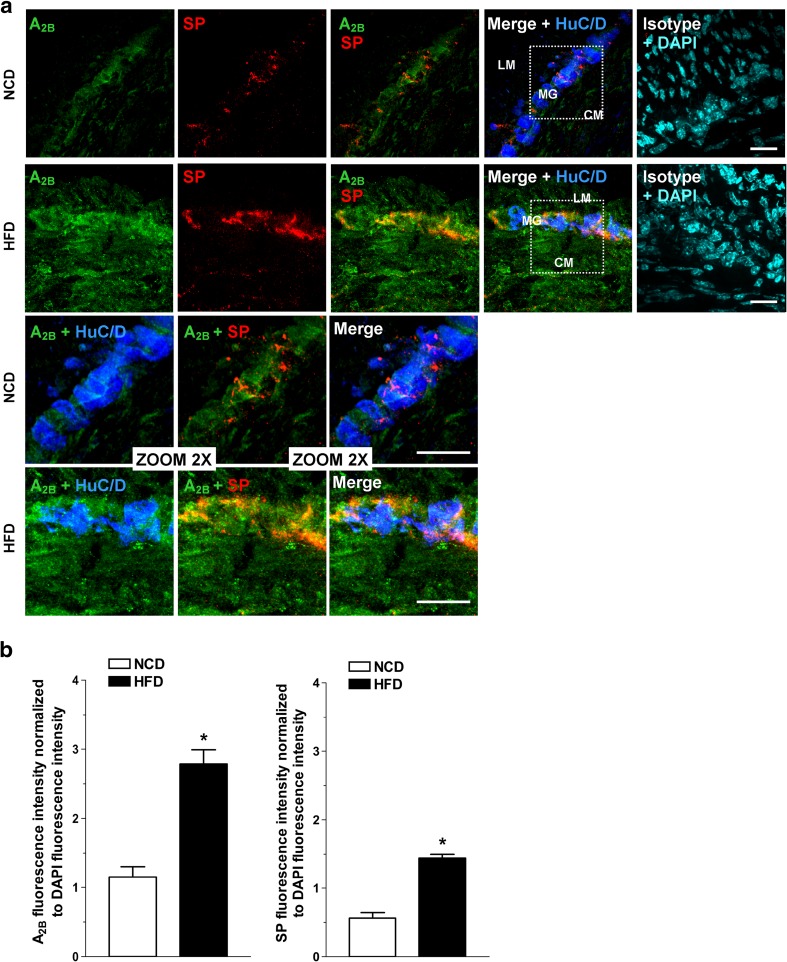
In NCD animals, immunohistochemical analysis of colonic cryosections revealed homogenous bundles of HuC/D+ neurons, aggregated in well-organized and detectable myenteric ganglia together with SP+ neurons, as shown in Fig. 4. Immunofluorescence of A2B receptors was detected in the NCD neuromuscular layer (Fig. 4). HFD increased the immunostaining of A2B receptors together with a higher immunoreactivity for SP in the neuromuscular compartment of colon cryosections from HFD mice (Fig. 4).
Fig. 4.
a Representative confocal microphotographs of HuC/D (blue), substance P (red), and A2B receptors (green) immunoreactivity in the neuromuscular compartment of colonic cryosections from mice fed with normocaloric diet (NCD) or high-fat diet (HFD). Cell nuclei were stained with DAPI (cyan; N = 5 mice/group). Bars = 25 μm. LM longitudinal muscle, CM circular muscle, MG myenteric ganglion. b Levels of A2B receptors or substance P fluorescence intensity normalized to DAPI fluorescence intensity in the neuromuscular compartment of colonic cryosections of NCD and HFD mice (n = 5 mice/group). *P < 0.05 versus NCD
Western blot assay
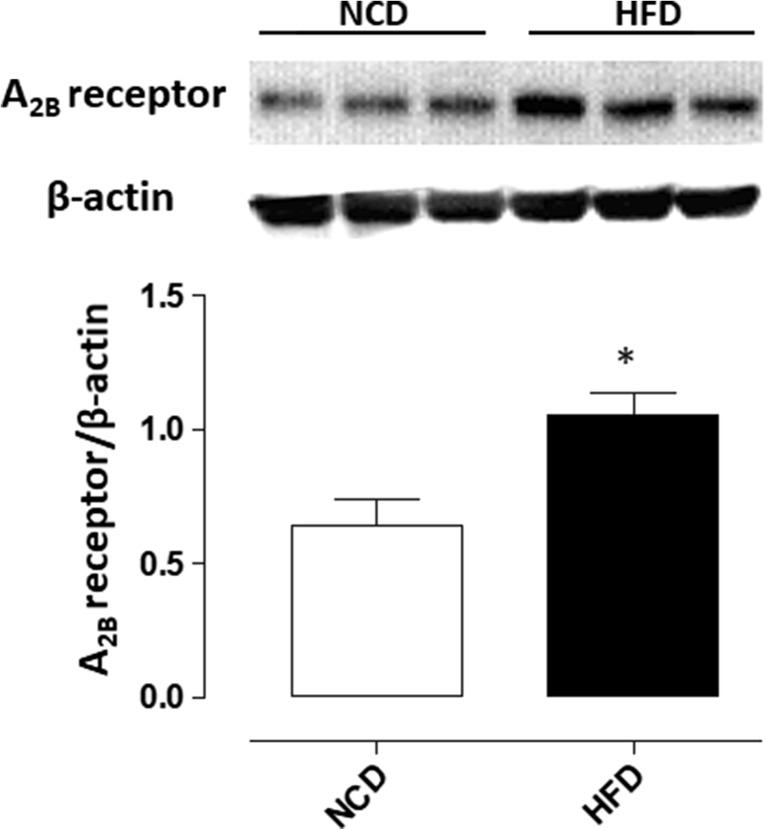
Western blot analysis showed a detectable expression of A2B receptors in colonic tissues from NCD-fed mice (Fig. 5). In tissues isolated from HFD animals, the expression of A2B receptors was significantly increased (Fig. 5).
Fig. 5.
Western blot analysis of A2B receptors in colonic tissues from normocaloric (NCD)-fed mice or animals treated with a high-fat diet (HFD). Each column represents the mean value ± SEM obtained from six animals. *P < 0.05 versus NCD
Effects of A2B receptor blockade on contractile activity of colonic longitudinal smooth muscle
During the equilibration period in standard Krebs solution, colonic preparations from NCD or HFD animals developed spontaneous contractile activity, which remained stable throughout the experiment and, in most cases, was low in amplitude and did not interfere with motor responses evoked by sES or exogenous substance P. Electrically evoked responses consisted of phasic contractions followed, in some cases, by after contractions of variable amplitude. Atropine (1 μM) abolished these phasic contractions or converted them into relaxations, and only after contractions were then evident (data not shown). Tetrodotoxin (1 μM) abolished the electrically induced contractions (data not shown).
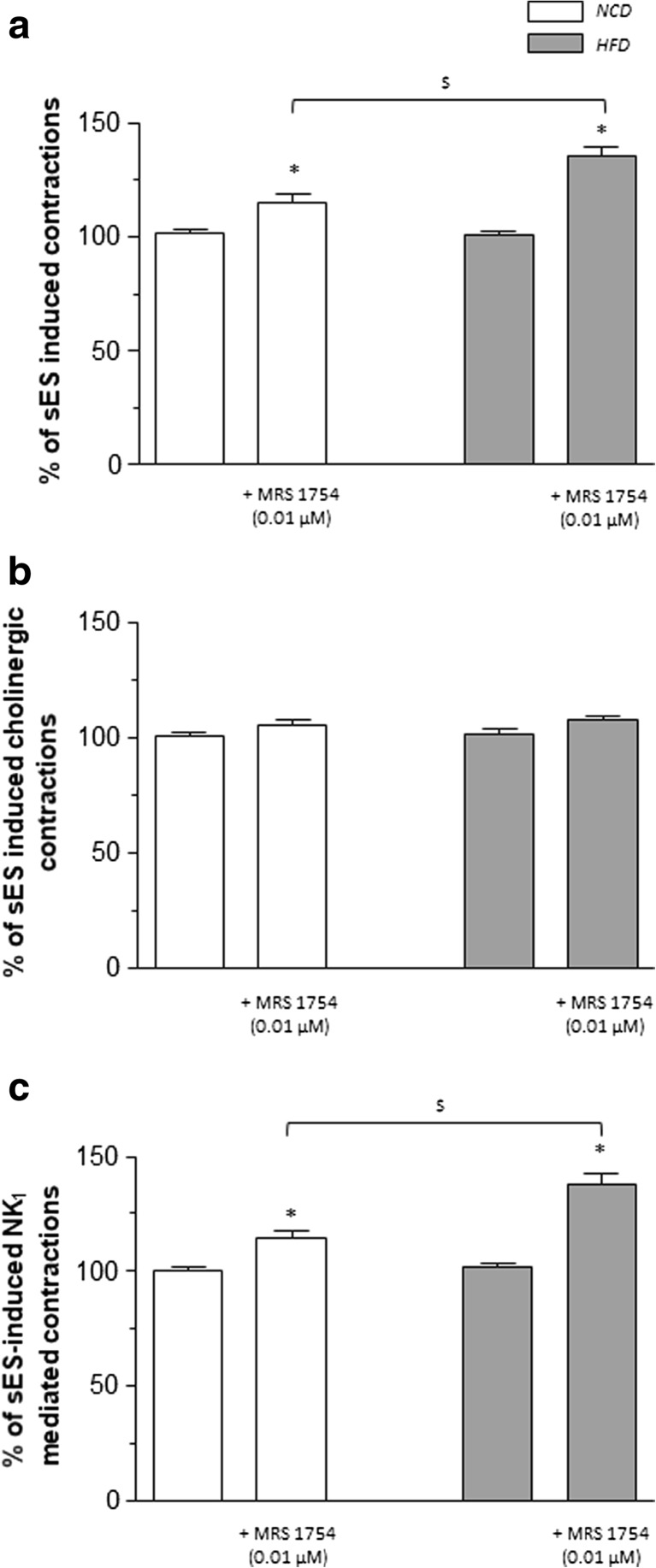
Under resting conditions, the A2B receptor antagonist MRS1754 (0.01 μM) did not affect the spontaneous contractile activity of colonic preparations from NCD or HFD mice. In colonic tissues from HFD mice maintained in standard Krebs solution, the pharmacological blockade of A2B receptors increased significantly the sES-evoked contractions, while a slight, despite significant effect was recorded in tissues from NCD mice (Fig. 6a).
Fig. 6.
Preparations of colonic longitudinal smooth muscle isolated from mice fed with normocaloric diet (NCD) or high-fat diet (HFD). Effects of MRS 1754 (0.01 μM) on contractions evoked by sES (0.5 ms, 10 Hz, 30 mA, 10 s) in colonic preparations maintained in standard Krebs solution (a) or added with guanethidine (10 μM), L-NAME (100 μM), L-732138 (NK1 receptor antagonist, 10 μM), GR159897 (NK2 receptor antagonist 1 μM), and SB218795 (NK3 receptor antagonist, 1 μM) (b), or Krebs solution containing guanethidine, L-NAME, atropine (1 μM), and the NK2 and NK3 receptor antagonists (c). Each column represents the mean ± SEM obtained from eight experiments. *P < 0.05 versus the respective values in the absence of MRS 1754; $ P<0.05
In colonic preparations from NCD or HFD mice incubated in Krebs solution supplemented with guanethidine, or added with guanethidine plus L-NAME, the effects of MRS1754 (0.01 μM) on sES-induced contractions were similar to those recorded in the presence of standard Krebs (+10.3 ± 1.8% in NCD and +34 ± 3.3% in HFD, in the presence of guanethidine) and (+13.6 ± 2.1% in NCD and +36.4 ± 2.7% in HFD, in the presence of guanethidine and L-NAME), thus arguing against a modulating action of A2B receptors on noradrenergic or nitrergic pathways.
In colonic tissues incubated in Krebs solution containing guanethidine, neurokinin (NK) receptor antagonists, and L-NAME, to record cholinergic contractions, the sES-evoked phasic contractions were abolished or markedly reduced by atropine. In this setting, the enhancing effect of MRS1754 on colonic preparations from HFD animals no longer occurred (Fig. 6b).
In colonic preparations from HFD mice maintained in Krebs solution containing guanethidine, L-NAME, atropine, and NK2 and NK3 receptor antagonists, electrical stimuli induced contractile responses, which were significantly increased in comparison with those recorded in NCD animals. The motor responses of colonic preparations from both NCD and HFD animals were incubated with NK1 receptor antagonist (not shown). Under these conditions, the enhancing effect of MRS 1754 (0.01 μM) on sES-evoked contractions was still evident (Fig. 6c), and it was almost completely abrogated by incubation with NK1 receptor antagonist (not shown).
In another series of experiments, the effects of A2B receptor blockade were tested on contractions evoked by direct activation of tachykininergic receptors on longitudinal smooth muscle. For this purpose, the effects of MRS1754 (0.01 μM) were tested on contractions evoked by exogenous substance P (1 μM) in the presence of tetrodotoxin. In this setting, MRS1754 did not elicit any effect against substance P-induced contractions in colonic tissues from both NCD and HFD mice (+5.8 ± 3.7 and +9.2 ± 4.1%, respectively).
Effects of A2B receptor activation on contractile activity of colonic longitudinal smooth muscle
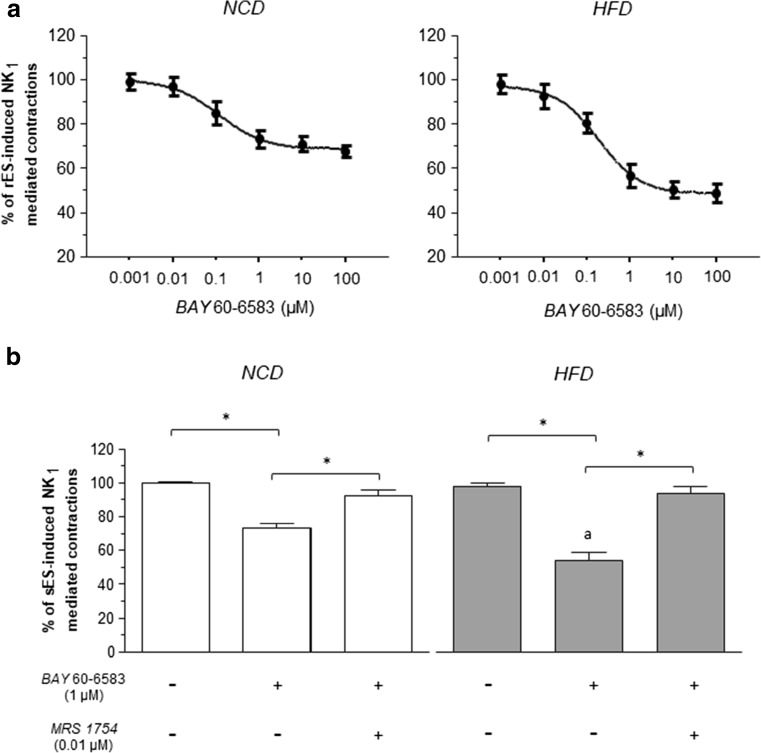
The effects of the A2B receptor agonist BAY 60-6583 (0.001–100 μM) were tested on rES-induced contractions in colonic preparations from NCD or HFD mice, which were maintained in Krebs solution containing dipyridamole (adenosine transport inhibitor, 0.5 μM) and adenosine deaminase (the enzyme responsible for adenosine catabolism, 0.5 U/mL) to minimize interference by endogenous adenosine, as well as guanethidine, L-NAME, atropine, and the NK2 and NK3 receptor antagonists. Under these conditions, the pharmacological stimulation of A2B receptors significantly decreased rES-evoked, NK1-mediated contractions (Fig. 7a). However, BAY60-6583 was more effective when tested on colonic preparations from HFD mice, as compared to NCD animals (Fig. 7a). Conversely, the pharmacological stimulation of A2B receptors did not exert any significant effect on contractions elicited by exogenous substance P in the presence of tetrodotoxin, both in HFD and NCD mice (−6 ± 2.8 and −8.4 ± 3.5%, respectively). The inhibitory effects of BAY 60-6583 (1 μM) on ES-induced contractions were significantly counteracted by incubation with MRS1754 (0.01 μM) (Fig. 7b).
Fig. 7.
Preparations of colonic longitudinal smooth muscle isolated from mice fed with normocaloric diet (NCD) or high-fat diet (HFD). a Effects of BAY 60-6583 (0.001–100 μM) on contractions evoked by rES (0.5 ms, 10 Hz, 30 mA, 10 s) in colonic preparations maintained in Krebs solution containing guanethidine, L-NAME, atropine (1 μM), GR159897 (NK2 receptor antagonist 1 μM) SB218795 (NK3 receptor antagonist, 1 μM) and added with dipyridamole (adenosine transport inhibitor, 0.5 μM) plus adenosine deaminase (the enzyme responsible for adenosine catabolism, 0.5 U/mL). b Column graphs showing the effects of BAY 60-6583 (1 μM), alone or in combination with MRS1754 (0.01 μM), on contractions elicited by sES (0.5 ms, 10 Hz, 30 mA, 10 s) in colonic preparations maintained in Krebs solution containing guanethidine, L-NAME, atropine (1 μM), and GR159897 (NK2 receptor antagonist 1 μM) SB218795 (NK3 receptor antagonist, 1 μM) and added with dipyridamole (adenosine transport inhibitor, 0.5 μM) plus adenosine deaminase (the enzyme responsible for adenosine catabolism, 0.5 U/mL). Each column represents the mean ± SEM obtained from six experiments. *P ˂ 0.05; a P ˂ 0.05 versus NCD plus BAY
Discussion
The present study was designed to specifically elucidate the contribution of adenosine to the control of neuromuscular activity in the distal colon from mice with NCD or HFD-induced obesity. Particular attention was paid at carefully evaluating the expression and distribution of A2B receptors in the colonic neuromuscular compartment, as well as the role of these receptors in the control of colonic neuromotility in the presence of obesity.
Our attention was focused on this receptor subtype since its critical involvement under detrimental conditions has been widely described [30]. Indeed, a number of studies have reported the involvement of A2B receptors in the pathophysiological mechanisms underlying several disorders [31], such as autoimmune encephalomyelitis [32], pulmonary inflammation [33, 34], intestinal inflammation [35, 36], diabetes [37, 38], and obesity [39]. It is worth mentioning also that HFD-induced obesity is currently regarded as an important tool for understanding the impact of high-fat Western diets on the development of obesity and related disorders [40]. In our hands, in line with previous reports [41–43], mice fed with HFD for 8 weeks showed a marked increase in body weight, followed by a marked alteration of several metabolic indexes, such as an increase in blood glucose cholesterol and triglycerides, thus corroborating the suitability of this experimental model.
Overall, the results of our investigations on distal colon point out that the colonic neuromuscular compartment of obese mice displayed a significant increase in substance P immunopositivity as well as a marked redistribution and enhanced immunoreactivity and expression of A2B receptors, as compared with NCD animals. In this setting, A2B receptors were found to mediate a marked tonic inhibitory control on excitatory tachykininergic motility, consistently with the evidence of their upregulation.
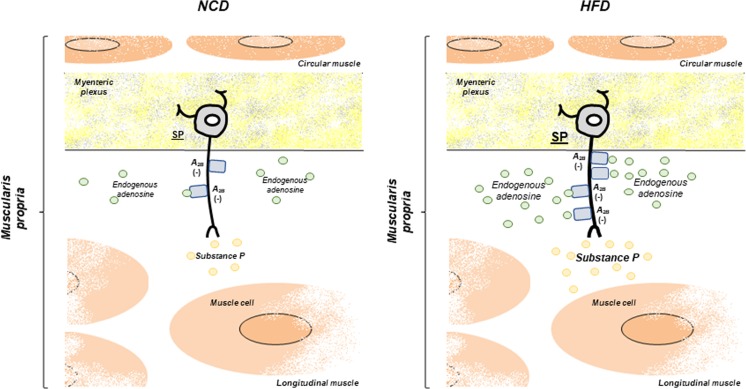
In NCD mice, in line with Chandrasekharan et al. [18], the presence of A2B receptors in the colonic neuromuscular layer was documented by immunofluorescence analysis that revealed their expression mainly in myenteric ganglia, with a faint distribution at level of smooth muscle. Our functional investigations disclosed a weak, despite significant, involvement of A2B receptor-mediated control by endogenous adenosine on electrically evoked contractions. Likewise, in NCD colonic tissues incubated with dipyridamole and adenosine deaminase to minimize interferences by endogenous adenosine, the selective A2B receptor agonist BAY 60-6583 exerted a moderate, albeit significant, inhibitory effect on contractions elicited by electrical stimuli. We then went on to evaluate the possible influence of A2B receptors on NCD colonic contractions elicited by direct muscular stimulations. For this purpose, the effects of A2B receptor ligands were tested on colonic contractions evoked by stimulation of NK1 receptors by exogenous substance P. Under these conditions, the contractions of colonic preparations were not affected by the A2B receptor ligands, suggesting that in the NCD colon, endogenous adenosine can exert modest modulating actions at neuronal level via A2B receptors (Fig. 8).
Fig. 8.
Schematic representation of the role played by endogenous adenosine, via A2B receptors, in regulating excitatory tachykininergic pathways in colonic tissues from mice fed with normocaloric (NCD) or high-fat diet (HFD). In the NCD colon, endogenous adenosine exerts modest modulating actions at neuronal level via A2B receptors. In the HFD mice, the increase in colonic A2B receptor expression represents a “purinergic brake” aimed at curbing the enhancement of colonic tachykininergic transmission
Previous investigations have shown that low-grade inflammation in the gut can alter digestive motility, through changes in the functions of enteric nerves, interstitial cells of Cajal (ICCs), or smooth muscle cells, thus pointing out a pathophysiological relationship between bowel inflammation and abnormalities in enteric motor activity [44]. In this context, a number of studies have shown that changes in enteric tachykininergic pathways are actively involved in the pathophysiology of motor digestive disorders associated with several inflammatory conditions [i.e., inflammatory bowel diseases (IBDs), diverticulitis, and irritable bowel syndrome] [12, 13]. In particular, an increased substance P release has been observed from neurons of myenteric and submucosal plexuses, as well as immune cells of the intestinal lamina propria (such as monocytes, macrophages, eosinophils, and lymphocytes), isolated from patients with IBDs, leading to the hypothesis that the enteric tachykininergic system participates to the pathogenesis of bowel motor dysfunctions associated with intestinal inflammation [45].
As anticipated above, obesity is related with a condition of chronic low-grade systemic inflammation, characterized by an abnormal production of pro-inflammatory cytokines and other mediators [46]. In particular, when considering innate immune cells, HFD-induced obesity is associated with a marked increment of macrophage infiltration into the adipose tissue, thought to be a major player in the dysregulation of metabolic and inflammatory processes [47]. Of note, macrophages infiltrating the adipose tissue are predominantly polarized towards a pro-inflammatory M1 phenotype, producing a cocktail of inflammatory signals and oxidative stress [48]. In line with this knowledge, we observed a marked increase in IL-6 and a significant degree of oxidative stress in colonic tissues from HFD mice. In this setting, we obtained also clear evidence about a marked increase in substance P immunopositivity in colonic myenteric ganglia and smooth muscle layer from HFD mice, suggesting an involvement of tachykininergic pathways in colonic motor dysfunctions associated with obesity. Previous studies revealed a tight correlation between the occurrence of bowel inflammatory disorders and the onset of intestinal motor disturbances sustained mainly by alterations of enteric tachykininergic pathways [49]. Our results, showing that the mild inflammation associated with HFD is related with an increased release of substance P from colonic tissues, are in keeping with these observations, and support a role for enteric tachykininergic pathways as determinants of colonic dysmotility associated with obesity.
In parallel with an increment of substance P immunopositivity, colonic tissues from HFD mice were characterized also by a marked increase in A2B receptor expression and immunoreactivity mainly in the neuromuscular compartment. This evidence is consistent with previous findings displaying an increased expression of A2B receptors, driven by TNF, in the inflamed colon from humans and mice [50]. Along the same lines, recent papers have shown an increased immunoreactivity for A2B receptors in the colonic smooth muscle layers of rats infected with herpes simplex virus type 1 [24] or in the presence of DNBS-induced colitis [19], thus suggesting an involvement of this receptor subtype in the pathophysiological mechanisms underlying inflammatory intestinal injury. Interestingly, over the years, the role played by A2B receptors in gut inflammation has been a matter of great debate and intense research. In particular, the discussion has been fostered by the evidence that, following initial encouraging results supporting an ameliorative effect of A2B receptor antagonists in mice with experimental colitis [36, 51], these results were questioned by Frick et al. [35], describing a protective effect associated with A2B receptor activation in the same model of intestinal inflammation.
In the present study, we demonstrated that the diet-induced obesity was characterized by an inflammatory condition at colonic level and, concomitantly, with an increased expression of A2B receptors in the neuromuscular compartment. Based on the above-mentioned evidences, it is conceivable that the low-grade inflammation observed in the presence of obesity, likely via the release of pro-inflammatory cytokines and/or a condition of oxidative stress, could determine the increased expression of A2B receptors. Such a hypothesis is supported by our preliminary experiments showing that HFD mice underwent to macrophage depletion, elicited by clodronate-liposome injection, displayed a decrease in inflammatory and oxidative stress parameters (IL-1β, IL-6, MDA) that occurred in parallel with a reduced colonic expression of A2B receptors (Antonioli et al., unpublished data).
An involvement of A2B and A3 receptors in regulating of colonic cholinergic motility in the presence of bowel inflammation has been previously demonstrated [19, 29]. In particular, the induction of experimental colitis resulted in an increased expression of both receptor subtypes, although with a scarce activation by endogenous adenosine [19, 29]. Further experiments revealed that such reduced recruitment of A2B and A3 receptors was ascribable to a decreased availability of endogenous adenosine in the receptor biophase as a consequence of a molecular interaction between the catabolic enzyme adenosine deaminase [19, 29]. In the present study, functional experiments on distal colon from NCD mice showed that incubation with the A2B receptor antagonist MRS 1754 determined an increase in electrically evoked contractions, which were markedly enhanced in preparations from HFD animals. These findings thus indicate that in the colon of HFD mice, the levels of endogenous adenosine in the biophase of A2B receptors are greater than those available in colonic tissues from NCD animals.
Of note, double-label immunofluorescence analysis revealed an increased immunoreactivity of A2B receptors and substance P in myenteric ganglia, suggesting a modulating activity of these receptors on enteric tachykininergic pathways. Such a relationship was indeed corroborated by our functional experiments, showing that electrically induced tachykininergic contractions were significantly enhanced in colonic preparations from HFD mice, and were further enhanced by A2B receptor blockade. Moreover, the pharmacological activation of A2B receptors with the agonist BAY 60-6583 reduced tachykininergic contractions evoked by electrical stimulation, but not by exogenous substance P. Taken together, these results support the view that HFD-induced obesity is associated with an increased expression of A2B receptors, which take a significant part in the inhibitory control of excitatory colonic tachykininergic nerve pathways by endogenous adenosine (Fig. 8).
A point that deserves a discussion is the putative role of glial cells in colonic contractile dysfunctions associated with HFD-induced obesity. Indeed, it has been widely appreciated that alterations of enteric glial cells (gliosis) are associated with inflammatory and functional disorders in the gut [52]. In this regard, since glial cells have been described to be a source of extracellular adenosine [53], a putative role of these cells in regulating A2B receptor engagement can be hypothesized. Accordingly, future experimental approaches, aimed at investigating this issue, represent a logical continuation of ongoing research on this topic.
In conclusion, the present findings highlight a novel and fascinating role of adenosine A2B receptors in the regulation of colonic neuromuscular functions in the presence of diet-induced obesity. In particular, our investigations showed for the first time an involvement of endogenous adenosine, via A2B receptors, in colonic dysmotility associated with obesity, through an involvement of excitatory tachykininergic pathways. In this context, we hypothesize that the increase in colonic A2B receptor expression could represent a sort of “purinergic brake” aimed at curbing the enhancement of colonic tachykininergic transmission. These observations, taken together with the increasing knowledge relating A2B receptors with the modulation of immune/inflammatory processes, might represent a promising basis for the development of novel pharmacological tools potentially useful for the therapeutic management of colonic motor dysfunctions associated with obesity.
Electronic supplementary material
Preparations of colonic longitudinal smooth muscle obtained from mice fed with normocaloric diet (NCD) or high-fat diet (HFD) maintained in standard Krebs solution. (A) Effects of increasing concentrations of MRS 1754 (0.001–1 μM) on contractions induced by electrical stimulation (ES, 10 Hz). (B) Effects of MRS 1754 (0.01 μM) on contractile responses to ES in the presence of 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; 0.01 μM), 4-(2-[7-Amino-2-(2-furyl) [1,2,4] triazolo[2,3-a] [1, 3, 5]triazin-5-ylamino]ethyl)phenol (ZM 241385; 0.01 μM) and N-[9-Chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide (MRS 1220; 0.1 μM). Each column represents the mean ± standard error of mean value obtained from six experiments. *P < 0.05 vs control. (GIF 111 kb).
Acknowledgements
This work was supported by grants from San Camillo Hospital, Treviso (Italy), to MCG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
LA, RC, MF, CP, GH, and CB designed the study; ET, DG, MCG, VC, IM, GO, NB, CS, CI, BC, and ZHN collected and analyzed the data; and LA, RC, MF, CS, CP, GH, and CB wrote the manuscript. All of the authors discussed the results and commented on the manuscript.
Compliance with ethical standards
Conflicts of interest
Luca Antonioli declares that he has no conflict of interest.
Carolina Pellegrini declares that she has no conflict of interest.
Matteo Fornai declares that he has no conflict of interest.
Erika Tirotta declares that she has no conflict of interest.
Daniela Gentile declares that she has no conflict of interest.
Laura Benvenuti declares that she has no conflict of interest.
Maria Cecilia Giron declares that she has no conflict of interest.
Valentina Caputi declares that she has no conflict of interest.
Ilaria Marsilio declares that she has no conflict of interest.
Genny Orso declares that she has no conflict of interest.
Nunzia Bernardini declares that she has no conflict of interest.
Cristina Segnani declares that she has no conflict of interest.
Chiara Ippolito declares that she has no conflict of interest.
Balázs Csóka declares that he has no conflict of interest.
Zoltán H. Németh declares that he has no conflict of interest.
György Haskó declares that he has no conflict of interest.
Carmelo Scarpignato declares that he has no conflict of interest.
Corrado Blandizzi declares that he has no conflict of interest.
Rocchina Colucci declares that she has no conflict of interest.
Ethical approval
The experiments were approved by the Ethical Committee for Animal Experimentation of the University of Pisa and by the Italian Ministry of Health (authorization no. 744/2015-PR).
Footnotes
L. Antonioli and C. Pellegrini equally contributed to the paper
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-017-9577-0) contains supplementary material, which is available to authorized users.
References
- 1.Soares A, et al. Intestinal and neuronal myenteric adaptations in the small intestine induced by a high-fat diet in mice. BMC Gastroenterol. 2015;15:3. doi: 10.1186/s12876-015-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7(1):66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho W, Spiegel BM. The relationship between obesity and functional gastrointestinal disorders: causation, association, or neither? Gastroenterol Hepatol (N Y) 2008;4(8):572–578. [PMC free article] [PubMed] [Google Scholar]
- 4.Fysekidis M, et al. Prevalence and co-occurrence of upper and lower functional gastrointestinal symptoms in patients eligible for bariatric surgery. Obes Surg. 2012;22(3):403–410. doi: 10.1007/s11695-011-0396-z. [DOI] [PubMed] [Google Scholar]
- 5.Le Pluart D, et al. Functional gastrointestinal disorders in 35,447 adults and their association with body mass index. Aliment Pharmacol Ther. 2015;41(8):758–767. doi: 10.1111/apt.13143. [DOI] [PubMed] [Google Scholar]
- 6.vd Baan-Slootweg OH, et al. Constipation and colonic transit times in children with morbid obesity. J Pediatr Gastroenterol Nutr. 2011;52(4):442–445. doi: 10.1097/MPG.0b013e3181ef8e3c. [DOI] [PubMed] [Google Scholar]
- 7.Bertrand RL, et al. A Western diet increases serotonin availability in rat small intestine. Endocrinology. 2011;152(1):36–47. doi: 10.1210/en.2010-0377. [DOI] [PubMed] [Google Scholar]
- 8.Nezami BG, et al. MicroRNA 375 mediates palmitate-induced enteric neuronal damage and high-fat diet-induced delayed intestinal transit in mice. Gastroenterology. 2014;146(2):473–483. doi: 10.1053/j.gastro.2013.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam YY, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7(3):e34233. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raybould HE. Gut microbiota, epithelial function and derangements in obesity. J Physiol. 2012;590(Pt 3):441–446. doi: 10.1113/jphysiol.2011.222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenman LK, Holma R, Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol. 2012;18(9):923–929. doi: 10.3748/wjg.v18.i9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neunlist M, et al. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52(1):84–90. doi: 10.1136/gut.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ter Beek WP, et al. Substance P receptor expression in patients with inflammatory bowel disease. Determination by three different techniques, i.e., storage phosphor autoradiography, RT-PCR and immunohistochemistry. Neuropeptides. 2007;41(5):301–306. doi: 10.1016/j.npep.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Antonioli L, et al. Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther. 2008;120(3):233–253. doi: 10.1016/j.pharmthera.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki M, et al. Molecular identification and pharmacological characterization of adenosine receptors in the guinea-pig colon. Br J Pharmacol. 2000;129(5):871–876. doi: 10.1038/sj.bjp.0703123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fozard JR, Baur F, Wolber C. Antagonist pharmacology of adenosine A2B receptors from rat, guinea pig and dog. Eur J Pharmacol. 2003;475(1–3):79–84. doi: 10.1016/S0014-2999(03)02078-8. [DOI] [PubMed] [Google Scholar]
- 17.Zizzo MG, Mule F, Serio R. Inhibitory responses to exogenous adenosine in murine proximal and distal colon. Br J Pharmacol. 2006;148(7):956–963. doi: 10.1038/sj.bjp.0706808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandrasekharan BP, et al. Adenosine 2B receptors (A(2B)AR) on enteric neurons regulate murine distal colonic motility. FASEB J. 2009;23(8):2727–2734. doi: 10.1096/fj.09-129544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonioli L, et al. Role of the A(2B) receptor-adenosine deaminase complex in colonic dysmotility associated with bowel inflammation in rats. Br J Pharmacol. 2014;171(5):1314–1329. doi: 10.1111/bph.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smemo S, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507(7492):371–375. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonioli L, et al. The blockade of adenosine deaminase ameliorates chronic experimental colitis through the recruitment of adenosine A2A and A3 receptors. J Pharmacol Exp Ther. 2010;335(2):434–442. doi: 10.1124/jpet.110.171223. [DOI] [PubMed] [Google Scholar]
- 22.Erben U, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7(8):4557–4576. [PMC free article] [PubMed] [Google Scholar]
- 23.Giron MC, et al. Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology. 2008;134(4):1116–1126. doi: 10.1053/j.gastro.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Zoppellaro C, et al. Adenosine-mediated enteric neuromuscular function is affected during herpes simplex virus type 1 infection of rat enteric nervous system. PLoS One. 2013;8(8):e72648. doi: 10.1371/journal.pone.0072648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orso G, et al. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J Clin Invest. 2005;115(11):3026–3034. doi: 10.1172/JCI24694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Antonioli L, et al. Involvement of the P2X7 purinergic receptor in colonic motor dysfunction associated with bowel inflammation in rats. PLoS One. 2014;9(12):e116253. doi: 10.1371/journal.pone.0116253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zizzo MG, et al. Adenosine negatively regulates duodenal motility in mice: role of A(1) and A(2A) receptors. Br J Pharmacol. 2011;164(6):1580–1589. doi: 10.1111/j.1476-5381.2011.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonioli L, et al. Control of enteric neuromuscular functions by purinergic A(3) receptors in normal rat distal colon and experimental bowel inflammation. Br J Pharmacol. 2010;161(4):856–871. doi: 10.1111/j.1476-5381.2010.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14(7):1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 31.Hasko G, et al. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009;30(6):263–270. doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei W, et al. Blocking A2B adenosine receptor alleviates pathogenesis of experimental autoimmune encephalomyelitis via inhibition of IL-6 production and Th17 differentiation. J Immunol. 2013;190(1):138–146. doi: 10.4049/jimmunol.1103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belikoff BG, et al. A2B adenosine receptor expression by myeloid cells is proinflammatory in murine allergic-airway inflammation. J Immunol. 2012;189(7):3707–3713. doi: 10.4049/jimmunol.1201207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun CX, et al. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116(8):2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frick JS, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182(8):4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolachala VL, et al. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135(3):861–870. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Csoka B, et al. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014;63(3):850–866. doi: 10.2337/db13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figler RA, et al. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60(2):669–679. doi: 10.2337/db10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston-Cox H, et al. The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One. 2012;7(7):e40584. doi: 10.1371/journal.pone.0040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol. 2012;821:421–433. doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim KA, et al. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10):e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laurila A, et al. High-fat, high-cholesterol diet increases the incidence of gastritis in LDL receptor-negative mice. Arterioscler Thromb Vasc Biol. 2001;21(6):991–996. doi: 10.1161/01.ATV.21.6.991. [DOI] [PubMed] [Google Scholar]
- 43.Kolbus D, et al. CD8+ T cell activation predominate early immune responses to hypercholesterolemia in ApoE(−)(/)(−) mice. BMC Immunol. 2010;11:58. doi: 10.1186/1471-2172-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan WI, Collins SM. Gut motor function: immunological control in enteric infection and inflammation. Clin Exp Immunol. 2006;143(3):389–397. doi: 10.1111/j.1365-2249.2005.02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25(6):503–511. doi: 10.1097/MOG.0b013e328331b69e. [DOI] [PubMed] [Google Scholar]
- 46.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249(1):218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghigliotti G, et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014;37(4):1337–1353. doi: 10.1007/s10753-014-9914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bing C. Is interleukin-1beta a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte. 2015;4(2):149–152. doi: 10.4161/21623945.2014.979661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fornai M, et al. Role of cyclooxygenase isoforms in the altered excitatory motor pathways of human colon with diverticular disease. Br J Pharmacol. 2014;171(15):3728–3740. doi: 10.1111/bph.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolachala V, et al. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci. 2005;62(22):2647–2657. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolachala V, et al. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol. 2008;155(1):127–137. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ochoa-Cortes F, et al. Enteric glial cells: a new frontier in neurogastroenterology and clinical target for inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22(2):433–449. doi: 10.1097/MIB.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 2010;17(7):1071–1082. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preparations of colonic longitudinal smooth muscle obtained from mice fed with normocaloric diet (NCD) or high-fat diet (HFD) maintained in standard Krebs solution. (A) Effects of increasing concentrations of MRS 1754 (0.001–1 μM) on contractions induced by electrical stimulation (ES, 10 Hz). (B) Effects of MRS 1754 (0.01 μM) on contractile responses to ES in the presence of 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; 0.01 μM), 4-(2-[7-Amino-2-(2-furyl) [1,2,4] triazolo[2,3-a] [1, 3, 5]triazin-5-ylamino]ethyl)phenol (ZM 241385; 0.01 μM) and N-[9-Chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide (MRS 1220; 0.1 μM). Each column represents the mean ± standard error of mean value obtained from six experiments. *P < 0.05 vs control. (GIF 111 kb).