Abstract
To report operative findings, postoperative course, and postimplantation performance in patients with cochlear malformations who underwent cochlear implantation. Seventeen patients with malformations which included enlarged vestibular aqueduct (n = 6), Mondini’s dysplasia (n = 5) common cavity deformity (n = 3) and incomplete partition type 2 (n = 3) underwent cochlear implantation with Nucleus 22 straight array device at our center. Operative findings described facial nerve anatomy and cerebrospinal fluid leak. Standard tests of speech perception were used to evaluate the postoperative performance for each subject. Operative findings included cerebrospinal fluid leak (thirteen patients) all of which were repaired successfully with graft. None had abnormal facial nerve anatomy. No surgical complications occurred. All the patients except two with common cavity had complete insertion. Electrode thresholds and discomfort levels were variable for several months after implantation. All patients demonstrated improved performance after implantation. Patients with enlarged vestibular aqueduct fared better than patients with other inner ear malformations. Cochlear implantation can be a successful method of rehabilitation in patients with congenital deafness who have cochlear malformations.
Keywords: Inner ear malformations, Cochleovestibular anomalies, Cochlear implantation, Cerebrospinal fluid gusher
Introduction
Cochlear implantation is an accepted method of auditory habilitation for profoundly hearing impaired children who do not benefit from amplification. Although most implanted patients have normal gross temporal bone anatomy, Jensen has estimated that 20% of children with congenital sensorineural hearing loss (SNHL) will have some abnormality of the inner ear [1]. These temporal bone anomalies are associated with a wide range of hearing acuity, varying degrees of progression of hearing loss, and presence or absence of related non-otological anomalies [2]. As a rule, the more severe the temporal bone deformity, the poorer the hearing [3].
Children with inner ear developmental malformations present a significant challenge to even the most experienced clinician due to uncertainty regarding surgical feasibility and performance outcomes [4]. Expectations from auditory performance after cochlear implant in patients with inner ear malformations are relatively less than those without. This may be due to the substantially reduced population of spiral ganglion cells [5–7]. Increased experience in cochlear implantation has led to more children with abnormal cochleovestibular anatomy being considered as candidates. This study was undertaken to evaluate the outcomes of cochlear implantation in children with cochleovestibular anomalies and to review any added difficulty encountered during their surgery.
Patients and Methods
This study was done in the department of ENT, SMS Medical College and Hospital, Jaipur, India, which is a tertiary care hospital and referral center for cochlear implants. It included seventeen patients with varying degrees of inner ear malformations who underwent implantation at ages ranging from 2 to 6 years. Of the seven children who were operated, six were girls and 11 were boys. The follow up varied from 18 to 24 months. Basic demographic data of all children and surgical findings are presented in Table 1.
Table 1.
Basic information and surgical findings of the patients
| S. no. | Age at surgery (in years) | Sex | Inner ear anomaly | Side of surgery | Surgical findings and complications |
|---|---|---|---|---|---|
| 1. | 4 | M | LVA | R | CSF gusher; controlled with packing |
| 2. | 5 | F | LVA | R | CSF gusher; controlled with packing |
| 3. | 2 | M | Mondini’s dysplasia | R | – |
| 4. | 4 | M | Common cavity deformity | R | CSF gusher; controlled with packing—Incomplete insertion |
| 5. | 4 | F | Common cavity deformity | L | Incomplete insertion |
| 6. | 3 | F | LVA | R | CSF gusher; controlled with packing |
| 7. | 3 | F | Mondini’s dysplasia | R | CSF gusher; controlled with packing |
| 8. | 3 | M | LVA | R | CSF gusher; controlled with packing |
| 9. | 4 | M | IP2 | R | CSF gusher; controlled with packing |
| 10. | 2 | M | LVA | R | CSF gusher; controlled with packing |
| 11. | 3 | F | LVA | R | CSF gusher; controlled with packing |
| 12. | 4 | M | Mondini’s dysplasia | R | CSF gusher; controlled with packing |
| 13. | 5 | M | Common cavity deformity | R | CSF gusher; controlled with packing |
| 14. | 5 | F | Mondini’s dysplasia | L | – |
| 15. | 3 | M | IP2 | R | CSF gusher; controlled with packing |
| 16. | 2 | M | IP2 | R | – |
| 17. | 4 | M | Mondini’s dysplasia | R | CSF gusher; controlled with packing |
M Male, F Female, LVA Large vestibular aqueduct, IP2 Incomplete partition 2, CSF Cerebrospinal fluid, L left, R right
Preoperative evaluation included otologic examination, audiological evaluation and a high resolution computed tomography (CT) of temporal bone as well as psychological assessment. Roentgenographic abnormalities were classified according to the scheme of Sennaroglu [8] wherein he has classified the cochlear malformations into labyrinthine aplasia, cochlear aplasia, common cavity, incomplete partition of the cochlea, hypoplasis and large vestibular aqueduct syndrome. Mondini’s dysplasia has a triad of incomplete partition type 2, an enlarged vestibular aqueduct and a minimally dilated vestibule [8–10]. There were six patients with large vestibular aqueduct (Fig. 1), five with Mondini’s deformity (Fig. 2), three with Incomplete Partition type 2 (Fig. 3) and 3 with common cavity deformity (Fig. 4). All children were using hearing aid but there was no significant benefit. Patients with anomalies of only semi-circular canals were excluded from the study.
Fig. 1.
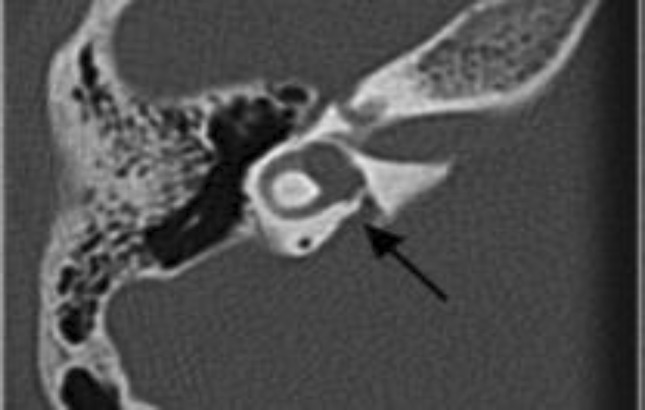
High resolution CT scan of a 4 year old boy with large vestibular aqueduct (LVA) who underwent successful cochlear implantation
Fig. 2.
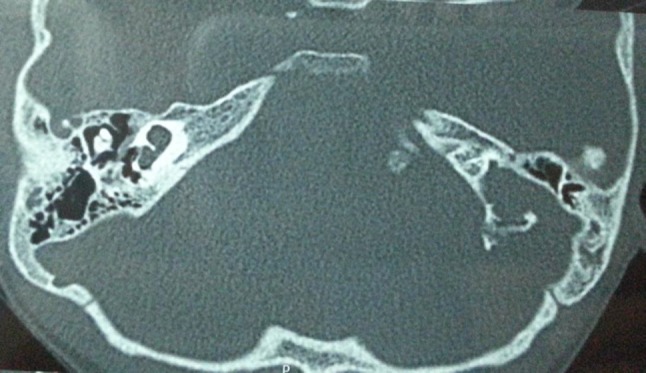
High resolution CT scan temporal bone (axial) of a 3-year old boy with Mondini’s dysplasia right side and Michel’s aplasia left side-underwent successful cochlear implantation on the right side with complete insertion of all the electrodes
Fig. 3.
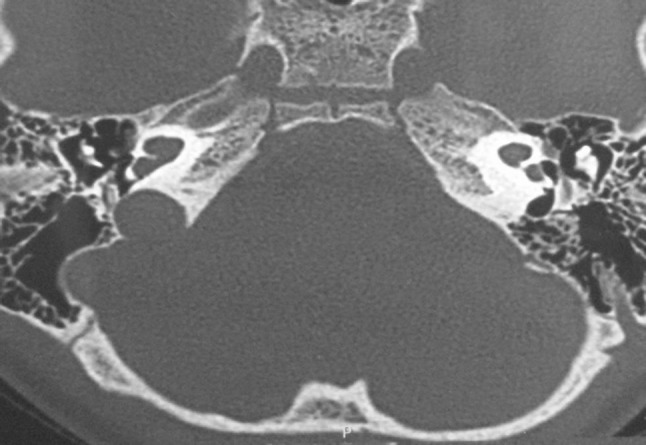
High resolution CT scan of a 3 year old male child with Incomplete Partition type 2 deformity-underwent successful cochlear implantation
Fig. 4.
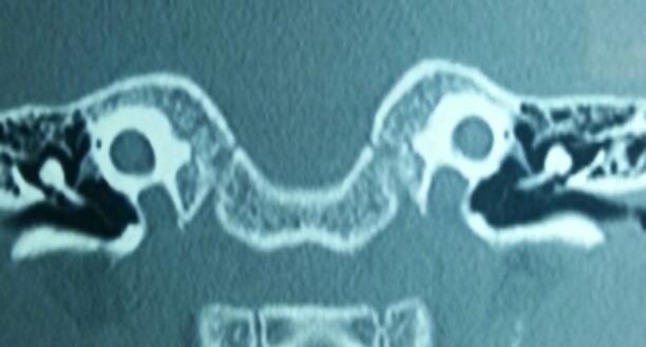
High resolution CT scan of a 4-year old child with bilateral common cavity deformity-underwent successful right cochlear implantation with complete insertion of all the electrodes
All the patients underwent cochlear implantation by transmastoid facial recess approach and Nucleus 22 straight array device was used. Post implantation, speech perception and production outcomes were measured using- Meaningful auditory integration scale (MAIS), Categories of auditory performance (CAP) and Speech intelligibility rating (SIR). All children were followed up at least once a month following initial activation for a total of 6 months and at 3 month intervals thereafter.
Results
Out of total 323 children who underwent cochlear implant at our center, the incidence of inner ear malformations was 7.43% which includes 17 patients with cochleovestibular anomalies and seven patients with isolated semicircular canal anomalies. Seventeen children with cochleovestibular anomaly underwent cochlear implantation. Complete electrode insertion was possible in all the cases except two with common cavity malformation. Cerebrospinal fluid gusher was encountered in 13 patients-six with large vestibular aqueduct, two with Mondini’s dysplasia, two with Incomplete Partition type 2 anomaly and three with common cavity deformity. This was successfully sealed using fascia in all the patients. There was no other significant intra operative or post operative complication.
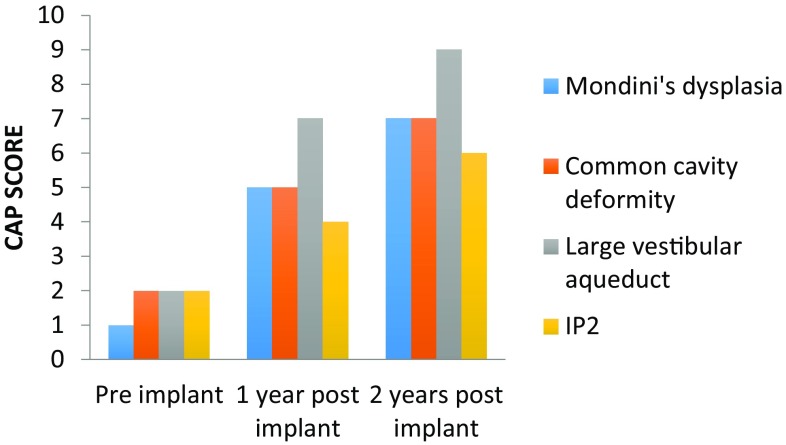
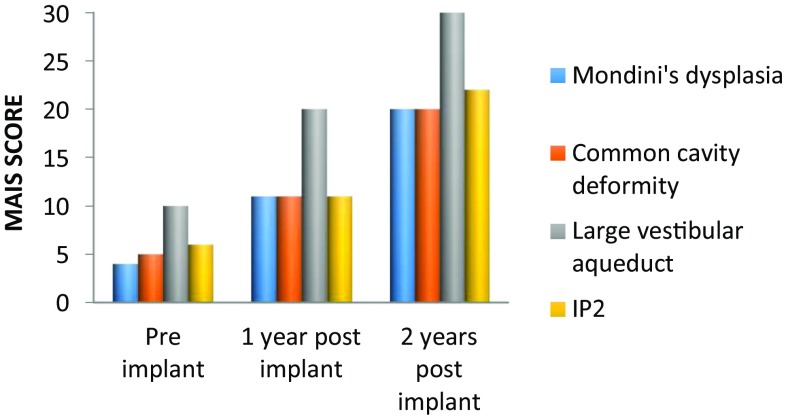
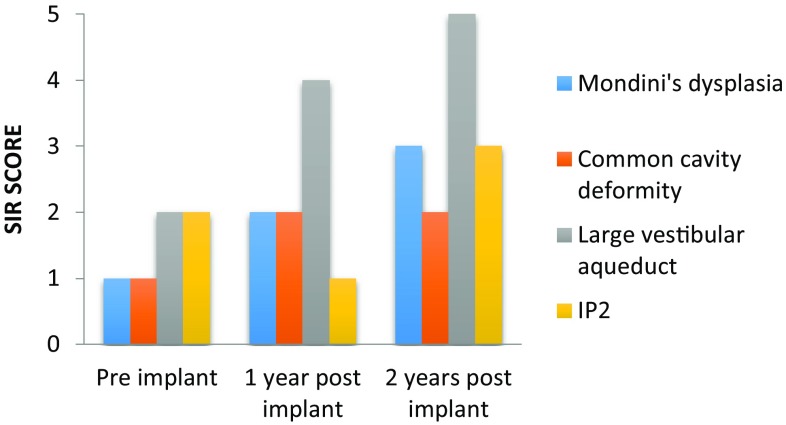
Post implantation, speech perception and production outcomes were measured using-Meaningful auditory integration scale (MAIS), Categories of auditory performance (CAP) and Speech intelligibility rating (SIR). The audiological performance at 1 and 2 years post implant are shown in Figs. 5, 6 and 7. Patients with LVAS did significantly better than those with other anomalies.
Fig. 5.
Categories of auditory performance (CAP) score
Fig. 6.
Meaningful auditory integration scale (MAIS)
Fig. 7.
Speech intelligibility rating (SIR) score
Discussion
The current study details the per-operative findings and complications along with the postoperative results in 17 patients with cochleovestibular anomalies who underwent cochlear implantation at our center from January 2011 to July 2014. All the patients were implanted using the transmastoid facial recess approach.
Out of these 17 patients 13 (76.47%) had CSF gusher which was controlled using cochleostomy packing. In a study conducted by Buchman et al. [4] they had CSF gusher in 21% patients all of which were sealed effectively. Luntz et al. [11] did 10 cochlear implants in 10 patients with inner ear malformations in which he had CSF gusher in 6 (60%) patients.
We did not encounter abnormal facial nerve anatomy in any of our cases. Though there are studies quoted in the literature where they have found a significant number of patients with abnormal facial nerve. Buchmann et al. [4] identified facial nerve anomalies in nine (32%) of 28 patients and were almost always associated with SCC aplasia or CC.
Papsin [12] reported that patients with cochleovestibular anomaly generally present fitting difficulties, such as low dynamic range, instable maps and facial nerve stimulation, especially if affected by common cavity and cochlear hypoplasia. Among patients with inner ear malformations, these problems may be less important in IP patients.
All the patients except two with common cavity had complete insertion of electrodes as compared to the study conducted by Buchman et al. [4] who had complete insertion in 89% of cases. None of our patients had any facial nerve abnormality and none of them developed any facial weakness postoperatively. All the patients underwent postoperative X-ray to ascertain the position of electrodes.
Audiological results postoperatively demonstrated that patients with LVA had comparable outcome as achieved in patients without any inner ear malformations. Their CAP score ranged from 7 to 9 postoperatively. Buchmann [4] in his study noted several interesting conclusions. First, children with the constellation of IP, EVA, and a dilated vestibule (i.e., Mondini’s malformation) perform very well on speech perception testing, with at least 10 (63%) of 16 patients achieving some degree of open-set speech recognition. Children with isolated EVA likewise perform well, with eight (89%) of nine achieving open-set recognition. This result corroborates with the result of our study in which audiological results were not affected by the presence of cochleovestibular anomalies. In our study results of patients with LVA is comparable to children with normal anatomy, however those with common cavity, Mondini’s dysplasia or IP2 anomaly do not fare so well.
Xia et al. [13] did follow-up for 36 months of 21 patients with common cavity deformity who underwent cochlear implantation, the average free-field hearing threshold was higher, and the scores for the CAP, SIR, IT-MAIS, and closed-set/open-set auditory speech perception tests were lower than in the control group (p < 0.05), which is similar to our results.
Bille et al. [14] compared the results of cochlear implantation in children with normal inner ear with those with inner ear malformations. The main outcome measures were category of auditory performance (CAP) and speech intelligibility rating (SIR). Eighteen children were diagnosed with cochlear malformations (12% of children receiving CI). No statistical differences regarding CAP and SIR scores were found between the two groups. Only one child was diagnosed with a common cavity and performed below average. Children with auditory neuropathy performed beyond average. Children with cochlear malformations performed equally to children without malformation in the long term.
To conclude, children with malformed cochleae demonstrate significant improvements in their speech recognition skills in comparison with their preoperative performance with hearing aids. Children with large vestibular aqueduct syndrome performed similar to patients with normal cochlea-vestibular anatomy. However, the patients with Mondini’s dysplasia and common cavity deformity and ossified cochlea fared less than their counterparts. The potentially increased difficulty in performing surgery and establishing optimal stimulation levels and the potential for poorer outcome in children with anomalous cochlea-vestibular anatomy must be weighed in candidacy decisions but do not preclude successful implantation and good outcome.
Compliance with Ethical Standards
Conflict of interests
All the authors declare that they have no conflict of interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Jensen S. Malformation of the inner ear in deaf children. Acta Radiol. 1969;286(suppl):1–97. [PubMed] [Google Scholar]
- 2.Park AH, Kou B, Hotaling A, Azar-Kia B, Leonetti J, Papsin B. Clinical course of pediatric congenital inner ear malformations. Laryngoscope. 2000;110:1715–1719. doi: 10.1097/00005537-200010000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Jackler RK, Luxford WM, House WF. Congenital malformations of the inner ear: a classifica- tion based on embryogenesis. Laryngoscope. 1987;97:2–14. doi: 10.1002/lary.5540971301. [DOI] [PubMed] [Google Scholar]
- 4.Buchman CA, Copeland BJ, Yu KK, et al. Cochlear Implantation in Children with Congenital Inner Ear Malformations. Laryngoscope. 2004;114:309–316. doi: 10.1097/00005537-200402000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Monsell EM, Jackler RK, Motta G, Lithicum FH. Congenital malformations of the inner ear. Laryngoscope. 1987;97(Suppl 40):18–24. doi: 10.1002/lary.5540971303. [DOI] [PubMed] [Google Scholar]
- 6.Otte J, Schuknecht HF, Kerr AG. Ganglion cell population in normal and pathological human cochlea. Laryngoscope. 1978;88:1231–1246. doi: 10.1288/00005537-197808000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Johnsson LG, Hawkins JE, Rouse RC, Kingley TC. Four variations of the Mondini inner ear malformations as seen in micro dissections. Am J Otolaryngol. 1984;5:242–257. doi: 10.1016/S0196-0709(84)80034-4. [DOI] [PubMed] [Google Scholar]
- 8.Sennaroglu L (2009) Cochlear implantation in inner ear malformations—a review article. Cochlear implants international [DOI] [PubMed]
- 9.Lo WW. What is a ‘Mondini’ and what difference does a name make? Am J Neuroradiol. 1999;20:1442–1444. [PMC free article] [PubMed] [Google Scholar]
- 10.Phelps PD, King A, Michaels L. Cochlear dysplasia and meningitis. Am J Otol. 1994;15:551–557. [PubMed] [Google Scholar]
- 11.Luntz M, Balkany T, Hodges NV, Telischi FF. Cochlear implant in children with congenital inner ear malformations. Arch Otol Head Neck Surg. 1997;123:974–977. doi: 10.1001/archotol.1997.01900090090013. [DOI] [PubMed] [Google Scholar]
- 12.Papsin BC. Cochlear implantation in children with anomalous cochleovestibular anatomy. Laryngoscope. 2005;115:1–26. doi: 10.1097/00005537-200501001-00001. [DOI] [PubMed] [Google Scholar]
- 13.Xia J, Wang W, Zhang D. Cochlear implantation in 21 patients with common cavity malformation. Acta Oto-Laryngol. 2015;135(5):459–465. doi: 10.3109/00016489.2014.990054. [DOI] [PubMed] [Google Scholar]
- 14.Bille J, Fink-Jensen V, Ovesen T. Outcome of cochlear implantation in children with cochlear malformations. Eur Arch Oto-Rhino-Laryngol. 2015;272(3):583–589. doi: 10.1007/s00405-014-2883-z. [DOI] [PubMed] [Google Scholar]