Abstract
To assess the role of sialendoscopy as a diagnostic and therapeutic modality in juvenile recurrent parotitis. Juvenile recurrent parotitis (JRP) is the second most frequent salivary gland disease in childhood and is characterized by recurrent non suppurative and non obstructive parotid inflammation. These attacks influence the quality of life and can even lead to gland destruction, and there are no definitive treatment to avoid them. Sialendoscopic dilatation is emerging as the new treatment modality in this aspect. Study design: retrospective study. Study setting: Department of Otorhinolaryngology in tertiary care hospital. 17 cases of juvenile recurrent parotitis (i.e. children of age group 3–11 years presenting with complaints of recurrent parotid region swelling and pain, sometimes associated with fever) were included in the study during October 2012–September 2015. All cases underwent sialendoscopy under general anaesthesia. Diagnostic (classifying the ductal lesion) and interventional sialendoscopic procedure (dilatation with instillation of steroid) were carried out in single sitting. Follow up was done for a minimum of 6 months (range 6–36 months). 17 patients with mean age of 5.6 years and gender distribution of 47:53 (boys:girls) underwent sialendoscopy for JRP. 8 patients presented with unilateral parotitis and 9 with bilateral. The mean number of attacks in previous 1 year were 9.2. Average time for procedure was 20 min. All cases had ductal stenosis and ductal mucosa was pale in 15 cases on endoscopy. 1 patient underwent repeat endoscopy after 2 years. 50% had complete resolution of symptoms and 6 patients had one mild (swelling not associated with fever which subsided on its own) attack after treatment. Follow up period ranged from 6 months to 3 years. No complications were observed. Sialendoscopy has emerged as a viable option for assessment and treatment of JRP. Dilatation of the parotid duct and steroid instillation has significantly reduced the morbidity of this condition.
Keywords: Juvenile recurrent parotitis (JRP), Sialendoscopy, Parotitis, Duct stenosis, Salivary duct obstruction
Introduction
Juvenile recurrent parotitis (JRP) is the second most frequent salivary gland disease in childhood and is characterized by recurrent attacks of non suppurative and non obstructive parotid inflammation. Each attack of parotitis is generally associated with intermittent painful swelling of one or both glands, often accompanied by local erythema and fever. The common age group involved is between 3 and 6 years and there is variable interval between two episodes. The pathogenesis is still unclear and multiple etiologies have been proposed. A familial form has been described with autosomal inheritance [1].
The main criterion for establishing the severity is the frequency of attacks. Each episode usually lasts for few days and may occur every 2–3 months. The treatment of acute phase is with antibiotics and analgesics whereas prophylactic antibiotics have no role. The prevention of recurring attacks represents the most dramatic and serious aspect of this pathology. JRP usually resolves spontaneously after puberty, however, in some cases the disease may continue leading to progressive loss of parenchymal function. Recurrences influence the quality of life and can also lead to progressive gland destruction, and consequently lead to major intervention such as parotidectomy [2].
Diagnosis is made by careful detailed history and clinical examination. Various imaging modalities are also used like ultrasound and MR sialography but there are no universal diagnostic guidelines.
Aim
To assess the role of sialendoscopy as a diagnostic and therapeutic modality in JRP.
Methods
A retrospective study of 17 children was done from October 2012 to September 2015 in department of Otorhinolaryngology in tertiary care hospital.
These patients of age group 3–11 years, presented with complaints of recurrent unilateral or bilateral parotid swelling and pain, usually associated with fever. They were managed conservatively with antibiotics and analgesics during the acute attack. Minimum of 6 attacks in previous 1 year was taken as criteria for endoscopic intervention.
Detailed history and clinical examination was done to establish diagnosis of JRP. The patients didn’t undergo any imaging procedure. All patients underwent sialendoscopy under general anaesthesia. Diagnostic sialendoscopy was done to classify the ductal pathology which was followed by interventional sialendoscopic procedure, wherein the duct was dilated using serial sizes of sialendoscopes with instillation of steroid, done in all cases in the same sitting.
The identification and dilatation of the parotid duct opening is the first step in this procedure. Identification can be improved using magnification via loops or microscope. Dilatation is most commonly performed using serial sizes of salivary probes (sizes 0000 to 8) and dilators of serial sizes (size 1–5). This was followed by diagnostic sialendoscopy using 0.9 mm all-in-one sialendoscope. Diagnostic sialendoscopy allowed minimally invasive exploration of the ductal system. The endoscope is coupled with irrigation tubing and the duct is continuously irrigated throughout the procedure to maintain patency, which has proven to be one of the therapeutic advantages of sialendoscopy. Salivary endoscopy is performed to visualize the main duct looking for mucosa condition, debris, areas of stenosis and obstructive sialoliths. A constant infusion of saline helps maintain a surgical endoscopic view. In parotid sialendoscopy, the masseter muscle can create a turn within the duct that is difficult to navigate and has been termed the “masseteric bend”. Pinching the cheek between the thumb and index finger with forward traction and using the other fingers of the hand to manipulate the salivary gland, it is often possible to straighten the duct and navigate the “masseteric bend”. A complete endoscopy includes visualization of the main duct as well as secondary and tertiary ductal systems.
Interventional sialendoscopy included ductal dilatation and introduction of pharmacological agents. The intervention used in these cases included dilatation of the duct using serial sizes of sialendoscopes (1.1, 1.3, 1.6 mm all-in-one sialendoscope). Finally, the parotid duct and gland was thoroughly lavaged with steroid (hydrocortisone) 50 mg (in normal saline dilution of 1:1) each side after dilatation.
After the procedure, injectable anitibiotic (amoxicillin–clavulanic acid) was given for 24 h. Massage of the parotid gland was also recommended in the first 24–48 h to decrease the postoperative swelling (which is usually due to use of saline solution for rinsing the sialendoscope while doing the procedure). Follow up was done after a period of 1 week, 1 month and then at 3 months interval. A minimum of 6 months of follow up was done (range 6–36 months). The patients were discharged same day with instructions for the use of parotid massage, antibiotics and pain medications for 1 week.
Results
17 children were clinically diagnosed and treated for JRP during October 2012–September 2015. The age of these children ranged from 3 to 11 years (mean age of 5.6 years). The study included 8 boys and 9 girls showing no sex preponderance.
The mean age when the symptoms began was 4.5 years. The first episode occurred approximately at age of 4.3 years in boys and 5 years in girls. Of these patients, 2 child had symptoms of the right parotid only, 6 had left parotitis and 9 had bilateral complaints alternatively or simultaneously (Table 1).
Table 1.
Clinical presentation
| Patient no./sex/age (years) | Side of involvement | Duration of symptoms (months) | Symptoms |
|---|---|---|---|
| 1/M/11 | Bilateral | 108 | Pain, swelling, fever |
| 2/F/5 | Bilateral | 36 | Pain, swelling, fever |
| 3/M/6 | Left | 12 | Pain, swelling, fever |
| 4/M/4 | Bilateral | 24 | Pain, swelling, fever |
| 5/F/5 | Right | 12 | Pain, swelling, fever |
| 6/F/7 | Bilateral | 36 | Pain, swelling, fever |
| 7/M/6 | Left | 6 | Swelling, fever |
| 8/F/3.5 | Bilateral | 10 | Pain, swelling, fever |
| 9/M/8 | Bilateral | 48 | Pain, swelling, fever |
| 10/F/4 | Bilateral | 6 | Swelling, fever |
| 11/M/3 | Bilateral | 12 | Pain, swelling, fever |
| 12/F/6 | Bilateral | 24 | Pain, swelling, fever |
| 13/F/6 | Left | 36 | Pain, swelling, fever |
| 14/F/4 | Right | 12 | Pain, swelling, fever |
| 15/M/8 | Left | 36 | Pain, swelling, fever |
| 16/M/3.5 | Left | 9 | Pain, swelling, fever |
| 17/F/6 | Left | 18 | Pain, swelling, fever |
The mean number of attacks in the preceding 1 year were 9.2.
Endoscopic Results
The average time of the endoscopic procedure was 20 min.
0.9 mm all-in-one scope was used for diagnostic purpose followed by dilatation with serial sizes (maximum size used 1.6 mm). The Stenson’s duct was examined in all cases and in 12 cases, the second generation branches were also navigated. Stenson’s duct was found stenosed in all cases. Also, the ductal mucosa was pale without the natural proliferation of blood vessels, seen in 11 cases. There was associated finding of mucoid flakes and debris in 5 cases (Fig. 1).
Fig. 1.
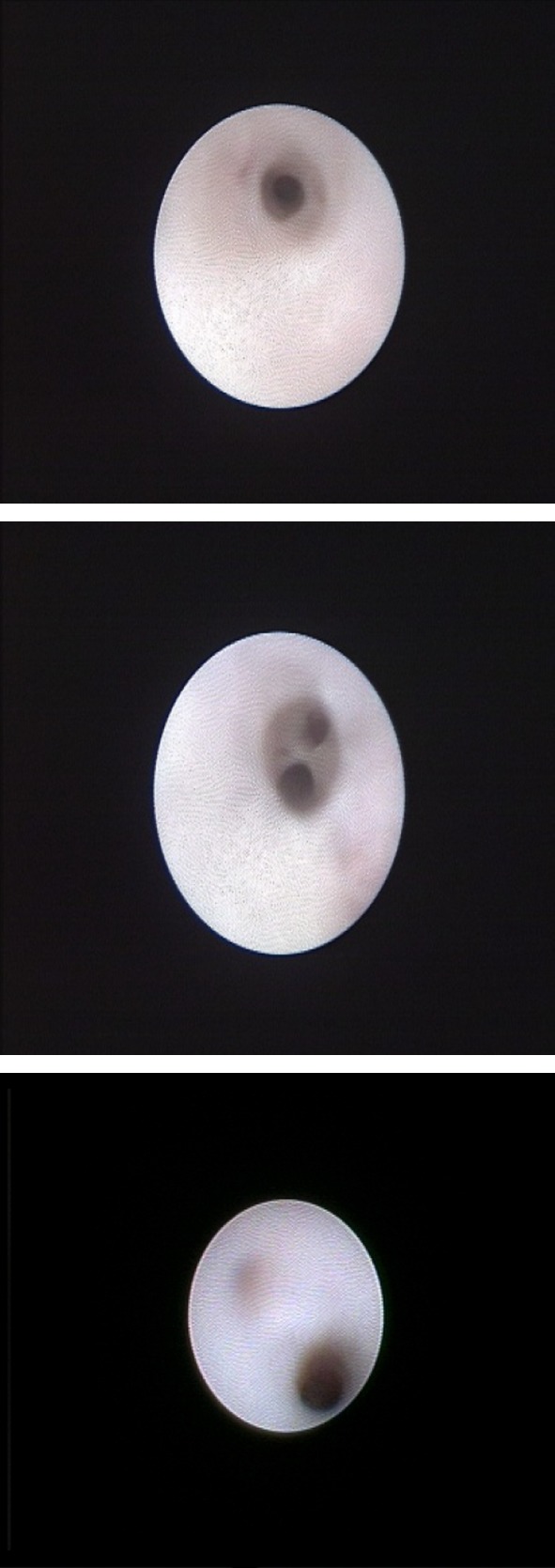
Sialendoscopic findings showing stenosed duct with pale mucosa
Stenosis of the stenson duct was classified according to the LSD (lithiasis, stenosis, dilation) grading system as given by Marchal [3]. Duct is labelled as stenosis grade S4 when there is generalized narrowing of the entire ductal system and there is difficulty in navigating the sialendoscope. The most common stenosis visualized was S4 in 10 cases followed by S3 in 5 cases, S2 in one case and S1 in one case.
In all cases, duct was dilated with serial sizes of sialendoscopes followed by instillation of steroid. The dilatation was done with sialendoscopes of sizes 1.1, 1.3, 1.6 mm. The maximum size used was 1.6 mm, however, the commonest size till which dilatation was possible was 1.3 mm. Hydrocortisone 50 mg was then flushed to lavage the duct and the gland. The patient remained hospitalised for 24 h. Massage of the parotid gland was recommended in first 24–48 h to decrease swelling due to irrigation fluid. Antibiotics were advised for 1 week.
Complications were minor like encountering acute masseteric bend that posed a challenge for navigating the scope in three patients. There were no major procedure-related complications such as ductal perforation, facial palsy or abscess after sialendoscopy in any of our patients.
Follow Up Results
The first follow up was done after 1 week of the procedure followed by 1, 3 months and then 6 months. A minimum of 6 months of follow up was done (range 6–36 months). The patients were examined for any recurrence of attacks of parotitis. 58.8% (10/17) patients had complete resolution of symptoms. The mean number of attacks in the follow up period of 1 year (for 12 cases) was 2.8 which showed a marked improvement from the mean number of attacks prior to sialendoscopy which was 9.2.
6 patients had a single mild episode of parotid swelling and pain after treatment (two had 6 months, two had 12 months, two had 18 months after treatment). These were resolved with 1 week course of antibiotic, hydration and analgesics. One patient had recurrent attacks every 6 months after endoscopic procedure (although these attacks were less severe and less frequent as compared to previous episodes). She was taken up for repeat endoscopic intervention. In the repeat procedure the duct was again dilated with sialendoscope of maximum size 1.6 mm and steroid was instilled. The patient has no further episodes of parotitis in past 1 year after the second sialendoscopic procedure (Table 2).
Table 2.
Follow-up results
| Patient no. | No. of attacks per year | Follow-up (months) | Repeat procedure | |
|---|---|---|---|---|
| Prior | After | |||
| 1 | 8 | 1 | 36 | No |
| 2 | 10 | 0 | 36 | No |
| 3 | 13 | 0 | 36 | No |
| 4 | 10 | 4 | 32 | No |
| 5 | 12 | 5 | 30 | Yes (once) |
| 6 | 10 | 1 | 36 | No |
| 7 | 10 | 0 | 30 | No |
| 8 | 8 | 5 | 24 | No |
| 9 | 10 | 0 | 24 | No |
| 10 | 8 | 0 | 24 | No |
| 11 | 10 | 0 | 18 | No |
| 12 | 12 | 0 | 18 | No |
| 13 | 10 | 0 | 18 | No |
| 14 | 10 | 0 | 12 | No |
| 15 | 7 | 0 | 12 | No |
| 16 | 6 | 2 | 12 | No |
| 17 | 8 | 0 | 6 | No |
Discussion
The development of minimally invasive procedures for diagnosing and treatment of paediatric salivary gland pathologies has led to profound implications with recognized significance. Sialendoscopy is emerging as the modality of choice in these pathologies.
Several studies report a JRP sex distribution favouring males whereas in our study, we found equal sex distribution. As in the literature in various studies, JRP occurred most commonly in age group of 3–6 years which is true for our study as well [4].
Imaging or histological examination reveals dilatation of the distal ducts of the parotid gland and punctuate sialectasis, usually without obstruction, leading to chronic inflammation of the glandular parenchyma. There are few theories in the literature regarding the etiology and pathophysiology of this phenomenon, including the possibility of ascending oral bacteria through Stenson’s duct, usually Gram positive aerobes, causing chronic infection and dilation of the distal ducts [5].
An initial low grade inflammation of the gland and ductal epithelium, secondary to dehydration or impaired salivary flow, results in stricture and columnar metaplasia which increases mucous secretions, resulting in decreased clearance of the more viscous saliva and further reduction in salivary flow, predisposing patient to recurrent parotitis [6]. Increase in MMP-2,9 and kallikrein from affected glands suggest a chronic inflammatory response [7].
Chronic inflammation of the gland leads to a lymphocytic periductal and intralobular infiltrate, which can have cytotoxic effect on the glandular parenchyma. Sialendoscopy supposedly breaks the cycle of inflammation by washing out intraductal debris and dilating stenosis and opening the ductal system due to pressure of irrigating fluid.
Many recommend performing sonography as the imaging method of first choice, in both the primary and follow-up stages. Sialography has the advantage in excluding ductal pathologies such as sialoliths and strictures. Computed tomography adds no further information and is not usually needed in these cases. Magnetic resonance imaging, specially magnetic resonance sialography, is now available for showing parotid ductal tree but rarely used in these cases. In our study, sialendoscopy appeared to be the diagnostic modality of choice. In a study by Nozaki et al. [8] two cases of stenosis were misdiagnosed as sialolithiasis by preoperative ultrasonography. During the endoscopic procedure, generalized ductal stenosis was seen. So, sialendoscopy has a greater sensibility and specificity than imaging. We feel that clinical history and physical examination are sufficient to provide an indication for endoscopic treatment, as recent studies have shown sialendoscopy to be a sufficient tool for the diagnosis of JRP [9].
Some studies have shown that the parotid system may be approximately the same size in children as in adults, and an ideal scope size has not been recommended for the management of JRP to date. However, our experience and previous studies report that the duct of a patient with JRP is likely to be stenotic, which would therefore call for a smaller endoscope to be used. Faure et al. [10] reported that a 1.3-mm sialendoscope can be used without difficulty for diagnostic sialendoscopy. However, we found that the 1.3-mm sialendoscope was technically more challenging to navigate as compared with the 0.9 and 1.1 mm endoscope. This may be due to the fact that we are treating a patient with diseased and stenotic ductal systems associated with JRP.
The most recognized sialendoscopic finding was represented by a pale, avascular and stenotic stenson duct. This gives an insight to the causative factors for JRP. The reduced ability to drain saliva would trigger an inflammatory vicious cycle which will lead to recurrent attacks. The global stenosis of the main duct and secondary branches of the ductal system seems more suggestive of a congenital origin [1].
The most common physical finding in exacerbations of JRP is an enlarged Stenson’s papilla with yellow plaques of coagulated proteins around the duct. The gland itself will be indurated but painful to palpation. Patients may also suffer from xerostomia, which can induce other related conditions like halitosis, cervical dental demineralization and decay, and mild dysphagia.
Our experience with sialendoscopy had promising results with technical success and subjective improvement in symptoms in all patients. It is comparable to the results shown by Nahlieli et al. [4], series of 26 cases of JRP treated by dilatation and abundant washing, resolution of symptoms in 92% cases. Similar study done by Quenin et al. [5] who examined 10 children with symptomatic JRP and initial ultrasound evaluation revealed a white duct without vascularity. Sialendoscope was used to dilate the duct with pressurized saline solution in all cases. Success rate as 89%. In 2009, Shacham et al. [11] included 70 children with JRP who were treated with sialendoscopic dilatation and lavage (saline followed by hydrocortisone) with a success rate of 93%.
In 2004, Nahlieli et al. [4] proposed an endoscopic technique for both the diagnosis and the treatment of JRP. Diagnosis was achieved by clinical history of two episodes of parotid swelling in a 12-month period, physical examination and ultrasound. Patients in this study underwent bilateral sialendoscopy of Stensen’s ducts regardless of laterality of symptoms, and lavage with 60 cc of normal saline was performed. All patients were then infused with 100 mg hydrocortisone through the sialendoscope. Results over the course of 14-year study were promising, with only nine of 70 patients having one subsequent episode of parotid swelling after treatment and only five requiring a repeat endoscopic treatment. Quenin et al. [5] reported a series of 10 patients in 2008. In this study, patients were diagnosed via clinical history, physical examination and an ultrasound. Indication for an endoscopic procedure in this study involved two episodes of parotid swelling in a 6-month period. He performed endoscopic intervention on the affected side only with saline and steroid irrigation. This study reported the need for only one repeat endoscopic procedure out of 10 patients with follow-up ranging from 2 to 24 months.
The main concern in this pathology is regarding the frequency of attacks which was found to be reduced after a single sialendoscopic dilatation and steroid instillation. Preoperatively the number of attacks were 9.2 in previous 1 year which was found to be greatly reduced to 2.8 attacks after the endoscopy. Also the severity of each attack was milder as compared to preoperative period. The challenge is, thus, to diagnose JRP as early as possible, to provide treatment, and to avoid the ultimate destruction of the gland.
In recent literature, interventional sialendoscopy is proving to be efficacious in treating JRP. The dimensions and placement of the endoscope alone, can help resolve sialectasis, and high pressure saline irrigation with or without corticosteroids, reduces the incidence of exacerbations and limits recurrence in many cases [12].
Historically, treatment of JRP included conservative management and in few cases aggressive surgical procedures. Conservative management includes antibiotics, analgesics, sialogogues and massage. Surgical intervention such as tympanic neurectomy and duct ligation have been tried. In few cases, the final treatment option is parotidectomy. But none of these measures proved useful in preventing attacks of JRP or treat JRP. Our technique’s benefit is the possibility to irrigate and to dilate the ductal system of the parotid gland under direct vision. Another advantage is the opportunity to inject medications in an intraductal manner under direct vision. The main aim of this treatment was to reduce the recurrent attacks of parotitis and prevent irreversible changes in the parotid glands.
Sialendoscopy is a safe and effective modality with low morbidity and fewer complications for management of JRP. The promising impact of sialendoscopy on the quality of life is the crucial clinical impact which is favouring sialendoscopy to slowly become the diagnostic and therapeutic modality of choice in JRP.
Compliance with Ethical Standards
Conflict of interest
All authors declare that he/she has no conflict of interest.
Ethical Approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and Animal Rights
This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Ried E, Douglas F, Crow Y, Hollman A, Gibson J. Autosomal dominant juvenile recurrent parotitis. J Med Genet. 1998;35(5):417–419. doi: 10.1136/jmg.35.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leerdam CM, Martin HC, Isaacs D. Recurrent parotitis of childhood. J Paediatr Child Health. 2005;41:631–634. doi: 10.1111/j.1440-1754.2005.00773.x. [DOI] [PubMed] [Google Scholar]
- 3.Marchal F, Chossegros C, Faure F, et al. Salivary stones and stenosis. A comprehensive classification. Rev Stomatol Chir Maxillofac. 2008;109(4):233–236. doi: 10.1016/j.stomax.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Nahlieli O, Shacham R, Eliav E. Juvenile recurrent parotitis: a new method of diagnosis and treatment. Paediatrics. 2004;114:9–12. doi: 10.1542/peds.114.1.9. [DOI] [PubMed] [Google Scholar]
- 5.Quenin S, Plouin-Gaudon I, Marchal F, Froehlich P, Disant F, Faure F. Juvenile recurrent parotitis: sialendoscopic approach. Arch Otolaryngol Head Neck Surg. 2008;134(7):715–719. doi: 10.1001/archotol.134.7.715. [DOI] [PubMed] [Google Scholar]
- 6.Maynard JD. Recurrent parotid enlargement. Br J Surg. 1965;52:784–789. doi: 10.1002/bjs.1800521021. [DOI] [PubMed] [Google Scholar]
- 7.Morales-Bozo I, Landaeta M, Urzúa-Orellana B, Retamales P. Association between the occurrence of matrix metalloproteinases 2 and 9 in parotid saliva with the degree of parotid gland damage in juvenile recurrent parotitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(3):377–383. doi: 10.1016/j.tripleo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Nozaki H, Harasawa A, Hara H, Kohno A, Shigeta A. Ultrasonographic features of recurrent parotitis in childhood. Pediatr Radiol. 1994;24(2):98–100. doi: 10.1007/BF02020162. [DOI] [PubMed] [Google Scholar]
- 9.Gary C, Kluka A, Schaitkin B, Walvekar R. Interventional sialendoscopy for treatment of juvenile recurrent parotitis. J Indian Assoc Pediatr Surg. 2011;16(4):132–136. doi: 10.4103/0971-9261.86865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faure F, Querin S, Dulguerov P, Froehlich P, Disant F, Marchal F. Pediatric salivary gland obstructive swelling: sialendoscopic approach. Laryngoscope. 2007;117(8):1364–1367. doi: 10.1097/MLG.0b013e318068657c. [DOI] [PubMed] [Google Scholar]
- 11.Shacham R, Droma EB, London D, Bar T, Nahlieli O. Long-term experience with endoscopic diagnosis and treatment of juvenile recurrent parotitis. J Oral Maxillofac Surg. 2009;67(1):162–167. doi: 10.1016/j.joms.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Canzi P, Occhini A, Pagella F, Marchal F, Benazzo M. Sialendoscopy in juvenile recurrent parotitis: a review of the literature. Acta Otorhinolaryngol Ital. 2013;33(6):367–373. [PMC free article] [PubMed] [Google Scholar]


