Abstract
The duration of adjuvant imatinib for high-risk patients with gastrointestinal stromal tumors (GISTs) is still controversial. Therefore, we retrospectively analyzed the data of high-risk patients with GISTs to investigate the appropriate duration. All 185 patients were divided into 4 groups: <1 year (Group A), 1–2 years (Group B), 2–3 years (Group C) and >3 years (Group D). The mean recurrence-free survival (RFS) in Groups A, B, and C were 44.3, 62.1, and 86.8 months, respectively (P < 0.001); the mean overall survival (OS) in Groups A, B and C was 75.2, 88.1, and 94.7 months, respectively (P = 0.009). The 5-year RFS in Groups A, B, C, and D was 15%, 26%, 83%, and 100%, respectively (P < 0.001); and the 5-year OS was 64%, 88%, 88%, and 100%, respectively (P < 0.001). The greatest impact on unfavorable outcomes was the tumor mitotic rate (HR, 2.01, 95% CI, 1.38–2.94; P < 0.001). Duration of adjuvant imatinib was the only favorable factor (HR, −0.95, 95% CI, 0.93–0.97; P < 0.001). For high-risk patients with high tumor size or mitotic rate, or non-gastric GISTs, we recommend that more than 3 years of adjuvant imatinib is feasible.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common gastrointestinal soft tissue malignancies1. In the past, the outcomes of patients suffering from GISTs were unfavorable because of the lack of response to other interventions except surgery, such as radiotherapy and chemotherapy. Complete surgical resection was the standard treatment for localized, primary GISTs, but approximately 40–50% have disease recurrence2. The study by Hirota and colleagues was the first to find that the activating mutations in KIT resulted in GISTs, significantly altering the biological understanding and management of this disease3. Now, we know that approximately 85% of GISTs contain an activating mutation in the KIT proto-oncogene, whereas 5–10% can have a mutation in the gene encoding platelet-derived growth factor receptor α (PDGFRα)4–8. Imatinib can inhibit activation of KIT and PDGFRα to treat GISTs. In 2000, imatinib was first used to treat metastatic GISTs effectively and has since been confirmed by successive research9–13. Imatinib has significantly changed the therapy of GISTs, and it is recommended as the standard first-line agent in the treatment of GISTs14,15. Adjuvant imatinib is the key treatment for postoperative high-risk patients with GISTs to decrease relapse16. Three large, randomized phase III trials have clearly demonstrated the benefit of adjuvant imatinib in high-risk patients with GISTs14,17,18. Consensus has been reached that adjuvant imatinib is necessary for high-risk patients with GISTs after surgery. However, there are still a number of controversies in the management of GISTs19,20. Some guidelines recommend that3 years of adjuvant imatinib for high-risk patients is effective to decrease recurrence21–24. The primary evidence is based upon a randomized trial (SSGXVIII/AIO) that showed that postoperative imatinib administered for 36 months can improve recurrence-free survival (RFS) and overall survival (OS) compared to 12 months for high-risk patients with GISTs18. However, before the randomized trial (SSGXVIII/AIO), two randomized trials recommended that adjuvant imatinib for 1 year could be effective to decrease recurrence (Z9001 ACOSOG) and that adjuvant imatinib for 2 years could prolong RFS for 1 year14,25,26. Based on three large, randomized trials, the longer duration of adjuvant imatinib was better. Is a 3-year duration of adjuvant imatinib sufficient for high-risk patients with GISTs? The relevant research is sparse, and to date, little prospective data available on the problem have been published. Therefore, our study was conducted to retrospectively analyze the clinical data of high-risk patients with GISTs after complete resection to investigate the appropriate duration of adjuvant imatinib.
Results
Characteristics
The 185 patients included 108 males and 77 females, and the mean age was 54.9 ± 12.0 years. The most frequent location of the primary tumor was the stomach (n = 124, 67.0%), followed by the small intestine (n = 49, 26.5%) and colorectal tract (n = 12, 6.5%). The baseline characteristics of the patients are shown in Table 1. We found that risk factors (tumor size, tumor site, tumor mitotic and GI bleeding) in the four groups had insignificant differences (Table 1). Among the 11 patients who did not receive imatinib for 12 months, 5 patients (4.7%) had intolerance or severe side effects and 6 patients did not take imatinib for economic reasons. Among the 95 patients with tolerance of imatinib, 18 patients (18.9%) interrupted treatment for side effects, such as diarrhea, constipation, and low leucocyte amount. However, the 18 patients recovered soon after stopping imatinib for one or more months, while 14 patients continued to take imatinib until the time was sufficient. The mean duration of follow-up, calculated from the data collection closure (March 2017), was 39 months (interquartile range [IQR], 23–59 months). The 5-year RFS rates and 5-year overall survival (OS) rate of all of patients were 36% and 78%, respectively.
Table 1.
Baseline characteristics of 185 patients with high-risk GISTs.
| Characteristics | All patients | Group A | Group B | Group C | Group D | P value |
|---|---|---|---|---|---|---|
| Age (M ± SD, years) | 54.8 ± 12.0 | 56.9 ± 12.8 | 53.7 ± 9.9 | 52.5 ± 10.5 | 52.1 ± 13.1 | 0.13 |
| Gender (M/F, n) | 77/108 | 39/51 | 15/25 | 15/15 | 8/17 | 0.53 |
| Tumor size (cm) | 8.6 ± 5.8 | 8.9 ± 6.3 | 7.9 ± 4.9 | 9.8 ± 7.1 | 7.2 ± 2.0 | 0.32 |
| Mitotic (/50 HPF) | 0.33 | |||||
| <5 | 36 | 21 | 6 | 4 | 5 | |
| 5–10 | 96 | 23 | 13 | 13 | 4 | |
| >10 | 53 | 46 | 21 | 13 | 16 | |
| Tumor sites (n) | 0.64 | |||||
| Gastric | 124 | 60 | 28 | 20 | 16 | |
| Small bowel | 49 | 21 | 11 | 9 | 8 | |
| Colorectal | 12 | 9 | 1 | 1 | 1 | |
| GI bleeding (Y/N, n) | 116/69 | 35/55 | 12/28 | 11/29 | 15/10 | 0.11 |
M ± SD: mean ± standard deviation.
GI bleeding: gastrointestinal bleeding.
Analysis and statistics of RFS and OS
We used the Kaplan–Meier method and log-rank test model to examine the duration of the adjuvant imatinib effect on RFS and OS in different groups. The mean RFS among all patients was 62.8 (95% CI, 56.5–69.1) months and the OS was 86.3 (95% CI, 81.4–91.0) months. The means of RFS in Groups A, B and C were 44.3 (95% CI, 36.5–52.0), 62.1 (95% CI, 50.5–73.8), and 86.8 (95% CI, 73.3–100.3) months, respectively (P < 0.001) (Fig. 1). The means of OS in these groups were 75.2 (95% CI, 66.4–84.0), 88.1 (95% CI, 79.7–96.5), and 94.7 (95% CI, 88.8–100.5) months, respectively (P = 0.009) (Fig. 2). None of the patients in Group D had recurrence; thus, the mean of PFS or OS could not be calculated. However, the 5-year RFS rates in Groups A, B, C, and D were 15%, 26%, 83%, and 100%, respectively (P < 0.001) (Fig. 1). The 5-year RFS of Group A was lower than that of Group B (χ 2 = 7.66 P = 0.006), and it was significantly different between Group B and Group C (χ 2 = 5.32 P = 0.02). The 5-year RFS of Group D was higher than that of Group C (χ 2 = 4.73 P = 0.03) (Fig. 1). The 5-year OS rates the groups were 64%, 88%, 88%, and 100% (P < 0.001), respectively (Fig. 2). The 5-year OS rate of Group A was lower than that of Group B (χ 2 = 4.54 P = 0.033), but it was insignificantly different between Groups C and B (χ 2 = 0.53 P = 0.47) and between Groups C and D (χ 2 = 1.76 P = 0.19). Group B vs. Group D did not show any significant difference (χ 2 = 2.97 P = 0.09) (Fig. 2).
Figure 1.
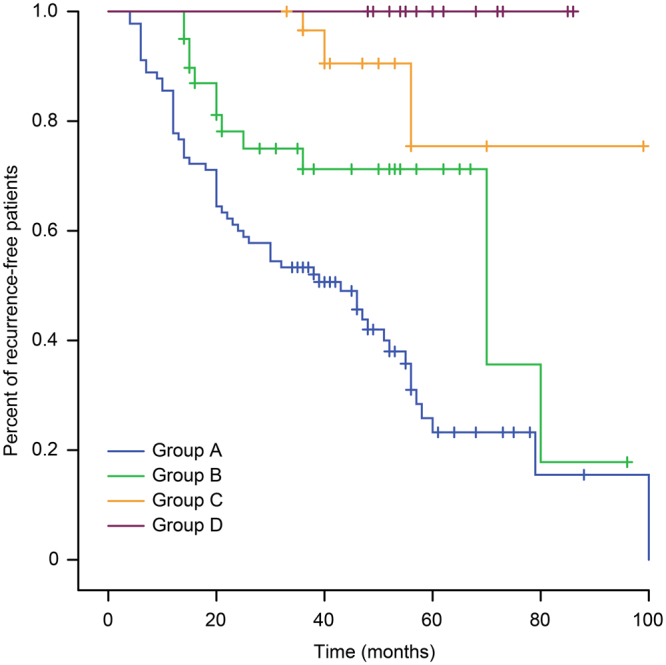
Kaplan–Meier estimates of the RFS of 185 patients in different groups.
Figure 2.
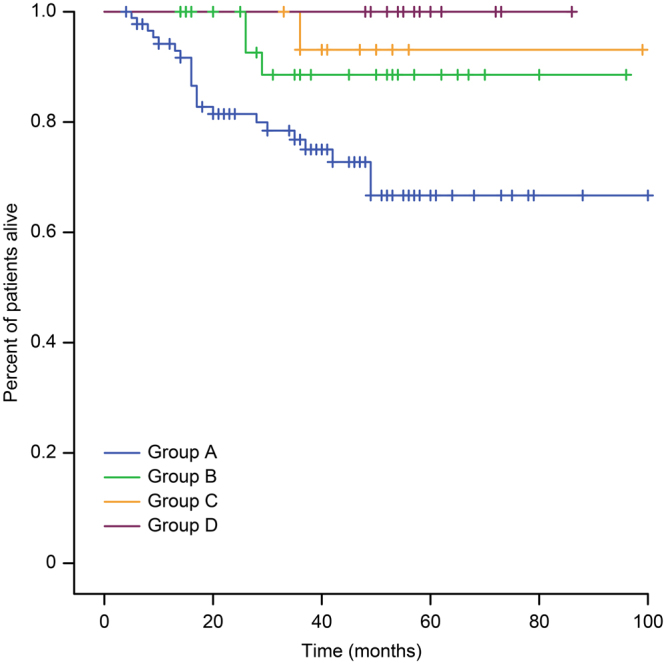
Kaplan–Meier estimates of the OS of 185 patients in different groups.
Cox proportional hazards model of the prognostic factors
In our study, death as a result of GISTs during our follow-up was minimal and could interfere with the accuracy of the statistics of OS, since the Cox proportional hazards model analyzed only multiple prognostic factors associated with the recurrence of high-risk patients with GISTs. To reduce the bias, we divided the duration of adjuvant imatinib, tumor size, tumor mitotic, tumor site and GI bleeding into different levels, as shown in Table 2. Through the multivariate analyses of risk factors with GISTs recurrence, GI bleeding was not associated with outcomes of high-risk patients with GISTs (P = 0.43) (Table 3). Tumor site was a risk factor for GIST recurrence (HR, 1.87, 95% CI, 1.13–3.10; P = 0.01) (Table 3), and tumor size was also associated with unfavorable outcomes (HR, 1.06, 95% CI, 1.02–1.11; P = 0.002) (Table 3). The greatest impact on unfavorable outcomes of high-risk patients with GISTs was the tumor mitotic rate (HR, 2.01, 95% CI, 1.38–2.94; P < 0.001) (Table 3). Duration of adjuvant imatinib was the only favorable factor with RFS (HR, −1.95, 95% CI, 1.93–1.97; P < 0.001) (Table 3).
Table 2.
Different levels of prognostic factors.
| Patient and Tumor | |||
|---|---|---|---|
| Factor | No. | (%) | Levels |
| Duration of adjuvant imatinib (m) | |||
| 0–11 | 90 | 48 | 0 |
| 12–24 | 40 | 22 | 1 |
| 25–36 | 30 | 16 | 2 |
| 37–48 | 3 | 2 | 3 |
| 49–60 | 15 | 8 | 4 |
| >60 | 7 | 4 | 5 |
| Tumor size (cm) | |||
| ≤5.0 | 56 | 30 | 1 |
| 5.1–10.0 | 78 | 42 | 2 |
| 10.1–15.0 | 37 | 20 | 3 |
| 15.0–20.0 | 10 | 7 | 4 |
| >20 | 4 | 2 | 5 |
| Tumor mitotic rate (count per 50 HPFs) | |||
| ≤5 | 32 | 17 | 1 |
| 6–10 | 53 | 28 | 2 |
| 11–15 | 36 | 20 | 3 |
| 16–20 | 27 | 15 | 4 |
| >20 | 37 | 20 | 5 |
| Tumor site | |||
| Stomach | 124 | 67 | 0 |
| Small intestine | 49 | 27 | 1 |
| Colon or rectum | 12 | 6 | 1 |
| GI bleeding | |||
| Yes | 116 | 63 | 1 |
| No | 69 | 37 | 0 |
Table 3.
Multivariate analyses of risk factors for RFS.
| Covariates | HR | 95%CI | P | |
|---|---|---|---|---|
| GI bleeding | 1.24 | 0.73 | 2.11 | 0.43 |
| Tumor mitotic rate | 2.01 | 1.38 | 2.94 | <0.001 |
| Tumor site | 1.87 | 1.13 | 3.10 | 0.01 |
| Tumor size | 1.06 | 1.02 | 1.11 | 0.002 |
| Duration of adjuvant imatinib | −1.95 | 1.93 | 1.97 | <0.001 |
HR: hazard ratio.
Discussion
The incidences of gastrointestinal stromal tumors (GISTs) have been increasing1,27,28. Surgery and adjuvant imatinib are the most important treatment for high-risk patients with GISTs. Imatinib, a small-molecule inhibitor of these tyrosine kinases, is the recommended first-line option for metastatic and unresectable GISTs according to the Clinical Practice Guidelines in 201129,30. However, the duration of adjuvant imatinib for high-risk patients who underwent complete resection has remained controversial.
Our retrospective study showed that the 5-year RFS rates in Groups A, B, C and D were 15%, 26%, 83%, and 100%, respectively (P < 0.001) (Fig. 1). The RFS of Group C was more favorable than that of Group B (P = 0.02), which was in line with previous studies18. Additionally, Group D was better than Group C (P = 0.03), which was the same as another retrospective study31. Based on previous studies and our retrospective data, adjuvant imatinib for three years was not sufficient for high-risk patients with GISTs, and a longer duration of adjuvant imatinib might decrease recurrence. In 2017, results from PERSIST-5 study reported by the ASCO conference indicated that 5-year adjuvant therapy may further prolong disease-free survival of intermediate and high risk patients. However, there were two main factors restricting long-term treatment. One was the side effects associated with imatinib, and another was the resistance of imatinib. Although clinical experience with imatinib demonstrated that many side effects were mild compared with other chemotherapy or targeted drugs, many studies reported that all patients treated with imatinib had at least one adverse event of any grade, and 21–43% experienced one or more Grade 3 or 4 adverse events at the 400 mg daily dose10,11,13,32. Thus, for a long duration of imatinib, a large proportion of patients had clinically significant toxicity, and treatment interruptions were not uncommon; these might ultimately alter the efficacy. The resistance of imatinib could be divided in two categories. Firstly, patients who progress within 6 months of an initial clinical response had primary resistance caused by an over-represented KIT exon 9 mutation or no detectable kinase mutation (wild-type tumor) in tumors6,33–35. The primary resistance to imatinib was minimal, but secondary resistance eventually developed in the majority of patients with imatinib36,37. The average time of secondary resistance was 20 months after treatment with imatinib38. Secondary resistance was found in 50–70% of the patients showing late progression. Secondary resistance occurred not only in late progression but also in the early progression, which had a great impact on the long duration of adjuvant imatinib for high-risk patients. Although knowledge about the mechanisms of resistance to imatinib has increased rapidly in recent years, it is unlikely that the whole spectrum of resistance mechanisms have now been elucidated; this has great importance, as it forms the foundation for treatment individualization and for the development of novel treatment approaches39,40. The optimal duration and scheduling of imatinib was unclear and 3 years was not sufficient for high-risk patients with GISTs. More research is still needed for the management of imatinib. Now, many researchers are exploring the optimal duration of adjuvant imatinib. For example, ‘Efficiency of Imatinib Treatment Maintenance or Interruption After 3 Years of Adjuvant Treatment in Patients with Gastrointestinal Stromal Tumors’ (NCT02260505) is sponsored by Centre Leon Berard, an open-label, randomized, multicenter phase III study aiming to determine the clinical impact of maintaining imatinib treatment beyond 3 years in the adjuvant setting for high-risk patients with GISTs.
In our study, most of the relapsed patients were discovered earlier for regular follow-up and were treated in a timely manner, e.g., reoperation or high-dose imatinib instead of sunitinib. This led to less patient deaths with GISTs recurrence (5-year OS, 78%), lower than that reported by other studies14,17,18,41. This suggests that regular follow-up is very important for high-risk patients with GISTs and can decrease the death rate for GISTs recurrence.
In the Cox proportional hazards model, duration of adjuvant imatinib was the only favorable factor with outcomes of high-risk patients with GISTs (P < 0.001) (Table 3). In 2012, contour maps were estimated by the risk of GIST recurrence after surgery by Joensuu H and are appropriate for estimation of individualized outcomes42–44. The high-risk GISTs classified by the modified NIH criteria were distributed into 30–100% of the 10-year risk of GIST recurrence in contour maps, indicating that the outcomes of high-risk GISTs had vast differences. Therefore, durations of adjuvant imatinib should be different for the same high-risk patients with GISTs. Adjuvant imatinib for three years might be enough for some patients, while it should be prolonged to decrease recurrence for others. Non-gastric GISTs (intestine and colon) were unfavorable compared with gastric GISTs20,45,46. Attending physicians should advise patients suffering non-gastric GISTs to prolong the duration of adjuvant imatinib.
In our data, the most important independent factor associated with an unfavorable outcome was the tumor mitotic rate (P < 0.001) (Table 3). When arranging the follow-up and planning duration of adjuvant imatinib, we should attach greater importance to high tumor mitotic rate than large tumor size. Even for the high tumor mitotic rate of median-risk GISTs, we should consider adjuvant imatinib for a short time. For patients with a high tumor mitotic rate, the follow-up after adjuvant imatinib might be arranged more frequently than for those with a low tumor mitotic rate.
Our retrospective study also found another interesting result about GI bleeding. GI bleeding was one of the most common presentations among patients with high-risk recurrence. However, whether GI bleeding indicates tumor rupture is unknown. From the Cox proportional hazards model of our data, we found that GI bleeding was not associated with the outcome of high-risk patients (P = 0.43) (Table 3). Some studies have reported that GI bleeding was an independent predictor of unfavorable prognosis47,48. However, some studies showed that GI bleeding did not seem to be associated with risk of recurrence49. A study reported that GI bleeding was a protective factor for GIST relapse and GI-bleeding patients who achieved a better prognosis50. It remains controversial whether GI bleeding was an independent predictor of unfavorable prognosis for patients with GISTs, and more comprehensive studies are needed.
There are certain limitations in our study. First, this is a retrospective study with a limited sample size and a large proportion of censored cases. Second, the study was conducted in one single central hospital. Third, mutational analysis of our data was incomplete before 2010, causing the lack of data on mutational status. Meanwhile, assessment of imatinib pharmacokinetics was used for all patients, and the lack of data was a limitation of our study. Finally, the short follow-up time might cause bias. Thus, a larger scale, multicenter, prospective study with a longer-term follow-up investigation is warranted. Despite these caveats, it appears that our findings can contribute to the management of imatinib in high-risk patients who underwent complete resection.
In summary, based on existing studies and our retrospective findings, we recommend that at least 3 years of adjuvant imatinib be standard care for high-risk patients with GISTs. For high-risk patients with a large tumor size, high tumor mitotic rate or non-gastric GISTs, we recommend that more than 3 years of adjuvant imatinib is also feasible but only if patients can tolerate the imatinib for a long duration. Individualized adjuvant imatinib for patients with GISTs and management of followup should be an important direction for future treatment of GISTs.
Methods
Patients
In this study, the data were retrospectively collected from 658 patients who underwent surgery for GISTs in West China Hospital, Sichuan University, from December 2009 to January 2015. The inclusion criteria were as follows: patients with KIT (CD117) positive in immunohistochemistry, patients without preoperative chemotherapy or imatinib, patients with high-risk GISTs confirmed by pathology, patients with complete resection and without distant metastasis, and patients beginning adjuvant imatinib between one and eight weeks after surgery. We used the modified NIH criteria as a risk-stratification scheme for GISTs; this has been generally accepted in clinical practice51–53. High-risk GISTs must meet at least one of the following features: maximum tumor diameter longer than 10.0 cm, mitotic count more than 10 mitoses per 50 high-power fields, tumor diameter above 5.0 cm and mitotic count over 5, and tumor rupture before surgery or during surgery. However, patients with tumor rupture were not included in our study because it might indicate incomplete resection36. The exclusion criteria were as follows: patients with other malignant diseases and patients who did not sign informed consent. By March 2017, we lost touch with 62 patients during the follow-up. Finally, after screening, a total of 185 patients were enrolled in the study from a total of 596 patients. The duration of adjuvant imatinib only included the time of patients receiving imatinib. The time, patients stopping imatinib for intolerance and side effects were not included. The dose of imatinib in patients who were tolerable (174 patients) was 400 mg qd, while 11 patients did not receive imatinib for 12 months, and 79 patients never took imatinib after surgery because of intolerance, side effects or other reasons. All patients were divided into 4 groups: <1 year (Group A, including 0–11 months, n = 90), 1–2 years (Group B, including 12–24 months, n = 40), 2–3 years (Group C, including 25–36 months, n = 30) and >3 years (Group D, including more than 36 months, n = 25) according to the duration of adjuvant imatinib. The primary endpoint was RFS and OS of the four groups. The risk recurrent factors of GISTs in our study were duration of adjuvant imatinib, tumor mitosis, tumor size, tumor site and gastrointestinal bleeding (GI bleeding), which was recently described in a study48,54–56. Through the results, we aimed to discover the appropriate duration of adjuvant imatinib for high-risk patients with GISTs.
Follow-up
Professional researchers followed up with the postoperative patients once every 1–3 months within 2 years and once every 3–6 months in the 3–5 year period after surgery via telephone calls and outpatient service. Contrast enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen and pelvis and CT of the chest or chest X-ray were required with the first dose of imatinib. The patients had CT or MRI of the abdomen and pelvis at 3- to 6-month intervals during the follow-up. Blood cell counts and chemistries were performed at 1- to 3-month intervals during the treatment period and subsequently at 6-month intervals after stopping imatinib.
Ethics statement
The study protocol was approved by the ethics committee of West China Hospital, Sichuan University. Written informed consent was obtained from the patients before beginning the study, even though the study is retrospective nature. The analysis did not involve interaction with human subjects or the use of personal identifying information. Patient records/information was anonymized and de-identified prior to analysis, and the methods were performed in accordance with the approved guidelines.
Statistical analyses
All statistical analyses were tested using SPSS version 20.0 (for MAC, IBM). We used the χ 2 test to compare categorical data and the t test or ANOVA to compare continuous data. The survival rate was compared using the Kaplan–Meier method, and the log-rank test model was used to detect differences in the survival curves of the various subgroups. The Cox proportional hazards model was used to analyze the prognostic factors of multiple factors associated with RFS. All P values were 2-sided, and P values < 0.05 were considered significant.
Acknowledgements
The authors thank the nursing and support staff at West China Hospital, Sichuan University. The study was supported by the Sichuan Provincial Science and Technology Support Project (2016SZ0047).
Author Contributions
Prof. Yong Zhou and Rui Zhao conceived the study together. All the authors contributed to the research and development process that resulted in this article. Rui Zhao, Yong Wang, Yuqian Huang, Yaping Cui, Yi Chen and Lin Xia performed follow-up tasks together for nearly 10 years. Rui Zhao wrote the manuscript under the guidance of Prof. Yong Zhou. All the authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yong Zhou, Email: nutritioner@hotmail.com.
Xiaoting Wu, Email: wxt1@medmail.com.cn.
References
- 1.Mucciarini C, et al. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC Cancer. 2007;7:230. doi: 10.1186/1471-2407-7-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joensuu H. Adjuvant treatment of GIST: patient selection and treatment strategies. Nat. Rev. Clin. Oncol. 2012;9:351–358. doi: 10.1038/nrclinonc.2012.74. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Miranda C, et al. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin. Cancer Res. 2012;18:1769–1776. doi: 10.1158/1078-0432.CCR-11-2230. [DOI] [PubMed] [Google Scholar]
- 5.Lux ML, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am. J. Pathol. 2000;156:791–795. doi: 10.1016/S0002-9440(10)64946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinrich MC, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J. Clin. Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich MC, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, et al. Frequent KIT mutations in human gastrointestinal stromal tumors. Sci. Rep. 2014;4:5907. doi: 10.1038/srep05907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joensuu H, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N. Engl. J. Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 11.Blanke CD, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J. Clin. Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 12.Verweij J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 13.Blanke CD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J. Clin. Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 14.DeMatteo RP, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frolov A, et al. Response markers and the molecular mechanisms of action of Gleevec in gastrointestinal stromal tumors. Mol. Cancer Ther. 2003;2:699–709. [PubMed] [Google Scholar]
- 16.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J. Clin. Oncol. 2002;20:1692–1703. doi: 10.1200/JCO.2002.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 17.Casali, P. G. et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): the EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J. Clin. Oncol. 31 (2013).
- 18.Joensuu H, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 19.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973–983. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 20.Demetri GD, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J. Natl. Compr. Canc. Netw. 2010;8(Suppl 2):S1–41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo DH, et al. Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res. Treat. 2016;48:1155–1166. doi: 10.4143/crt.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poveda A, et al. GEIS 2013 guidelines for gastrointestinal sarcomas (GIST) Cancer Chemother. Pharmacol. 2014;74:883–898. doi: 10.1007/s00280-014-2547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poveda A, et al. SEOM Clinical Guideline for gastrointestinal sarcomas (GIST) (2016) Clin. Transl. Oncol. 2016;18:1221–1228. doi: 10.1007/s12094-016-1579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Mehren M, et al. Soft TissueSarcoma, Version 2.2016 clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2016;14:758–786. doi: 10.6004/jnccn.2016.0078. [DOI] [PubMed] [Google Scholar]
- 25.Corless CL, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J. Clin. Oncol. 2014;32:1563–1570. doi: 10.1200/JCO.2013.51.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang YK, et al. Two-year adjuvant imatinib mesylate after complete resection of localized, high-risk GIST with KIT exon 11 mutation. Cancer Chemother. Pharmacol. 2013;71:43–51. doi: 10.1007/s00280-012-1970-3. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era - a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 28.Sandvik OM, Soreide K, Kvaloy JT, Gudlaugsson E, Soreide JA. Epidemiology of gastrointestinal stromal tumours: single-institution experience and clinical presentation over three decades. Cancer Epidemiol. 2011;35:515–520. doi: 10.1016/j.canep.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Barnes T, Reinke D. Practical management of imatinib in gastrointestinal stromal tumors. Clin. J. Oncol. Nurs. 2011;15:533–545. doi: 10.1188/11.CJON.533-545. [DOI] [PubMed] [Google Scholar]
- 30.Italiano A, et al. Patterns of care, prognosis, and survival of patients with metastatic gastrointestinal stromal tumors (GIST) refractory to first-line imatinib and second-line sunitinib. J. Clin. Oncol. 2011;29:10044. doi: 10.1200/jco.2011.29.15_suppl.10044. [DOI] [PubMed] [Google Scholar]
- 31.Lin JX, et al. Is 3-years duration of adjuvant imatinib mesylate treatment sufficient for patients with high-risk gastrointestinal stromal tumor? A study based on long-term follow-up. J Cancer Res Clin Oncol. 2017;143:727–734. doi: 10.1007/s00432-016-2334-x. [DOI] [PubMed] [Google Scholar]
- 32.Dempsey DT. Yearbook of Gastroenterology. 2009. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial; pp. 150–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinrich MC, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J. Clin. Oncol. 2006;24:4764–4774. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 34.Debiec-Rychter M, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer. 2004;40:689–695. doi: 10.1016/j.ejca.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Tamborini E, et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology. 2004;127:294–299. doi: 10.1053/j.gastro.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Parfitt JR, Streutker CJ, Riddell RH, Driman DK. Gastrointestinal stromal tumors: a contemporary review. Pathol. Res. Pract. 2006;202:837–847. doi: 10.1016/j.prp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Heinrich MC, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of Imatinib Mesylate for Treatment of Advanced Gastrointestinal Stromal Tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J. Clin. Oncol. 2008;26:5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Younus J, Verma S, Franek J, Coakley N. & Sarcoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. Sunitinib malate for gastrointestinal stromal tumour in imatinib mesylate-resistant patients: recommendations and evidence. Curr. Oncol. 2010;17:4–10. doi: 10.3747/co.v17i4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamborini E, et al. Functional analyses and molecular modeling of two c-Kit mutations responsible for imatinib secondary resistance in GIST patients. Oncogene. 2006;25:6140–6146. doi: 10.1038/sj.onc.1209639. [DOI] [PubMed] [Google Scholar]
- 40.Sleijfer S, Wiemer E, Verweij J. Drug Insight: gastrointestinal stromal tumors (GIST)–the solid tumor model for cancer-specific treatment. Nat. Clin. Pract. Oncol. 2008;5:102–111. doi: 10.1038/ncponc1037. [DOI] [PubMed] [Google Scholar]
- 41.Gao XD, et al. Role of surgery in patients with focally progressive gastrointestinal stromal tumors resistant to imatinib. Sci. Rep. 2016;6:22840. doi: 10.1038/srep22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joensuu H, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 43.Schmieder M, et al. Comparison of different risk classification systems in 558 patients with gastrointestinal stromal tumors after R0-resection. Front. Pharmacol. 2016;7:504. doi: 10.3389/fphar.2016.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yip D, et al. Controversies in the management of gastrointestinal stromal tumors. Asia Pac. J. Clin. Oncol. 2014;10:216–227. doi: 10.1111/ajco.12187. [DOI] [PubMed] [Google Scholar]
- 45.Gold JS, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeMatteo RP, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112:608–615. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 47.Yacob M, Inian S, Sudhakar CB. Gastrointestinal stromal tumours: review of 150 cases from a single centre. Indian J. Surg. 2015;77:505–510. doi: 10.1007/s12262-013-0899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, et al. Prognostic impact of gastrointestinal bleeding and expression of PTEN and Ki-67 on primary gastrointestinal stromal tumors. World J. Surg. Oncol. 2014;12:89. doi: 10.1186/1477-7819-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmebakk T, et al. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br. J. Surg. 2016;103:684–691. doi: 10.1002/bjs.10104. [DOI] [PubMed] [Google Scholar]
- 50.Yin Z, et al. Clinicopathological and prognostic analysis of primary gastrointestinal stromal tumor presenting with gastrointestinal bleeding: a 10-year retrospective study. J Gastrointest Surg. 2017;21:792–800. doi: 10.1007/s11605-017-3385-2. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher CDM, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum. Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 52.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 53.Rutkowski P, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur. J. Surg. Oncol. 2011;37:890–896. doi: 10.1016/j.ejso.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 54.DeMatteo RP, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann. Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scarpa M, et al. A systematic review on the clinical diagnosis of gastrointestinal stromal tumors. J. Surg. Oncol. 2008;98:384–392. doi: 10.1002/jso.21120. [DOI] [PubMed] [Google Scholar]
- 56.Caterino S, et al. Gastrointestinal stromal tumors: correlation between symptoms at presentation, tumor location and prognostic factors in 47 consecutive patients. World J. Surg. Oncol. 2011;9:13. doi: 10.1186/1477-7819-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


