Abstract
Supported metal clusters containing only a few atoms are of great interest. Progress has been made in synthesis of metal single-atom catalysts. However, precise synthesis of metal dimers on high-surface area support remains a grand challenge. Here, we show that Pt2 dimers can be fabricated with a bottom–up approach on graphene using atomic layer deposition, through proper nucleation sites creation, Pt1 single-atom deposition and attaching a secondary Pt atom selectively on the preliminary one. Scanning transmission electron microscopy, x-ray absorption spectroscopy, and theoretical calculations suggest that the Pt2 dimers are likely in the oxidized form of Pt2Ox. In hydrolytic dehydrogenation of ammonia borane, Pt2 dimers exhibit a high specific rate of 2800 molH2 molPt −1 min−1 at room temperature, ~17- and 45-fold higher than graphene supported Pt single atoms and nanoparticles, respectively. These findings open an avenue to bottom–up fabrication of supported atomically precise ultrafine metal clusters for practical applications.
Controlled fabrication of few-atoms supported catalysts is a major challenge in the synthesis of nanomaterials. Here, the authors show a bottom-up approach to precisely synthesize platinum dimers supported on graphene, which display higher catalytic activity and stability than single atoms and nanoparticles.
Introduction
Supported metal catalysts are among the most important categories of heterogeneous catalysts in many reactions including chemical upgrading, automobile exhaust treatment, Fischer-Tropsch synthesis, biomass conversions, and many other processes1–7. Decreasing metal particle size is desirable for improving metal utilization, since catalytic reactions take place on the surface of metal nanoparticles (NPs). When a metal cluster contains only a few metal atoms, it could have a discrete energy band structure, tightly correlated with the number of metal atoms. Changing one atom in the ultrafine cluster might largely alter the electronic structure and drastically change its catalytic properties. Such atom-dependent catalytic behaviors have been successfully demonstrated by the model catalysts of mass-selected metal clusters, which were fabricated by soft landing of mass-selected ions from their physical vapor under ultrahigh vacuum conditions8–14. However, such complicated approach is only limited to model catalyst studies and is not applicable to high-surface area supports for practical applications.
Recently, synthesis of supported metal single-atom catalysts (SACs) has been extensively explored and a number of successful examples have been demonstrated15–23. Nonetheless, synthesis of atomically precise ultrafine metal clusters, such as dimers, on high-surface area supports, remains a grand challenge. The decisive limitation is the lack of precise control over the aggregation process, which often causes metal NPs formation with a broad size distribution. Protecting metal clusters with a strong ligand can certainly inhibit metal aggregation to a large extent, such as in the case of thiolate-protected Au magic clusters24. However, these strong protective ligands typically poison the metal clusters, and decrease their catalytic activities considerably25–30. Alternatively, Gates et al. demonstrated that precisely defined iridium and rhodium clusters were achieved by grafting the corresponding carbonyl complexes with a specific number of metal atoms onto oxide supports31–33. But the success is limited. As a consequence, a general bottom–up approach to synthesize atomically precise metal clusters on high-surface area supports is still missing. Atomic layer deposition (ALD) relies on two sequential self-limiting surface reactions at the molecular level, which are separated by inert gas purging34–36. This unique character makes ALD possible to bottom–up construct catalytic materials on a high-surface area substrate uniformly and precisely37–39.
Here, we show that Pt2 dimers can be bottom–up fabricated on a graphene support by depositing Pt on phenol-related oxygen anchor sites atom-by-atom in a sequential manner using Pt ALD. The dominant presence of isolated Pt1 single atoms and Pt2 dimers in the corresponding samples were confirmed by both aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and X-ray absorption fine structure spectroscopy (XAFS). Their structures were determined through a combination of density function theory (DFT) calculations and XAFS spectra simulations. In hydrolysis of ammonia borane (AB) for hydrogen generation, graphene supported Pt2 dimers (Pt2/graphene) exhibited a striking activity, which is ~17- and 45-fold higher than that of graphene supported Pt1 single atoms and Pt NPs, respectively. Compared to Pt1 single atoms and Pt NPs, the decreased adsorption energies of both AB and H2 molecules on Pt2 dimers are likely the major reason for the high activity. More importantly, the Pt2 dimers were stable under the current reaction condition and in the inert environment at below 300 °C.
Results
Synthesis and morphology of Pt1/graphene and Pt2/graphene
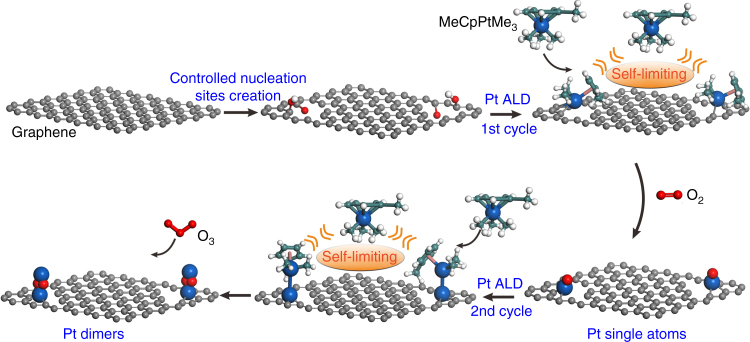
Based on our recent strategy22, the nucleation sites of isolated phenols or phenol–carbonyl pairs suggested by Shenoyl et al40. for Pt ALD were first created on pristine graphene nanosheets through acid oxidation followed by high-temperature thermal reduction, as illustrated in the schematic model in Fig. 1. The reduced graphene oxide support was defect-rich multilayered graphene films with a thickness of about a few nanometers (Supplementary Fig. 1). It had a surface area of 570 m2/g. Next, Pt ALD was performed on the graphene support by alternately exposing trimethyl(methylcyclopentadienyl)-platinum(IV) (MeCpPtMe3) and molecular O2 at 250 °C. The self-limiting surface reactions between MeCpPtMe3 and the support ensure nucleation of one MeCpPtMe3 molecule on one phenol-related nucleation site during the saturated MeCpPtMe3 exposure (Supplementary Fig. 2). It should be noted that Pt nucleation on graphene defect sites, such as edges and line defects, was inhibited at 250 °C, although it is possible at 300 °C (Supplementary Table 1)41. A similar temperature effect on inhibiting metal ALD on oxides was also observed by Elam et al42. Next, the ligands were removed through a combustion reaction during the O2 exposure step43–45 and Pt1 single atoms are formed (denoted as Pt1/graphene).
Fig. 1.
Schematic illustration of bottom–up synthesis of dimeric Pt2/graphene catalysts. Controlled creation of isolated anchor sites on pristine graphene; one cycle of Pt ALD on the anchor sites for Pt single atoms formation by alternately exposing MeCpPtMe3 and molecular O2 at 250 °C; second cycle of Pt ALD on the Pt1/Graphene to selectively deposit the secondary Pt atoms on the preliminary ones for Pt2 dimers formation at 150 °C. The balls in cyan, white, red, and blue represent carbon, hydrogen, oxygen, and platinum while the ball in gray represents carbon atoms in the graphene support
Next, the formed Pt1 single atoms was further utilized as nucleation sites for anchoring the secondary MeCpPtMe3 molecule in the following cycle. Again, the steric hindrance between MeCpPtMe3 molecules restricts chemisorbing one MeCpPtMe3 molecule only on one isolated Pt1 atom. However, we noticed that a considerable amount of Pt NPs were formed after two successive cycles of Pt ALD at 250 °C (denoted as 2cPt/graphene, Supplementary Fig. 3). Therefore, the deposition temperature was decreased to 150 °C for the second ALD cycle to avoid any metal aggregation. Meanwhile, ozone (O3), a stronger oxidizing regent was utilized to remove the ligand efficiently to form Pt2 dimers (Pt2/graphene) (Fig. 1)46. The formed Pt1 single atoms and Pt2 dimers are expected to be in the oxidized forms, since they were exposed to O2 and O3 during synthesis, respectively.
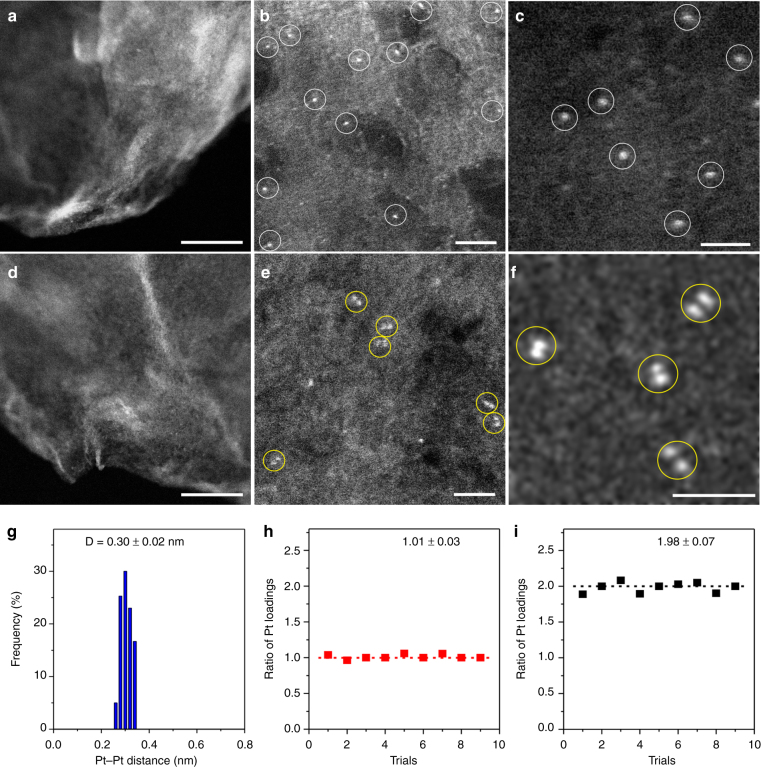
Aberration-corrected HAADF-STEM measurements were carried out to investigate the morphologies of the single-atom Pt1/graphene and dimeric Pt2/graphene catalysts. Compared to the naked graphene (Supplementary Fig. 1), HAADF-STEM images illustrated that one cycle of Pt ALD on the graphene support at 250 °C resulted in a formation of atomically dispersed Pt1 atoms without presence of any visible clusters or NPs (Fig. 2a–c, and Supplementary Fig. 4). These Pt1 single atoms were well isolated from each other with a distance >2 nm in average, which is significantly larger than the effective diameter of the MeCpPtMe3 molecule of ~0.96 nm47, confirming the steric effect during synthesis. Similar to our recent study of Pd1 single-atom growth on graphene22, we found that complete removal of other oxygen-contained functional groups, such as carboxyl groups, from graphene by carefully tuning the reduction temperature and time, is the key to eliminate any Pt clusters or NPs formation (Supplementary Fig. 5). These findings suggest that metal atoms anchored on carboxyl groups have a general weak interaction with the graphene support, thus aggregate aggressively to larger metal NPs under the ALD conditions, in line with literature48.
Fig. 2.
Morphology of the single-atom Pt1/graphene and dimeric Pt2/graphene catalysts. Aberration-corrected HAADF-STEM images of Pt1/graphene (a–c) and dimeric Pt2/graphene (d, e). Scale bars, 20 nm (a, d), 2 nm (b, e), and 1 nm (c, f). Pt single atoms in b and c and dimers in e and f are highlighted by white and yellow circles, respectively. g Statistical Pt–Pt distance in the observed Pt2 dimers. h The ratio of Pt loading of in Pt-MeCpPtMe/graphene to that in MeCpPtMe/graphene, and i the ratio of Pt loading in Pt2/graphene to that in Pt1/graphene in nine independent trials determined by ICP-AES
After performing another cycle of Pt ALD on Pt1/graphene at 150 °C (Pt2/graphene), Pt2 dimers were dominantly formed along with a certain number of Pt1 single atoms (Fig. 2d–f, and Supplementary Fig. 6), where neither Pt clusters nor NPs were observed. Very interestingly, we noticed that Pt2 dimers frequently rotated by specified angles of 30, 60, and 90° under the electron beam during STEM measurements and then split into two isolated Pt1 atoms (see more details in Supplementary Figs. 7–9). Such characteristic rotations might be related with the geometry of the graphene support and the size of carbon defect by considering the aforementioned Pt2 dimer structure (Supplementary Fig. 10). This observation provides strong evidence of the presence of Pt2 dimers rather than the projection coincidence of two isolated Pt1 atoms at different Z positions. Statistical analysis of more than 80 pairs of Pt2 dimers showed a Pt–Pt distance of 0.30 ± 0.02 nm for Pt2 dimers (Fig. 2g), which is significantly longer than the Pt–Pt bond in Pt bulk. This indicates that the Pt2 dimers are in the oxidized form as expected.
To step-wise elucidate the selective deposition of secondary Pt atom onto the preliminary ones for the formation of Pt2 dimers in the second ALD cycle, a set of control experiments were further performed using inductively coupled plasma-atomic emission spectroscopy (ICP-AES). First, the influence of the Pt precursor ligand on the second Pt ALD cycle was examined. In this case, half cycle of Pt ALD was executed on the graphene support by performing the MeCpPtMe3 pulse step only (denoted as MeCpPtMe/graphene) at 250 °C. After that, the ALD reactor was cooled to near room temperature and half amount of the MeCpPtMe/graphene sample was taken out of reactor for ICP-AES analysis; while the rest of the sample was put back to the ALD reactor quickly to perform the second cycle (Pt-MeCpPtMe/graphene) at 150 °C. In nine independent trials, the ICP-AES results showed that the ratio of the Pt loadings of Pt-MeCpPtMe/graphene to those of the corresponding MeCpPtMe/graphene were all very close to one (Fig. 2h). Therefore, there was no additional Pt deposited on MeCpPtMe/Graphene, reflecting the saturated self-limiting reaction character of ALD34–36. Second, we found that exposure of pristine graphene to O2 at 250 °C did not cause any detectable Pt deposition either (Supplementary Table 1). This is very important to ensure that the oxygen pulse at 250 °C in the first ALD cycle did not create any additional nucleation sites. Taken together, the Pt1 single atoms formed on graphene, confirmed by HAADF-STEM in Fig. 2a–c, are the only nucleation sites for the following ALD cycle. It is worthy to note that Pt1 single atoms well isolated from each other could be crucial to make all the Pt1 single atoms accessible for chemisorbing the second MeCpPtMe3 molecule in the second ALD cycle without steric hindrance.
During the second ALD cycle, one MeCpPtMe3 molecule anchors on one Pt1 atom in the Pt1/graphene sample due to the steric hindrance effect, which doubles the Pt loading. This was confirmed by the ratios of two for the Pt loadings of Pt2/Graphene to Pt1/graphene in nine independent trials (Fig. 2i and Supplementary Table 2), hence providing strong evidence of the formation of Pt2 dimers. The Pt1 single atoms observed in Fig. 2e and Supplementary Figs. 6–9 were likely formed by uncoupling of Pt2 dimers under the high flux electron beam during STEM measurements49. On the other hand, once Pt NPs were formed during ALD, the ratio of Pt loading of the two-ALD cycle sample to the one-cycle sample was apparently off from the stoichiometric value of two (Supplementary Table 2).
Performing an additional cycle on Pt2/graphene to form Pt3 trimers might be possible. However, we noticed that the O3 in the second cycle can create additional nucleation sites on graphene. As a consequence, selective deposition was not achieved for the third cycle and resulted in a mixture of Pt1, Pt2 and Pt3. Therefore, we mainly focused on the Pt2 dimers in this work.
XAFS characterization and DFT calculations
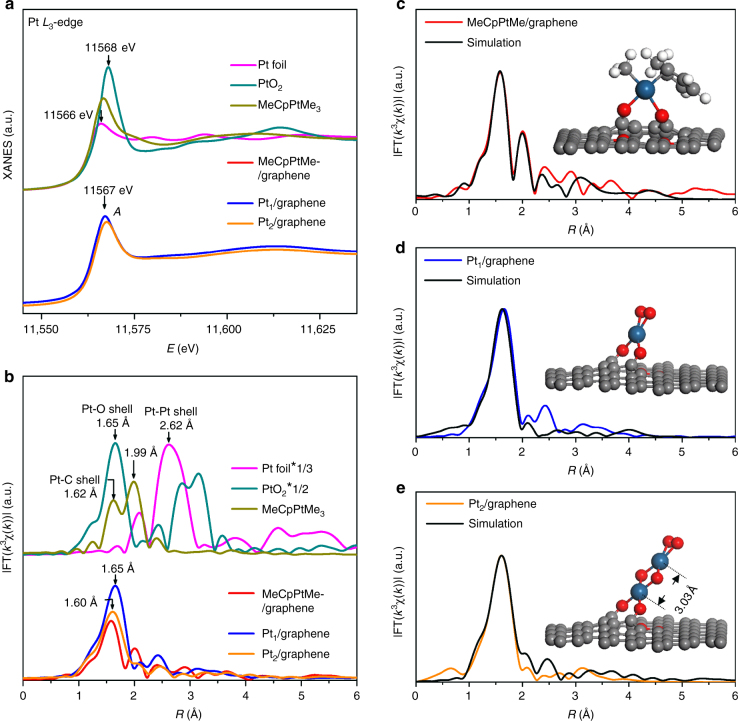
Figure 3a shows the X-ray absorption near-edge structure (XANES) spectra of MeCpPtMe/graphene, Pt1/graphene, and Pt2/graphene at the Pt L 3-edge, along with Pt foil, PtO2, and MeCpPtMe3 as references. Evidently, the XANES white line peaks of these three samples (11,567 eV) located at between Pt foil and PtO2, indicating that the Pt in MeCpPtMe/graphene, Pt1 single atoms, and Pt2 dimers were all in a similar oxidation state between Pt0 and Pt4+. The MeCpPtMe3 reference sample exhibits two well-resolved peaks at 1.62 and 1.99 Å in the Fourier transformed (FT) k 3χ(k) curve in the real-space (R-space) (Fig. 3b), assigned to the shorter Pt–C bonds (1.99−2.14 Å) in the three Pt–Me groups and longer Pt–C bonds (2.26−2.36 Å) in the Pt–MeCp group, respectively (Supplementary Fig. 11a)47,50. Apparently, the two split peaks in the MeCpPtMe/graphene curve suggests that the MeCp group stayed on Pt in this sample. This observation is in line with both the experimental result of the formation of MeCpPtMe2 surface species on oxides43,51 and theoretical calculations where MeCpPtMe3 on epoxydated and hydroxylated graphene surfaces liberates either one or two methyl groups depending on the available surface groups52.
Fig. 3.
XAFS structural characterization and spectra simulations. a The XANE spectra and b the K2-weighted Fourier transform spectra of MeCpPtMe/graphene, Pt1/graphene, and Pt2/graphene at the Pt L3-edge. The reference samples of Pt foil, PtO2, and MeCpPtMe3 are also shown for comparison. Comparison of the EXAFS simulations based on the corresponding DFT calculated structural models (insets) with the experimental EXAFS spectra of MeCpPtMe/graphene (c), Pt1/graphene (d) and Pt2/graphene (e). The ball in gray, white, red, and dark blue represent carbon, hydrogen, oxygen, and platinum, respectively
The first shell FT peak in the Pt1/graphene spectrum had a higher intensity and slightly shifted to 1.65 Å, while the peak at 1.99 Å disappeared. Clearly, the MeCp ligand was combusted off after the O2 exposure step at 250 °C. The first shell peak is assigned to Pt−O and/or Pt−C coordinations. Similar to MeCpPtMe/graphene, a very weak peak at 2.4 Å was visible in the spectrum of Pt1/graphene. However, this peak is significantly different from the Pt–Pt coordination peak (at ~2.62 Å) in Pt foil, thus assigned to the second nearest C/O neighbors of Pt. This suggests the absence of Pt NPs in Pt1/graphene, consistent with our STEM observation (Fig. 2a–c and Supplementary Fig. 4). The dimeric Pt2/graphene sample showed a similar FT curve with Pt1/graphene, implying a similar local C/O coordinations in these two samples. In the Pt2/graphene spectrum, there was no discernible peak for the Pt–Pt coordination, suggesting the Pt2 dimers are in the oxidized form after the ozone exposure step at 150 °C.
Considering the difficulties in discriminating the C/O neighbors by EXAFS fittings, we resorted to the combination of DFT calculations with EXAFS simulations to determine the optimized structures of these three samples. Here, a graphene support containing a carbon vacancy along with either isolated phenol group or phenol–carbonyl pairs40 was employed as the reduced graphene oxide surface. The structural models optimized by DFT calculations were further examined by EXAFS simulations.
Regarding the previous work52, the structures of MeCpPtMe2 and MeCpPtMe were both considered for the MeCpPtMe/graphene sample (Supplementary Fig. 11). Compared to the MeCpPtMe3 molecule, the five Pt–C bonds in the Pt–MeCp group in these two structures both changed significantly. According to the EXAFS simulations for these two structures, MeCpPtMe bonded to the graphene support through two interfacial O atoms might be the promising structure for the MeCpPtMe/graphene sample (Fig. 3c and Supplementary Figs. 11b and 12). Compared to the spectrum of MeCpPtMe3, the remarkably attenuated peak at 1.99 Å in the MeCpPtMe/graphene spectrum is due to the considerable distortion of the MeCp group.
When oxygen combusts off the ligand, additional oxygen chemisorbs on the Pt1 atom in the Pt1/graphene sample53. Indeed, Pt1 atom with one chemisorbed O2 molecule at the terminal position (the Pt–O bond distance: 2.00 Å) and two O atoms at the interface (the Pt–O bond distance: 2.02 Å) produces an EXAFS spectrum in good agreement with the experimental result (Fig. 3d and Supplementary Fig. 12). On the contrary, the Pt1 atom with one O and one C atom at the interface generates split FT peaks in the first shell, in contrast with the experimental results (Supplementary Fig. 13). Nonetheless, this structure might not be completely ruled out.
During the second Pt ALD cycle, a secondary Pt atom anchors on the preliminary one and then becomes oxidized during the O3 exposure step. Taking this information into account, a Pt2O6 chain structure with O atoms alternating between the terminal and bridge positions was constructed (inset in Fig. 3e). After optimization, our calculations show that the Pt–Pt bond distance in the Pt2O6 chain is 3.03 Å (the inset of Fig. 3e), consistent with the experimental results very well (Fig. 2g). The lengths of the Pt–O bonds in the Pt2O6 chain are very close to each other, ~1.93–2.03 Å. Moreover, XAFS spectrum simulation for this Pt2O6 chain structure also agrees very well with the experimental result (Fig. 3e and Supplementary Fig. 12). This chain structure is found to be similar to the suggested structure models for PtxOy (x = 1–3) clusters by Schneider et al. previously54. Interestingly, we also noticed that the tilted angle of the Pt2O6 chain could vary largely from ~8 to ~50°, depending on both the size of carbon vacancies and the configurations of two interfacial O atoms (Supplementary Fig. 14). The largely varied angles tilted from the graphene support, explain well the different Pt–Pt bond distances in the Pt2 dimers observed by STEM (Fig. 2e–g). Again, the structure model of Pt2 dimers with one O and one C atom at the interface might not be completely ruled out.
Catalytic activity
AB with satisfactory air stability and remarkably high hydrogen content of 19.6 wt%, has been regarded as a promising hydrogen storage media for portable applications55. Here, hydrolysis of AB for hydrogen production was utilized as a probe reaction to investigate the catalytic properties of Pt1 single atoms and Pt2 dimers. According to literature, this reaction is depicted as the following Eq. (1):56
| 1 |
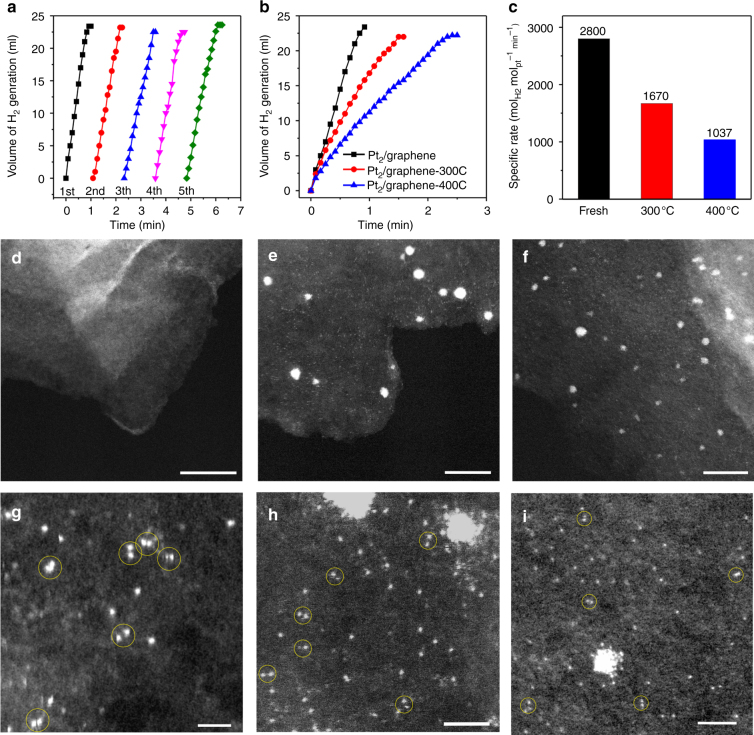
As shown in Fig. 4a, the single-atom Pt1/graphene catalyst generated 9.8 mL H2 gas only in 10.8 min, which is ~42% of the theoretical volume of 23.4 mL according to the Eq. (1). In sharp contrast, the dimeric Pt2/graphene catalyst released 23.4 mL H2 vigorously in only 0.9 min, indicating the depletion of AB. The activity of 2cPt/graphene, synthesized by two successive cycles of Pt ALD on graphene at 250 °C, was rather close to Pt1/graphene, by generating 7.5 mL H2 in 10.5 min. Obviously, Pt2/graphene and 2cPt/graphene were distinctly different in structure. As a comparison, the activities of the Pt NP catalysts of Pt/graphene-WI, Pt/carbon, and Pt/SiO2, as well as the commercial PtO2 were tested. The Pt/graphene-WI catalyst with a Pt particle size of 1.8 ± 0.5 nm (Supplementary Fig. 15 and Table 3), showed a very poor activity of 6.6 mL H2 release in 15.2 min. The commercial Pt/carbon catalyst with a Pt particle size of 2.3 ± 0.7 nm (Supplementary Fig. 16 and Table 3) was considerably better, generating 23.4 mL H2 in 9.7 min. The Pt/SiO2 ALD catalyst with a Pt particle size of 1.9 ± 0.3 nm (Supplementary Fig. 17 and Table 3) was also very active, releasing 23.4 mL H2 in 6.8 min. The commercial PtO2 powder (Supplementary Fig. 18) generated ~ 21 mL H2 in 15 min. In this case, a reduction of PtO2 into Pt occurred during the reaction, in line with literature56.
Fig. 4.
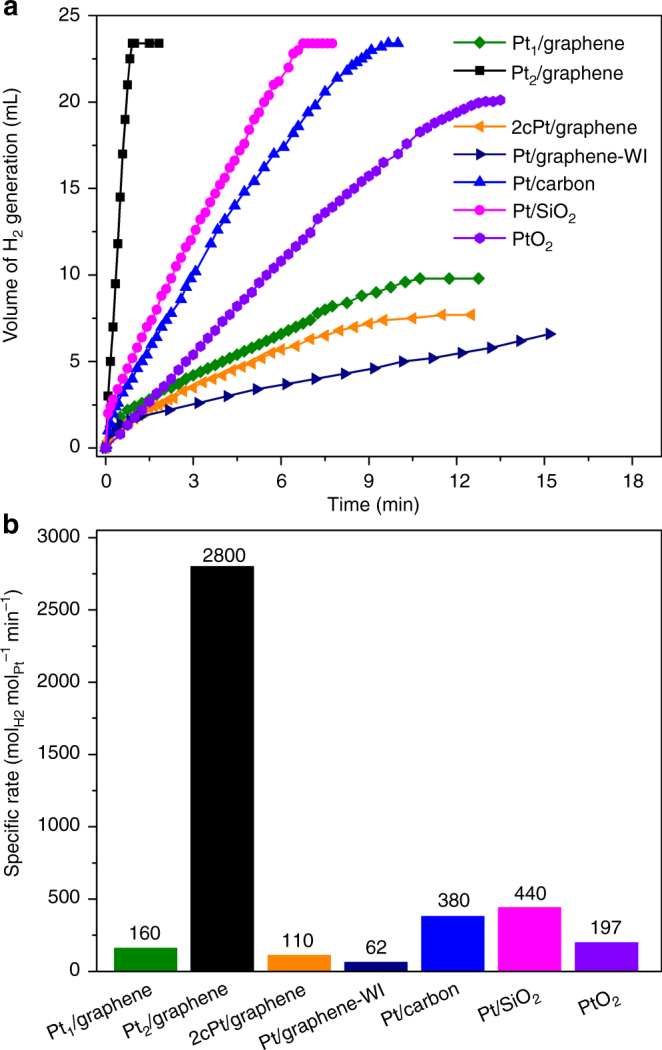
Catalytic activities of various Pt catalysts in AB hydrolysis. a Plots of time vs normalized volume of hydrogen gas generated from the AB hydrolysis reaction over the single-atom Pt1/graphene and dimeric Pt2/graphene catalysts. Pt NPs samples of 2cPt/graphene, Pt/graphene-WI, Pt/carbon, Pt/SiO2, and commercial PtO2 powder were also evaluated as a comparison. b The specific rates over these samples based on the mole of Pt
The specific rates of these samples were calculated based on the Pt contents. The rates were 160 and 110 molH2 MolPt −1 min−1, for Pt1/graphene and 2cPt/graphene, respectively (Fig. 4b and Supplementary Fig. 19). For the Pt NP samples, the rates were 62, 380, and 440 molH2 MolPt −1 min−1, for Pt/graphene-WI, Pt/carbon, and Pt/SiO2, respectively, close to the values for Pt catalysts reported in the literature (Supplementary Table 4). The rate of PtO2 was 197 molH2 MolPt −1 min−1. Obviously, hydrolytic dehydrogenation of AB on Pt catalysts is a structure sensitive reaction, the size, and electronic properties of Pt NPs might both play important roles57,58. In sharp contrast with the above samples, the Pt2 dimers exhibited the highest rate of 2800 molH2 MolPt −1 min−1 ever reported in literature, which was ~17 and 45 times higher than the corresponding single-atom Pt1/graphene and Pt/graphene-WI samples, respectively. When the mole ratio of Pt to the AB substrate was increased, Pt2/graphene could preserve the high specific rate to a large extent (Supplementary Fig. 20). Note that all the Pt samples produced a similar product of BO2 − in the spent reaction solution, according to the identical 11B resonance at 8.9 ppm (Supplementary Fig. 21)56.
DFT calculations were further carried out to get a deeper insight into the vast activity difference between Pt1/graphene and Pt2/graphene. Since AB is known as an excellent reducing agent59, and could likely stripe off the terminal dioxygen of Pt1/graphene and Pt2/graphene (the insets of Fig. 3d, e) during the reaction, partially reduced structures without the terminal dioxygen were considered for both Pt1/graphene and Pt2/graphene (the insets of Fig. 5a). The reduced samples are denoted as Pt1/graphene-R and Pt2/graphene-R, respectively. First, we compared the projected density of states of the 5d orbitals of the Pt atom in Pt1/graphene-R and the top Pt atom in Pt2/graphene-R. It was found that the unoccupied 5d states of the top Pt atom in Pt2/graphene-R locates at a considerably higher energy position of 0.87 eV above Fermi level (E f) than that of the Pt atom in Pt1/graphene-R (0.40 eV), which indicates that Pt1/graphene-R is more prone to accept electrons than Pt2/graphene-R (Fig. 5a). This result is in line with the recent literature where Åstrand et al. reported that Pt1 single atom had a more strong ability to accept electrons than the top Pt atom in Pt2 dimer, thereby showing stronger CO adsorption on Pt1 60,61.
Fig. 5.
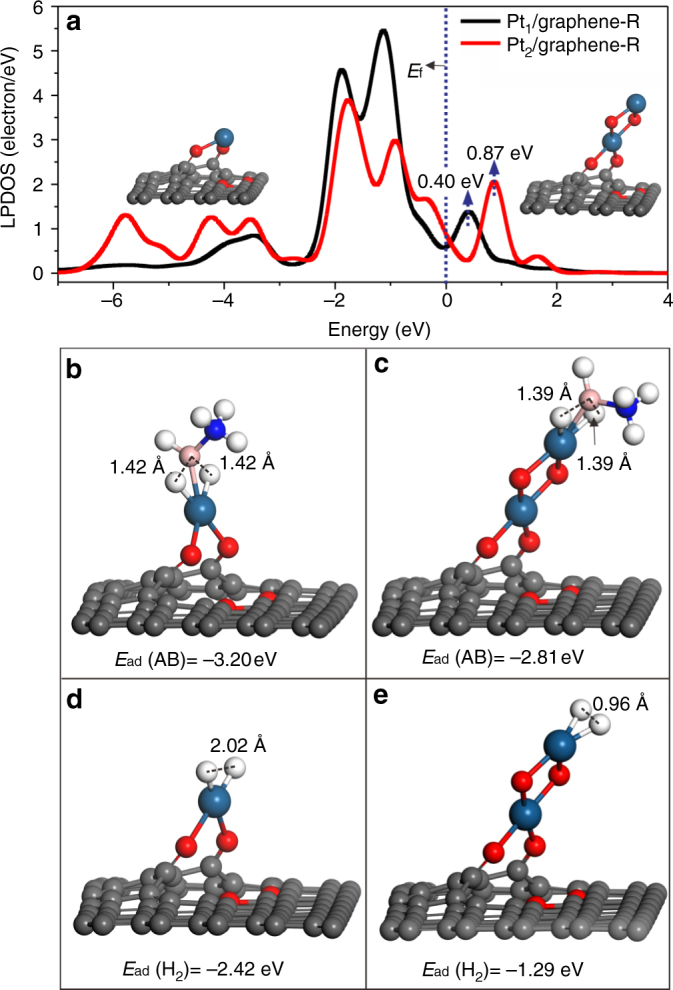
Electronic properties as well as AB and H2 adsorptions. a The local partial density of state (LPDOS) projected on the Pt 5d orbitals of Pt1/graphene-R and the top Pt atom in Pt2/graphene-R. The local configurations for AB adsorption on Pt1/graphene-R (b), Pt2/graphene-R (c). The local configurations for H2 adsorption on Pt1/graphene-R (d), Pt2/graphene-R (e). The ball in gray, white, pink, blue, red, and dark blue represent carbon, hydrogen, boron, nitrogen, oxygen, and platinum, respectively
When AB is adsorbed on Pt1/graphene-R, two B–H bonds were significantly elongated from 1.22 to 1.42 Å, with a strong adsorption energy of −3.20 eV (Fig. 5b). On Pt2/graphene-R, the elongation of the two B–H bonds was slightly less, to 1.39 Å, and the AB adsorption energy was considerably weaker, about −2.81 eV (Fig. 5c). The adsorption of AB on Pt (111) was also investigated as a comparison (Supplementary Fig. 22). We found that AB could quickly dissociate to three H atoms and BNH3 species without any barrier. The AB adsorption energy is −3.97 eV, significantly stronger than that on Pt1 single atom and Pt2 dimer. The strong AB adsorption on Pt (111) revealed by DFT calculations agrees well with the literature57, where Pt NP catalyst deactivation induced by B poisoning was observed during the AB hydrolysis reaction. Bearing this in mind, we further examined the recycling stabilities of the Pt1/graphene, Pt NP, and PtO2 catalysts and measured the B contents in the used samples using ICP-AES. Indeed, catalyst deactivations and considerable amounts of B adsorption were observed on all the used samples (Supplementary Figs. 23 and 24), in line with the literature57. In addition, sintering and leaching of Pt were also noticed on Pt1/graphene, Pt/graphene-WI, and Pt/SiO2 (Supplementary Fig. 25). Therefore, the considerable weaker adsorption of AB on Pt2 dimer could be one key factor for its high activity as shown in Fig. 4.
H2 adsorptions on Pt1/graphene-R and Pt2/graphene-R were also investigated as a descriptor of hydrogen desorption from the catalyst surface during the AB hydrolysis reaction (Fig. 5d, e). It was again found that H2 adsorption on Pt1/graphene-R (−2.42 eV) is remarkably stronger than that on Pt2/graphene-R (−1.29 eV). More interestingly, we found that H2 chemisorbs dissociatively on Pt1/graphene-R, but molecularly on Pt2/graphene-R, indicated by the H–H bond distance of 2.02 and 0.96 Å, respectively. Such molecular adsorption of H2 on the Pt2 dimer with a moderate adsorption energy favors H2 desorption during the AB hydrolysis reaction, thereby further boosting the catalytic activity. Taken together, compared to Pt1 single atom, the higher energy position of the unoccupied state of the Pt 5d orbital of the top Pt in the Pt2 dimer might play an important role in weakening the adsorption of both AB and H2 molecules, thus facilitating the activity remarkably.
Stability of Pt2 dimers on graphene
In sharp contrast with all other Pt samples shown in Supplementary Fig. 23, the dimeric Pt2/graphene catalyst exhibited a very high stability in the AB hydrolysis reaction during the recyclability test for five cycles (Fig. 6a). STEM measurements showed that there was no any visible Pt NPs formation and Pt2 dimers remained as the main features in the used sample (Fig. 6d, g). The Pt1 single atoms shown in Fig. 6g were likely produced by the electron beam during STEM measurements (Supplementary Figs. 7–9), since there was no apparent activity decrease. When Pt2/graphene was annealed at high temperatures in helium, the activity declined considerably (Fig. 6b), but rates still remained as high as1670 and 1037 molH2 MolPt −1 min−1 for the sample annealed at 300 (Pt2/graphene-300C) and 400 °C (Pt2/graphene-400C), respectively (Fig. 6c). Obviously, significant amounts of Pt2 dimers were survived after the high-temperature treatments. Indeed, HAADF-STEM revealed a mixture of Pt2 dimers, Pt1 single atoms, and Pt NPs in both Pt2/graphene-300C (Fig. 6e, h) and Pt2/graphene-400C (Fig. 6f, i). Please keep in mind that these STEM images might significantly underestimate the portion of Pt2 dimers in the samples owing to the possible beam damage during STEM measurements (Supplementary Figs. 7–9).
Fig. 6.
Stability of the dimeric Pt2/graphene catalyst. a Five recycles in hydrolytic dehydrogenation of AB at room temperature over the dimeric Pt2/graphene catalyst by adding additional 0.325 mmol of pure AB into the reaction flask after each run. b Plots of time vs volume of hydrogen gas generated from AB hydrolysis and c the corresponding specific rates at room temperature over the dimeric Pt2/graphene catalysts after different pretreatments: as-prepared, annealing in helium at 300 and 400 °C for 1 h, respectively. d, g Representative HAADF-STEM images of the used Pt2/graphene catalyst after the recyclability test, scale bars, 20 nm (d), 1 nm (g). e, h Representative HAADF-STEM images of the Pt2/graphene catalyst after annealing in helium at 300 °C for 1 h, scale bars, 10 nm (e), 2 nm (h). f, i Representative HAADF-STEM images of the Pt2/graphene catalyst after annealing in helium at 400 °C for 1 h, scale bars, 10 nm (f), 2 nm (i). Pt2 dimers in g–i are highlighted by yellow circles
In conclusion, we have successfully demonstrated that Pt2 dimers can be bottom–up constructed on graphene with a high-surface area. We found that the type of surface nucleation sites, selective deposition, the self-limiting nature of ALD, and the high stabilities of Pt1 single atoms and Pt2 dimers are the keys factors for the Pt2 dimers synthesis. The dominant presence of Pt2 dimers on graphene in the oxidized form of Pt2Ox were confirmed by a combination of aberration-corrected HAADF-STEM, ICP-AES, and XAFS and DFT calculations. Rotating and uncoupling of Pt2 dimers under the electron beam during STEM measurements, provide direct evidence of the presence of Pt2 dimers on graphene. In the AB hydrolysis reaction, the dimeric Pt2/graphene catalyst exhibited a strikingly high activity, which was ~17- and 45-fold higher than graphene supported Pt1 single atoms and Pt NPs, respectively. The lower adsorption energies of AB and H2 on the Pt2 dimers than that on Pt1 single atoms or Pt NPs are likely the major reasons for the high activity. Importantly, the dimeric Pt2/graphene catalyst showed a high stability under the current reaction conditions and in the inert environment at below 300 °C. Finally, our findings point out a new avenue to bottom–up synthesis of atomically precise ultrafine metal (and/or metal oxide) clusters on high-surface area supports for advanced catalysis.
Methods
Materials
Trimethyl(methylcyclopentadienyl)platinum(IV) (MeCpPtMe3, 98%), chloroplatinic acid (H2PtCl6, ≥99.9%, trace metals basis), ammonia borane (97%), the commercial PtO2 (≥99.9%, 70 m2/g), and Pt/carbon catalysts (the Pt content, 5.0 wt%) were all purchased from Sigma Aldrich. Silica gel was purchased from Alfa Aesar (Brunauer, Emmett, and Teller (BET) surface area 300 m2/g). Pristine graphene nanosheet (99.5%) was bought from Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Sciences. All materials were used as received without further purification.
Preparation of reduced graphene oxide
Pristine graphene nanosheet was first oxidized to graphene oxide according to the procedure described previously62. In brief, 0.6 g graphene nanosheet and 0.3 g sodium nitrate was sequentially added into concentrated sulfuric acid (H2SO4, 15 mL) and stirred at room temperature for 22 h. Then the mixture was cooled down to 0 °C to add 1.8 g potassium permanganate (KMnO4). After stirring at room temperature and 35 °C for 2 and 3 h, respectively, the mixture was heated to 98 °C and kept at this temperature for another 30 min. Next, it was cooled down to 40 °C, and 90 mL of water and 7.5 mL of hydrogen peroxide (H2O2, 30%) were slowly added into the mixture. After that the precipitate was filtered out by washing with HCl (5%) and ultrapure water, it was dried in a vacuum oven at 45 °C overnight. The dry material was grinded to obtain graphene oxide powder. Finally, the reduced graphene oxide was obtained by thermal deoxygenation of graphene oxide powder at 1050 °C for 2 min under helium at a flow rate of 50 mL/min.
Synthesis of Pt1/graphene
Pt ALD was carried out on a viscous flow reactor (GEMSTAR-6 Benchtop ALD, Arradiance) by alternatively exposing to MeCpPtMe3 precursor and O2 (99.999%) at 250 oC46,63,64. Ultrahigh purity N2 (99.999%) was used as the carrier gas at a flow rate of 200 mL/min. The Pt precursor was heated to 65 °C to get a sufficient vapor pressure. The reactor inlets were held at 110 °C to avoid any precursor condensation. The timing sequence was 90, 120, 60, and 120 sec for the MeCpPtMe3 exposure, N2 purge, O2 exposure, and N2 purge, respectively (90-120-60-120).
Synthesis of Pt2/graphene
The second Pt ALD cycle was performed on the Pt1/graphene SAC at 150 °C. Here, O3 was used as the oxidant to make sure that the precursor ligand can be fully removed65. The timing sequence was 90-120-60-120.
Synthesis of 2cPt/graphene
Two consecutive cycles of Pt ALD was also performed on the reduced graphene oxide at 250 °C using the same timing sequence.
Synthesis of Pt/SiO2
Pt ALD was performed on the silica gel support for one cycle at 250 °C using the same timing sequence.
Synthesis of Pt/graphene-WI
A Pt/graphene NP catalyst was synthesized by the wetness impregnation method (Pt/graphene-WI). In this case, 100 mg graphene support was slowly added into a 1.93 × 10−2 M H2PtCl6 aqueous solution (0.9 mL). Then, the mixture was stirred for 30 min, and then dried in air at room temperature for 12 h. The dried material was first calcined in air at 120 °C for 12 h, and then reduced in 10% H2 in argon at 300 °C for another 2 h to get the Pt/graphene-WI catalyst.
Catalyst characterization
The Pt loadings in these samples were determined by ICP-AES measurements; therein all samples were dissolved in hot fresh aqua regia. The BET surface area was measured on a Micromeritics ASAP 2020 system. Raman spectra were recorded on a LabRAM HR Raman spectrometer with a 514 nm Ar laser in backscattering geometry. Aberration-corrected HAADF-STEM measurements were taken on a JEM-ARM200F instrument (University of Science and Technology of China) at 200 keV. XAFS measurements at the Pt L 3-edge (11,564 eV) were performed in the transmission mode with the Si (111) monochromator at the BL14W1 beamline of the Shanghai Synchrotron Radiation Facility (SSRF), China. The storage ring of SSRF worked at 3.5 GeV with a maximum current of 210 mA.
XAFS data analysis and simulation
The acquired EXAFS data were processed according to the standard procedures using the ATHENA module implemented in the IFEFFIT software packages61.The EXAFS oscillation functions χ(k) were obtained by subtracting the post-edge background from the overall absorption spectra and then normalized with respect to the edge-jump step. The R bkg value of 1.0 was used for all samples. Subsequently, k 3-weighted χ(k) fucntions in the k range of 2.2–13.5 Å−1 were FT to the R space by using a Hanning window of dk = 3.0 Å−1.
EXAFS simulations were performed with the FEFF8.4 code62 using the structural models suggested by DFT calculations. The simulated EXAFS χ(k) functions were also k 3-weighted and FT into the R-space by using the same k range of 2.2–13.5 Å−1 as that in the experimental data. During simulations, the coordination numbers were set to the values of the model structures generated by DFT calculations. The amplitude reduction factor S 0 2 was fixed at the value of 0.86 which was determined by fitting the reference metal Pt foil. The Debye–Waller factors for the nearest Pt−O/C and Pt−Pt pairs were set at the typical values of 0.0030 and 0.0065 Å2 determined from the fittings of PtO2 and Pt foil references, respectively, and they were set at 0.008 Å2 for all the other distant paths which contributed barely discernible signals as seen from the experimental data in the R-space. To further improve the match between the simulation and the experimental data, for the MeCpPtMe/graphene sample, the two nearest Pt–C and Pt–O interatomic distances were optimized to 2.00 and 2.02 Å, respectively, both of which are within ~ 2% error level as compared to the optimized structure by DFT calculations (2.05 and 2.06 Å, respectively). For the other two samples, the simulated EXAFS spectra based on the DFT-generated structures match well with the experimental data, thus no further structure optimization was performed during EXAFS simulations.
DFT Calculations
All spin-polarized calculations were performed by using the DFT method. The DFT Semi-core Pseudopotential method66 with a double numerical basis set together with polarization functions (DNP) were adopted to form the Perdew-Burke-Ernzerhof (PBE) exchange-correlation functional within the generalized gradient approximation67, implemented in DMol3 package (DMol3 is a density functional theory quantum mechanical package available from Accelrys Software Inc.)68. A DFT-D semi-empirical correction with Tkatchenko-Scheffler (TS) method is applied with the PBE functional to account for the dispersion interaction. Conductor-like screening model (COSMO) with a dielectric constant of 78.54 is adopted to consider the water solvent effect regarding the adsorption of molecule and fragment in AB hydrolysis. A smearing of 0.001 Ha to the orbital occupation is applied to achieve electronic convergence. The real-space global cutoff radius is set to be 4.5 Å. A hexagonal supercell containing (8 × 8) unit cells of graphene monolayer with about 17 Å vacuum layer was used as a support. The convergence tolerances of energy, force, and displacement for the geometry optimization were 1 × 10−5 Ha, 0.002 Ha/Å, and 0.005 Å, respectively. 1 × 1 × 1 k-points grid is used to describe the Brillouin zone for geometric optimization. The adsorption energy is defined by the formula: E ads(AB) = E (AB/catalyst)–(E catalyst + E AB) and E ads(H2) = E (H2/catalyst)–(E catalyst + E H2,gas), where E (AB/catalyst), E (H2/catalyst), and E catalyst are the total energies for the optimized equilibrium configurations of catalyst with and without AB or H2, respectively; and E AB (E H2,gas) is the energy of the AB (gas phase H2) molecule in its ground state. For Pt(111), the supercell is (4×4).
Hydrolytic dehydrogenation of AB
As a probe reaction, hydrolytic dehydrogenation of AB was performed in a three-necked flask at 27 °C under atmospheric pressure. The flask was immersed in a water bath to control the reaction temperature. 10 mg of the Pt2/graphene catalyst was used, while the weight of other catalysts was adjusted to keep the same amount of Pt with Pt2/graphene. Typically, 5 mL aqueous AB solution (6.5 × 10−2 M) were introduced into the glass container via a syringe. For the commercial PtO2 catalyst, the mole ratio of Pt to AB was kept as the same with other samples, and the result was normalized to other samples based the amount of Pt. The AB solution and the catalyst were well-mixed by using a magnetic stirrer at a speed of 800 r/min to eliminate any mass-transfer issue. The generated volume of H2 was measured by a water-filled gas burette, where the volume of water discharged was converted into the volume of hydrogen generated69.
The specific rates (r) of these catalysts were calculated according to the Eq. (2):
| 2 |
Here is the mole of generated H2, while is the total mole of Pt in the sample. t is the reaction time in min.
Data availability
All the relevant data are available from the authors upon request.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21473169, 21673215, 21533007, and 21233007), Innovative Research Groups of the National Natural Science Foundation of China (11621063), the One Thousand Young Talents Program under the Recruitment Program of Global Experts, the Young Scientists Fund of the National Natural Science Foundation of China (11404314), Anhui Provincial Natural Science Foundation (1708085MA06), and the Fundamental Research Funds for the Central Universities (WK2060030017). The calculations were performed on the supercomputing system in USTC-SCC and Guangzhou-SCC. We gratefully thank the BL14W1 beamline at the Shanghai Synchrotron Radiation Facility (SSRF), China.
Author contributions
J.L. conceived the idea and designed the experiments. H.Y. performed catalyst synthesis and catalytic performance evaluations. Y.L. performed the STEM measurements. S.W., Z.S., H.C., W.L., and T.Y. performed the XAFS measurements. W.Z., H.W., and J.Y. performed the DFT calculations. C.W., J.L., and X.H. assisted catalyst characterization and catalytic performance tests. J.L. wrote the manuscript. All the authors contributed and commented on the manuscript. H.Y. and Y.L. contributed equally to this work.
Competing interests
The authors declare no competing financial interests.
Footnotes
Huan Yan and Yue Lin contributed equally to this work.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-017-01259-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shiqiang Wei, Email: sqwei@ustc.edu.cn.
Junling Lu, Email: junling@ustc.edu.cn.
References
- 1.Bartholomew, C. H. & Farrauto, R. J. Fundamentals of Industrial Catalytic Processes. 2nd edn, (Wiley, 2006).
- 2.Funabiki M, Yamada T, Kayano K. Auto exhaust catalysts. Catal. Today. 1991;10:33–43. doi: 10.1016/0920-5861(91)80072-H. [DOI] [Google Scholar]
- 3.Guo Z, et al. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014;43:3480–3524. doi: 10.1039/c3cs60282f. [DOI] [PubMed] [Google Scholar]
- 4.Shanks BH. Conversion of biorenewable feedstocks: new challenges in heterogeneous catalysis. Ind. Eng. Chem. Res. 2010;49:10212–10217. doi: 10.1021/ie100487r. [DOI] [Google Scholar]
- 5.Khodakov AY, Chu W, Fongarland P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007;107:1692–1744. doi: 10.1021/cr050972v. [DOI] [PubMed] [Google Scholar]
- 6.Alonso DM, Wettstein SG, Dumesic JA. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012;41:8075–8098. doi: 10.1039/c2cs35188a. [DOI] [PubMed] [Google Scholar]
- 7.Calle-Vallejo F, Koper MTM, Bandarenka AS. Tailoring the catalytic activity of electrodes with monolayer amounts of foreign metals. Chem. Soc. Rev. 2013;42:5210–5230. doi: 10.1039/c3cs60026b. [DOI] [PubMed] [Google Scholar]
- 8.Kaden WE, Wu TP, Kunkel WA, Anderson SL. Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces. Science. 2009;326:826–829. doi: 10.1126/science.1180297. [DOI] [PubMed] [Google Scholar]
- 9.Palmer RE, Pratontep S, Boyen HG. Nanostructured surfaces from size-selected clusters. Nat. Mater. 2003;2:443–448. doi: 10.1038/nmat897. [DOI] [PubMed] [Google Scholar]
- 10.Li ZY, et al. Three-dimensional atomic-scale structure of size-selected gold nanoclusters. Nature. 2008;451:46–48. doi: 10.1038/nature06470. [DOI] [PubMed] [Google Scholar]
- 11.Abbet S, et al. Acetylene cyclotrimerization on supported size-selected Pd-n clusters (1<=n<=30): one atom is enough. J. Am. Chem. Soc. 2000;122:3453–3457. doi: 10.1021/ja9922476. [DOI] [Google Scholar]
- 12.Yoon B, et al. Charging effects on bonding and catalyzed oxidation of CO on Au-8 clusters on MgO. Science. 2005;307:403–407. doi: 10.1126/science.1104168. [DOI] [PubMed] [Google Scholar]
- 13.Nesselberger M, et al. The effect of particle proximity on the oxygen reduction rate of size-selected platinum clusters. Nat. Mater. 2013;12:919–924. doi: 10.1038/nmat3712. [DOI] [PubMed] [Google Scholar]
- 14.Vajda S, et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 2009;8:213–216. doi: 10.1038/nmat2384. [DOI] [PubMed] [Google Scholar]
- 15.Yang XF, et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013;46:1740–1748. doi: 10.1021/ar300361m. [DOI] [PubMed] [Google Scholar]
- 16.Yang M, et al. Catalytically active Au-O(OH)(x)-species stabilized by alkali ions on zeolites and mesoporous oxides. Science. 2014;346:1498–1501. doi: 10.1126/science.1260526. [DOI] [PubMed] [Google Scholar]
- 17.Flytzani-Stephanopoulos M. Gold atoms stabilized on various supports catalyze the water-gas shift reaction. Acc. Chem. Res. 2014;47:783–792. doi: 10.1021/ar4001845. [DOI] [PubMed] [Google Scholar]
- 18.Qiao BT, et al. Single-atom catalysis of CO oxidation using Pt-1/FeOx. Nat. Chem. 2011;3:634–641. doi: 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- 19.Peterson, E. J. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014). [DOI] [PubMed]
- 20.Vile G, et al. A stable single-site palladium catalyst for hydrogenations. Angew. Chem. Int. Ed. 2015;54:11265–11269. doi: 10.1002/anie.201505073. [DOI] [PubMed] [Google Scholar]
- 21.Liu PX, et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science. 2016;352:797–801. doi: 10.1126/science.aaf5251. [DOI] [PubMed] [Google Scholar]
- 22.Yan H, et al. Single-atom pd-1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 2015;137:10484–10487. doi: 10.1021/jacs.5b06485. [DOI] [PubMed] [Google Scholar]
- 23.Ding K, et al. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science. 2015;350:189–192. doi: 10.1126/science.aac6368. [DOI] [PubMed] [Google Scholar]
- 24.Jin RC, Zeng CJ, Zhou M, Chen YX. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 2016;116:10346–10413. doi: 10.1021/acs.chemrev.5b00703. [DOI] [PubMed] [Google Scholar]
- 25.Yoskamtorn T, et al. Thiolate-mediated selectivity control in aerobic alcohol oxidation by porous carbon-supported Au-25 clusters. ACS Catal. 2014;4:3696–3700. doi: 10.1021/cs501010x. [DOI] [Google Scholar]
- 26.Fang J, et al. The support effect on the size and catalytic activity of thiolated Au-25 nanoclusters as precatalysts. Nanoscale. 2015;7:6325–6333. doi: 10.1039/C5NR00549C. [DOI] [PubMed] [Google Scholar]
- 27.Das S, et al. Reductive deprotection of monolayer protected nanoclusters: an efficient route to supported ultrasmall au nanocatalysts for selective oxidation. Small. 2014;10:1473–1478. doi: 10.1002/smll.201302854. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, et al. Ligand-stabilized and atomically precise gold nanocluster catalysis: a case study for correlating fundamental electronic properties with catalysis. Chem. Eur. J. 2013;19:10201–10208. doi: 10.1002/chem.201300600. [DOI] [PubMed] [Google Scholar]
- 29.Nie XT, Qian HF, Ge QJ, Xu HY, Jin RC. CO oxidation catalyzed by oxide-supported Au-25(SR)(18) nanoclusters and identification of perimeter sites as active centers. ACS Nano. 2012;6:6014–6022. doi: 10.1021/nn301019f. [DOI] [PubMed] [Google Scholar]
- 30.Wu ZL, et al. Thiolate ligands as a double-edged sword for CO oxidation on CeO2 Supported Au-25(SCH2CH2Ph)(18) Nanoclusters. J. Am. Chem. Soc. 2014;136:6111–6122. doi: 10.1021/ja5018706. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z, et al. Size-dependent catalytic activity of supported metal-clusters. Nature. 1994;372:346–348. doi: 10.1038/372346a0. [DOI] [Google Scholar]
- 32.Argo AM, Odzak JF, Lai FS, Gates BC. Observation of ligand effects during alkene hydrogenation catalysed by supported metal clusters. Nature. 2002;415:623–626. doi: 10.1038/415623a. [DOI] [PubMed] [Google Scholar]
- 33.Gates BC. Supported metal-clusters - synthesis, structure, and catalysis. Chem. Rev. 1995;95:511–522. doi: 10.1021/cr00035a003. [DOI] [Google Scholar]
- 34.George SM. Atomic layer deposition: an overview. Chem. Rev. 2010;110:111–131. doi: 10.1021/cr900056b. [DOI] [PubMed] [Google Scholar]
- 35.Puurunen RL. Surface chemistry of atomic layer deposition: a case study for the trimethylaluminum/water process. J. Appl. Phys. 2005;97:121301. doi: 10.1063/1.1940727. [DOI] [Google Scholar]
- 36.Knez M, Niesch K, Niinisto L. Synthesis and surface engineering of complex nanostructures by atomic layer deposition. Adv. Mater. 2007;19:3425–3438. doi: 10.1002/adma.200700079. [DOI] [Google Scholar]
- 37.Lu JL, Elam JW, Stair PC. Atomic layer deposition-Sequential self-limiting surface reactions for advanced catalyst “bottom-up” synthesis. Surf. Sci. Rep. 2016;71:410–472. doi: 10.1016/j.surfrep.2016.03.003. [DOI] [Google Scholar]
- 38.O’Neill BJ, et al. Catalyst design with atomic layer deposition. ACS Catal. 2015;5:1804–1825. doi: 10.1021/cs501862h. [DOI] [Google Scholar]
- 39.Detavernier C, Dendooven J, Sree SP, Ludwig KF, Martens JA. Tailoring nanoporous materials by atomic layer deposition. Chem. Soc. Rev. 2011;40:5242–5253. doi: 10.1039/c1cs15091j. [DOI] [PubMed] [Google Scholar]
- 40.Bagri A, et al. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010;2:581–587. doi: 10.1038/nchem.686. [DOI] [PubMed] [Google Scholar]
- 41.Kim K, et al. Selective metal deposition at graphene line defects by atomic layer deposition. Nat. Commun. 2014;5:4781. doi: 10.1038/ncomms5781. [DOI] [PubMed] [Google Scholar]
- 42.Lu JL, et al. Toward atomically-precise synthesis of supported bimetallic nanoparticles using atomic layer deposition. Nat. Commun. 2014;5:3264. doi: 10.1038/ncomms4264. [DOI] [PubMed] [Google Scholar]
- 43.Kessels WMM, Knoops HCM, Dielissen SAF, Mackus AJM, van de Sanden MCM. Surface reactions during atomic layer deposition of Pt derived from gas phase infrared spectroscopy. Appl. Phys. Lett. 2009;95:013114. doi: 10.1063/1.3176946. [DOI] [Google Scholar]
- 44.Aaltonen T, Rahtu A, Ritala M, Leskela M. Reaction mechanism studies on atomic layer deposition of ruthenium and platinum. Electrochem. Solid State Lett. 2003;6:C130–C133. doi: 10.1149/1.1595312. [DOI] [Google Scholar]
- 45.Mackus AJM, Leick N, Baker L, Kessels WMM. Catalytic combustion and dehydrogenation reactions during atomic layer deposition of platinum. Chem. Mater. 2012;24:1752–1761. doi: 10.1021/cm203812v. [DOI] [Google Scholar]
- 46.Hamalainen J, Ritala M, Leskela M. Atomic layer deposition of noble metals and their oxides. Chem. Mater. 2014;26:786–801. doi: 10.1021/cm402221y. [DOI] [Google Scholar]
- 47.Xue ZL, et al. Characterization of (Methylcyclopentadienyl)trimethylplatinum and low-temperature organometallic chemical vapor-deposition of platinum metal. J. Am. Chem. Soc. 1989;111:8779–8784. doi: 10.1021/ja00206a002. [DOI] [Google Scholar]
- 48.Gong T, et al. Activated carbon supported palladium nanoparticle catalysts synthesized by atomic layer deposition: genesis and evolution of nanoparticles and tuning the particle size. J. Phys. Chem. C. 2015;119:11544–11556. doi: 10.1021/jp5130102. [DOI] [Google Scholar]
- 49.Lacroix LM, Arenal R, Viau G. Dynamic HAADF-STEM observation of a single-atom chain as the transient state of gold ultrathin nanowire breakdown. J. Am. Chem. Soc. 2014;136:13075–13077. doi: 10.1021/ja507728j. [DOI] [PubMed] [Google Scholar]
- 50.Shen, J., Muthukumar, K., Jeschke, H. O. & Valenti, R. Physisorption of an organometallic platinum complex on silica: an ab initio study. New J. Phys. 14 (2012).
- 51.Setthapun W, et al. Genesis and evolution of surface species during pt atomic layer deposition on oxide supports characterized by in situ xafs analysis and water-gas shift reaction. J. Phys. Chem. C. 2010;114:9758–9771. doi: 10.1021/jp911178m. [DOI] [Google Scholar]
- 52.Karasulu B, Vervuurt RHJ, Kessels WMM, Bo AA. Continuous and ultrathin platinum films on graphene using atomic layer deposition: a combined computational and experimental study. Nanoscale. 2016;8:19829. doi: 10.1039/C6NR07483A. [DOI] [PubMed] [Google Scholar]
- 53.Moses-DeBusk M, et al. CO oxidation on supported single pt atoms: experimental and ab initio density functional studies of co interaction with Pt Atom on theta-Al2O3(010) surface. J. Am. Chem. Soc. 2013;135:12634–12645. doi: 10.1021/ja401847c. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y, Shelton WA, Schneider WF. Thermodynamic equilibrium compositions, structures, and reaction energies of PtxOy (x = 1-3) clusters predicted from first principles. J. Phys. Chem. B. 2006;110:16591–16599. doi: 10.1021/jp0614446. [DOI] [PubMed] [Google Scholar]
- 55.Hamilton CW, Baker RT, Staubitz A, Manners I. B-N compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009;38:279–293. doi: 10.1039/B800312M. [DOI] [PubMed] [Google Scholar]
- 56.Chandra M, Xu Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia-borane. J. Power Sources. 2006;156:190–194. doi: 10.1016/j.jpowsour.2005.05.043. [DOI] [Google Scholar]
- 57.Chen WY, et al. Mechanistic insight into size-dependent activity and durability in pt/cnt catalyzed hydrolytic dehydrogenation of ammonia borane. J. Am. Chem. Soc. 2014;136:16736–16739. doi: 10.1021/ja509778y. [DOI] [PubMed] [Google Scholar]
- 58.Chen WY, et al. Unique reactivity in Pt/CNT catalyzed hydrolytic dehydrogenation of ammonia borane. Chem. Commun. 2014;50:2142–2144. doi: 10.1039/c3cc48027e. [DOI] [PubMed] [Google Scholar]
- 59.Andrews GC, Crawford TC. Synthetic utility of amine borane reagents in the reduction of aldehydes and ketones. Tetrahedron Lett. 1980;21:693–696. doi: 10.1016/S0040-4039(00)71448-1. [DOI] [Google Scholar]
- 60.Mahmoodinia M, et al. Structural and electronic properties of the Pt-n-PAH complex (n=1, 2) from density functional calculations. Phys. Chem. Chem. Phys. 2014;16:18586–18595. doi: 10.1039/C4CP02488E. [DOI] [PubMed] [Google Scholar]
- 61.Mahmoodinia M, Astrand PO, Chen D. Influence of carbon support on electronic structure and catalytic activity of pt catalysts: binding to the CO Molecule. J. Phys. Chem. C. 2016;120:12452–12462. doi: 10.1021/acs.jpcc.6b02001. [DOI] [Google Scholar]
- 62.Hummers WS, Offeman RE. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958;80:1339–1339. doi: 10.1021/ja01539a017. [DOI] [Google Scholar]
- 63.King JS, et al. Ultralow loading Pt nanocatalysts prepared by atomic layer deposition on carbon aerogels. Nano Lett. 2008;8:2405–2409. doi: 10.1021/nl801299z. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y, King DM, Liang XH, Li JH, Weimer AW. Optimal preparation of Pt/TiO2 photocatalysts using atomic layer deposition. Appl. Catal. B Environ. 2010;101:54–60. doi: 10.1016/j.apcatb.2010.09.005. [DOI] [Google Scholar]
- 65.Hamalainen J, Puukilainen E, Sajavaara T, Ritala M, Leskela M. Low temperature atomic layer deposition of noble metals using ozone and molecular hydrogen as reactants. Thin Solid Films. 2013;531:243–250. doi: 10.1016/j.tsf.2013.01.091. [DOI] [Google Scholar]
- 66.Delley B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B. 2002;66:155125. doi: 10.1103/PhysRevB.66.155125. [DOI] [Google Scholar]
- 67.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 68.Delley B. From molecules to solids with the DMol(3) approach. J. Chem. Phys. 2000;113:7756–7764. doi: 10.1063/1.1316015. [DOI] [Google Scholar]
- 69.Jiang HL, Xu Q. Catalytic hydrolysis of ammonia borane for chemical hydrogen storage. Catal. Today. 2011;170:56–63. doi: 10.1016/j.cattod.2010.09.019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the relevant data are available from the authors upon request.