Abstract
Abstract
Glioblastoma (GBM) is the most aggressive primary brain tumor with a median survival of less than 15 months, emphasizing the need for better treatments. Immunotherapy as a treatment for improving or aiding the patient’s own immune defense to target the tumor has been suggested for GBM. A randomized clinical trial of adoptive cell transfer using ALECSAT (Autologous Lymphoid Effector Cells Specific Against Tumor Cells) is currently ongoing in Sweden. Here we performed a paired pre-clinical study to investigate the composition and in vitro effect of ALECSAT and identify determinants for the effect using autologous GBM-derived cancer stem cells (CSC), immunocytochemistry and flow cytometry. We show a clear dose-response relationship of ALECSAT on CSC, suggesting that the number of infused cells is of importance. In addition, the in vitro effect of ALECSAT on CSC correlated significantly to the blood count of T helper (Th) cells in the patient indicating a potential benefit of collecting cells for ALECSAT preparation at an even earlier stage when patients generally have a better blood count. The factors identified in this study will be important to consider in the design of future immunotherapy trials to achieve prolonged survival.
Abbreviations: ALECSAT, Autologous Lymphoid Effector Cells Specific Against Tumor Cells; CTA, Cancer/testis antigens; CSC, Cancer stem cells; CTL, Cytotoxic T lymphocytes; FBS, Fetal bovine serum; GBM, Glioblastoma; HGG, High-grade glioma; MHC, Major histocompatibility complex; NK cells, Natural killer cells; PCA, Principal component analysis; SOX2, SRY (sex determining region Y)-box 2; Th cells, T helper cells; Treg cells, T regulatory cells; TMZ, Temozolomide
Introduction
Glioblastoma (GBM), a type of high-grade glioma (HGG), is the most common malignant brain tumor in adults with a very poor prognosis [1], [2], [3]. The median survival is less than 15 months despite multimodal treatment with maximal safe surgical tumor resection followed by the so called Stupp regimen comprising radiotherapy and concomitant and adjuvant chemotherapeutic treatment with the alkylating agent temozolomide (TMZ) [4], [5]. New and better treatments to improve the prognosis are needed and immunotherapy, which incorporates different methods to induce, boost or restore the immune system of the patient, has been suggested for GBM and several clinical trials are currently ongoing [6]. The rationale for employing immunotherapy for GBM is supported by the association between elevated number of infiltrating T cells in GBM tumors and a better prognosis [7]. Further, infusion of CD8+ cytotoxic T lymphocytes (CTL) and CD4+ T helper (Th) cells into mice with brain tumors prolonged the survival [8]. However, GBM tumors display numerous immunoevasive features to avoid elimination [9], such as immune suppression by T regulatory (Treg) cells [10], [11], decreased expression of major histocompatibility complex (MHC), and a general low immunogenicity [12]. Another issue is quiescent/non-proliferating cells such as cancer stem cells (CSC), which are thought to be among the drivers behind tumor recurrence in GBM and are therefore of particular importance to target during treatment [13], [14]. In vitro studies have demonstrated that activated natural killer (NK) cells, i.e. cytotoxic lymphocytes which unlike CTL are not antigen-specific, are highly effective against CSC derived from GBM [15] and that NK cells preferentially target CSC [16].
The efficacy and safety of an immunotherapy treatment called ALECSAT (Autologous Lymphoid Effector Cells Specific Against Tumor Cells) is investigated as an add-on therapy to radiotherapy and TMZ in newly diagnosed GBM in an ongoing clinical randomized phase II multi-center trial in Sweden (clinical trial identifier; NCT-02799238). ALECSAT is based on the type of immunotherapy known as adoptive cell transfer where, in this case, autologous cytotoxic NK cells and CTL are amplified and activated ex vivo from a blood sample prior to injection. Given the relatively few published studies on immune-mediated eradication of CSC in an autologous setting, we performed a parallel pre-clinical study to examine the effect of ALECSAT on autologous GBM-derived CSC in vitro. The aim was to identify potentially adjustable parameters affecting the in vitro effect of ALECSAT that may be of clinical relevance. In this study we describe several key factors to consider in future immunotherapy studies to optimize study design and eventually achieve prolonged patient survival.
Materials and Methods
Tumor Collection and Cell Culture
Fresh tissue from tumors was collected from the patients' tumor resection at Sahlgrenska University Hospital after informed consent from the patients. The tumor tissue was dissociated and cells were cultured as described previously [17] up to at least passage five prior to in vitro ALECSAT treatments. All CSC lines were derived from the primary tumor except GU-HGG-160, which was established from the recurring tumor. As non-cancer cells controls, we used adult stem-like cells, GU-NS-6, derived from human ependyma from a patient undergoing endoscopic surgery for a brain cyst after signed informed consent and the cells were cultured on laminin in neural stem cell media supplemented with 10% fetal bovine serum (FBS). In addition, BJ cells (human fibroblasts) (ATCC) cultured in Minimum Essential medium (Life Technologies) supplemented with 10% FBS and split every 2 to 4 days using Trypsin-EDTA were used.
ALECSAT Production
ALECSAT was developed and produced by CytoVac A/S (Hørsholm, Denmark) according to their patented technology (WO 2008081035 A1, Anti-tumor vaccine derived from normal chemically modified cells). Briefly, lymphocytes and monocytes were isolated from a peripheral blood sample from each patient and the monocytes were cultured and differentiated into dendritic cells. Autologous activated Th cells were generated by co-culture of mature dendritic cells and lymphocytes. The Th cells were employed as antigen presenting cells by inducing the expression of antigens, predominantly the cancer/testis antigens (CTA), in the cells through treatment with 5-aza-2′-deoxycytidine, a DNA-demethylation agent. Non-activated lymphocytes were then stimulated by the CTA-expressing activated Th cells and the effector cells were expanded in number. Each dose of ALECSAT contained 107 to 109 cells and the production took 20 to 26 days.
Clinical Treatment Schedule
The patients received standard treatment according to the Stupp regimen with ALECSAT as an add-on therapy; maximal safe tumor resection and blood donation for the first ALECSAT treatment followed by external radiotherapy (daily fractions of 2 Gy, 5 days per week up to a total dose of 60 Gy) and oral TMZ (75 mg/m2) daily for approximately six weeks. The patients received the first ALECSAT treatment after completion of radiotherapy and simultaneously donated blood for the next treatment. After a 4- to 5-week break on completion of radiotherapy, patients received adjuvant TMZ treatment, typically six cycles (150-200 mg/m2 daily for 5 days every 28 days), alongside three doses of ALECSAT at four week intervals followed by single doses approximately every three months.
In vitro Cytotoxicity Assay Using ALECSAT
The day before ALECSAT treatment, 5 000 or 10 000 cells to be treated (CSC or control cells) were seeded in 96-well plates in triplicates for each condition. ALECSAT cells, provided by CytoVac A/S, were shipped at room temperature in growth media, centrifuged upon arrival, resuspended in culture media (for the cells to be treated) and counted with a Countess™ Automated Cell Counter (Invitrogen). ALECSAT cells were serially diluted to ratios ranging from 20 times more ALECSAT cells than seeded GU-HGG cells (20:1) to ten times fewer ALECSAT cells compared to seeded GU-HGG cells (1:10). Half of the media was removed from the cells in the 96-well plates and replaced with media containing ALECSAT at varying ratios with triplicates for each condition, and 5μM EdU (Invitrogen) when proliferation was assessed. Treated cells were fixed with 4% paraformaldehyde after 24 hours ALECSAT treatment. Surplus of ALECSAT was frozen in CellBanker (Amsbio) with 20% FBS for flow cytometry analysis at a later time point.
Immunostaining, Quantification and Analyzes
Fixed cells were permeabilized with triton-x and, if applicable, treated according to the manufacturer's instructions to expose EdU staining. Primary antibodies (mouse monoclonal Nestin, R&D MAB1259, 1:500; rabbit SOX2, Abcam ab97959; 1:1000; rabbit vimentin, Abcam ab45939, 1:900), were added after a blocking step and incubated overnight at 4°C. Secondary antibodies, goat, conjugated to Alexa dyes (Molecular Probes), 1:1000, were left on for one hour at room temperature. Finally, cell nuclei were stained with DAPI (Sigma Aldrich). Plates were scanned with the Operetta (Perkin Elmer) and analyzed with the Harmony software. The number of cells was automatically counted with triplicates for each condition. Error bars in the graphs denote standard deviation. The effect of ALECSAT was estimated as the slope of the linear region of the dose response curve (logarithmic x-axis) for each treatment. P values are included where appropriate and P < .05 was considered significant. The Bonferroni correction was used to adjust for multiple testing. Pearson correlation (r) was calculated for correlations.
Flow Cytometry Analysis of ALECSAT and Blood Samples
Frozen ALECSAT cells were thawed and washed in warm media prior to flow cytometry analysis. ALECSAT cells were stained with the following anti-human antibodies: CD3-PerCP-Cy5.5 (SK7), CD4-PE (SK3), CD8-APC (RPA-T8), CD16-APC-Cy7 (3G8) and CD56-PE-Cy7 (NCAM16.2) all from BD Biosciences. Samples were analyzed using a 4-laser BD LSRFortessa SORP (405, 488, 532 and 640 nm; BD Biosciences) and data was analyzed in FACSDiva Version 7 (BD Biosciences) or FlowJo version 10 (TreeStar).
Results
Patient and Cell Line Information
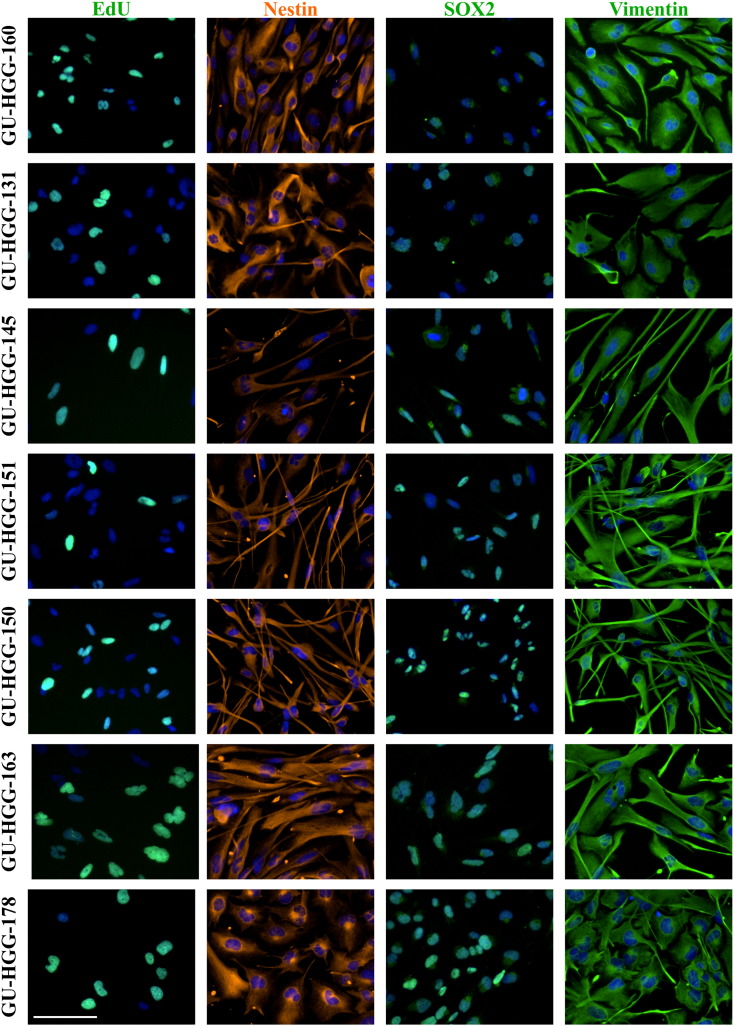
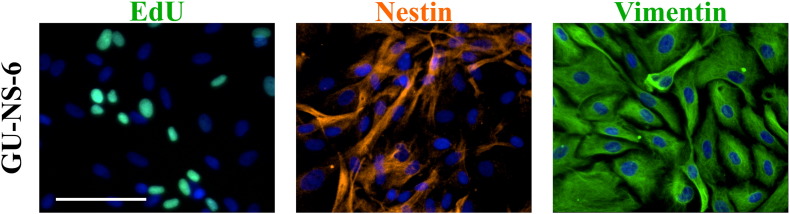
In this study we examined the in vitro effect of ALECSAT on autologous GBM-derived CSC cultured under serum-free conditions in stem cell media to retain the features of the tumor it was derived from [17]. We also performed flow cytometry analysis on the blood donated for production of ALECSAT and the final ALECSAT product (Figure 1A). All patients enrolled in the study were diagnosed with primary GBM (see Table 1 for patient data). The treatment protocol consisted of repeated doses of ALECSAT as add-on therapy to standard treatment (radiotherapy and TMZ; Figure 1B). We successfully derived CSC lines from seven of the first nine patients (78%) in the study for which tissue material was available. All CSC lines were verified by immunocytochemistry as proliferative (EdU incorporation) and positive for the stem cell markers Nestin and SOX2, and the neural progenitor marker Vimentin (Supplementary Figure S1). GU-NS-6 was proliferative, positive for Vimentin and had a heterogeneous expression of Nestin (Supplementary Figure S2).
Figure 1.
Experimental setup. (A) Seven patients who received 1-4 ALECSAT treatments were included in the study. The blood donated for ALECSAT production, and the final ALECSAT product, was analyzed with flow cytometry (FC). Autologous cell lines of cancer stem cells (CSC) derived from tumor tissue were established and the cells were treated with ALECSAT for 24 hours, fixed, stained and evaluated with high content imaging. (B) The patients received radiotherapy and temozolomide (TMZ) according to standard treatment and also received several doses of ALECSAT as an add-on therapy.
Table 1.
Patient Data
| Patient ID | Gender (M/F) |
Age at Diagnosis (Years) |
Diagnosis |
|---|---|---|---|
| GU-HGG-160 | M | 41 | Gliosarcoma |
| GU-HGG-131 | M | 57 | Glioblastoma |
| GU-HGG-145 | M | 56 | Glioblastoma |
| GU-HGG-151 | F | 44 | Glioblastoma |
| GU-HGG-150 | F | 40 | Glioblastoma |
| GU-HGG-163 | F | 68 | Glioblastoma |
| GU-HGG-178 | F | 49 | Glioblastoma |
Supplementary Figure S1.
Protein expression of cancer stem cells. The cell lines are proliferative, as seen by EdU incorporation, and express the stem cell markers Nestin, SOX2 and Vimentin. Scale bar: 100 μm.
Supplementary Figure S2.
Protein expression of normal adult stem-like cells. The cell line is proliferative (EdU), and have a heterogenous expression of the stem cell marker Nestin and homogenous expression of Vimentin. Scale bar: 100 μm.
Analysis of ALECSAT Content with Flow Cytometry
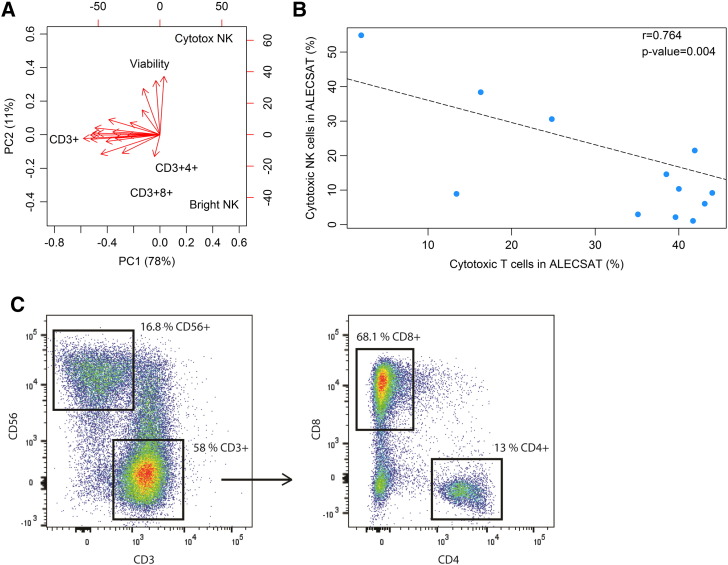
First, we characterized the content of ALECSAT products (n = 19) with flow cytometry. Principal component analysis (PCA) of these data revealed both inter- and intra-patient variation of the product infused to the patients, particularly regarding the percentage of CD3+ T cells (range 21-87%; Figure 2A). As illustrated in Figure 2B, there was an inverse correlation between the proportion of NK cells and CTL cells in ALECSAT, indicating the presence of cytotoxic cells regardless of composition. The composition of a representative treatment of average lymphocyte proportions is displayed in Figure 2C. For the occasional patient, we observed an increase in CD3+ (T cells), Th, (CD3+4+), CTL (CD3+8+) and NK cells at later ALECSAT treatments compared to the first but there was no discernible overall trend (data not shown).
Figure 2.
Content of ALECSAT. (A) Principal component analysis (PCA) of the viability and content of ALECSAT treatments (n = 19) for the following cell types; CD3+ (T cells), CD3+4+ (T helper cells), CD3+8+ (cytotoxic T lymphocytes; CTL), CD3-56+ (bright NK cells) and CD3-16+56+ (cytotoxic NK cells). (B) The proportion of cytotoxic NK cells and CTL in ALECSAT are inversely correlated. Each dot represents one ALECSAT product. (C) Flow cytometry plot and gating of the content of one ALECSAT treatment of average composition.
ALECSAT Displays Broad and Robust Cytotoxicity
Next, we investigated the effect of ALECSAT on autologous CSC and non-cancer cells (adult neural stem-like cells; GU-NS-6 and fibroblasts; BJ) at the same effector/target ratios of ALECSAT. The CSC and both control cell lines displayed susceptibility to ALECSAT, demonstrating a broad cytotoxic effect of ALECSAT (Figure 3A). The BJ cells were more resistant than CSC to ALECSAT (Figure 3B). The cytotoxic effect on BJ cells varied between ALECSAT treatments and was significantly correlated (r = 0.996, P = .05) to the proportion of cytotoxic NK cells in the ALECSAT (data not shown). This was in contrast to the adult stem-like cells GU-NS-6, which were treated with ALECSAT containing very low levels of cytotoxic NK cells but high levels of CTL, suggesting that CTL could eliminate adult neural stem-like cells but not BJ cells. We also examined if ALECSAT affected the proliferation of CSC and found that ALECSAT decreased the proportion of proliferating cells among the surviving cells with increasing doses after 24 hours of treatment (Figure 3C).
Figure 3.
Cytotoxicity of ALECSAT on a range of cell types. (A) The percentage of remaining cells after ALECSAT treatment, compared to untreated cells, of cancer stem cells (CSC) and a paired non-cancer cell line (BJ, fibroblasts, or GU-NS-6, adult neural stem-like cells) for the highest ALECSAT dose examined for each pair. (B) The BJ cells are more resistant than the CSC and a higher dose of ALECSAT is required to eliminate all BJ cells. * denote a significant difference (P < .05) in the response of CSC and BJ at the indicated concentration. (C) The proportion of proliferating cells decrease with increasing doses of ALECSAT. * denote significant differences (P < .05) between the control (untreated GU-HGG) and ALECSAT for each cell line.
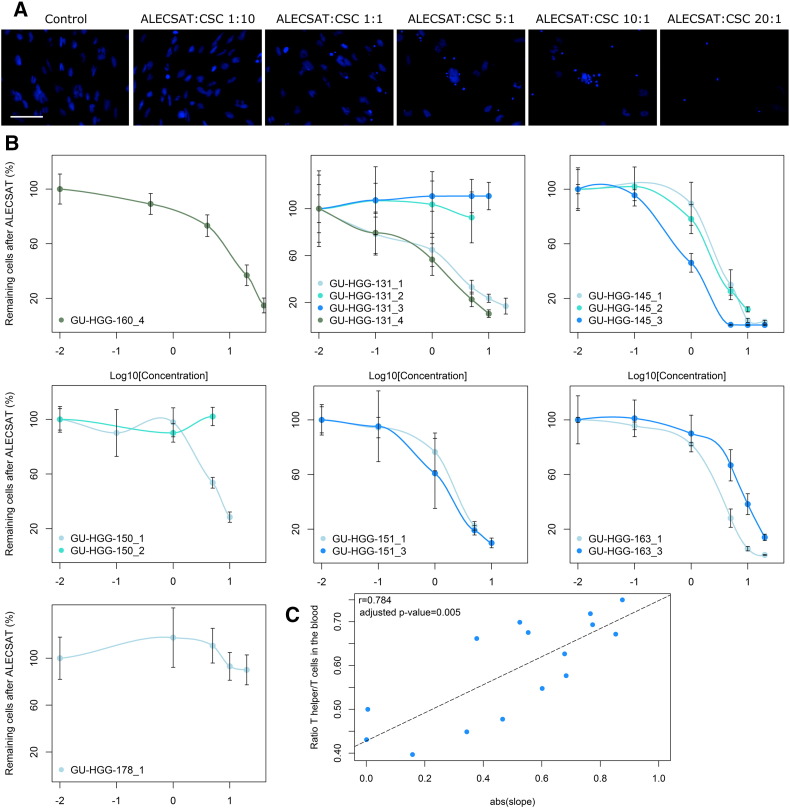
ALECSAT Eliminates Cancer Stem Cells in a Dose-Dependent Manner and the Effect Correlates to the Blood Count of T Helper Cells
We noted that already after 24 hours ALECSAT mediated almost complete elimination of CSC at the higher ALECSAT to CSC ratios (Figure 4A). The susceptibility to the ALECSAT product varied between the CSC lines but also between the treatments for the same patient. Some treatments had no effect on the CSC, but the majority exhibited a dose-response relationship to increasing concentrations of ALECSAT (Figure 4B). The effect of ALECSAT was measured as the slope of the linear region of the dose-response curves for each treatment and was found to correlate significantly with the number of Th cells (CD3+4+) alone, the ratio of Th/T cells, the ratio of CTL/T cells (inverse correlation) and Th/CTL cells in the patient’s blood from which the ALECSAT was produced (Figure 4C and Table 2). There was no correlation between the effect of ALECSAT and the content of the ALECSAT treatments.
Figure 4.
In vitro dose response correlates to patient’s blood values at the time of donation for ALECSAT. (A) Example image of the effect of increasing amounts of ALECSAT on cancer stem cells (CSC). Cell nuclei are stained with DAPI (blue) and the scale bar is 100 μm. (B) The cell lines respond in a dose-dependent manner to increasing amounts of ALECSAT for most treatments. The x-axis is the 10-logarithm of the ALECSAT:CSC ratio (concentration). Each cell line is presented in a separate graph and the number of the ALECSAT treatment is indicated after the cell line. (C) The slope in the linear region of the dose response curves were extracted and they correlated significantly with the ratio of T helper/T cells (Th; CD3+4+) in the patients’ blood at the time of donation for ALECSAT.
Table 2.
Correlation Between Blood Count and the in vitro Effect of ALECSAT on Cancer Stem Cells
| Cell Type | Correlation (r) | Adjusted P Value |
|---|---|---|
| T cells | 0.418 | 1.000 |
| Th cells | 0.710 | .030 * |
| Th/T cells | 0.784 | .005 * |
| CTL/T | −0.742 | .015 * |
| CTL | −0.153 | 1.000 |
| Th/CTL | 0.735 | .018 * |
Pearson correlation between the blood count of immune cells at donation and the in vitro effect of ALECSAT on cancer stem cells (measured as the slope in the linear region of dose response curves). The P value was adjusted for multiple testing (n = 10) with the Bonferroni correction. * denotes significant correlations at significance <0.05. Th, T helper cells; CTL, cytotoxic T lymphocytes.
Discussion
The dismal prognosis of GBM significates the dire need of new treatments. The diffuse and infiltrating nature of GBM complicates complete tumor resection and immunotherapy has been suggested as an attractive option to eradicate the remaining tumor cells as its cytotoxic effect is tumor specific. Immunotherapy in the form of NK cells has shown great promise of targeting CSC, which are believed to be one of the main culprits behind recurrence [13], [14]. Several clinical immunotherapy trials are currently ongoing, including ALECSAT NCT-02799238, which evaluates the effect of the immunotherapy ALECSAT as an add-on therapy to standard treatment for primary GBM. However, many questions regarding the optimal type of immunotherapy and the biological key factors affecting patient survival remain unanswered.
Our results show that ALECSAT has a robust and broad cytotoxic effect targeting GBM-derived CSC and the two tested control cell lines. In addition to the cytotoxic effect of ALECSAT, we also documented a decrease in proliferation of the surviving population of CSC. The highest doses investigated were very effective against CSC, achieving a complete response, which is encouraging given the resistance of these cells to conventional therapy [14]. Autologous NK cells have previously been described capable of completely eliminating GBM stem cells at ratios (20:1 or 40:1) [15] similar to those in our study (10:1 or 20:1). We observed a strong dose-response relationship of ALECSAT on CSC for the majority of the treatments, which is of potential clinical relevance as 107 - 109 ALECSAT cells are infused into the patient at each treatment. This is further supported by a study of melanoma in mice where an increasing number of injected T cells correlated significantly with larger tumor regression [18]. From this we hypothesize that patients infused with the highest amount of cells will have the greatest response. However, in order to obtain higher cell numbers, cells need to be cultured for a longer time ex vivo, which as a result may induce senescence [19], [20] thus limiting the feasible cell number of quality cells for infusion. We conclude that further studies are needed to define the optimal amount of infused T cells in individual patients.
There was no significant correlation between the size of the lymphocyte populations in ALECSAT and its in vitro effect, suggesting that several cytotoxic mechanisms are involved. Considering the inverse correlation between NK and CTL cells in ALECSAT, it is tempting to speculate that both cell types can exert effector functions. We did find significant correlations between the in vitro effect of ALECSAT on CSC and the patients’ blood count (at the time of donation for ALECSAT production). These correlations particularly involved Th cells. Since there was no significant correlation between the number of CTL and the ALECSAT effect, the inverse correlation of CTL/T cells does not automatically imply that fewer CTL cells result in a worse effect. It is more likely that the inverse correlation of CTL/T cells reflects the significant correlation with Th cells, both in absolute numbers and as a ratio of Th/T cells, as the proportion of CTL must be low if the proportion of Th cells is high. These findings highlight the importance of Th cells, which have previously been shown necessary for the recruitment and cytolytic effect of CTL and to augment the effect of CTL [8], [21], [22], [23], [24]. However, for the majority of the patients we noted a trend of decreasing number of Th cells in the blood during treatment, as previously reported in a HGG study correlating low CD4 counts to shorter survival and also showing a toxic effect of TMZ and radiation on CD4+ cells [25], [26]. The observed correlation between Th cell count in the blood and the in vitro effect of ALECSAT suggests a potential therapeutic benefit of further advancing the blood donations and injections to an even earlier stage during treatment.
In conclusion, we have shown that ALECSAT is highly effective against CSC in vitro and that there is a clear dose-response relationship. Our data identify the number of infused cells as an important parameter for efficacy of ALECSAT that may potentially prolong patient survival. Our results further show that the number of Th cells in the patient’s blood, which correlate to the effect on the CSC, are of particular importance and suggest that optimal benefit from ALECSAT treatment could be obtained by advancing treatment to an earlier phase when blood values are generally better. These factors together will be of importance for the design of future immunotherapy trials.
The following are the supplementary data related to this article.
Declarations
Ethics Approval and Consent to Participate
The study was approved by the regional ethics committee (Dnr 604-12, 927-14, 1001-15) and was carried out in accordance with the relevant guidelines and regulations.
Declaration of Interest
Conflicts of interest: none.
Funding
This work was supported by the Swedish Cancer Society, the Swedish Research Council, the Swedish Society for Medical Research, the Wenner-Gren foundations, the Assar Gabrielsson foundation and Lions Cancerfond Väst. Bertil Rydenhag holds funding from AFA Försäkring and The Health Medical Care Committee at Västra Götaland of Sweden, (regional FoU-support) nr: VGFOUREG-566741.
Acknowledgments
Acknowledgements
We thank CytoVac A/S for providing ALECSAT, and Christoffer Bom and Erén Svensson for administrative support.
References
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro-Oncology. 2013;15(Suppl. 2):ii1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fekete B, Werlenius K, Orndal C, Rydenhag B. Prognostic factors for glioblastoma patients--a clinical population-based study. Acta Neurol Scand. 2016;133:434–441. doi: 10.1111/ane.12481. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial, The Lancet. Oncology. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.Tivnan A, Heilinger T, Lavelle EC, Prehn JH. Advances in immunotherapy for the treatment of glioblastoma. J Neuro-Oncol. 2017;131:1–9. doi: 10.1007/s11060-016-2299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker RC. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 8.Hoepner S, Loh JM, Riccadonna C, Derouazi M, Maroun CY, Dietrich PY, Walker PR. Synergy between CD8 T cells and Th1 or Th2 polarised CD4 T cells for adoptive immunotherapy of brain tumours. PLoS One. 2013;8:e63933. doi: 10.1371/journal.pone.0063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–232. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- 10.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, II, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs JF, Idema AJ, Bol KF, Nierkens S, Grauer OM, Wesseling P, Grotenhuis JA, Hoogerbrugge PM, de Vries IJ, Adema GJ. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro-Oncology. 2009;11:394–402. doi: 10.1215/15228517-2008-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, Mortini P, Ferrone S, Doglioni C, Marincola FM. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res. 2010;16:800–813. doi: 10.1158/1078-0432.CCR-09-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, Griffero F, Marubbi D, Spaziante R, Bellora F. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 16.Ames E, Canter RJ, Grossenbacher SK, Mac S, Chen M, Smith RC, Hagino T, Perez-Cunningham J, Sckisel GD, Urayama S. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J Immunol. 2015;195:4010–4019. doi: 10.4049/jimmunol.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenger A, Larsson S, Danielsson A, Elbaek KJ, Kettunen P, Tisell M, Sabel M, Lannering B, Nordborg C, Schepke E. Stem cell cultures derived from pediatric brain tumors accurately model the originating tumors. Oncotarget. 2017;8:18626–18639. doi: 10.18632/oncotarget.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med. 2010;207:2469–2477. doi: 10.1084/jem.20092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Church SE, Jensen SM, Antony PA, Restifo NP, Fox BA. Tumor-specific CD4+ T cells maintain effector and memory tumor-specific CD8+ T cells. Eur J Immunol. 2014;44:69–79. doi: 10.1002/eji.201343718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arina A, Karrison T, Galka E, Schreiber K, Weichselbaum RR, Schreiber H. Transfer of Allogeneic CD4+ T Cells Rescues CD8+ T Cells in Anti-PD-L1-Resistant Tumors Leading to Tumor Eradication. Cancer Immunol Res. 2017;5:127–136. doi: 10.1158/2326-6066.CIR-16-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, Williams L, Foster T, Sepkowitz KA, Chapman PB. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]