Abstract
Background
There are many similarities with regard to contributing cytokines in patients with psoriasis and osteoporosis. A theory of probable relationship between these two entities has been proposed but there is no concordant consensus. The aim of this study was to evaluate bone mineral density (BMD) in patients with psoriasis.
Method and materials
This cross-sectional study of BMD was conducted with 64 eligible patients with psoriasis who were referred to the dermatology clinic of Razi hospital in Tehran, Iran in between 2011 and 2012.
Results
The mean T score of femoral neck was –1.17 and –0.49 in men and women, respectively, which was statistically significant (p = .047). The mean T score of the lumbar spine was –0.93 and –0.30 in men and women, respectively, but not statistically significant (p = .058). In total except with the exclusion of the study site (femur or lumbar), men and women did not have a statistically significant difference with regard to osteoporosis or osteopenia in BMD (p = .114). The Pearson correlation coefficient demonstrated a moderate inverse relationship between age and T score of the femoral neck and lumbar spine (r = –0.419 and –.406, respectively), which was statistically significant (p = .001). Although there was no statistically significant relationship between the Psoriasis Area and Severity Index (PASI) and T scores of the femoral neck (p = .596), a positive and weak correlation was observed between the PASI and T scores for the lumbar spine, which was statistically significant (r = 0.269; p = .03).
Conclusion
Patients with psoriasis had decreased bone density, which was more significant in men. Prevalence of osteoporosis showed no statistically significant difference when compared with the healthy population in Iran.
Keywords: plaque-type psoriasis, bone mineral density, osteoporosis, osteopenia, T-score
Introduction
Psoriasis is a chronic, inflammatory disorder of the joints, skin, and immune system with a prevalence of approximately 2% to 4% in the general population. Several recent studies have implicated systemic inflammatory cytokines with the pathogenesis of psoriasis and suggested that they are responsible for higher internal involvement such as cardiovascular disease, diabetes, and dyslipidemia. These studies show that psoriasis is a complex disorder (Langley et al., 2005, Neimann et al., 2006, Ridker, 2010, Schön and Henning-Boehricke, 2005).
At present, despite screening and prevention programs, osteoporosis remains a hidden problem that is less often detected (Poole and Compston, 2006). Many cytokines including interferon-gamma, interleukin-6, and TNF-α have been identified in the pathogenesis of osteoporosis and these are the same as those involved in the inflammation of psoriasis. Some studies have proposed a hypothesis of association between these two disorders (De Martinis et al., 2006, Kastelan et al., 2006).
A review of the literature revealed that there are few studies on the association between cutaneous psoriasis and osteoporosis and their results are inconsistent. Most of the existing research only focused on the impact of psoriatic arthritis on the generalized decrease in bone mineral mass (Frediani et al., 2001). Interestingly, in contrast with the usual higher average rates of osteopenia-osteoporosis among women in the health population, a few studies observed a prevalence among men with psoriasis (Dreiher et al., 2009, Hofbauer et al., 2006).
This study was designed and conducted to evaluate the changes in bone density in patients with chronic plaque-type psoriasis (with or without arthritis) and probable gender-related predilection among a group of Iranian patients who were referred to a dermatology referral center at the Razi Hospital in Tehran, Iran.
Methods and materials
This cross-sectional study enrolled 64 patients with psoriasis who were referred to the Razi Dermatology Center in Tehran, Iran between 2011 and 2012. The patients did not have a history of other chronic inflammatory disorders, endocrine disorders (thyroid, parathyroid, and gonadal), chronic kidney disease, or liver disease. For each patient, a questionnaire on demographic data (age, sex), daily physical activity (subjective as acceptable or not), smoking, drug history recorded, and body indices including weight and height were measured. The severity of the skin disease on the basis of Psoriasis Area and Severity Index (PASI) score and the joint involvement on the basis of the number of joints were determined and recorded. Blood samples were tested for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), 25-hydroxycholecalciferol (25 [OH]) vitamin D3 (Vit D3), calcium, phosphorus, and parathyroid hormone levels at the laboratory center of the Razi hospital.
Bone mineral density (BMD) using the dual-energy X-ray absorptiometry (DEXA) method of the lumbar spine (L1-L4) and femoral neck was performed at the Sina Hospital that is affiliated with the Tehran University of Medical Sciences and the results were recorded as T-scores and compared between male and female patients.
According to the World Health Organization’s definition, T score values between –1 and –2.5 are considered osteopenia and values smaller than or equal to –2.5 as osteoporosis. Data were analyzed using SPSS (IBM, North Castle, NY) Version 18 and for the descriptive analysis, mean, standard deviation, and frequency were calculated with the t and analysis of variance (ANOVA) tests to compare different variable groups. Pearson’s correlation coefficient or nonparametric equivalents were used for the quantitative data analysis. All tests were interpreted with a significance level of 5%, and in the case of insignificant results, we reported the power of each test.
The study protocol was approved by the Ethics Committee of the Tehran University of Medical Sciences and after a complete explanation of the study aims to patients, written informed consent forms were obtained.
Results
Of the 64 patients enrolled in the study, 34 patients (53.1%) were male and 30 patients (46.9%) were female with a mean age of 43.9 ± 16.8 years. The mean body mass index (BMI) of patients was 27.12 ± 4.93 kg/m2. Forty patients (62.5%) were overweight (BMI > 25). The demographic and laboratory data of patients are shown in Table 1.
Table 1.
Demographic and laboratory data of patients
| Value | |
|---|---|
| Number | 64 |
| Sex | |
| Male | 34 (53.1%) |
| Female | 30 (46.9%) |
| Age (years) | |
| Mean ± SD | 43.9 ± 16.8 |
| Min-Max | 13-79 |
| Body mass index (kg/m2) | |
| Mean ± SD | 27.12 ± 4.93 |
| Min-Max | 17-44 |
| Activity | 18 (28.1%) |
| Cigarette smoking | 13 (20.3%) |
| Disease duration (years) | |
| Mean ± SD | 27.12 ± 4.93 |
| Min-Max | 1-40 |
| PASI score | |
| Mean ± SD | 5.5 ± 4.7 |
| Min-Max | 0.4-26 |
| Psoriatic arthritis | 2 (3.1%) |
| Serum total calcium (mg/dl) | |
| Mean ± SD | 9.9 ± 0.5 |
| Min-Max | 7.6-10.9 |
| Serum total phosphorus (mg/dl) | |
| Mean ± SD | 4.7 ± 0.8 |
| Min-Max | 2.7-6.7 |
| Serum parathyroid hormone (ng/dl) | |
| Mean ± SD | 33.9 ± 16.4 |
| Min-Max | 7-84 |
| 25 (OH) Vit D3 (ng/dl) | |
| Mean ± SD | 17.2 ± 11.6 |
| Min-Max | 2.4-56 |
| ESR | |
| Mean ± SD | 15.8 ± 6.2 |
| Min-Max | 5-30 |
| CRP | |
| Positive | 8 (12.5%) |
| Negative | 56 (87.5%) |
25 (OH) Vit D3, 25-hydroxycholecalciferol and vitamin D3; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PASI, Psoriasis Area and Severity Index; SD, standard deviation.
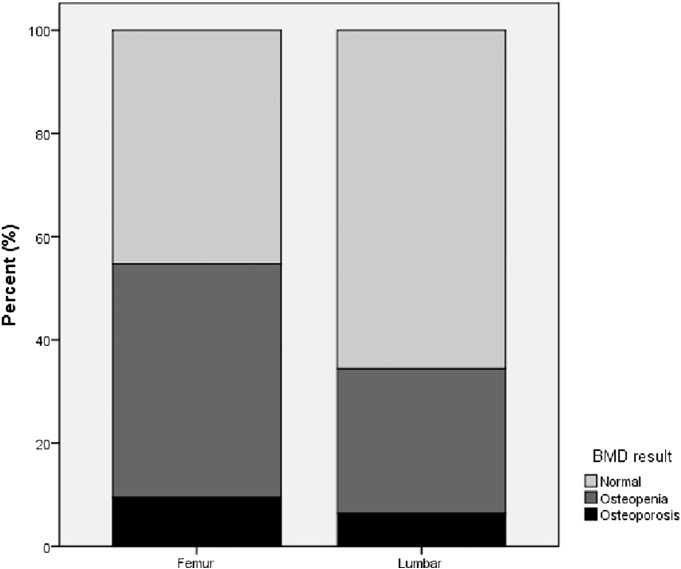
Twenty-nine patients (45.3%) had femoral neck osteopenia and 6 patients (9.4%) had femoral neck osteoporosis. A BMD study of the lumbar spine showed that 18 patients (28.1%) had osteopenia and four patients (6.3%) had osteoporosis (Fig 1). A total of 28 patients (43.8%) had a decrease of bone mass (osteopenia) in either one of the two sites (femoral or lumbar), and 8 patients (12.5%) had osteoporosis in at least one of the two sites (Fig 2).
Fig. 1.
Prevalence of osteoporosis and osteopenia in patients’ femoral neck and lumbar spine.
Fig. 2.
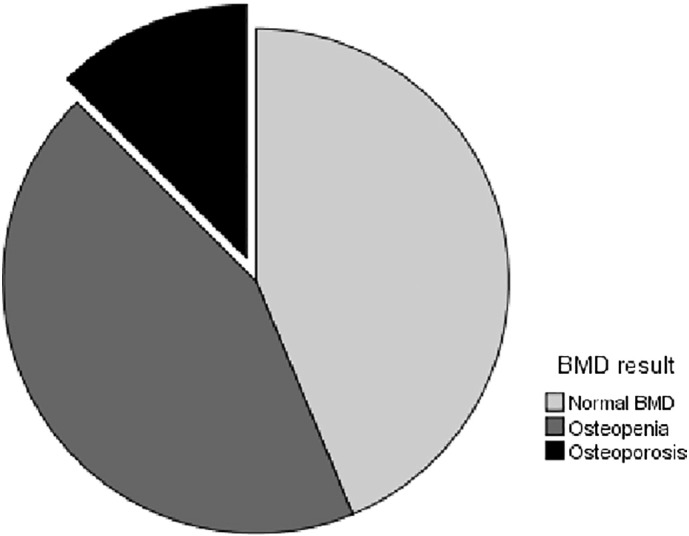
Prevalence of osteoporosis and osteopenia in patients with psoriasis.
The mean T score of femoral neck was –1.17 and –0.49 in men and women, respectively. The difference was –0.68 (95% confidence interval (CI), –0.01 to –1.36), which was statistically significant (p = .047). The mean T score of the lumbar spine was –0.93 and –0.30 in men and women, respectively, and the mean difference was –0.63 (95 CI, –0.02 to –1.28) but not statistically significant (p = .058).
A total of 17 male patients (50%) and 11 female patients (36.7%) had osteopenia. Six male patients (17.6%) and two female patients (6.7%) had osteoporosis. In these cases, there was no significant difference between the men and women (p = .114).
The Pearson correlation coefficient demonstrated a moderate inverse relationship between age and T score of the femoral neck and lumbar spine (r = – .419 and –.406, respectively), which was significant in both cases (p = .001). Table 2 shows the average density of bone mass in patients by age and sex.
Table 2.
Mean bone mineral density on the basis of age and sex
| Age group |
T score Femur |
T score Lumbar |
||
|---|---|---|---|---|
| Male Patients (n = 34) | Female Patients (n = 30) | Male Patients (n = 34) | Female Patients (n = 30) | |
| < 20 years | –0.84 (1) | –0.10 (2) | –0.82 (1) | –0.74 (2) |
| 20-29 years | –0.16 (3) | –0.66 (4) | –0.26 (3) | 0.13 (4) |
| 30-39 years | –0.56 (6) | 0.02 (4) | 0.06 (6) | 0.53 (4) |
| 40-49 years | –1.1 (14) | 0.74 (13) | –0.74 (14) | 0.40 (13) |
| 50-59 years | –1.64 (7) | –1.21 (5) | –1.30 (7) | –0.93 (5) |
| 60-69 years | –2.33 (2) | –3.68 (1) | –1.16 (2) | –0.60 (1) |
| ≥ 70 years | –3.13 (1) | –1.55 (1) | –1.36 (1) | –1.59 (1) |
No significant correlation was observed between BMI and T score of the lumbar spine and femoral neck (p = .083 and p = .274, respectively). The mean BMI of patients with osteopenia or osteoporosis also had no significant difference when compared with the BMI of health subjects (p = .382).
The mean T score of the femoral neck and lumbar spine in patients who smoke was lower than that of healthy subjects (mean difference, 0.35 and 0.63, respectively) although these differences were not statistically significant (p = .413 and p = .124, respectively). In addition, no significant differences were observed in the prevalence of smoking among osteoporotic, osteopenic, and normal subjects on the basis of BMD (p = .572).
Furthermore, no significant correlation was observed between disease duration and T score of the femoral neck and lumbar spine (p = –.133 and p = .150, respectively) and there was no statistically significant difference between the mean disease duration in patients with osteoporotic, osteopenic and normal BMD (p = .859).
Although there was no statistically significant relationship between PASI and T scores of the femoral neck (p = .596), a correlation was observed between PASI and T scores of the lumbar spine, which was positive and weak but significant (r = .269, p = 0.03). A significant difference was not observed between PASI scores of patients with osteoporotic, osteopenic, and normal BMD (p = .159).
With regard to inflammatory markers and cytokines, there was no significant correlation between ESR and T score of the femoral neck or lumbar spine (p = .230 and p = .844, respectively) and no significant difference was observed in the ESR of patients with an osteoporotic, osteopenic, or normal BMD (p = .473).
No significant difference was observed about the mean T score of the femoral neck and lumbar spine in patients with positive or negative CRP levels (p = .589 and p = .608, respectively). The prevalence of positive CRP levels among patients with osteoporotic, osteopenic, and normal BMD was not different (p = .520).
During the investigation, no significant correlation between serum total calcium and T score of the femoral neck and lumbar spine (p = .780 and p = .053, respectively) was found. No difference in serum calcium levels between patients with osteoporotic, osteopenic, and normal BMD was observed (p = .537).
Serum phosphorus and bone density of the femoral neck and lumbar spine were not correlated (p = .965 and p = .785, respectively) and phosphorus serum levels among patients with osteoporotic, osteopenic, and normal BMD did not differ (p = .920).
A significant relationship between the level of parathyroid hormone and T score of the lumbar spine or femoral neck was not observed (p = .816 and p = .785, respectively) and the hormone levels between patients with osteoporotic, osteopenic, and normal BMD showed no significant difference (p = .671).
Finally, the levels of 25 (OH) and Vit D3 did not differ statistically significant with regard to T score of the femoral neck or lumbar spine (p = .953 and p = .172, respectively) and vitamin D levels were not significantly different between patients with osteoporotic, osteopenic, and normal BMD (p = .636).
Table 3 demonstrates the different variables that affect the correlation coefficient of the femoral neck and lumbar spine’s T score. Table 4 shows the frequencies of these factors in patients with osteoporotic, osteopenic, and normal BMD.
Table 3.
Pearson’s correlation coefficient of the femoral neck and lumbar spine’s T score and risk factors of osteoporosis
| Femur T score | Lumbar T score | Age | BMI | Disease Duration | PASI | Calcium | Phosphorus | Parathyroid Hormone | 25(OH) Vit D3 | ESR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Femur T score | 1 | ||||||||||
| Lumbar T score | .665a | 1 | |||||||||
| Age | –.419a | –.406a | 1 | ||||||||
| BMI | .219 | .139 | .203 | 1 | |||||||
| Disease Duration | –.133 | .150 | –.120 | .057 | 1 | ||||||
| PASI | .068 | .269 | –.097 | .079 | .198 | 1 | |||||
| Calcium | .040 | .273 | –.324a | –.022 | .040 | .027 | 1 | ||||
| Phosphorus | .006 | .039 | –.200 | .069 | –.146 | –.013 | .547a | 1 | |||
| Parathyroid Hormone | –.033 | –.046 | .137 | –.043 | –.021 | –.056 | –.260 | –.224 | 1 | ||
| 25(OH) Vit D3 | –.008 | –.194 | .159 | .150 | –.101 | –.120 | .334a | .103 | –.284a | 1 | |
| ESR | –.171 | –.028 | .137 | –.161 | –.049 | .191 | .004 | .007 | –.108 | –.020 | 1 |
25 (OH) Vit D3, 25-hydroxycholecalciferol and vitamin D3; BMI, body mass index; ESR, erythrocyte sedimentation rate; PASI, Psoriasis Area and Severity Index.
p value < .05.
Table 4.
Frequencies of factors that affect the bone density in patients with psoriasis and osteoporosis, osteopenia, and normal BMD
| Osteoporosis | Osteopenia | Normal BMD | p value | |
|---|---|---|---|---|
| Number (%) | 8 (12.5%) | 28 (43.8%) | 28 (43.8%) | |
| Age | 57.2 ± 20.8 | 47.5 ± 16.1 | 36.5 ± 12.8 | .002 |
| Sex | ||||
| Male | 6 (17.6%) | 17 (50%) | 11 (32.4%) | .114 |
| Female | 2 (6.7%) | 11 (36.7%) | 17 (56.7%) | |
| BMI | 25.1 ± 3.3 | 26.9 ± 3.6 | 27.8 ± 6.2 | .382 |
| Activity | 1 (5.6%) | 10 (55.6%) | 7 (38.9%) | .387 |
| Cigarette smoking | 2 (15.4%) | 7 (53.8%) | 4 (30.8%) | .572 |
| Disease duration | 8 ± 13.2 | 7.7 ± 9.1 | 6.7 ± 4.3 | .859 |
| PASI | 2.9 ± 2.1 | 6.5 ± 4.1 | 5.3 ± 5.5 | .159 |
| Calcium | 9.6 ± 1.4 | 9.9 ± 0.4 | 9.8 ± 0.4 | .537 |
| Phosphorus | 4.6 ± 1.6 | 4.5 ± 0.8 | 4.4 ± 0.8 | .920 |
| Parathyroid Hormone | 41.0 ± 11.0 | 33.1 ± 14.1 | 33.9 ± 19.8 | .671 |
| 25(OH)Vit D3 | 19.8 ± 8.1 | 18.3 ± 13.4 | 15.4 ± 9.7 | .636 |
| ESR | 14.7 ± 4.6 | 16.8 ± 5.4 | 14.7 ± 7.3 | .473 |
| CRP | 2 (25%) | 3 (37.5%) | 3 (37.5%) | .520 |
25(OH)Vit D3, 25-hydroxycholecalciferol and vitamin D3; BMD, bone mineral density; BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PASI, Psoriasis Area and Severity Index.
Discussion
After observation of the similarities between cytokines that affect patients with psoriasis and osteoporosis, the theory of a probable relationship between these two entities has strongly emerged (Kastelan et al., 2006). The present study evaluated patients with psoriasis with regard to bone density and showed a decreased BMD, especially in men, although not statistically significant. Unfortunately, due to the lack of a control group, it was not possible to compare the results with healthy controls. However, when using the results of a national study on the general population of Iran and after matching the age and sex categories, we could determine that the BMD of patients with psoriasis on the basis of T scores shows a reduction. For example, in our study, the mean T scores of the femoral neck in 10 male patients of age 50 to 59 years old and 7 female patients of the same age group were –1.64 and –1.2, respectively. In a national Iranian study by Larijani et al. on osteoporosis in male and female patients of age 50 to 59 years old, these rates were 0.91 and 0.96, respectively (Bagheri et al., 2011, Larijani et al., 2004, Larijani et al., 2005). These T scores are also available for other sex- and age-matched groups in Iran in studies on the femoral neck and lumbar spine (Bagheri et al., 2011, Larijani et al., 2004, Larijani et al., 2005). It appears that the amount of reduction in the bone density of patients with psoriasis compared with age- and sex-matched groups in a national study is not statistically significant and not in the range of osteoporosis.
In a large meta-analysis study on 21 related studies that analyzed 9657 cases, the prevalence of femoral neck and lumbar spine osteoporosis in Iran was approximately 18% (Bagheri et al., 2011), which was near the results of our study but not comparable because 12.5% of our patients had osteoporosis in at least one of these two areas.
Other studies on the association between psoriasis and BMD showed conflicting results. A study by Millard et al. (2001) on the Z score of patients with chronic plaque-type psoriasis compared with healthy controls showed no significant difference. Also, Balato et al. (2012) examined the prevalence of osteoporosis among patients with psoriasis in Italy and did not report a significant difference in BMD with the healthy population of Italy. One study (Keller et al., 2013) showed that patients with osteoporosis had a higher prevalence of psoriasis compared with patients without osteoporosis (1.5% vs. 0.87%). The prevalence of osteoporosis in Iranian studies has been reported generally lower than those in European and American studies (Larijani et al., 2004) but our study demonstrated a higher prevalence of osteoporosis in patients with psoriasis than in other studies (e.g., 5% in Balato et al., (2012) and 12.5% in our study), which shows the importance of demographic and climatic factors as well as races.
Overall, in most studies, women are at higher risk for osteoporosis, bone density loss, and fracture (Bagheri et al., 2011, Larijani et al., 2004) but in our study, a BMD reduction in men with psoriasis was higher than in women with psoriasis with 17.6% of men and 6.7% of women showing osteoporosis. The study by Dreiher et al. (2009) also showed that the prevalence of osteoporosis in men with psoriasis was higher than that in women and even after controlling for confounding variables, osteoporosis had a higher and stronger association with the male sex (odds ratio [OR]: 1.35 in men; 0.90 in women).
In the study by Hofbauer et al. (2006), 10.2% of men with psoriatic arthritis showed osteoporosis but this rate among women was only 1.75%. This can be explained perhaps by the fact that osteoporosis in women is usually due to an estrogen deficiency and postmenopausal women are generally at a higher risk for osteoporosis. In contrast, osteoporosis in men is usually caused by an underlying systemic disorder and much more a limitation of physical activities. In psoriasis, a great amount of inflammatory cytokines and immunological events can be an underlying cause of osteoporosis and women with psoriasis are typically at premenopausal age (Dreiher et al., 2009).
The findings by Hofbauer and Dreiher are comparable with those of our study with regard to different sex predilection of bone density loss in patients with psoriasis. In this study, none of the underlying factors (nutritional factors such as intake of calcium, phosphorus and vitamin D3, personal habits such as daily exercise and smoking) showed a significant difference in the association of psoriasis and osteoporosis although the mean T score of the femoral neck and lumbar spine in patients who smoked was lower. In the study by Balato et al. (2012), patients with psoriasis and osteoporosis or osteopenia also had no significant difference with regard to these risk factors.
Logically, the greater severity of the skin involvement and the longer exposure to the inflammatory cytokines causes the higher degree of bone loss and higher risk of developing osteoporosis; however, our study found no association between disease duration and/or PASI score with the prevalence of osteopenia and osteoporosis in patients with psoriasis. The reduction of bone density in patients with psoriasis is associated with the number of involved joints. ESR and CRP levels are a sign of underlying inflammation but these factors did not show any increase in patients with psoriasis and osteoporosis comparable with the results of the Dreiher and Hofbauer studies (Dreiher et al., 2009, Hofbauer et al., 2006).
Limitations and recommendations
The most important limitation of this study was a lack of any age- and sex-matched healthy controls. We also did not exclude patients with older ages such as menopausal women, patients with a higher BMI, or smokers who could be confounders of our study. It is highly recommended that other, larger, and well-designed controlled studies are conducted with more focus on probable confounders.
Conclusion
In this study, patients with psoriasis had decreased BMD, which was more significant in men even though the prevalence of osteoporosis in patients showed no statistically significant difference with the health population of Iran (Bagheri et al., 2011; p = .114). However, the design of well-controlled studies that are matched for age, sex, and race are essential. Screening studies for osteoporosis should be considered in patients with psoriasis and especially in men with severe and widespread disease who also have other metabolic disorders.
Footnotes
Conflicts of interest: The authors have no conflict of interest to disclose.
Funding sources: This study was funded by the Vice Chancellor of Research at the Tehran University of Medical Sciences in Iran.
References
- Bagheri P., Haghdoost A., Dortaj E., Halimi L., Vafayi Z., Farhangnia M. Ultra analysis of prevalence of osteoporosis in Iranian women: A systematic review and meta-analysis. Iranian J Endocrinol Metabol. 2011;3:315–325. [Google Scholar]
- Balato N., Balato A., Gallo L., Napolitano M., Patruno C., Ayala F. Psoriasis and osteoporosis: Data from a Southern Italian population. Arch Osteoporosis. 2012;7(1-2):321–323. doi: 10.1007/s11657-012-0112-1. [DOI] [PubMed] [Google Scholar]
- De Martinis M., Di Benedetto M.C., Mengoli L.P., Ginaldi L. Senile osteoporosis: Is it an immune-mediated disease? Inflamm Res. 2006;55:399–404. doi: 10.1007/s00011-006-6034-x. [DOI] [PubMed] [Google Scholar]
- Dreiher J., Weitzman D., Cohen A.D. Psoriasis and osteoporosis: A sex-specific association? J Invest Dermatol. 2009;129(7):1643–1649. doi: 10.1038/jid.2008.432. [DOI] [PubMed] [Google Scholar]
- Frediani B., Allegri A., Falsetti P., Storii L., Bisongo S., Baldi F. Bone mineral density in patients with psoriatic arthritis. J Rheumatol. 2001;28:138–143. [PubMed] [Google Scholar]
- Hofbauer L.C., Schoppet M., Christ M., Teichmann J., Lange U. Tumor necrosis factor-related apoptosis-inducing ligand and osteoprotegerinserum levels in psoriatic arthritis. Rheumatology. 2006;45:1218–1222. doi: 10.1093/rheumatology/kel108. [DOI] [PubMed] [Google Scholar]
- Kastelan D., Kastelan M., Massari L.P., Korsic M. Possible association of psoriasis and reduced bone mineral density due to increased TNF-α and IL-6 concentrations. Med Hypotheses. 2006;67:1403–1405. doi: 10.1016/j.mehy.2006.04.069. [DOI] [PubMed] [Google Scholar]
- Keller J.J., Kang J.H., Lin H.C. Association between osteoporosis and psoriasis: Results from the Longitudinal Health Insurance Database in Taiwan. Osteoporos Int. 2013;24:1835–1841. doi: 10.1007/s00198-012-2185-5. [DOI] [PubMed] [Google Scholar]
- Langley R.G., Krueger G.G., Griffiths C.E. Psoriasis: Epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64:ii18–23. doi: 10.1136/ard.2004.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larijani B., Hossein-Nezhad A., Mojtahedi A., Pajouhi M., Bastanhagh M.H., Soltani A. Normative data of bone mineral density in healthy population of Tehran, Iran: A cross sectional study. BMC Musculoskelet Disord. 2005;6:38. doi: 10.1186/1471-2474-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larijani B., Mohajeri Tehrani M.R., Hamidi Z., Soltani A.A.F., Pajouhi M. Osteoporosis, global and Iranian aspects. Iranian J Publ Health. 2004;33:1–17. [Google Scholar]
- Millard T.P., Antoniades L., Evans A.V., Smith H.R., Spector T.D., Barker J.N. Bone mineral density of patients with chronic plaque psoriasis. Clin Exp Dermatol. 2001;26:446–448. doi: 10.1046/j.1365-2230.2001.00855.x. [DOI] [PubMed] [Google Scholar]
- Neimann A.L., Shin D.B., Wang X., Margolis D.J., Troxel A.B., Gelfand J.M. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Poole K.E., Compston J.E. Osteoporosis and its management. BMJ. 2006;333:1251–1256. doi: 10.1136/bmj.39050.597350.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P.M. Psoriasis, inflammation, and vascular risk: A problem more than skin deep? Eur Heart J. 2010;31(8):902–904. doi: 10.1093/eurheartj/ehq042. [DOI] [PubMed] [Google Scholar]
- Schön M., Henning-Boehricke W. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]