Abstract
Radiation-induced morphea (RIM) is a rare and underrecognized complication of radiation therapy that most commonly occurs in women after treatment for breast cancer. Although not fully understood, RIM is hypothesized to arise from an increase in cytokines that stimulate collagen production and extracellular matrix formation. Most documented cases of RIM occur 1 year after radiation therapy and are localized to areas that were treated for breast cancer. We report on a case of a female patient with stage IB endometrial adenocarcinoma who was treated with 24 Gray of adjuvant brachytherapy. The patient developed a diffuse morpheaform, pruritic eruption only at distant sites from the brachytherapy treatment field. Although treatment for RIM is generally unsatisfactory, our patient experienced improvement in the pruritus and a regression of the lesions while applying topical 0.1% tacrolimus ointment and 0.1% triamcinolone creme. An early diagnosis of RIM can prevent extensive workup, guide treatment, and improve quality of life for patients.
Keywords: radiation-induced morphea, postirradiation morphea
Introduction
Radiation-induced morphea (RIM) is a rare and often underrecognized complication of radiation therapy that most frequently occurs in women who are treated for breast cancer (Spalek et al., 2015). Its early diagnosis can prevent extensive workup, improve quality of life, and help initiate early intervention for these patients (Spalek et al., 2015). We report on a unique and rare case of a female patient with endometrial adenocarcinoma who was treated with 24 Gy of adjuvant brachytherapy and who developed histologically confirmed, circumscribed superficial morphea at distant sites from the irradiated field.
Case
A 69-year-old Caucasian woman presented to the dermatology clinic with a 1-year history of a firm, pink rash on the bilateral flanks and left breast. The rash was occasionally pruritic but not painful. The patient had a history of stage IB endometrial adenocarcinoma that was treated with total laparoscopic hysterectomy, bilateral salpingo-oophorectomy, and adjuvant vaginal cuff brachytherapy 4 to 6 weeks prior to the development of the rash. She received a total dose of 24 Gy from six fractions delivered over 17 days. No laboratory samples were tested for autoantibodies in the absence of other findings that were suggestive of systemic sclerosis. She did not have a history of autoimmune disease, Raynaud’s phenomena, systemic symptoms, sclerodactyly, nail fold capillary changes, or arthralgia nor did these develop at any point during the course of treatment.
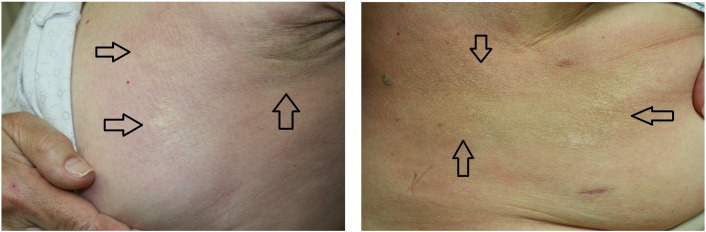
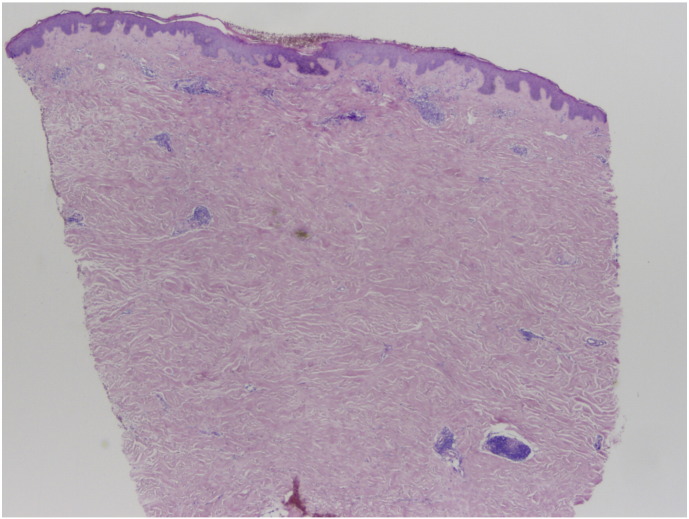
A physical examination revealed scattered 1 to 4 cm, white, indurated, and firm plaques with surrounding erythema on the left breast and bilateral flanks (Fig. 1). There was no substantial wrinkling of the skin and no dell to suggest lichen sclerosus. A punch biopsy was performed on the left flank. Histopathology test results revealed a squared-off punch biopsy with thickened and sclerotic-appearing collagen in the dermis that extended around the eccrine units (Fig. 2). A dermal perivascular and interstitial infiltrate that was composed of small lymphocytes and plasma cells was evident. There were no interface changes at the dermo-epidermal junction.
Fig. 1.
On the left breast (left) and right flank (right) of the patient are scattered 1 to 4 cm white, indurated, and firm plaques with surrounding erythema that are consistent with radiation-induced morphea. The flank lesion becomes hyperpigmented as it extends posteriorly. A few guide arrows are included to identify lesions. A total of three lesions are appreciated on the left breast and another three lesions on the right flank.
Fig. 2.
Histopathologic test results reveal a relatively unremarkable epidermis. In the dermis, thickened and sclerotic-appearing collagen is evident and extends around the eccrine units. A dermal perivascular and interstitial infiltrate that is composed of small lymphocytes and plasma cells is seen. (Hematoxylin and eosin stain, original magnification × 2).
On the basis of these findings and the patient’s clinical history, a diagnosis of a circumscribed, superficial subtype of RIM was made. Topical 0.1% tacrolimus ointment and 0.1% triamcinolone creme were both applied twice daily over the course of 1 week and stabilized the disease until the patient no longer noted pruritus. The patient felt that the lesions, although no different in size, were less red and inflamed after initiation of the treatment and she currently remains on the topical therapy.
Discussion
RIM is a rare complication of radiation therapy that occurs 1 in 500 patients (Akay et al., 2010, Spalek et al., 2015). RIM has been postulated to arise in the setting of abnormal fibroblast activity that results in collagen deposition and extensive fibrosis (Akay et al., 2010, Spalek et al., 2015). Although the exact pathogenesis is unknown, RIM is hypothesized to occur from upregulation of Th2 cytokines and transforming growth factor-beta (TGF-β), which enhance collagen deposition and extracellular matrix production (Spalek et al., 2015).
RIM typically occurs within 1 year of radiation but ranges of 1 month to 32 years have been reported (Akay et al., 2010, Spalek et al., 2015). RIM is characterized by an inflammatory phase and a sclerosing phase (Akay et al., 2010, Spalek et al., 2015). The inflammatory phase consists of perivascular and periadnexal lymphocyte infiltration with collagen thickening (Akay et al., 2010, Spalek et al., 2015). Subsequently, the sclerosing phase features significant fibrosis with loss of periadnexal adipose and inflammatory infiltrate (Akay et al., 2010, Spalek et al., 2015).
Clinically, these lesions start as erythematous and edematous plaques that become indurated with pigment changes during the sclerosing phase (Akay et al., 2010, Spalek et al., 2015). Histopathologic testing is critical to make the diagnosis as it was for our case as well. Histopathologic test results showed thickened and sclerotic-appearing collagen, a dermal perivascular and interstitial infiltrate, and an uninvolved epidermis, which distinguished it from radiation dermatitis, cancer recurrence, new cancer lesion, or radiation recall.
Sixty-six cases of RIM have been described over 25 years by Spalek et al. (2015). Although the majority of cases occurred in women after treatment for breast cancer, two cases were associated with endometrial adenocarcinoma (Akay et al., 2010, Spalek et al., 2015, Ullén and Björkholm, 2003). There was no correlation with radiation dose or fractionation (Spalek et al., 2015). RIM generally occurs within the radiation field but rarer cases demonstrate involvement beyond the treated area (Akay et al., 2010, Spalek et al., 2015). There are a few reported cases of distant site morphea in response to radiation therapy (Ardern-Jones and Black, 2003, Balegar et al., 2016). One case of endometrial cancer that was treated with brachytherapy and external beam radiation therapy featured lesions that extended from the site of treatment to the right lower abdomen and the upper and lower leg (Akay et al., 2010). Our case uniquely features distant site involvement in response to adjuvant vaginal cuff brachytherapy for endometrial adenocarcinoma. More interestingly, the area around the primary treatment site remains unaffected.
Morphea distant from the treatment site may support the hypothesis that RIM arises in part from neoantigen formation (Akay et al., 2010, Spalek et al., 2015). Ardern-Jones and Black (2003) noted widespread morphea in a patient who was treated for breast cancer and postulated that radiation therapy induces fibroblast or endothelial cell neoantigen formation, which activates T cells. These T cells could cause a systemic release of TGF-β, which causes RIM by activating fibroblasts and inducing collagen formation (Ardern-Jones and Black, 2003). Although morphea that is unrelated to radiation treatment cannot be fully excluded, the timing of the eruption diminishes the likelihood of a sporadic case. With the salient understanding that morphea develops as the result of a cutaneous trigger, we hypothesize that brachytherapy was the incipient factor in the development of disease at distant sites from the irradiated area in our patient.
Treatment of RIM is generally unsatisfactory (Spalek et al., 2015). However, the first-line option for localized disease is topical tacrolimus with secondary treatments including potent topical steroid medications and phototherapy before progressing to systemic therapies (Fett and Werth, 2011). Our patient was prescribed topical 0.1% tacrolimus ointment and 0.1% triamcinolone creme, which were both applied twice daily with consequent stabilization of the disease and cessation of the pruritus.
Conclusion
The early diagnosis of RIM, an often underrecognized complication that most frequently occurs in female patients after breast radiation therapy, can prevent extensive workup (Spalek et al., 2015). Importantly, any treatment should be initiated early to prevent irreversible fibrosis, atrophy, and improve patient quality of life (Spalek et al., 2015). Although morphea is an entity that is otherwise well-known to dermatologists, both dermatologists and radiation oncologists should be aware of the possibility of morpheaform eruptions after radiation therapy, even distal to the site of radiation.
Footnotes
Sources of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Akay B.N., Sanli H., Heper A.O. Postirradiation linear morphoea. Clin Exp Dermatol. 2010;35:e106–e108. doi: 10.1111/j.1365-2230.2009.03717.x. [DOI] [PubMed] [Google Scholar]
- Ardern-Jones M.R., Black M.M. Widespread morphoea following radiotherapy for carcinoma of the breast. Clin Exp Dermatol. 2003;28:160–162. doi: 10.1046/j.1365-2230.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- Balegar S., Mishra D.K., Chatterjee S. Generalized Morphea following radiotherapy for an intracranial tumor. Indian J Dermatol. 2016;61:581. doi: 10.4103/0019-5154.190132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett N., Werth V.P. Update on morphea: Part II. Outcome measures and treatment. J Am Acad Dermatol. 2011;64:231–242. doi: 10.1016/j.jaad.2010.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalek M., Jonska-Gmyrek J., Gałecki J. Radiation-induced morphea - A literature review. J Eur Acad Dermatol Venereol. 2015;29:197–202. doi: 10.1111/jdv.12704. [DOI] [PubMed] [Google Scholar]
- Ullén H., Björkholm E. Localized scleroderma in a woman irradiated at two sites for endometrial and breast carcinoma: a case history and a review of the literature. Int J Gynecol Cancer. 2003;13:77–82. doi: 10.1046/j.1525-1438.2003.13006.x. [DOI] [PubMed] [Google Scholar]