Abstract
Throughout pregnancy, the body undergoes a variety of physiologic changes. The cutaneous findings can be most noticeable and often worrisome to both physicians and patients. Obstetricians and dermatologists must be able to differentiate between changes that are benign and those that may be pathologic. Most physicians recognize benign changes that are commonly described in literature such as hyperpigmentation, melasma, striae gravidarum, and telogen effluvium; however, they may be unaware of changes that tend to be less frequently discussed. This comprehensive review provides a broad overview of the physiologic cutaneous changes that occur during pregnancy as described in the literature over the past 10 years.
Keywords: pregnancy, hyperpigmentation, acne, melasma
Introduction
Pregnancy induces a variety of hormonal, immunologic, and metabolic changes that exert significant effects on a woman’s body. Altered levels of circulating hormones, increased intravascular volume, and compression from the enlarging uterus underlie the complex physiological adaptations that are essential for the development of the fetus. These factors also contribute to the variety of cutaneous changes that may concern patients and physicians. Obstetricians and dermatologists must be able to differentiate normal physiologic findings from pathologic lesions and rashes. A misdiagnosis of benign changes can lead to unnecessary stress and intervention.
We provide a comprehensive review of the physiologic changes of the skin that occur during pregnancy as characterized in the literature over there past 10 years. The pathologic dermatoses of pregnancy will not be covered in this review because we will focus on the characterization and clinical presentation of benign physiologic changes in pigmentation, nevi, mucosa, connective tissue, hair, nails, and breasts as well as vasculature and glands as they relate to dermatologic findings.
Methods
A comprehensive search of the literature was performed to identify all articles that described changes in pregnancy or gestation combined with the search terms dermatol-, skin, cutaneous, integumentary system, pigmentation, nevus, mucosa, gingiva, oral, vulva, vagina, gland, acne, pruritus, hair, or nail. The search produced 7338 articles in total, which were filtered to include only studies that were performed on humans and those written in English.
Only manuscripts that were published between July 1, 2007 and July 1, 2017 were included. A total of 972 manuscripts were reviewed to exclude those unrelated to the topic. Manuscripts that described pathologic changes or treatment interventions were included only if physiologic changes were also described. At the end of this selection, 25 manuscripts were considered eligible for inclusion in this review as either review articles, studies, or clinical case reports. Studies were assessed and findings were categorized according to type of change to provide a comprehensive review of all relevant cutaneous physiological changes that occur throughout gestation.
Pigmentation
The most commonly reported skin change during pregnancy is hyperpigmentation, which develops in some form in 85% to 90% of pregnant women, typically during the second half of the pregnancy (Bieber et al., 2017, Fernandes and Amaral, 2015, Geraghty and Pomeranz, 2011, Rathore et al., 2011, Tyler, 2015, Van Onselen, 2012). The exact mechanism of hyperpigmentation of the skin is not well understood; however, it is commonly attributed to a combination of hormonal factors, genetic predisposition, and ultraviolet exposure (Bieber et al., 2017, Geraghty and Pomeranz, 2011). Melanocytes may be more sensitive to the elevated levels of α- and β-melanocyte-stimulating hormone, estrogen, progesterone, and β-endorphins. These hormones likely stimulate the production of melanin, which underlie the hyperpigmentation that is seen clinically (Bieber et al., 2016). Upregulation of tyrosinase by human placental lipids may further potentiate melanin synthesis (Bieber et al., 2017, Tyler, 2015).
Although generalized hyperpigmentation can occur, more commonly affected are those areas that are already physiologically darker such as the areolas (termed secondary areolas [Bieber et al., 2017]), nipples, genitalia, axillae, periumbilical area, and inner thighs. Intertriginous areas and skin folds may also darken. Patients can develop new acanthosis nigricans or have exacerbation of preexisting lesions. Linea nigra describes the darkening of the linea alba, which is a line that runs along the midline of the lower abdomen and suprapubic area. Darkening here is seen most commonly from the umbilicus to the pubic symphysis (Bieber et al., 2017, Geraghty and Pomeranz, 2011, Tyler, 2015, Van Onselen, 2012).
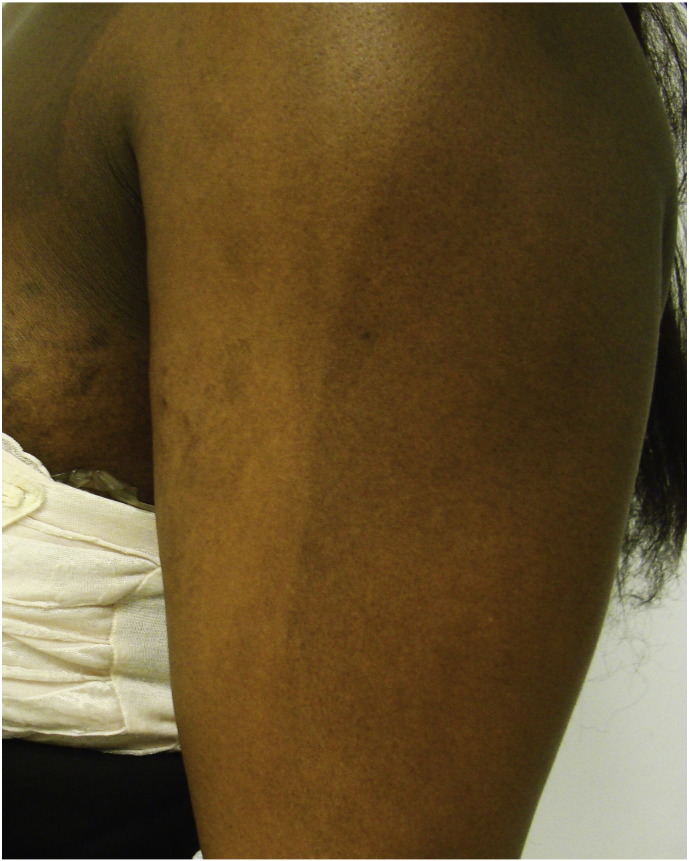
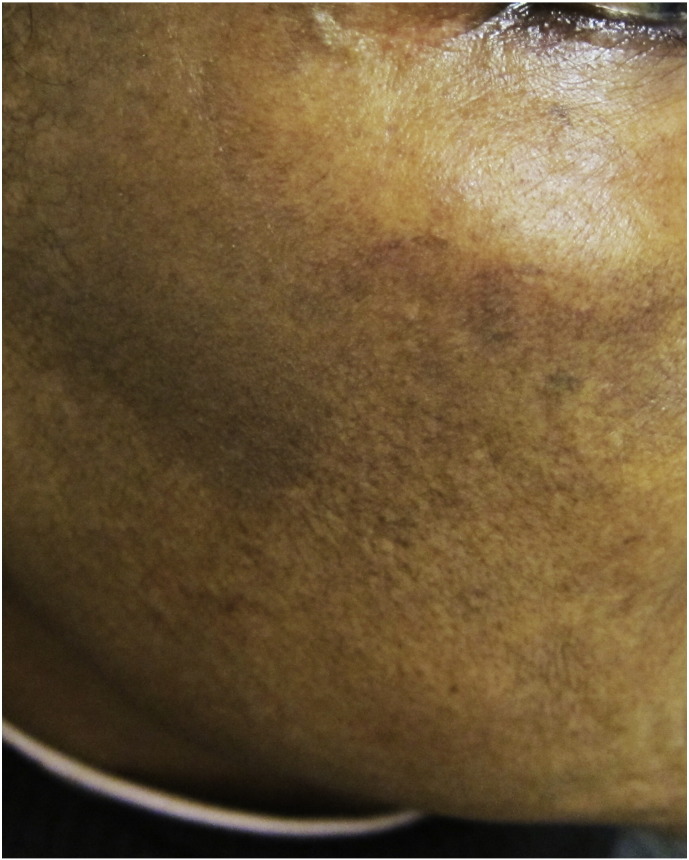
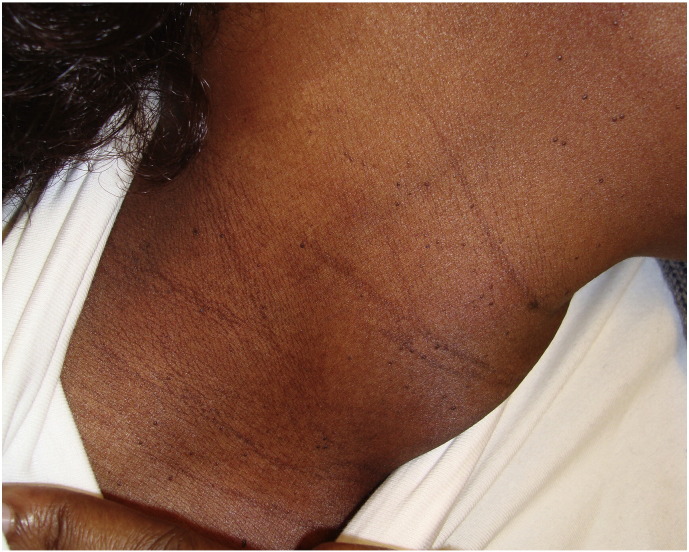
Pigmentary lines of demarcation (Fig. 1), which are known as Voigt or Futcher lines, are rare phenomena that are characterized by abrupt, linear boundaries between areas of lighter and darker skin. They tend to follow the distribution of the peripheral cutaneous nerves (Bieber et al., 2017, Ponnapula and Boberg, 2010, Rathore et al., 2011, Tyler, 2015). Melasma (Fig. 2), also called the mask of pregnancy, reportedly occurs in up to 70% of gravid women and typically in patients with darker skin (Bieber et al., 2017, Farage and Maibach, 2011, Farage et al., 2009, Fernandes and Amaral, 2015, Geraghty and Pomeranz, 2011, Rathore et al., 2011, Soutou and Aractingi, 2015, Turcic et al., 2009, Tyler, 2015, Van Onselen, 2012).
Fig. 1.
Pigmentary lines of demarcation
Fig. 2.
Melasma
Nevi
Historically, melanocytic nevi were thought to darken during pregnancy; however, studies have revealed that this phenomenon is uncommon during pregnancy and requires further research (Bieber et al., 2016). Although color changes are uncommon, nevi are likely to undergo some widening in diameter when they are located in areas that are affected by skin stretching and particularly the chest and abdomen (Akturk et al., 2007, Bieber et al., 2016, Goldberg and Maloney, 2013). Several reports describe the transient dermoscopic changes in nevi such as new dot formation, thickening of the pigment network, darkening of globules, and increasing numbers of vessels, which are not necessarily suggestive of melanoma (Akturk et al., 2007, Bieber et al., 2016, Goldberg and Maloney, 2013).
Histologic changes in nevi that are consistent with increased rates of mitotic activity have also been described. These include increased numbers of dermal mitoses and what have been termed “superficial micronodules of pregnancy,” which are rounded clusters of large epithelioid melanocytes with prominent nucleoli, abundant pale eosinophilic cytoplasm, and occasional fine melanosomes. The significance of these remains unclear because they are also found in patients who are not pregnant (Bieber et al., 2016, Chan et al., 2010). Although physiologic changes of nevi do occur, any changes in their clinical appearance that elicit concern for malignancy warrant an immediate biopsy, which can be performed safely with the use of lidocaine regardless of the location or trimester of pregnancy (Goldberg and Maloney, 2013).
Vasculature
Increased venous hydrostatic pressure can result in a nonpitting edema, which most commonly affects the lower extremities but involvement of the face and hands has also been described (Rathore et al., 2011, Soutou and Aractingi, 2015, Van Onselen, 2012). This benign edema can be relieved by bed rest, leg elevation, compression stockings, or sleeping in the left lateral decubitus position (Ponnapula and Boberg, 2010). Persistent edema, particularly of the face and hands, can be a sign of preeclampsia and warrants further examination.
Varicosities can arise throughout the body and most commonly involve the saphenous vein (Fernandes and Amaral, 2015, Ponnapula and Boberg, 2010, Rathore et al., 2011, Soutou and Aractingi, 2015, Tyler, 2015). The gravid uterus may compress the femoral and pelvic vessels, thereby increasing venous pressure and contributing to the development of varicose veins. Venous dilation typically returns to baseline in the postpartum period and is unrelated to the number pregnancies (Engelhorn et al., 2010).
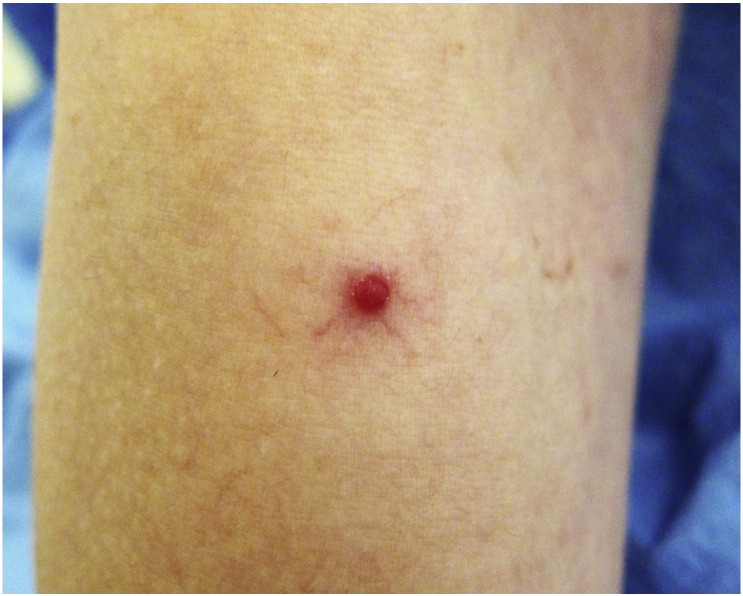
Elevated levels of estrogen during gestation can increase the number and appearance of telangiectasias including spider angiomas (Fig. 3) and unilateral nevoid telangiectasias (Fernandes and Amaral, 2015, Geraghty and Pomeranz, 2011, Ponnapula and Boberg, 2010, Rathore et al., 2011). These can be particularly noticeable in patients with lighter skin. They appear in areas that are drained by the superior vena cava, which include the face, neck, upper chest, and arms. Preexisting hemangiomas, subcutaneous hemangioendotheliomas, glomangiomas, petechiae, and purpura may worsen or new lesions may develop (Fernandes and Amaral, 2015, Geraghty and Pomeranz, 2011).
Fig. 3.
Spider angioma
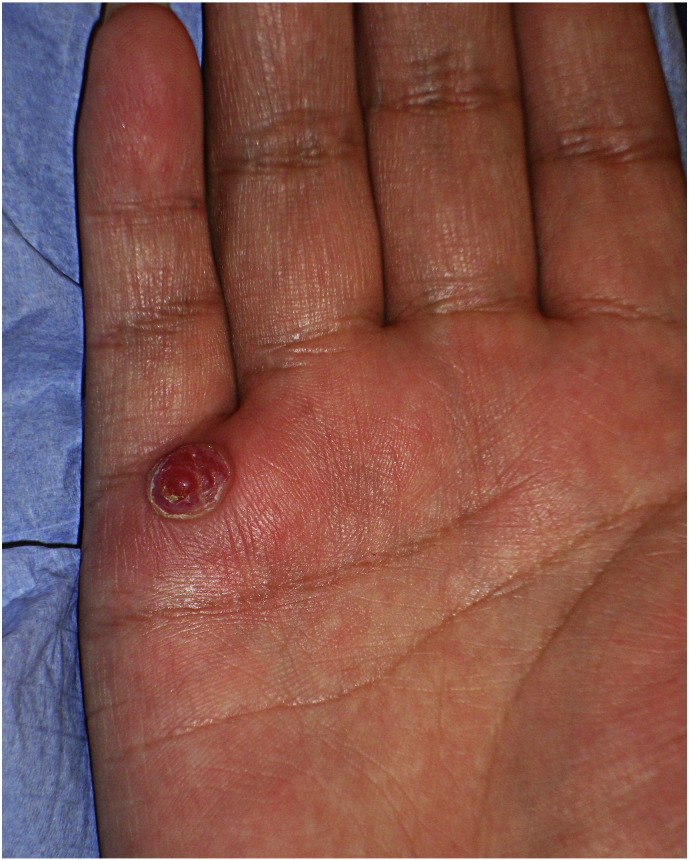
Pyogenic granuloma of pregnancy (Fig. 4), also termed granuloma gravidarum or pregnancy tumor, is a benign hyperplasia of capillaries that presents as a rapidly growing, lobulated or pedunculated lesion with a color that ranges from pink to red to purple. This painless lesion occurs in 0.5% to 5% of pregnant women and may bleed but usually regresses spontaneously after childbirth (Van Onselen, 2012, Ramos et al., 2016, Silva de Araujo Figueiredo et al., 2017, Tyler, 2015).
Fig. 4.
Pyogenic granuloma of pregnancy
Within the first trimester, palmar erythema can present as a diffusely mottled appearance of the palms (Geraghty and Pomeranz, 2011, Rathore et al., 2011, Soutou and Aractingi, 2015, Tyler, 2015, Van Onselen, 2012). Vasomotor instability can manifest as flushing, pallor, hot and cold sensations, and cutis marmorata (Geraghty and Pomeranz, 2011, Soutou and Aractingi, 2015, Turcic et al., 2009).
Mucosa
Although frequently discussed by our gynecologic and dental colleagues, mucosal changes are often excluded in the dermatologic literature. Within 4 to 8 weeks, the increased blood flow, vascularity, and edema produce a softening and bluish hue to the vagina and cervix, which is termed Goodell’s and Chadwick’s signs (Geraghty and Pomeranz, 2011, Soutou and Aractingi, 2015).
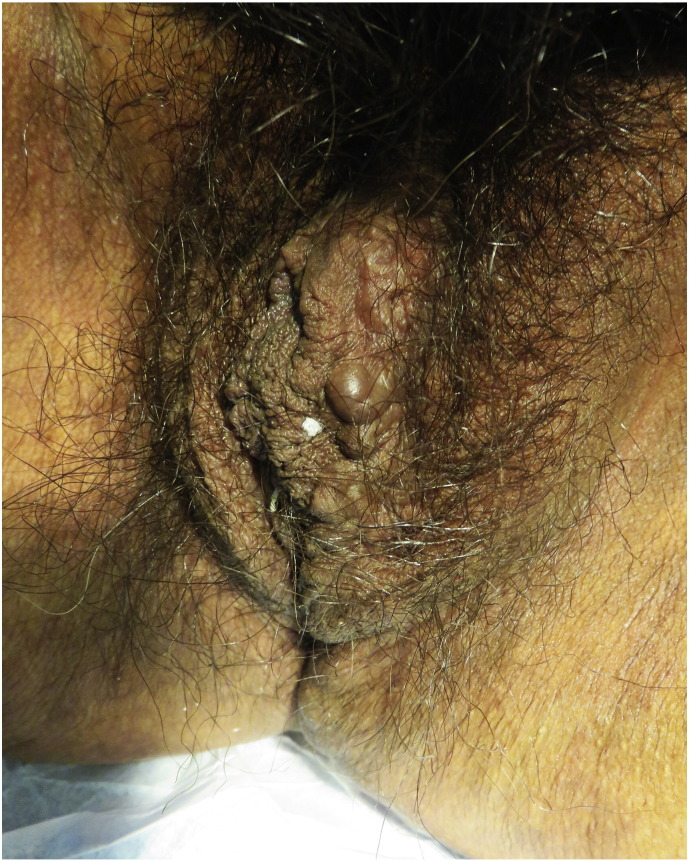
More likely to be observed by a dermatologist is the Jacquemier’s sign, which describes varicosities of the vagina and vulva (Fig. 5; Soutou and Aractingi, 2015). Hemangiomas are frequently reported to develop on the vulvovaginal mucosa (Farage and Maibach, 2011, Ramos et al., 2016, Soutou and Aractingi, 2015, Turcic et al., 2009). Constipation and prolonged straining during delivery can predispose gravid women to hemorrhoids (Soutou and Aractingi, 2015).
Fig. 5.
Vulvar varicosity
As early as the second month of gestation, hyperemia and edema of the gingival tissues may occur and result in gingivitis (Ramos et al., 2016, Rathore et al., 2011, Silva de Araujo Figueiredo et al., 2017). Frequently described in the dental literature, gingivitis manifests as increased probing depths, inflammation, bleeding, and sensitivity. Mucosal pyogenic granulomas of pregnancy can form on the lip and gingiva (Ramos et al., 2016, Silva de Araujo Figueiredo et al., 2017, Tyler, 2015, Van Onselen, 2012). Dry mouth from decreased salivary secretion or congestion from sinus and nasal hyperemia may also develop (Geraghty and Pomeranz, 2011).
Glands
Eccrine and sebaceous gland activity typically increases during pregnancy but apocrine gland activity decreases (Geraghty and Pomeranz, 2011, Soutou and Aractingi, 2015, Tyler, 2015). Increased eccrine gland activity occurs throughout the body except on the palms and may present clinically as hyperhidrosis and miliaria (Ponnapula and Boberg, 2010, Soutou and Aractingi, 2015, Tyler, 2015, Van Onselen, 2012). Heightened sebaceous gland activity promotes the enlargement of Montgomery tubercles, which are small papules on the areolas that provide lubrication to the nipples and areolas for breastfeeding (Geraghty and Pomeranz, 2011, Higgins et al., 2013, Stone and Wheeler, 2015, Tyler, 2015). The effects of enhanced sebaceous activity on acne vulgaris are variable with conflicting reports that describe both the improvement and worsening of acne (Bogdan et al., 2017, Tyler, 2015, Van Onselen, 2012). The combination of elevated sebum production and increased intravascular volume may represent a plausible mechanism for the pregnancy glow described by patients.
Connective tissue
Hormonal influences, genetics, and physical stretching of the skin can disrupt the dermal connective tissue and result in the development of stretch marks called striae distensae (Bogdan et al., 2017, Fernandes and Amaral, 2015, Geraghty and Pomeranz, 2011, Goldberg and Maloney, 2013, Rathore et al., 2011, Soutou and Aractingi, 2015, Turcic et al., 2009, Van Onselen, 2012). Striae distensae affects more than 63% of gravid women and are one of the most commonly described changes during pregnancy (Rathore et al., 2011). They appear in the sixth and seventh months of pregnancy as pink or purple atrophic bands along skin tension lines, typically on the breasts, abdomen, hips, buttocks, and thighs. Over time, the striae become paler and less noticeable but do not disappear entirely. The physical stretch of skin and mucosa during this time may also affect the scalp, abdominal, anal, or vulvar pruritus (Geraghty and Pomeranz, 2011).
Women may experience worsening of neurofibromas, keloids, leiomyomas, dermatofibromas, and cellulite also called gynoid lipodystrophy (Geraghty and Pomeranz, 2011). Skin tags (Fig. 6) that develop throughout the pregnancy are termed molluscum fibrosum gravidarum and may appear on the face, neck, chest, axillae, inframammary areas, inguinal folds, and medial thighs (Geraghty and Pomeranz, 2011, Soutou and Aractingi, 2015, Turcic et al., 2009, Tyler, 2015).
Fig. 6.
Skin tags
Hair
Most gravid women undergo some degree of hirsutism and/or hypertrichosis (Soutou and Aractingi, 2015, Tyler, 2015). Women with darker hair seem to have a greater degree of hirsutism with more pronounced hair growth on the upper lip, chin, and cheeks (Van Onselen, 2012). Hypertrichosis along the midline suprapubic area may also be noted (Geraghty and Pomeranz, 2011). The new, soft, fine hairs may disappear around 6 months after delivery but the coarse hair typically persists.
Hair may appear thicker throughout pregnancy and studies have confirmed an increased diameter of the hair shaft (Tosti et al., 2009). Hair thickening may also be the result of decreased shedding because some follicles remain in the anagen phase of the hair cycle longer than normal throughout pregnancy (Farage et al., 2009, Farage and Maibach, 2011, Gizlenti and Ekmekci, 2014, Rathore et al., 2011, Soutou and Aractingi, 2015, Tosti et al., 2009, Tyler, 2015). This is evidenced by the increased number of hairs in the anagen phase at the end of pregnancy compared with those hairs at the beginning of pregnancy (Gizlenti and Ekmekci, 2014).
The hormonal alterations that occur after delivery cause these hairs to transition into the telogen phase simultaneously. Subsequently, diffuse hair loss can occur several months to even a year after pregnancy (Farage et al., 2009, Gizlenti and Ekmekci, 2014, Tosti et al., 2009, Turcic et al., 2009, Tyler, 2015). This phenomenon, called telogen effluvium, can be distressing for the patient; however, hair regrows in 6 to 15 months, at which time the hair cycle returns to asynchrony (Gizlenti and Ekmekci, 2014, Tosti et al., 2009). Although frequently discussed in the literature, telogen effluvium is suggested to be an infrequent cause of hair shedding (Gizlenti and Ekmekci, 2014). Even less common is male pattern hair loss that occurs later during pregnancy whereby hair regrowth is unfortunately unpredictable in these cases (Tyler, 2015).
Nails
Studies report that nail alterations occur in 2% to 40% of gravid women (Erpolat et al., 2016, Rathore et al., 2011). The most commonly occurring changes are leukonychia, onychoschizia, and ingrown toenails but increased nail growth, onycholysis, melanonychia, and subungual keratosis are also described (Erpolat et al., 2016, Ponnapula and Boberg, 2010, Soutou and Aractingi, 2015, Turcic et al., 2009, Tyler, 2015, Van Onselen, 2012). Benign, uniform, symmetrical hyperpigmentation of the multiple nails is reported during pregnancy with fading that occurs postpartum (Bieber et al., 2017); however, irregular pigmentation with cuticle involvement should be referred to a dermatologist for evaluation of possible melanoma.
Breast
Breast development throughout pregnancy may result in enlargement, tenderness, increased prominence of veins, striae, areolar enlargement, erectile nipples, and/or nipple sensitivity (Higgins et al., 2013, Stone and Wheeler, 2015, Turcic et al., 2009). As previously discussed, the nipple and areola may undergo hyperpigmentation, development of a secondary areola, and enlargement of the Montgomery glands or tubercles (Higgins et al., 2013, Soutou and Aractingi, 2015). Pregnancy-associated hyperkeratosis of the nipple and areola may also occur and appear as bilateral, yellow-to-tan, hyperkeratotic and/or warty papules that usually involve the top of the nipple (Higgins et al., 2013). The lesion can be persistent postpartum, and treatment is challenging (Higgins et al., 2013).
Conclusion
The physiologic changes that occur during pregnancy are the result of hormonal and metabolic adaptations that are necessary to support the developing fetus. They also produce a variety of cutaneous findings, which are easily apparent to both patient and physician. Recognition of the normal cutaneous changes allows obstetricians and dermatologists to offer appropriate reassurance and prevent unnecessary anxiety and intervention. Treatment of these benign changes is predominantly cosmetic, but many of their treatments have not been studied during pregnancy or lactation.
Furthermore, these changes typically resolve postpartum. Thus, it may be appropriate to postpone nonurgent and cosmetic interventions until after delivery. Educating patients on the physiologic nature of these changes in the skin and related structures can often alleviate distress that patients may experience.
Footnotes
Conflicts of interest: None.
Funding sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Akturk A.S., Bilen N., Bayramgurler D., Demirsoy E.O., Erdogan S., Kiran R. Dermoscopy is a suitable method for the observation of the pregnancy-related changes in melanocytic nevi. J Eur Acad Dermatol Venereol. 2007;21:1086–1090. doi: 10.1111/j.1468-3083.2007.02204.x. [DOI] [PubMed] [Google Scholar]
- Bieber A.K., Martires K.J., Driscoll M.S., Grant-Kels J.M., Pomeranz M.K., Stein J.A. Nevi and pregnancy. J Am Acad Dermatol. 2016;75:661–666. doi: 10.1016/j.jaad.2016.01.060. [DOI] [PubMed] [Google Scholar]
- Bieber A.K., Martires K.J., Stein J.A., Grant-Kels J.M., Driscoll M.S., Pomeranz M.K. Pigmentation and pregnancy: Knowing what is normal. Obstet Gynecol. 2017;129:168–173. doi: 10.1097/AOG.0000000000001806. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Iurian S., Tomuta I., Moldovan M. Improvement of skin condition in striae distensae: Development, characterization and clinical efficacy of a cosmetic product containing Punica granatum seed oil and Croton lechleri resin extract. Drug Des Devel Ther. 2017;11:521–531. doi: 10.2147/DDDT.S128470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.P., Chan M.M., Tahan S.R. Melanocytic nevi in pregnancy: Histologic features and Ki-67 proliferation index. J Cutan Pathol. 2010;37:843–851. doi: 10.1111/j.1600-0560.2009.01491.x. [DOI] [PubMed] [Google Scholar]
- Engelhorn C.A., Cassou M.F., Engelhorn A.L., Salles-Cunha S.X. Does the number of pregnancies affect patterns of great saphenous vein reflux in women with varicose veins? Phlebology. 2010;25:190–195. doi: 10.1258/phleb.2009.009057. [DOI] [PubMed] [Google Scholar]
- Erpolat S., Eser A., Kaygusuz I., Balci H., Kosus A., Kosus N. Nail alterations during pregnancy: A clinical study. Int J Dermatol. 2016;55:1172–1175. doi: 10.1111/ijd.13316. [DOI] [PubMed] [Google Scholar]
- Farage M.A., Maibach H.I. Morphology and physiological changes of genital skin and mucosa. Curr Probl Dermatol. 2011;40:9–19. doi: 10.1159/000321042. [DOI] [PubMed] [Google Scholar]
- Farage M.A., Neill S., MacLean A.B. Physiological changes associated with the menstrual cycle: A review. Obstet Gynecol Surv. 2009;64:58–72. doi: 10.1097/OGX.0b013e3181932a37. [DOI] [PubMed] [Google Scholar]
- Fernandes L.B., Amaral W.N. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822–826. doi: 10.1590/abd1806-4841.20153570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty L.N., Pomeranz M.K. Physiologic changes and dermatoses of pregnancy. Int J Dermatol. 2011;50:771–782. doi: 10.1111/j.1365-4632.2010.04869.x. [DOI] [PubMed] [Google Scholar]
- Gizlenti S., Ekmekci T.R. The changes in the hair cycle during gestation and the post-partum period. J Eur Acad Dermatol Venereol. 2014;28:878–881. doi: 10.1111/jdv.12188. [DOI] [PubMed] [Google Scholar]
- Goldberg D., Maloney M. Dermatologic surgery and cosmetic procedures during pregnancy and the post-partum period. Dermatol Ther. 2013;26:321–330. doi: 10.1111/dth.12072. [DOI] [PubMed] [Google Scholar]
- Higgins H.W., Jenkins J., Horn T.D., Kroumpouzos G. Pregnancy-associated hyperkeratosis of the nipple: A report of 25 cases. JAMA Dermatol. 2013;149:722–726. doi: 10.1001/jamadermatol.2013.128. [DOI] [PubMed] [Google Scholar]
- Ponnapula P., Boberg J.S. Lower extremity changes experienced during pregnancy. J Foot Ankle Surg. 2010;49:452–458. doi: 10.1053/j.jfas.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Ramos E.S., Martins N.R., Kroumpouzos G. Oral and vulvovaginal changes in pregnancy. Clin Dermatol. 2016;34:353–358. doi: 10.1016/j.clindermatol.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Rathore S.P., Gupta S., Gupta V. Pattern and prevalence of physiological cutaneous changes in pregnancy: A study of 2000 antenatal women. Indian J Dermatol Venereol Leprol. 2011;77:402. doi: 10.4103/0378-6323.79741. [DOI] [PubMed] [Google Scholar]
- Silva de Araujo Figueiredo C., Goncalves Carvalho Rosalem C., Costa Cantanhede A.L., Abreu Fonseca Thomaz E.B., Fontoura Nogueira da Cruz M.C. Systemic alterations and their oral manifestations in pregnant women. J Obstet Gynecol Res. 2017;43:16–22. doi: 10.1111/jog.13150. [DOI] [PubMed] [Google Scholar]
- Soutou B., Aractingi S. Skin disease in pregnancy. Best practice & research. Clin Obstet Gynecol. 2015;29:732–740. doi: 10.1016/j.bpobgyn.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Stone K., Wheeler A. A review of anatomy, physiology, and benign pathology of the nipple. Ann Surg Oncol. 2015;22:3236–3240. doi: 10.1245/s10434-015-4760-4. [DOI] [PubMed] [Google Scholar]
- Tosti A., Piraccini B.M., Sisti A., Duque-Estrada B. Hair loss in women. Minerva Ginecol. 2009;61:445–452. [PubMed] [Google Scholar]
- Turcic P., Bukvic Mokos Z., Jurakic Toncic R., Blagaic V., Lipozencic J. Dermatologic medication in pregnancy. ADC. 2009;17:40–47. [PubMed] [Google Scholar]
- Tyler K.H. Physiological skin changes during pregnancy. Clin Obstet Gynecol. 2015;58:119–124. doi: 10.1097/GRF.0000000000000077. [DOI] [PubMed] [Google Scholar]
- Van Onselen J. Skin changes during pregnancy. Part 1. J Fam Health Care. 2012;22:28–30. [PubMed] [Google Scholar]