Abstract
Improved treatment outcomes for the endometrial cancer patient requires precision methods to investigate the biology of this disease and clinically relevant models to test treatment drugs. Hence, we applied a personalized platform to investigate whether in vitro and in vivo models could accurately predict effective treatment regimens. We successfully expanded ascites‐derived tumor cells from an endometrial cancer patient with malignant ascites using ascites collected prior to chemotherapy treatment. Hematoxylin–eosin and immunohistochemistry staining of ascites‐derived tumor cells confirmed the source of endometrial cancer cells. Ascites‐derived tumor cells were sensitive to cisplatin and doxorubicin single‐agent treatments in CCK‐8 assay and 3‐D culture, a condition that more closely mimics the in vivo environment. We further showed that ascites‐derived tumor cells from this patient could form tumors in NOD/SCID mice with preserved morphological characteristics. A remarkable concordance between the clinical response of cisplatin and the results of in vitro and in vivo drug tests reflected the reliability of our personalized approach in this case. Together, our results indicated that an effective platform for ex vivo and in vivo culture of ascites‐derived tumor cells from our endometrial cancer patient could be applied to identify treatment options, and may be commonly used in treating cancer patients with malignant ascites in the future.
Keywords: Ascites, drug sensitivity test, endometrial cancer, ex vivo culture, patient‐derived xenograft model
An estimated 64 400 new uterine cancer cases and 21 800 cancer‐associated deaths occurred in China in 2015.1 Uterine serous cancer, an aggressive subtype of endometrial cancer, accounts for almost 10% of all uterine carcinomas.2 Approximately one‐third of patients with endometrial cancer have peritoneal spread, which can lead to ascites in the advanced disease.3 Treatment strategies are limited and consist of surgical resection, adjuvant platinum‐based chemotherapy, and radiotherapy.2, 3, 4 Improved treatment outcomes for the endometrial cancer patient require precision methods to investigate the biology of this disease and clinically relevant models to test treatment drugs.1, 4, 5 Therefore, there is an urgent need to develop a feasible and reliable method to help predict the effective treatment response.
In this study, we describe the case of a 62‐year‐old woman with endometrial cancer and breast cancer. In May 2015, malignant ascites was detected and confirmed by abdominal cavity puncture. To test effective treatment regimens, we collected ascites‐derived tumor cells from the chemotherapy‐naive ascites to investigate whether in vitro and in vivo models could accurately predict the patient's treatment responses. Previous research6 reported that primary cultures of ascites‐derived tumor cells were better suited for experimental studies than established cancer cell lines due to irreversible changes of the biological properties. In addition, patient‐derived xenograft models (PDX) may be optimal for studying tumor heterogeneity and currently represent a powerful tool for assessing cancer‐associated mechanisms.6, 7 Therefore, we also developed PDX to test their response to preclinical drug treatment so that our patient was not exposed to drugs that they were not likely to respond to. Together, both ex vivo culture and a PDX model of ascites‐derived tumor cells were a reliable platform for identifying individual treatment options in our case.
Materials and Methods
Patient and sample collection
The study was undertaken at the Second Hospital of Dalian Medical University (Dalian, China). The study protocol was approved and documented by the Ethics Committee of the Second Hospital of Dalian Medical University. All the procedures were carried out in accordance with the Declaration of Helsinki.
A 62‐year‐old woman, who had undergone a diagnostic curettage due to menorrhagia, was diagnosed with moderate differentiation of endometrial adenocarcinoma with serous components. Subsequently, a laparoscopic hysterectomy with pelvic washing, bilateral adnexectomy, and pelvic lymphadenectomy was carried out. Postoperative pathology showed the residual cancer with infiltration more than one‐half of the myometrium. Unfortunately, after 1 month, the patient underwent a breast conservative surgery due to a left breast invasive ductal carcinoma (Fig. 1). From 2014 until May 2015, she was then treated with chemotherapy, radiotherapy, and adjuvant tamoxifen because the immunohistochemistry (IHC) analysis of the primary breast cancer revealed that it was estrogen receptor‐positive (>50%), progesterone receptor‐positive (>40%), and human epidermal growth factor receptor 2‐negative. Extended adjuvant tamoxifen therapy had to be terminated due to predominant ascites. Instead, the patient received i.p. carboplatin and achieved partial response as assessed by computed tomography scans using the RECIST criteria (1.1). In June 2016, a thyroid tumor was detected by ultrasound. In February 2017, metastases appeared in many areas of the body. Unfortunately, the patient died shortly thereafter.
Figure 1.
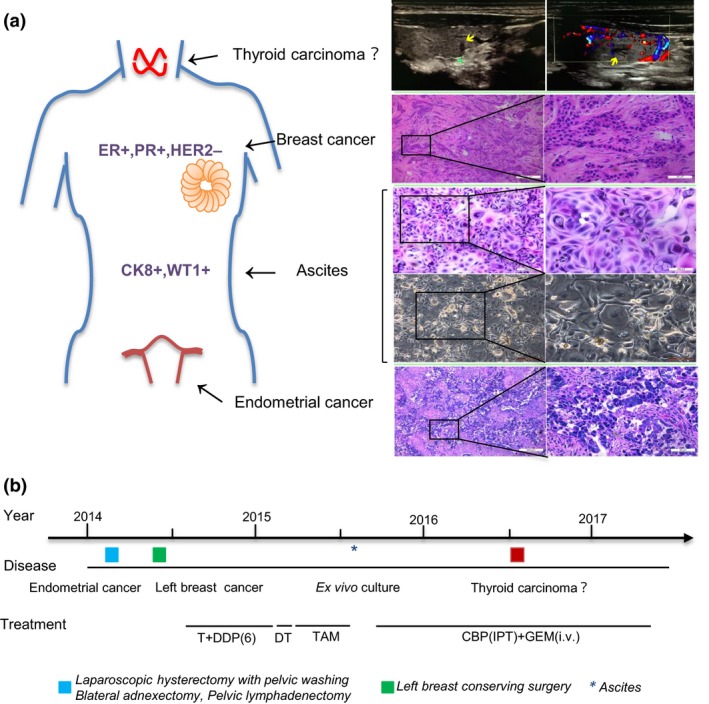
Tumor sites and treatment timelines in a 62‐year‐old woman with endometrial cancer and breast cancer. (a) Different tumor localizations for endometrial cancer, left breast cancer, malignant ascites, and clinical suspicion of thyroid carcinoma. Right panel, representative images of H&E staining and ultrasound (Yellow arrows mean tumor lesions). CK8, cytokeratin 8; ER, estrogen receptor; HER2, human epidermal growth factor receptor‐2; PR, progesterone receptor; WT1, Wilms’ tumor suppressor gene 1. (b) Treatment timeline. CBP, carboplatin; DDP(6), six cycles of cisplatin; DT, radiotherapy; GEM, gemcitabine; IPT, i.p. perfusion therapy; i.v., i.v. injection; T, paclitaxel; TAM, tamoxifen.
Ascites were obtained from this patient at the time of abdominal puncture. Ascites were collected in sterile tubes and centrifuged. Enriched cells were grown in a 100 mm2 dish only supplemented with ascites. To eliminate fibroblast contamination, the upper fluids were removed and placed in a new dish after 30 min.8, 9 Ten milliliters of ascites were added to 10 mL RPMI‐1640 medium (Gibco, Carlsbad, CA, USA) with 20% FBS, 20 mmol/L L‐glutamine, and 1% penicillin–streptomycin. These cells were incubated at 37°C in a 5% CO2‐containing atmosphere.8 All experiments were carried out on less than three‐passage cells and medium was replaced every 48 h.
Immunohistochemistry
Cells were seeded on coverslips in 12‐well plates and incubated in 1 mL medium for 48 h. Coverslips were washed with cold PBS and fixed with methanol. The tumor tissue samples were fixed in 4% paraformaldehyde (pH 7.0) before embedded in paraffin. The slides were incubated overnight with primary antibody cytokeratin 8 (CK8), Wilms’ tumor suppressor gene 1 (WT1), phosphatase and tensin homolog (PTEN), phosphorylated AKT (pAKT) (Ser473), pERK (Thr202/Tyr204), phosphorylated S6 ribosomal protein (pS6RP) (Ser235/236), Ki67 (Cell Signaling Technology, Danvers, MA, USA), and cancer antigen 125 (CA125) (Zhongshan Goldenbridge Biotechnology Company, Beijing, China). The DAB kit was purchased from Zhongshan Goldenbridge Biotechnology. All procedures were carried out according to the manufacturer's instructions.
Drug sensitivity assay
Cells were seeded into 96‐well plates and incubated for 48 h to allow optimal growth before exposure to the indicated drugs. Each drug was added at five different concentrations, and each concentration represented a twofold increase from the previous concentration. Cell viability was tested after 3 days of drug exposure. Signaling from the viable cells was assessed using the CCK‐8 assay (Dojindo Molecular Technologies, Tokyo, Japan).
Three‐dimensional sphere culture
Briefly, primary tumor cells were seeded on 96‐well plates coated with 100 μL Matrigel (BD Biosciences, Bedford, MA, USA). Cells were grown in RPMI‐1640 medium with 10% FBS, 20 mmol/L L‐glutamine, 1% penicillin–streptomycin, and 2% Matrigel, and allowed to attach for 3 days. Subsequently, these cells were incubated in medium containing the indicated drugs. The specific medium was replaced every 3 days.
Growth of ascites‐derived cancer cells in NOD/SCID mice
Tumor cells were centrifuged at 1000 g for 5 min. The cell precipitation was resuspended in 300 μL specific medium and mixed with 300 μL Matrigel (BD Biosciences) and kept on ice. Total 600 μL specific medium/Matrigel was injected s.c. into one flank of 9–12‐week‐old female NOD/SCID mice. The animals were monitored weekly, and tumor volume was calculated as tumor length × tumor width2 / 2. Tumor fragments were passaged into female NOD/SCID mice and the remaining tumor tissues were harvested for IHC analysis.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Statistical analysis
Two‐tailed Student's t‐test was used to compare between two groups. All experiments were repeated in triplicate. P‐value <0.05 was considered statistically significant.
Results
Establishing primary culture of ascites‐derived tumor cells
To evaluate the feasibility of primary culture of ascites‐derived tumor cells, we cultured these tumor cells from the chemotherapy‐naive ascites to investigate whether ascites‐derived tumor cells could proliferate in vitro. The ascites samples were collected from an endometrial cancer patient at the initial diagnosis of malignant ascites and before i.p. chemotherapy (Fig. 1). To minimize contamination with fibroblasts (unwanted cell types), the upper medium was removed and placed into a new dish after 30 min.8, 9 We successfully established ascites‐derived tumor cell cultures within 1 week. The common morphology of the ascites‐derived tumor cell primary cultures was a monolayer and characteristic of epithelial cells (Fig. 1a). Hematoxylin–eosin staining confirmed the source of endometrial cancer cells (Figs. 1a, 2). All experiments were undertaken during one to three passages. These cells gradually underwent senescence after six passages.
Figure 2.
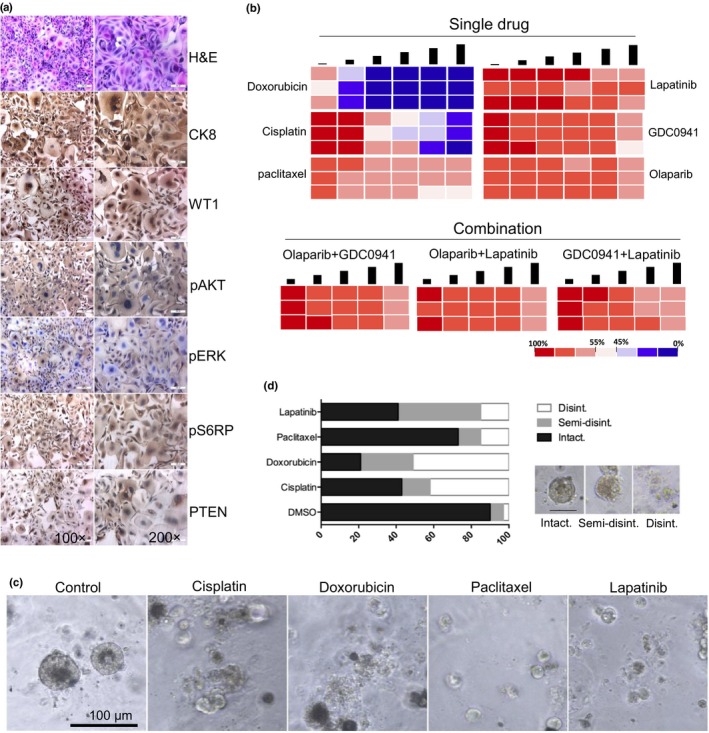
Representative images and drug sensitivity of cultured ascites‐derived tumor cells from a 62‐year‐old woman with endometrial cancer and breast cancer. (a) Representative images of H&E and immunohistochemistry staining. CK8, cytokeratin 8; pS6RP, phosphorylated S6 ribosomal protein; PTEN, phosphatase and tensin homolog; WT1, Wilms’ tumor suppressor gene 1. (b) Heat maps representing cell viability after treatment of selected anticancer drugs. Each concentration represents a twofold increase from the previous dose, with each concentration tested in triplicate. Conditions were optimized for highly repeatable testing of viability. (c) Ascites‐derived tumor cells were grown in 3‐D culture and treated with indicated drugs. Over 25 structures were scored for each drug. (d) Representative images of scored structures: intact, semi‐disintegrated, and disintegrated (Disint.).
Characteristics of ascites‐derived tumor cells
To further investigate the ascites‐derived tumor cells and confirm their origin of endometrial cancer, immunohistochemistry (IHC) staining was carried out on cells. The analysis of IHC clearly showed positive staining of epithelial marker CK8, WT1, and PTEN.
(Fig. 2a). Samples were positive to CK8 and WT1 excluding the mesothelial and fibroblastic origin of the cells. We also observed the similar IHC staining of primary endometrial cancer tissue obtained from the same patient (Fig. 3). Additional positive staining for pAKT, pERK, and pS6RP was also observed (Fig. 2a). Taken together, these experiments indicated the epithelial origin of the ascites‐derived tumor cells, and they showed the clinical characteristics of endometrial cancer.
Figure 3.
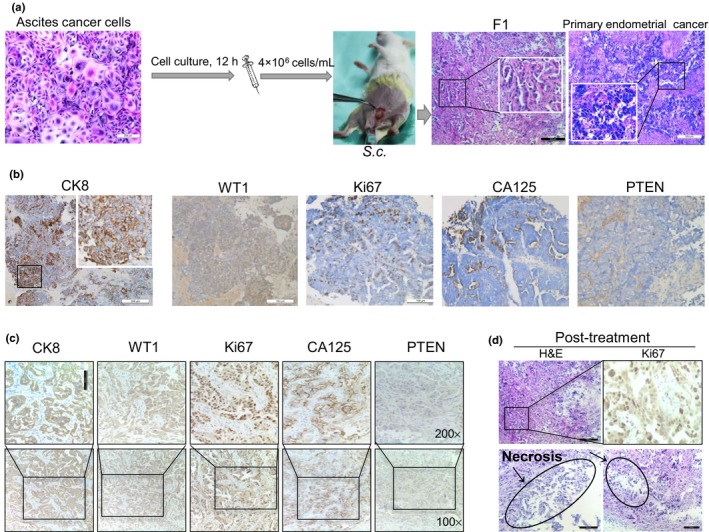
Ascites‐derived tumor cells are tumorigenic and patient‐derived xenografts mirror patient response to therapy in a 62‐year‐old woman with endometrial cancer and breast cancer. (a) Ascites‐derived tumor cells enriched from the patient were injected into NOD/SCID mice. Representative images of H&E staining are shown. (b,c) Representative images of immunohistochemistry staining in primary endometrial cancer (b) and the patient‐derived xenograft model (c). CA125, cancer antigen 125; CK8, cytokeratin 8; PTEN, phosphatase and tensin homolog; WT1, Wilms’ tumor suppressor gene 1. (d) Prominent central necrosis in the treated group and immunohistochemistry staining of anti‐Ki67.
Personalized therapeutic results of ascites‐derived tumor cells
We tested the sensitivity of cultured ascites‐derived tumor cells to several clinically used single drugs (cisplatin, paclitaxel, and doxorubicin) and drugs for targeted inhibition (PI3K inhibitor GDC0941 and lapatinib), according to positive staining for pAKT, pERK, and pS6RP. Our next candidate drug was the poly(ADP‐ribose) polymerase inhibitor olaparib that was recently approved by the FDA to treat platinum‐sensitive ovarian cancer with mutations in BRCA genes. Of all six drugs tested, paclitaxel and doxorubicin showed the best anticancer effect, while the other regimens just showed modest inhibitory effects on tumor cell viability tests (Fig. 2b). Subsequently, we also assessed drug response to cisplatin, paclitaxel, doxorubicin, and lapatinib in 3‐D culture, a condition that more closely mimics the in vivo environment. When cultured in 3‐D Matrigel, ascites‐derived tumor cells were still sensitive to cisplatin and doxorubicin single‐agent treatments (Fig. 2c,d). Drug sensitivity measurements were blinded to clinical history and patient treatment selections were not informed by drug sensitivity tests, but our drug sensitivity test results were concordant with clinical histories, namely sensitivity to cisplatin. Together, ex vivo culture of ascites‐derived tumor cells may serve as a predictive model for preclinical assessment of potential effective treatment regimens in our case.
Ascites‐derived tumor cells are tumorigenic
To exploit the tumorigenicity of ascites‐derived tumor cells, we injected 4 × 106 tumor cells into the mammary fat pad of immunosuppressed NOD/SCID female mice (Fig. 3a). The palpable tumors were detected within 2 months of implantation. We assessed the H&E and IHC of PDX in comparison to their corresponding clinical specimens and ascites‐derived tumor cells. We observed typical endometrial cancer morphology in both diagnostic specimens and PDX (Fig. 3a). This tumor expressed CK8, WT1, and PTEN in accordance with ascites‐derived tumor cells and primary endometrial cancer (Fig. 3b,c). We frequently observed mitotic cells (Ki67‐positive ~70%). Notably, IHC of PDX showed the secretory CA125 (Fig. 3c), which was consistent with laboratory tests that showed elevated levels of the serum and ascites CA125. Therefore, these data supported that the common origin of PDX and the parental tumor, and PDX represented the clinical endometrial cancer.
Response of PDX to cisplatin
To further assess the drug sensitivity of PDX to cisplatin, an in vivo chemosensitivity experiment was carried out by the s.c. transplantation of tumor fragments (F1) into NOD/SCID female mice. When tumors reached 100 mm3, they were randomized by sequential assignment to cisplatin and vehicle treatment groups. Tumor volume was monitored, blinded to treatment group. Through analysis of the growth curves, the tumor sizes of the treated group were found to be significantly smaller than those of the control group. We observed prominent central necrosis in the treated group and a lower level of cell proliferation (staining with Ki67) (Fig. 3d). These data implied the uniformity among in vitro and in vivo results and the clinical outcomes, and further reflected the reliability of our personalized approach in this patient.
Ex vivo culture of ascites‐derived tumor cells is a reliable platform
The availability of comprehensive ascites‐derived tumor cell phenotypes brings with it the challenge of identifying therapeutic targets that are likely to be beneficial to an individual patient. To explore this opportunity, we cultured ascites‐derived tumor cells as a platform for a personalized approach to predict potential effective treatment drugs (Fig. 4). In parallel, PDX aided research into tumor biology and pharmacology without long‐term manual manipulation of cell cultures in vitro (Fig. 4a,b,f). Patient‐derived xenograft models retained sufficient fidelity regarding histology, transcriptome, and genome.6, 7 These data validated that our reliable platform could serve as a preclinical model in this study, and may facilitate delivery of personalized medicine for endometrial cancer patients with malignant ascites in the future.
Figure 4.
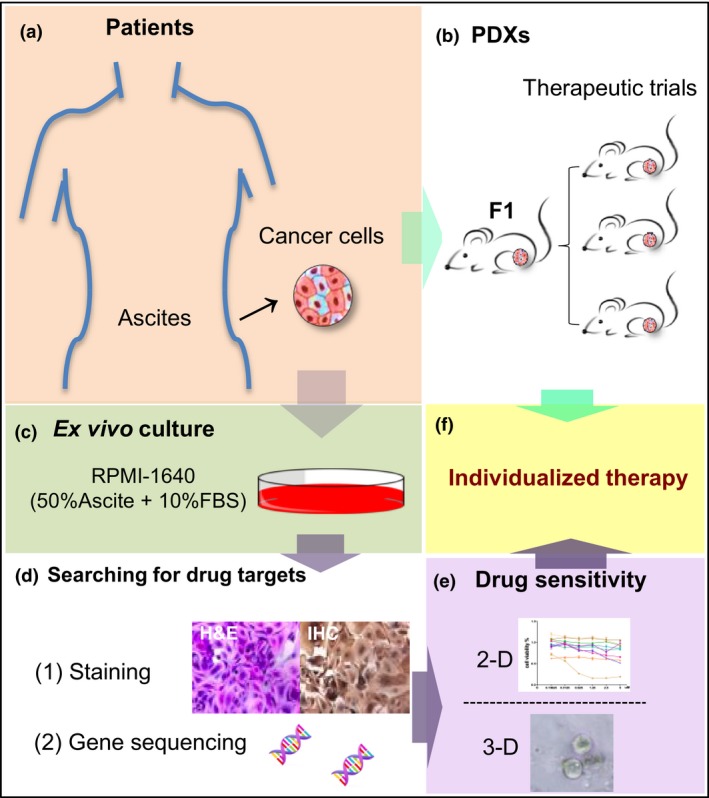
A reliable platform for cancer individualized medicine. The ex vivo culture of ascites‐derived tumor cells and in vivo models were developed for predicting potential effective treatment regimens. All methods ended in a recommendation for a specific therapy for a patient with malignant ascites on an individualized basis. Gene sequencing and immunohistochemistry (IHC) staining were part of a comprehensive approach to precise matching of novel therapies to patients. PDX, patient‐derived xenograft.
Discussion
Here we describe an effective platform for ex vivo and in vivo expansion of ascites‐derived tumor cells from an endometrial cancer patient with malignant ascites. We found that there was a remarkable concordance between the patient's response to cisplatin and the results of our drug sensitivity test. All these data supported that this platform could be applied to identify treatment options for our patient. To our knowledge, this is the first time that ascites‐derived tumor cells from a patient with endometrial cancer have been cultured in vitro and in vivo for drug sensitivity testing.
In 2006, Shepherd et al.8 refined the protocol of obtaining primary epithelial ovarian cancer cells from ascites fluid. The isolation of ascites‐derived tumor cells could be completed in 1 h, and primary tumor cells were further expanded in vitro for several weeks. Golan et al. successfully established ascites‐derived cell cultures of ascites fluid samples obtained from pancreatic ductal adenocarcinoma patients.9 Puiffe and colleagues10 addressed the ability of ovarian cancer‐derived ascites in terms of cell invasion, growth, and spheroid formation. Kar et al.11 reported that survivin siRNA increased the sensitivity of primary cultures of ovarian cancer cells shed in ascites to paclitaxel. In our case, although drug sensitivity measurements of ascites‐derived tumor cells were blinded to clinical history, our results were concordant with the patient's clinical responses. We believed that the ex vivo culture platform could help us to understand this lethal disease, and predict potential effective treatment regimens.
Prior to this study, the viability and tumor‐initiating capacity of ascites‐derived tumor cells was assumed but unknown. Here, we showed that ascites‐derived tumor cells from a patient with endometrial cancer could form tumors in NOD/SCID mice with preserved morphological characteristics. Patient‐derived xenografts are considered superior to traditional cell line xenografts as they may be more similar to parental tumors, particularly in terms of intertumor and intratumor heterogeneity.12, 13, 14, 15 Strikingly, our PDX faithfully recapitulated responses of donor patients to cisplatin. In this study, the ascites‐derived tumor cells were tumorigenic and the tumors they formed imitated donor patients’ tumor characteristics.
This platform had several disadvantages, including: (i) long‐term ex vivo culture in artificial conditions that did not accurately imitate complex biological conditions and may lose tumor heterogeneity;16, 17, 18 and (ii) treatment schedule delays and high costs may limit the clinical application of PDX. At least 3 months were required to develop PDX that may be used for preclinical study.6, 12 Our next aim is to identify the most appropriate conditions and methods to optimize the in vitro expansion efficiency and tumor‐formation rates in PDX. We firmly believe that this platform may be commonly used in treating cancer patients in the future.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We thank all patients enrolled in this study. We are indebted to the clinical teams who collected patient data and samples, and to Professor Pixu Liu, Professor Hailing Cheng, and Dr. Yuan Zhang for their guidance in the core technique. This work was supported by the National Natural Science Foundation of China (Nos. 81071127, 81471751, and 81673762 to Zuowei Zhao; No. 81650018 to Man Li), and the Provincial Natural Science Foundation of Liaoning (No. 2014023025 to Man Li).
Cancer Sci 108 (2017) 2352–2357
Funding Information
The National Natural Science Foundation of China, (Grant / Award Number: ‘No.81071127, NO.81471751, NO.81673762, No.81650018’) Provincial Natural Science Foundation of Liaoning, (Grant / Award Number: ‘No.2014023025’).
Contributor Information
Zuowei Zhao, Email: dmuzhaozuowei@163.com.
Man Li, Email: dmuliman@163.com.
References
- 1. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Genome Atlas Research . Kandoth C, Schultz N et al Integrated genomic characterization of endometrial carcinoma. Nature 2013; 497(7447): 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cherniack AD, Shen H, Walter V et al Integrated molecular characterization of Uterine carcinosarcoma. Cancer Cell 2017; 31: 411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Jonge MM, Mooyaart AL, Vreeswijk MP et al Linking uterine serous carcinoma to BRCA1/2‐associated cancer syndrome: a meta‐analysis and case report. Eur J Cancer 2017; 72: 215–25. [DOI] [PubMed] [Google Scholar]
- 5. Hodgkinson CL, Morrow CJ, Li Y et al Tumorigenicity and genetic profiling of circulating tumor cells in small‐cell lung cancer. Nat Med 2014; 20: 897–903. [DOI] [PubMed] [Google Scholar]
- 6. Byrne AT, Alférez DG, Amant F et al Interrogating open issues in cancer precision medicine with patient‐derived xenografts. Nat Rev Cancer 2017; 17: 254–68. [DOI] [PubMed] [Google Scholar]
- 7. Friedman AA, Letai A, Fisher DE, Flaherty KT. Precision medicine for cancer with next‐generation functional diagnostics. Nat Rev Cancer 2015; 15: 747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shepherd TG, Thériault BL, Campbell EJ, Nachtigal MW. Primary culture of ovarian surface epithelial cells and ascites‐derived ovarian cancer cells from patients. Nat Protoc 2006; 1: 2643–9. [DOI] [PubMed] [Google Scholar]
- 9. Golan T, Atias D, Barshack I et al Ascites‐derived pancreatic ductal adenocarcinoma primary cell cultures as a platform for personalised medicine. Br J Cancer 2014; 110: 2269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puiffe ML, Le Page C, Filali‐Mouhim A et al Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 2007; 9: 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kar R, Palanichamy JK, Banerjee A et al Survivin siRNA increases sensitivity of primary cultures of ovarian cancer cells to paclitaxel. Clin Transl Oncol 2015; 17: 737–42. [DOI] [PubMed] [Google Scholar]
- 12. Hidalgo M, Amant F, Biankin AV et al Patient‐derived xenograft models: an emerging platform for translational cancer research[J]. Cancer Discov 2014; 4: 998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khaled WT, Liu P. Cancer mouse models: Past, present and future. Semin Cell Dev Biol 2014; 27: 54‐60. [DOI] [PubMed] [Google Scholar]
- 14. Boone JD, Dobbin ZC, Straughn JM Jr, Buchsbaum DJ. Ovarian and cervical cancer patient derived xenografts: the past, present, and future. Gynecol Oncol 2015; 138: 486–91. [DOI] [PubMed] [Google Scholar]
- 15. Gołąb K, Grose R, Trzonkowski P et al Utilization of leukapheresis and CD4 positive selection in Treg isolation and the ex‐vivo expansion for a clinical application in transplantation and autoimmune disorders. Oncotarget 2016; 7: 79474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morrison G, Lenkala D, LaCroix B et al Utility of patient‐derived lymphoblastoid cell lines as an ex vivo capecitabine sensitivity prediction model for breast cancer patients. Oncotarget 2016; 7: 38359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kulasinghe A, Perry C, Warkiani ME et al Short term ex‐vivo expansion of circulating head and neck tumour cells. Oncotarget 2016; 7: 60101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mukhopadhyay A, Elattar A, Cerbinskaite A et al Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly (ADP‐ribose) polymerase inhibitors. Clin Cancer Res 2010; 16: 2344–51. [DOI] [PubMed] [Google Scholar]


