Abstract
Dog spontaneously develop prostate cancer (PC) like humans. Because most dogs with PC have a poor prognosis, they could be used as a translational model for advanced PC in humans. Stem cell‐derived 3‐D organoid culture could recapitulate organ structures and physiology. Using patient tissues, a human PC organoid culture system was established. Recent study has shown that urine cells also possess the characteristic of stem cells. However, urine cell‐derived PC organoids have never been produced. Therefore, we generated PC organoids using the dog urine samples. Urine organoids were successfully generated from each dog with PC. Each organoid showed cystic structures and resembled the epithelial structures of original tissues. Expression of an epithelial cell marker, E‐cadherin, and a myofibloblast marker, α‐SMA, was observed in the urine organoids. The organoids also expressed a basal cell marker, CK5, and a luminal cell marker, CK8. CD49f‐sorted basal cell organoids rapidly grew compared with CD24‐sorted luminal cell organoids. The population of CD44‐positive cells was the highest in both organoids and the original urine cells. Tumors were successfully formed with the injection of the organoids into immunodeficient mice. Treatment with a microtubule inhibitor, docetaxel, but not a cyclooxygenase inhibitor, piroxicam, and an mTOR inhibitor, rapamycin, decreased the cell viability of organoids. Treatment with a Hedgehog signal inhibitor, GANT61, increased the radiosensitivity in the organoids. These findings revealed that PC organoids using urine might become a useful tool for investigating the mechanisms of the pathogenesis and treatment of PC in dogs.
Keywords: Dog, organoid, prostate cancer, stem cell, urine
Prostate cancer (PC) is the most common cancer in men worldwide.1 Because dogs are known to spontaneously develop PC, like humans, and have some similarities in the pathogenesis of the disease,2 they may be a suitable translational model for advanced PC in humans.
Human PC mainly exhibits low grade and slow progression, which hardly leads to the cause of death.3 In contrast, dog PC possesses an aggressive nature and exhibits local invasion and widespread metastasis.4 Prostate‐specific antigen (PSA) protein in the blood is used for diagnosis of PC in humans.5 However, it is not available for the diagnosis of dog PC due to the lack of PSA produced from the prostate cells.6 The prevalence of dog PC is 0.2–0.6%7 and the survival time after definitive diagnosis is quite short (from weeks to months).8 Therefore, the mechanisms of the causes of dog PC remain unclear and an appropriate treatment protocol for dog PC has never been established.
3‐D organoid culture is derived from self‐renewing stem cells, which accurately recapitulate in vivo architecture, functions and genetic signatures. It also could be useful for cancer research and personalized therapy.9 Recently, prostate organoid culture systems were established from primary prostate and advanced PC tissues.10 In addition, recent studies demonstrated that urine cells could be used for the bladder repair.11 Urine cells possess the capacity of multipotent differentiation12 and express stem cell markers, such as CD44 and CD29, after culturing in the media.13 Nevertheless, organoid culture using urine cells from PC patients has never been conducted.
In the present study, we cultured the cells of urine samples from dogs with PC using the 3‐D organoid culture method. Then, we, for the first time, established the system of urine‐derived organoid culture and demonstrated that the organoids could be useful for the analysis of the cell components, structures, origins and tumorigenesis of dog PC as well as the application of chemotherapy and radiotherapy for dog PC.
Materials and Methods
Materials
To generate organoids, cells of urine samples were cultured with modified media as described previously.14, 15 The components were as follows: Advanced DMEM with 50% Wnt, Noggin and R‐Spondin conditioned medium; GlutaMax; B‐27 supplement; 100 μg/mL Primocin (Thermo Fisher Scientific, Waltham, MA, USA); 1 mM N ‐Acetyl‐ l ‐cysteine; 10 mM nicotinamide (Sigma‐Aldrich, St. Louis, MO, USA); 50 ng/mL mouse EGF (PeproTech, Rocky Hill, NJ, USA); 500 nM A83‐01 (Adooq Bioscience, Irvine, CA, USA); 3 μM SB202190; and 10 μM Y‐27632 (Cayman, Ann Arbor, MI, USA). Anti‐cancer drugs were as follows: rapamycin (Sigma‐Aldrich); piroxicam; docetaxel; GANT61 (Cayman). Antibody sources were as follows: E‐cadherin; glioma‐associated oncogene homolog (GLI)‐2 (R&D System, Minneapolis, MN, USA); CD45 (Abgent, San Diego, CA, USA); vimentin (Sigma‐Aldrich); α‐smooth muscle actin (SMA) (DAKO, Glostrup, Denmark); CK5; androgen receptor (AR); GLI‐1; PTCH1 (GeneTex Irvine, CA, USA); CD44 (Bethyl Laboratories, Montgomery, TX, USA); ki67 (DAKO); CK8 (Biolegend, San Diego, CA, USA); COX‐2 (Santa Cruz, Dallas, TX, USA); phosphorylation of Akt (Cell Signaling, Beverly, MA, USA); total Akt; β‐III tublin; p53 (Cell Signaling); and actin (Sigma‐Aldrich). Secondary antibodies were as follows: Alexa Fluor 488 goat anti‐mouse IgG; Alexa Fluor 488 goat anti‐rabbit IgG; Alexa Fluor 568 goat anti‐rabbit IgG; Alexa Fluor 488 donkey anti‐goat IgG (Thermo Fisher Scientific Inc); HRP‐conjugated anti‐rabbit IgG; HRP‐conjugated anti‐goat IgG (Cayman); and HRP‐conjugated anti‐mouse IgG (Millipore, Temecula, CA, USA). Fluorescence‐activated cell sorting (FACS) antibodies were as follows: FITC‐conjugated CD44 (1M7); allophycocyanin (APC)‐conjugated CD49f (G0H3) (Biolegend); FITC‐conjugated CD133 (13A4) (eBioscience, San Diego, CA, USA); and FITC‐conjugated CD24 (M1/69) (BD Bioscience, San Jose, CA, USA). Isotype control antibodies were as follows: APC‐conjugated anti‐rat IgG and FITC‐conjugated anti‐rat IgG (Biolegend).
Urine sample from prostate cancer dog
Urine samples were mainly obtained from dogs at the Department of Small Animal Clinical Science, Joint Faculty of Veterinary Medicine, Yamaguchi University Graduate School of Medicine (Yamaguchi, Japan). The rest of the samples were given by The Laboratory of Veterinary Radiology, Graduate School of Life and Environmental Sciences, Osaka Prefecture University (Osaka, Japan), the Laboratory of Veterinary Clinical Oncology, Faculty of Applied Biological Sciences, Gifu University (Gifu, Japan), the Laboratory of Veterinary Surgery, Graduate School of Agricultural and Life Sciences, The University of Tokyo (Tokyo, Japan) and the Laboratory of Small Animal Surgery 2 School of Veterinary Medicine, Kitasato University (Aomori, Japan). All dogs were diagnosed with prostate tumors. The urine samples were used for the organoids culture. Eight samples of urine samples from eight dogs were attempted to produce organoids (Table 1). Five organoids were successfully generated and expanded rapidly. Organoids from one sample grew so slowly that they were discarded. The rest of the samples were contaminated. Among them, we showed the data for four samples in this study. Written informed consent for this study was obtained from all the dog owners.
Table 1.
Sample information
| Case ID | Age (year old) | Breed | Sample Date | Stage | Prior Therapy | Organoid growth | Other information |
|---|---|---|---|---|---|---|---|
| PC16001 | 11 | Chihuahua | 09‐12‐2016 | T3N1M1 | Lansoprazole, Enrofloxacin, Firocoxib | Good | This sample was used for experiment. |
| PC16002 | 13 | Yorkshire Terrier | 16‐12‐2016 | T3N0M0 | Cephalexin, Robenacoxib | Contaminated | |
| PC17001 | 13 | Yorkshire Terrier | 11‐01‐2017 | T3N0M0 | Piroxicam | Good | This sample was used as D1. |
| PC17002 | 14 | Miniature Dachshund | 04‐03‐2017 | T3N1M0 | Piroxicam, Amoxicillin, Famotidine, Carbazochrome sodium sulfonate, Tranexamic acid | Good | This sample was used as D2. |
| PC17003 | 10 | Toy Poodle | 24‐03‐2017 | T3N2M1 | Firocoxib,Potassium Clavulanate | Good | This sample was used as D3. |
| PC17004 | 13 | Labrador Retriever | 17‐04‐2017 | T2N0M0 | Piroxicam | Bad | |
| PC17005 | 14 | Mix | 20‐04‐2017 | T3N1M0 | Piroxicam | Good | |
| PC17006 | 14 | Miniature Dachshund | 20‐04‐2017 | T2N0M0 | Antibiotics | Contaminated |
Modified from Owen LN. 1980. TNM Classification of Tumours in Domestic Animals. Geneva: World Health Organization. T0: no evidence of a primary tumor, T1: Superficial papillary tumor, T2: tumor invading the prostate wall, with induration, T3: tumor invading neighboring organs; N0: no regional lymph node involvement, N1: regional lymph node involved, N2: regional lymph node and juxtaregional lymph node involved; M0: no evidence of metastasis, M1: distant metastasis present.
Generation of urine sample organoids
The urine samples from dogs with PC were centrifuged at 600 g for 3 min. After the pellets were washed with cold HEPES buffered saline (HBS) and centrifuged at 600 g for 3 min, they were mixed with Matrigel (BD Bioscience) on ice and seeded on 24‐well plates. After solidifying the gel at 37°C for 30 min, the media was added and cultured. Organoids were passaged every 7–14 days by using a 5‐mM EDTA/HBS solution at 1:2–4 split.
Cell culture
Dog mammary tumor cells, CIP‐p and CIP‐m, and dog osteosarcoma cells, C‐HOS, were cultured in RPMI‐1640 supplemented with 10% FBS (Thermo Fisher Scientific) as described previously.16
H&E staining of organoids
After the organoids were fixed with 4% paraformaldehyde (PFA) at 4°C overnight, they were embedded in paraffin. After deparaffinization, 4 μm‐thick sections were stained with H&E as described previously.15, 17 The images were obtained using a light microscope (BX‐53; Olympus, Tokyo, Japan).
Immunofluorescence staining of organoids
Immunofluorescence staining of organoids was performed as described previously.18 After the organoids were fixed with 4% PFA for 1 h and dehydrated with 30% sucrose solution at 4°C overnight, they were embedded in OCT compound. The frozen sections were made and blocked with 1% BSA/PBS at room temperature for 1 h. They were then incubated with a primary antibody (E‐cadherin; 1:100, CD44; 1:100, AR; 1:100, vimentin; 1:200, α‐SMA; 1:200, CD45; 1:50, ki67; 1:100) at 4°C overnight. After incubation with a secondary antibody (1:500 or 1:1000) at room temperature for 1 h, they were observed with a confocal microscope (LSM 800; ZEISS, Copenhagen, Germany).
Immunohistochemical staining of organoids
Immunohistochemical staining of organoids was performed as described previously.18 After the deparaffinized sections were treated with 3% peroxidase for 15 min, they were blocked with 1% BSA/PBS at room temperature for 1 h. They were then incubated with primary antibodies (CK5; 1:100, CK8; 1:100; ki67; 1:100) at 4°C overnight. They were washed three times with PBS for 5 min. After incubation with secondary antibodies (1:500) at room temperature for 1 h, they were washed three times with PBS for 5 min. They were observed using a light microscope (BX‐53).
Flow cytometry
After the organoids were trypsinized for 15 min, 2 × 105 cells were collected into 96‐well plates. After the cells were washed with FACS buffer (2% FBS/PBS), they were stained with antibodies (CD24; 1:50, CD49f; 1:50, CD44; 1:100, CD133; 1:100) for 30 min. APC‐conjugated anti‐rat IgG and FITC‐conjugated anti‐rat IgG antibodies were used as isotype control. Cells were incubated with propidum iodide before flow cytometric analysis to exclude dead cells. The samples were analyzed using BD Accuri C6 (BD Biosciences) as described previously.16 The cell population data were analyzed using BD Accuri software (BD Bioscience) and the positive cells were determined based on each isotype control. Cell sorting was performed using Sony Cell Sorter (SH800, Sony, Tokyo, Japan) after staining the cells using the method as described above.
Aldehyde dehydrogenase activity assay
Aldehyde dehydrogenase (ALDH) activity was assessed by an ALDEFLUOR kit (STEM CELL Technologies, Vancouver, BC, Canada). After organoids were trypsinized for 15 min, 1 × 106 cells were collected into a 1.5‐mL tube. After centrifugation at 600 g for 3 min, the cells were resuspended in 1 mL of ALDEFLUOR assay buffer. Next, 5 μL of activated reagent was added to the tube and 0.5 mL of mixed cell solution was transferred to a negative control tube containing 5 μL of DEAB reagent. The rest is used as a test tube. Each tube was incubated for 30 min at 37°C. Following incubation, each tube was centrifuged at 250 g for 5 min and the supernatant was removed. The cell pellets were resuspended in 0.5 mL ALDEFLUOR Assay Buffer. The ALDH‐active cells were analyzed using BD Accuri C6 (BD Biosciences) and determined based on negative control. The data were analyzed using BD Accuri software.
Mouse xenograft assay
NOD.CB17 mouse Prkdc scid/J mice were obtained from Japan SLC (Shizuoka, Japan). All studies involving mice were conducted according to the guide to animal use and care of the Yamaguchi University and approved by the ethics committee. After the organoids were subcutaneously injected into the back of NOD/SCID mice (5–6 weeks old, 1 × 106 cells each) as described previously,16 the tumor volumes were observed. After the mice were euthanized by exsanguination, the tumor tissues were isolated and the tumor sizes were measured. The tissues were used for H&E, immunofluorescence and immunohistochemical staining.
Cell viability assay of organoids
Cell viability assay of organoids was performed as described previously.15 After the organoids were trypsinized for 15 min and filtered using a 70‐μm cell strainer (Falcon, Cary, NC, USA), 5 × 103 cells of urine‐derived organoids were seeded into 10 μL of Matrigel on a 96‐well culture plate and incubated for 24 h. Then, they were treated with piroxicam, rapamycin or docetaxel at varying concentrations for 4 days. Cell viability was examined by a cell counting using an alamarBlue kit (Thermo Fisher Scientific). The fluorescence (emission wavelength; 585 nm) was determined using a standard plate reader (Beckman Coulter, Irvine, CA, USA).
Western blotting
Western blotting was performed as described previously.16, 19 Protein lysates were obtained by homogenizing the cells with Triton‐based lysis buffer (50 mM Tris‐HCl [pH 8.0], 5 mM EDTA, 5 mM EGTA, 1% Triton X100, 1 mM Na3VO4, 20 mM sodium pyrophosphate and Roche Complete protease inhibitor mixture). Loading proteins (10–20 μg) were separated by SDS‐PAGE (10%) and transferred to a nitrocellulose membrane (Wako, Osaka, Japan). After blocking with 3% BSA or 0.5% skim milk, the membranes were incubated with primary antibodies (COX‐2; 1:500, P‐Akt; 1:500, total Akt; 1:200, total actin; 1:500, β‐III tublin; 1:200, p53; 1:200, PTCH1; 1:200, GLI‐1; 1:200, GLI‐2; 1:200) at 4°C overnight. The membranes were incubated with secondary antibodies (1:10,000 dilution, 1 h) and ECL Pro (PerkinElmer, Freiburg, Germany). The results were visualized using LAS‐3000 (Fujifilm, Tokyo, Japan) and quantified using ImageJ densitometry analysis software (National Institutes of Health, Bethesda, MD, USA).
Radiation exposure
After the organoids were trypsinized for 15 min and filtered using a 70‐μm cell strainer (Falcon), 5 × 103 cells of the tumor organoids were seeded into 10 μL of Matrigel on a 96‐well culture plate and incubated for 24 h. They were then treated with irradiation at 2–8 Gy using an X‐ray generating system (MX‐80 Labo; MediXtec Japan, Chiba, Japan). The X‐ray was delivered at a dose rate of 0.22 Gy/min (40 kVp and 0.06 mA). After 4 days, cell viability was examined by cell counting using alamarBlue kit (Thermo Fisher Scientific).
Statistical analysis
Data are shown as means ± SEM. Statistical evaluations were performed by using Student's t‐test between two groups. Values of P < 0.05 were considered statistically significant.
Results
Generation of urine‐derived organoids
Because most dogs with PC exhibit invasion and metastasis,4 we hypothesized that the cells from urine samples are useful for PC organoid culture. To prove this, we collected the urine samples from dogs with PC and generated urine‐derived organoids (Fig. 1a). After collecting and washing the cells with HBS solution without any digestion treatment, they were seeded into Matrigel and cultured in stemness‐stimulated media containing Wnt, EGF, Noggin and R‐spondin as reported previously.14 We observed that the cells in the urine sample from each dog gradually formed organoids at days 7–14. After passaging several times, the organoids continually increased over 28 days (Fig. 1b). We also observed that the structures of urine‐derived organoids resembled their original tumor epithelium (Fig. 1c). To identify the cell components of urine sample‐derived organoids, we performed immunofluorescence staining. Expression of an epithelial cell marker, E‐cadherin, was observed exclusively in the organoids (Fig. 1d). In contrast, expression of a cancer stem cell marker, CD44, was heterogeneously observed (Fig. 1e). Although AR signaling is essential for PC initiation and progression in humans, AR expression is downregulated in dog PC tissues.20 As expected, AR expression was faintly observed in the organoids (Fig. 1f). Interestingly, expression of a fibroblast cell marker, vimentin (Fig. 1g), and a myofibroblast marker, α‐SMA (Fig. 1h) was observed in the surroundings of organoids. Expression of a leukocyte marker, CD45 (Fig. 1i), and a proliferating cell marker, ki67, (Fig. 1j) was also observed in the organoids. These results suggest that urine‐derived organoids could recapitulate the tumor microenvironment of dog PC tissues.
Figure 1.
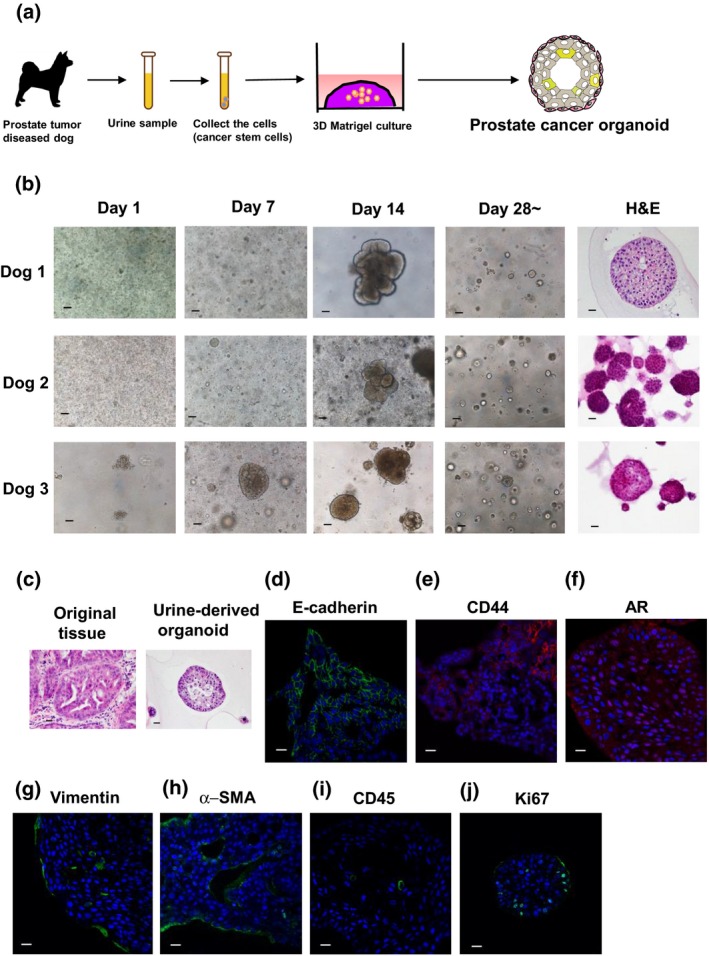
Generation of urine‐derived organoids. Schematic experimental design of a 3‐D culture system using urine sample‐derived prostate cancer organoids (a). The urine samples from prostate tumor‐diseased dogs were collected. After centrifuging and washing the pellets with HEPES buffered saline (HBS) several times, they were seeded in Matrigel for organoid formation. Pictures were taken at days 1, 7, 14 and over 28 days after seeding the cells. Representative images for phase contrast and H&E (culture day 14–21) of each dog‐derived organoid are shown (b). Scale bar: 200 μm (phase contrast), 50 μm (H&E). H&E stainings of an original tumor tissue and the urine‐derived organoids were shown (c). Culture day 21. Scale bar: 50 μm. Expression of an epithelial cell marker, E‐cadherin (d), a cancer stem cell marker, CD44 (e), a prostate cell marker, androgen receptor (AR) (f), a fibroblast cell marker, vimentin (g), a myofibroblast marker, α‐smooth muscle actin (SMA) (h), a leukocyte marker, CD45 (i) and a proliferating cell marker, ki67 (j). Representative photomicrographs were shown (n = 4). Culture day 21–30. Scale bar: 50 μm (d–j).
Cell origins of urine‐derived prostate cancer organoid
Prostate epithelial cells consist of basal, luminal and a few neuroendocrine cells. Basal cells are regarded as stem cell components and are located at the basement membrane; they are identified by expression of CK5. The luminal cells are the secretory components of the gland, which express CK8.21 To clarify which cells regulate tumor progression in dog PC, we performed the sorting of basal and luminal cells (Fig. 2a). We first observed that CK5 and CK8 were expressed in the urine‐derived organoids (Fig. 2b). In the flow cytometry experiments, we also checked the population of a basal cell marker, CD49f (36.1%). and a luminal cell marker, CD24 (45.5%). in the organoids (Fig. 2c). After isolating basal and luminal cells from the organoids by using CD49f and CD24 antibodies, the same number (2.5 × 104/well) of each cell was seeded. The CD49f‐sorted organoids grew rapidly, while the CD24‐sorted organoids hardly grew (Fig. 2d). The organoid‐forming efficiency of CD49f‐sorted organoids was significantly higher than that of CD24‐sorted organoids (Fig. 2e). The CD49f‐sorted organoids had solid sphere structures. In contrast, CD24‐sorted organoids had glandular structures (Fig. 2f). These results correspond with human prostate sorted cell‐derived organoids as reported previously22 and imply that basal cells mainly regulate tumor initiation and progression of dog PC.
Figure 2.
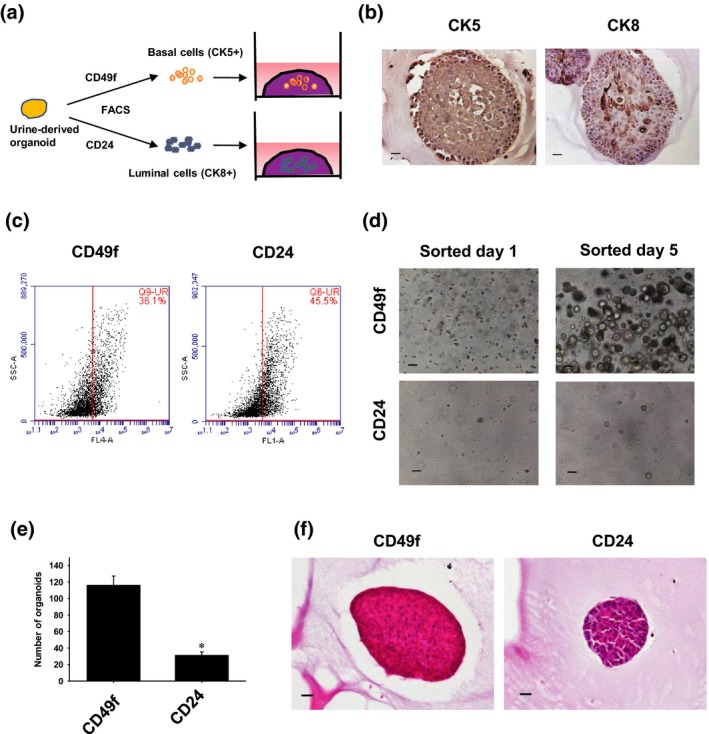
Cell origins of urine‐derived prostate cancer organoids. Schematic experimental design of an analysis of the cell components of the organoids (a). After the cultured urine‐derived organoids were tripsinized, they were sorted for basal and luminal cells by flow cytometry using specific cell markers, CD49f and CD24. The sorted cells were cultured and used for experiments. Expression of a basal cell marker, CK5, and a luminal cell marker, CK8, in the organoids (b). Representative photomicrographs are shown (n = 3). Scale bar: 50 μm. Expression of a basal cell marker, CD49f, and a luminal cell marker, CD24, in the organoids (c). After the organoids were tripsinized, they were treated with antibodies. The population of positive cells was analyzed by flow cytometry (n = 4). Representative images of flow cytometry are shown. Comparison of the organoid growth between basal and luminal cell organoids. After the organoids were sorted using CD49f and CD24 antibodies and seeded at 2.5 × 104 cells/well in the Matrigel, the organoid formation was observed (d). The number of organoids was counted (e, n = 6). *P < 0.05 versus CD49f organoid. Representative images for H&E staining of sorted organoids are shown (f). Culture day 14. Scale bar: 50 μm.
Population of cancer stem cell markers in the urine‐derived prostate cancer organoids
In the PC tissues, several cancer stem cell markers, such as CD44 and CD133, were expressed and regulate tumor progression.23 Active ALDH‐expressing prostate cancer cells are also associated with tumor formation ability.24 To clarify the populations of cancer stem cell markers in the organoids and the cells in the urine, we performed flow cytometry (Fig. 3a). The population of CD44, CD133‐positive and ALDH‐active cells in the organoids was significantly higher than that of the cells in urine (Fig. 3b–d). In the urine‐derived organoids, the population of CD44‐positive cells was the highest (Fig. 3b, 34.0 ± 2.6%) compared with CD133‐positive cells (Fig. 3c, 3.1 ± 0.5%) and ALDH‐active cells (Fig. 3d, 6.4 ± 0.8%). Similarly, CD44‐positive cells in the urine were the highest (9.6 ± 1.3%) compared with CD133‐positive cells (Fig. 3c, 3.1 ± 0.4%) and ALDH‐active cells (Fig. 3d, 0.5 ± 0.2%). In the normal urine, CD44 positive‐cells existed but were lower (Fig. 3e, 2.4 ± 0.5%) than those of dogs with PC. These findings imply that CD44 is essential for the generation of urine‐derived organoids.
Figure 3.
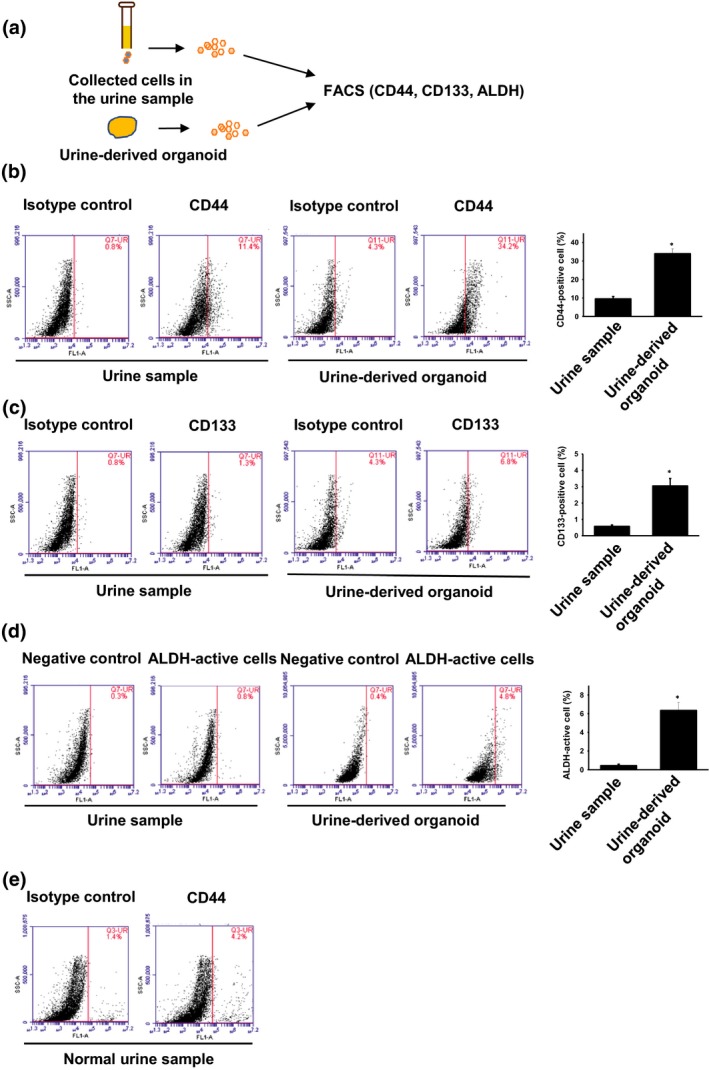
Population of cancer stem cell markers in the urine‐derived prostate cancer organoids (a). Schematic experimental design of a comparison of cancer stem cell markers (CD44, CD133 and aldehyde dehydrogenase [ALDH]) between the organoids and the original cells of urine samples (a). After the cultured urine‐derived organoids or collected cells in the urine samples were tripsinized, they were subject to flow cytometry. Expression of CD44 (b, n = 4) and CD133 (c, n = 4) in the organoids and the cells of urine samples were analyzed. Representative images of flow cytometry are shown. ALDH‐active cells were analyzed by using an ALDEFLUOR kit (d, n = 4). *P < 0.05 versus urine sample. Expression of CD44 (e, n = 3) in the cells of normal urine samples was analyzed.
Tumorigenesis by urine‐derived prostate cancer organoid
We next examined whether urine‐derived organoids form tumor in vivo (Fig. 4a). Within 8 weeks, tumors (>1 cm in diameter) were successfully formed following injection of the organoids into immunodeficient mice (Fig. 4b) but there was no metastasis. We also observed that both granule and solid‐like cell structures exist in the tumor tissues (Fig. 4c). This mixed tumor histology was also observed in a previous report on the injection of human PC organoids.22 To identify the cell components of the tumor tissues, we performed immunofluorescence and immunohistochemical staining. Expression of E‐cadherin was observed in the tumor tissues (Fig. 4d). Expression of α‐SMA was observed in the surroundings of granule‐like cells (Fig. 4e). CK5 (Fig. 4f), CK8 (Fig. 4g) and ki67 (Fig. 4h)‐positive cells were also observed in the granule‐like cells. In contrast, expression of AR and PSA was not observed in the tumor tissues (data not shown). These findings suggest that urine‐derived organoids reproduce tumor tissues in vivo, which exhibit similar features of original tumor tissues of dogs with PC.
Figure 4.
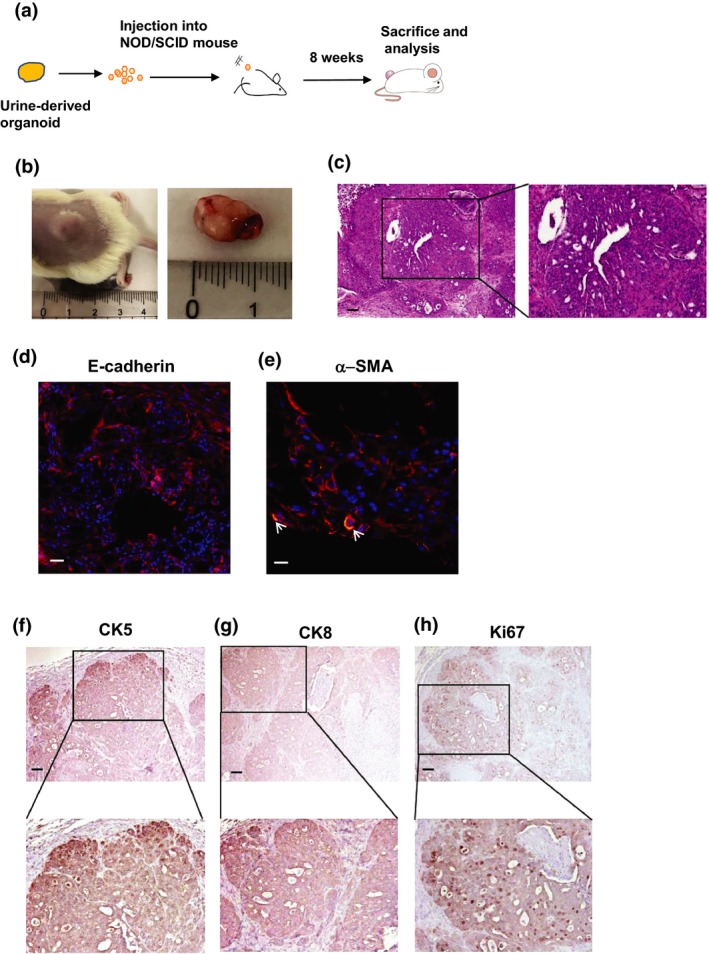
Tumorigenesis by urine‐derived prostate cancer organoids. Schematic experimental design of a xenograft experiment of urine‐derived organoids (a). The tripsinized cells were subcutaneously injected into the back of NOD/SCID mice (n = 6). After 8 weeks, the formed tumor was observed and removed for H&E, immunohistochemistry and immunofluorescence staining. Observation of organoid‐injected tumor formation (b). Representative images for H&E staining of the tumor tissues are shown (c). The enlarged image is shown on the right. Scale bar: 100 μm. Expression of E‐cadherin (d), α‐SMA (e), vimentin (f), CK5 (g), CK8 (h) and ki67 (i). Representative photomicrographs are shown (n = 4). Scale bar: 100 μm (c, d, f–h), 50 μm (e). Image of α‐SMA (green) was merged with E‐cadherin (red). Arrows show the representative positive cells (e). Each enlarged image is shown at the bottom (f–h).
Effects of anti‐cancer drugs on urine‐derived prostate cancer organoids
Because most dogs with PC are diagnosed at advanced stages and the tumors are highly metastatic, the prognosis is poor.25 Although most dogs were treated with COX inhibitors, which extended their survival period to some extent,25 remission was not achieved in any cases. To find an effective therapy for dogs with PC, we examined whether anti‐cancer drugs for human PC have effects on the cell viability of dog PC organoids. To examine the responsiveness of organoids to anti‐cancer drugs, we performed a 96‐well Matrigel cell viability assay as described previously (Fig. 5a).15 Treatment with a COX inhibitor, piroxicam, had no effect on cell viability of organoids in each dog culture (Fig. 5b,c). PTEN is a tumor suppressor gene, negatively controlling activation of PI3K/Akt signal, which regulates cell growth, survival and division.26 Inhibition of PTEN is also associated with progression and recurrence of PC.27, 28 Rapamycin inhibits mTOR activity, a signal downstream of PI3K/Akt, which leads to the inhibition of proliferation and survival in cancer cells.29 Docetaxel is a microtubule stabilizing taxane that prevents formation of mitotic spindles, which leads to mitotic arrest and cell death through activation of p53.30 In men with PC, treatment with docetaxel improves survival times.31 Although treatment with rapamycin decreased cell viability of organoids in each culture at the concentration of 0.01–0.1 μM in a dose‐dependent manner, high concentration of rapamycin (1, 10 μM) had no additional effects, and proliferating cells of organoids remained (Fig. 5b,c). In contrast, docetaxel decreased cell viability of organoids in a dose‐dependent manner (Fig. 5b,c). Interestingly, dog 1's (D1) organoids exhibited more resistance to docetaxel compared with D2 and D3 (Fig. 5c). To check the expression level of the target protein of anti‐cancer drugs, we performed western blotting (Fig. 5d,e). The expression level of COX‐2 was higher in the organoids compared with other dog cancer cells (mammary tumor cells, CIP‐p and CIP‐m, and osteosarcoma cells, C‐HOS) (Fig. 5d,f). In contrast, the phosphorylation level of Akt was lower than in other cells, which might cause the resistance to rapamycin (Fig. 5d). In D1 organoids, expression of β‐III tublin was higher than in D2 and D3 organoids. In contrast, expression of p53 was lower (Fig. 5e,f). Because increased β‐III tublin and decreased p53 are correlated with resistance to docetaxel,32, 33 D1 organoids might be resistant to docetaxel owing to upregulation of β‐III tublin and downregulation of p53 expression.
Figure 5.
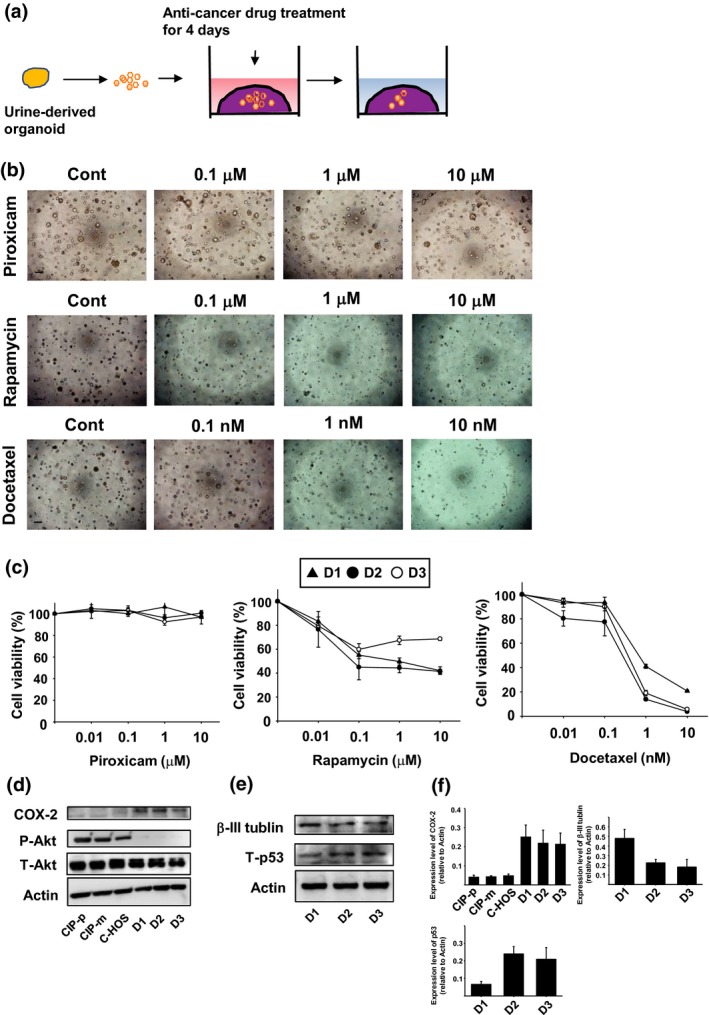
Effects of anti‐cancer drugs on urine‐derived prostate cancer organoids. Schematic experimental design of an anti‐cancer drug treatment for the organoids (a). After the organoids were seeded into Matrigel, they were treated with piroxicam (0.01–10 μM), rapamycin (0.01–10 μM) or docetaxel (0.01–10 nM) for 4 days (n = 6 each for three dogs [D1, D2, D3]). Representative phase contrast images of urine‐derived organoids treated with piroxicam, rapamycin or docetaxel are shown (b). Scale bar: 500 μm. Cell viability was determined using an alamarBlue assay and 100% represents cell viability of each control (c). Expression and activity of the target proteins for piroxicam and rapamycin in the organoids and dog cancer cell lines (mammary tumor cells, CIP‐p and CIP‐m, and osteosarcoma cells, C‐HOS) (d). After the organoids were seeded, COX‐2 expression or Akt phosphorylation was determined by western blotting (n = 3). Equal protein loading was confirmed using total Akt and actin antibody. Expression of the targets for docetaxel in the organoids (e). After the organoids were seeded, expression of β‐III tublin and p53 was determined by western blotting (n = 3). Equal protein loading was confirmed using total actin antibody. Expression level of COX‐2, β‐III tublin and p53 was analyzed using ImageJ software and quantified based on the ratio of expression level to total actin (f, n = 3).
Effects of irradiation on urine‐derived prostate cancer organoids
Radiotherapy has been attempted for dogs with PC. We therefore checked whether irradiation has effects on cell viability of the organoids. To examine the responsiveness of organoids to irradiation, we performed a 96‐well Matrigel cell viability assay after 4 days of irradiation (Fig. 6a). Treatment with irradiation decreased the cell viability of organoids in each culture in a dose‐dependent manner (Fig. 6b,c). The Hedgehog signal plays a significant role in the course of body development, which is also involved in PC development and resistance to therapy.34 Hedgehog signals are activated by the binding of Hedgehog ligands and the receptor, PTCH1. Activated Hedgehog signals allow GLI‐1 proteins to translocate to the nucleus and promote transcription of target genes, such as GLI‐2.34 To compare the expression level of these Hedgehog signal‐related proteins between these organoids, we performed western blotting (Fig. 6d). The expression level of PTCH1, GLI‐1 and GLI‐2 was lower in D1 organoids compared with D2 and D3 organoids. We therefore investigated the combination of a GLI‐1 inhibitor, GANT61, and irradiation in D3 organoids, expressing higher level of GLI‐1 expression. Treatment with GANT61 significantly increased radiosensitivity of the organoids at 2–6 Gray (Gy) (Fig. 6e). These results suggest that urine organoids could be useful for checking the radiosensitivity of PC in dogs. It it also shown that Hedgehog signals might mediate the resistance of dog PC to radiotherapy.
Figure 6.
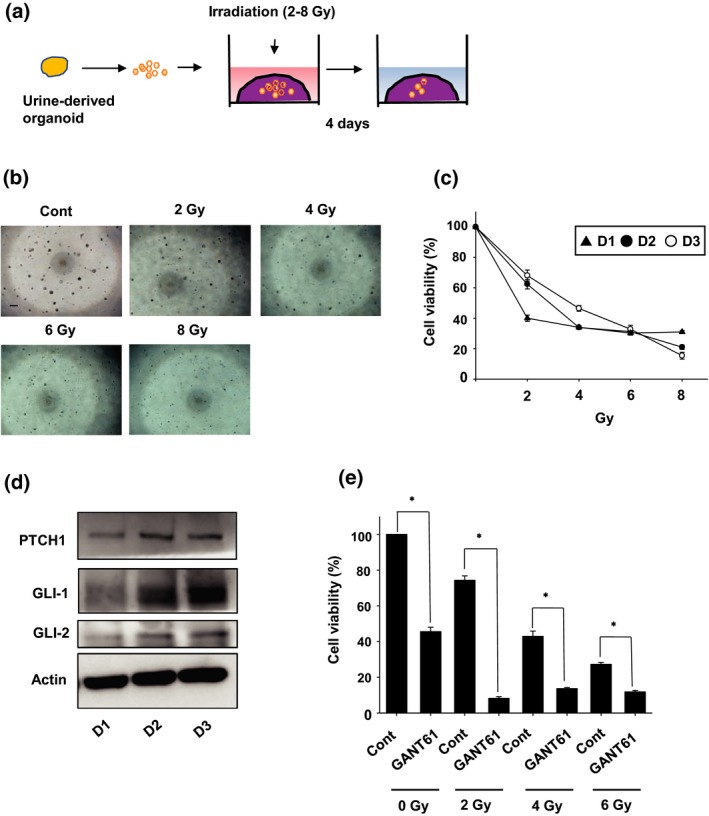
Effects of irradiation on urine‐derived prostate cancer organoids. Schematic experimental design of a radiation exposure for the organoids (a). After the organoids were seeded into Matrigel, they were exposed with radiation (2–8 Gray (Gy)) (n = 6 each for three dogs (D1, D2, D3)). Representative phase contrast images of the organoids after 4 days of irradiation are shown (b). Scale bar: 500 μm. Cell viability was determined using an alamarBlue assay and 100% represents cell viability of each control (c). Expression level of Hedgehog signal related‐proteins in the organoids. After the organoids were seeded, expression of PTCH1, GLI‐1 and GLI‐2 was determined by western blotting (d, n = 3). Equal protein loading was confirmed using total actin antibody. Effects of a Hedgehog signal inhibitor, GANT61, on the radiosensitivity of D3 organoids (e). After D3 organoids were seeded into Matrigel, they were exposed with radiation (2–6 Gy) in the presence or absence of GANT61 (10 μM) for 4 days (n = 6). After 4 days of irradiation, cell viability was determined using an alamarBlue assay and 100% represents cell viability of control. P < 0.05 versus control at each irradiation.
Discussion
In the present study, we, for the first time, established dog PC organoids by using a urine sample from PC diseased dogs. The major findings of the present study are as follows: (i) urine‐derived organoids consist of epithelial cells, myofibloblasts, cancer stem cells and a few leukocytes (Fig. 1); (ii) basal cells in the organoids play a significant role in organoid formation and growth (Fig. 2); (iii) the population of CD44‐positive cells in the organoids is the highest compared with CD133‐positive cells and ALDH‐active cells (Fig. 3); (iv) injection of the organoids into NOD/SCID mice was successful in forming tumors (Fig. 4); (v) Treatment with docetaxel but not piroxicam and rapamycin decreased the cell viability of organoids (Fig. 5); and (vi) treatment with GANT61 increased the sensitivity of radiation in the organoids (Fig. 6). Collectively, our results indicate that dog PC organoids might help in investigating the mechanisms of tumor growth and also provide new insights for dog PC treatment.
The cell origins of PC are still debated. In human PC tissues, luminal phenotype characterized by atypical glands and an absence of basal cells was observed,35 suggesting that prostate cancer originates from luminal cells. In PC mouse models, it was shown that both basal and luminal cells serve as original cells for PC.36, 37 In contrast, it is reported that only basal cells have the capacity for transformation in human PC.38, 39 In the present study, we showed that the basal cell‐sorted organoids grow rapidly compared with luminal cell‐sorted organoids (Fig. 2). A recent study also demonstrated that xenograft experiments using human c‐Myc/Akt‐expressing basal cell organoids showed more aggressive histology than luminal cell organoids.22 This finding corresponds with the data in the present study. We therefore suppose that basal cells might be the origin and mainly mediate the progression of PC in dogs.
CD44 is a transmembrane glycoprotein that plays a role in cell adhesion.40 Its expression correlates with cancer metastasis and poor prognosis.41, 42 Several studies show that the population of CD44‐positive cells of PC cells is associated with tumor‐initiating cells.41, 43 Liu et al. (2011) show that CD44 is negatively regulated by microRNA (miR)34a in PC.44 They also show that overexpression of miR34a inhibits PC metastasis through the decrease in the expression of CD44. In the present study, we showed that the population of CD44 is the highest among stem cell markers in both urine‐derived organoids and the original cells in urine samples (Fig. 3b–d). Interestingly, the population of each stem cell marker‐positive cell in the organoids was higher than that of cells in urine samples (Fig. 3b–d). It was also shown that the population of CD44 in the PC dog‐derived urine cells was higher than in normal cells (Fig. 3b,e). These results suggest that CD44 expression might mainly regulate the initiation and progression of dog PC organoids. In addition, miR34a might become novel therapeutic targets for dog PC metastasis.
In human PC patients, androgen deprivation is used as the primary therapy.45 However, hormonal therapy is not likely to be effective in most dogs because androgen plays a relatively minor role in PC in dogs.8, 46 In the present study, urine‐derived organoids hardly expressed AR (Fig. 1f), which corresponds with a previous report on AR expression in dog PC tissues.20 Because the expression level of COX‐2 was elevated in dog PC tissues,25 the dogs were usually treated with COX inhibitors, such as piroxicam or carprofen. While COX inhibitors extended the survival periods to some extent,25 the direct effects on PC cells remain unknown. In the present study, we demonstrated that piroxicam had no effects on the cell viability of dog PC organoids (Fig. 5b,c). In contrast, docetaxel decreased cell viability in a dose‐dependent manner (Fig. 5b,c). Docetaxel inhibits depolymerization of tubulin through binding, which ultimately leads to apoptosis via activation of p53.33 Prostate tumor cells bearing wild‐type p53 are more sensitive to docetaxel treatment compared to those with mutant or null p53.33 In the present study, p53 expression was correlated with the sensitivity of docetaxel (Fig. 5e). We therefore speculate that docetaxel treatment might be more effective for the dogs with PC bearing wild‐type p53.
In the present study, we investigated the radiosensitivity of urine‐derived PC organoids. The cell viability was decreased in a dose‐dependent manner (Fig. 6b). In a previous study, the cell viability of human PC cell lines was decreased by 2–6 Gy irradiation for 4 days.47 We therefore suppose that the radiosensitivity of dog PC organoids was almost the same level as them. Hedgehog signals are involved in PC development, progression and resistance to therapy.34, 48 Previous studies showed that Hedgehog signals are involved in radiation resistance of hepatocellular and pancreatic cancer cell lines.49, 50 It is also reported that a GLI‐1 inhibitor, GANT61, improved radiosensitivity in a human PC cell line, 22Rv1 cells.47 Although radiotherapy was conducted in dogs with PC, the combination effects of GANT61 on dogs with PC were still unclear. We therefore investigated whether the combination of GANT61 and irradiation has effects on the cell viability of dog PC organoids. As expected, GANT61 increased the radiosensitivity in Hedgehog signal‐related proteins expressing organoids (Fig. 6d,e). Because treatment with GANT61 at 2 Gy was more effective than at 4 and 6 Gy, we speculate that high exposure of radiation affects activity of Hedgehog signals, which might lead to the low sensitivity to GANT61. Further investigations using urine‐derived dog PC organoids might help to explore a promising therapeutic strategy for enhancing the radiation response of dogs with PC.
In conclusion, for the first time, dog PC organoids were produced using urine samples. The organoids recapitulated the tumor microenvironment and showed tumorigenesis in vivo. It was also suggested that basal cells of the organoids and CD44‐positive cells mediate organoid formation and growth. Further studies on the urine sample‐derived organoid culture system could contribute to the treatment of PC in dogs.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
We thank Dr Kuo and Dr Salahudeen for kindly providing Wnt, Noggin and R‐spondin‐producing cells. This study was supported in part by a Grant‐in‐Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS; grant number 17K15370).
Cancer Sci 108 (2017) 2383–2392
Funding Information
Grant‐in‐Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (17K15370).
References
- 1. Siegel RL, Sahar L, Portier KM, Ward EM, Jemal A. Cancer death rates in US congressional districts. CA Cancer J Clin 2015; 65: 339–44. [DOI] [PubMed] [Google Scholar]
- 2. Palmieri C, Lean FZ, Akter SH, Romussi S, Grieco V. A retrospective analysis of 111 canine prostatic samples: histopathological findings and classification. Res Vet Sci 2014; 97: 568–73. [DOI] [PubMed] [Google Scholar]
- 3. Bullock TL, Andriole GL Jr. Emerging drug therapies for benign prostatic hyperplasia. Expert Opin Emerg Drugs 2006; 11: 111–23. [DOI] [PubMed] [Google Scholar]
- 4. Waters DJ, Bostwick DG. Prostatic intraepithelial neoplasia occurs spontaneously in the canine prostate. J Urol 1997; 157: 713–6. [PubMed] [Google Scholar]
- 5. Carlsson SV, Roobol MJ. Improving the evaluation and diagnosis of clinically significant prostate cancer in 2017. Curr Opin Urol 2017; 27: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leroy BE, Northrup N. Prostate cancer in dogs: comparative and clinical aspects. Vet J 2009; 180: 149–62. [DOI] [PubMed] [Google Scholar]
- 7. Bell FW, Klausner JS, Hayden DW, Feeney DA, Johnston SD. Clinical and pathologic features of prostatic adenocarcinoma in sexually intact and castrated dogs: 31 cases (1970–1987). J Am Vet Med Assoc 1991; 199: 1623–30. [PubMed] [Google Scholar]
- 8. Cornell KK, Bostwick DG, Cooley DM et al Clinical and pathologic aspects of spontaneous canine prostate carcinoma: a retrospective analysis of 76 cases. Prostate 2000; 45: 173–83. [DOI] [PubMed] [Google Scholar]
- 9. Bartfeld S, Clevers H. Stem cell‐derived organoids and their application for medical research and patient treatment. J Mol Med 2017; 95: 729–38. [DOI] [PubMed] [Google Scholar]
- 10. Karthaus WR, Iaquinta PJ, Drost J et al Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 2014; 159: 163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qin D, Long T, Deng J, Zhang Y. Urine‐derived stem cells for potential use in bladder repair. Stem Cell Res Ther 2014; 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, McNeill E, Tian H et al Urine derived cells are a potential source for urological tissue reconstruction. J Urol 2008; 180: 2226–33. [DOI] [PubMed] [Google Scholar]
- 13. Bharadwaj S, Liu G, Shi Y et al Multipotential differentiation of human urine‐derived stem cells: potential for therapeutic applications in urology. Stem Cells 2013; 31: 1840–56. [DOI] [PubMed] [Google Scholar]
- 14. Drost J, Karthaus WR, Gao D et al Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc 2016; 11: 347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Usui T, Sakurai M, Enjoji S et al Establishment of a novel model for anticancer drug resistance in three‐dimensional primary culture of tumor microenvironment. Stem Cells Int 2016; 2016: 7053872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kake S, Usui T, Ohama T, Yamawaki H, Sato K. Death‐associated protein kinase 3 controls the tumor progression of A549 cells through ERK MAPK/c‐Myc signaling. Oncol Rep 2017; 37: 1100–6. [DOI] [PubMed] [Google Scholar]
- 17. Usui T, Okada M, Hara Y, Yamawaki H. Death‐associated protein kinase 3 mediates vascular inflammation and development of hypertension in spontaneously hypertensive rats. Hypertension 2012; 60: 1031–9. [DOI] [PubMed] [Google Scholar]
- 18. Usui T, Sakurai M, Kawasaki H, Ohama T, Yamawaki H, Sato K. Establishment of a novel three‐dimensional primary culture model for hippocampal neurogenesis. Physiol Rep 2017; 5: pii: e13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujiwara N, Usui T, Ohama T, Sato K. Regulation of Beclin 1 protein phosphorylation and autophagy by protein phosphatase 2A (PP2A) and death‐associated protein kinase 3 (DAPK3). J Biol Chem 2016; 291: 10858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rivera‐Calderon LG, Fonseca‐Alves CE, Kobayashi PE et al Alterations in PTEN, MDM2, TP53 and AR protein and gene expression are associated with canine prostate carcinogenesis. Res Vet Sci 2016; 106: 56–61. [DOI] [PubMed] [Google Scholar]
- 21. Packer JR, Maitland NJ. The molecular and cellular origin of human prostate cancer. Biochem Biophys Acta 2016; 1863: 1238–60. [DOI] [PubMed] [Google Scholar]
- 22. Park JW, Lee JK, Phillips JW et al Prostate epithelial cell of origin determines cancer differentiation state in an organoid transformation assay. Proc Nat Acad Sci USA 2016; 113: 4482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin X, Farooqi AA, Qureshi MZ, Romero MA, Tabassum S, Ismail M. Prostate cancer stem cells: viewing signaling cascades at a finer resolution. Arch Immunol Ther Exp (Warsz) 2016; 64: 217–23. [DOI] [PubMed] [Google Scholar]
- 24. van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Pelger RC, van der Pluijm G. The aldehyde dehydrogenase enzyme 7A1 is functionally involved in prostate cancer bone metastasis. Clin Exp Metas 2011; 28: 615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sorenmo KU, Goldschmidt MH, Shofer FS, Goldkamp C, Ferracone J. Evaluation of cyclooxygenase‐1 and cyclooxygenase‐2 expression and the effect of cyclooxygenase inhibitors in canine prostatic carcinoma. Vet Comp Oncol 2004; 2: 13–23. [DOI] [PubMed] [Google Scholar]
- 26. Milella M, Falcone I, Conciatori F et al PTEN: multiple functions in human malignant tumors. Front Oncol 2015; 5: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bertram J, Peacock JW, Fazli L et al Loss of PTEN is associated with progression to androgen independence. Prostate 2006; 66: 895–902. [DOI] [PubMed] [Google Scholar]
- 28. Yoshimoto M, Cunha IW, Coudry RA et al FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer 2007; 97: 678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Nat Acad Sci USA 2001; 98: 7037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morse DL, Gray H, Payne CM, Gillies RJ. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol Cancer Ther 2005; 4: 1495–504. [DOI] [PubMed] [Google Scholar]
- 31. Mendiratta P, Armstrong AJ, George DJ. Current standard and investigational approaches to the management of hormone‐refractory prostate cancer. Rev Urol 2007; 9(Suppl 1): S9–19. [PMC free article] [PubMed] [Google Scholar]
- 32. Ploussard G, Terry S, Maille P et al Class III beta‐tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel‐based chemotherapy. Cancer Res 2010; 70: 9253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu C, Zhu Y, Lou W et al Functional p53 determines docetaxel sensitivity in prostate cancer cells. Prostate 2013; 73: 418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gonnissen A, Isebaert S, Haustermans K. Hedgehog signaling in prostate cancer and its therapeutic implication. Int J Mol Sci 2013; 14: 13979–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Humphrey PA. Histological variants of prostatic carcinoma and their significance. Histopathology 2012; 60: 59–74. [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Kruithof‐de Julio M, Economides KD et al A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 2009; 461: 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang ZA, Mitrofanova A, Bergren SK et al Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell‐of‐origin model for prostate cancer heterogeneity. Nat Cell Biol 2013; 15: 274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science 2010; 329: 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stoyanova T, Cooper AR, Drake JM et al Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal‐like cells. Proc Nat Acad Sci USA 2013; 110: 20111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naor D, Sionov RV, Ish‐Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res 1997; 71: 241–319. [DOI] [PubMed] [Google Scholar]
- 41. Patrawala L, Calhoun T, Schneider‐Broussard R et al Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006; 25: 1696–708. [DOI] [PubMed] [Google Scholar]
- 42. Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer 2008; 98: 756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005; 65: 10946–51. [DOI] [PubMed] [Google Scholar]
- 44. Liu C, Kelnar K, Liu B et al The microRNA miR‐34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011; 17: 211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin AM, Small EJ. Prostate cancer update: 2006. Curr Opin Oncol 2007; 19: 229–33. [DOI] [PubMed] [Google Scholar]
- 46. Obradovich J, Walshaw R, Goullaud E. The influence of castration on the development of prostatic carcinoma in the dog. 43 cases (1978–1985). J Vet Intern Med 1987; 1: 183–7. [DOI] [PubMed] [Google Scholar]
- 47. Gonnissen A, Isebaert S, McKee CM, Dok R, Haustermans K, Muschel RJ. The hedgehog inhibitor GANT61 sensitizes prostate cancer cells to ionizing radiation both in vitro and in vivo. Oncotarget 2016; 7: 84286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karhadkar SS, Bova GS, Abdallah N et al Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 2004; 431: 707–12. [DOI] [PubMed] [Google Scholar]
- 49. Shafaee Z, Schmidt H, Du W, Posner M, Weichselbaum R. Cyclopamine increases the cytotoxic effects of paclitaxel and radiation but not cisplatin and gemcitabine in Hedgehog expressing pancreatic cancer cells. Cancer Chemother Pharmacol 2006; 58: 765–70. [DOI] [PubMed] [Google Scholar]
- 50. Chen YJ, Lin CP, Hsu ML, Shieh HR, Chao NK, Chao KS. Sonic hedgehog signaling protects human hepatocellular carcinoma cells against ionizing radiation in an autocrine manner. Int J Radiat Oncol Biol Phys 2011; 80: 851–9. [DOI] [PubMed] [Google Scholar]


