Abstract
Adult T‐cell leukemia–lymphoma (ATL) is a mature T‐cell malignancy associated with human T‐cell leukemia virus type 1 (HTLV‐1) infection. Japan is the most endemic country for HTLV‐1 and ATL in the world. Recent nationwide studies of Japanese blood donors reported that HTLV‐1 carriers spread from endemic areas to non‐endemic areas. Therefore, the latest information on nationwide epidemiological and clinical data for ATL is necessary to guide clinical practice. We undertook a multicenter, hospital‐based survey of newly diagnosed ATL patients from 2010 to 2011. A total of 996 patients with ATL were registered from 126 hospitals across Japan. Of those, 922 (487 men and 435 women) were included in the analysis. The median age at diagnosis was 68 years (interquartile range, 60–75 years). Overall, 67.2% of ATL was diagnosed in the Kyushu–Okinawa area. The most common subtype was acute (49.5%), followed by lymphoma (25.7%), chronic (14.2%), and smoldering (10.6%). Lymphoma type was more prevalent in men (60%), whereas chronic was more prevalent in women (60%). Half of patients with lymphoma type were aged over 70 years, whereas one‐third of patients with the chronic type were aged under 60 years. All of these characteristics were different from those of the previous nationwide surveys in the 1980s and 1990s. This survey clarified that half of current patients with ATL are aged over 68 years who were unable to receive intensive cytotoxic therapies. New less toxic agents for aged patients and further strategies to prevent the development of ATL from HTLV‐1 carrier status are needed.
Keywords: Adult T‐cell leukemia–lymphoma, ATL, HTLV‐1, human T‐cell leukemia virus type 1, nationwide survey
Adult T‐cell leukemia–lymphoma (ATL) is a peripheral T‐cell malignancy associated with human T‐cell leukemia virus type 1 (HTLV‐1).1, 2 It is estimated that HTLV‐1 infects approximately 10 million people worldwide,3 with endemic foci in the Caribbean basin, South America, sub‐Saharan Africa, and Japan. Japan has the highest prevalence of HTLV‐1 and ATL in the world,3 with approximately 1 million HTLV‐1 carriers4 and 1000 deaths from ATL annually.5, 6 Human T‐cell leukemia virus type 1 infection is also associated with inflammatory diseases including HTLV‐1‐associated myelopathy/tropical spastic paraparesis7 and HTLV‐1 uveitis.8 Although the majority of HTLV‐1 carriers remain asymptomatic during their lifetime, it is estimated that the annual incidence of ATL among HTLV‐1 carriers is approximately 60 per 100 000 carriers, with the lifetime risk being approximately 5% for men and 3% for women in Japan.9
In Japan, soon after the discovery of ATL in 1977,1 nationwide studies were undertaken every 2 years during the 1980s and 1990s to identify the epidemiological and clinical features of HTLV‐1 carriers and ATL patients.10, 11, 12, 13, 14, 15, 16, 17, 18 The studies reported the heterogeneous distribution of HTLV‐1 carriers and ATL patients, the diversity of the clinical features of ATL, and the natural history. In 1991, diagnostic criteria were established for ATL and the four clinical subtypes: smoldering, chronic, lymphoma, and acute.19 However, since the last nationwide study was completed in 1998,18 no such studies have been carried out in Japan, except for a simplified survey in 2008.20
In 2010, the Japan Ministry of Health, Labour and Welfare encouraged researchers to update current data for HTLV‐1 carriers and ATL patients.21 The epidemiological features of HTLV‐1 carriers have been updated already by two nationwide studies using HTLV‐1 test results from blood donors, which found that HTLV‐1 carriers have spread from endemic areas to non‐endemic areas.4, 22 However, the nationwide epidemiological features of ATL have not yet been updated.
In the nearly 20 years since the last detailed nationwide study of ATL in 1998,18 both population demographics and diagnostic techniques have changed. Also, with the development of phenotypic and molecular diagnostic tests,23, 24 hematologists and pathologists are now able to make a clear differential diagnosis between ATL and other T‐cell malignancies. Therefore, it is important to determine the current epidemiological features of ATL in Japan. To address this important issue, we conducted a nationwide survey of ATL in Japan. The aim of this study was to evaluate how epidemiological and clinical characteristics of ATL have changed over time in Japan.
Methods
Study design and subjects
We undertook a multicenter, hospital‐based survey during the study period 2013–2016. Study subjects were patients newly diagnosed with ATL between January 2010 and December 2011. To characterize the differences in the clinical and epidemiological characteristics of ATL between the 1980s–1990s and the present day, we followed similar methods to those of previous studies.10, 11, 12, 13, 14, 15, 16, 17, 18 The current study was carried out in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committee and institutional review board of the National Cancer Center in Japan (approval no. 2012‐293). The ethical committee waived the need for written informed consent because of the retrospective and anonymous data collection.
First, we extracted 2500 hospitals with more than 100 beds and with departments of hematology and/or dermatology from the list of medical institutions authorized to treat patients with health insurance coverage in the Regional Bureau of Health and Welfare (http://www.mhlw.go.jp/kouseiroudoushou/shozaiannai/chihoukouseikyoku.html), which cover all 47 prefectures in Japan. Next, we sent a questionnaire to the 2500 hospitals to ask whether or not there were any patients who had been newly diagnosed with ATL between 2010 and 2011, and whether the hospital was willing to participate in the study. Approval to participate in this study was also obtained from each of the participating hospitals, based on their institutional policies.
The first questionnaires were returned from 607 hospitals (24.3%); of those, 173 (28.5%) agreed to participate in the study after approval of the study protocol by the regional Institutional Review Board at each hospital. We then sent a set of study sheets, which contained items of epidemiological and clinical data, to the participating hospitals. A reminder letter was mailed if the study sheet had not been returned. Data collection was completed by the end of September 2016. The data collection and management were carried out by a professional clinical research support office (Ata‐Life Inc., Tokyo, Japan).
The study sheet was designed to collect epidemiological data pertaining to sex, birth year, birth‐place and hospital location in Japan (classified by 47 prefectural codes), blood transfusion history before 1986 (HTLV‐1 screening for blood donors started in 1986), familial history of HTLV‐1 carriers, HTLV‐1 uveitis, HTLV‐1‐associated myelopathy/tropical spastic paraparesis, and ATL, and history of any infectious diseases, skin diseases, malignancies, and autoimmune diseases. The 47 prefectural codes were grouped into six areas (Hokkaido–Tohoku, Kanto, Chubu–Hokuriku, Kinki, Chugoku–Shikoku, and Kyushu–Okinawa) (Fig. S1).
Clinical data consisted of the following six parts: (i) basic information: date of first diagnosis, age at diagnosis, clinical subtype,19 diagnosis basis site, “B” symptoms, performance status, Ann‐Arbor stage, anti‐HTLV‐1‐antibody positivity, and other clinical data; (ii) information on disease extent: site and number of lesions of involved lymph nodes and extranodal lesions and types of skin lesions;25 (iii) comorbidities; (iv) peripheral blood counts including ATL cells and lymphoid cells; (v) serum levels of biochemical tests; and (vi) immunophenotypic profiles of ATL cells.
In each participating hospital, the diagnosis of ATL and the subtype classification was made by clinicians. A central review was carried out on all the returned study sheets by five skilled hematologists in the field of ATL, based on criteria for the cytologically or histologically confirmed T‐cell lymphoid malignancy2 and for the subtype classification of ATL.19
Statistical analyses
All statistical analyses were carried out using SAS software, version 9.4 (SAS Institute, Cary, NC, USA). Continuous variables were presented as the median with ranges, interquartile range, or mean and compared using Wilcoxon's rank‐sum test or the Kruskal–Wallis test. Continuous variables were also categorized into several groups as necessary. Differences in the frequency of categorical variables were compared using the χ2‐test or Fisher's exact test. The agreement for diagnosis of subtypes between participating hospitals and central review was evaluated using a weighted kappa coefficient. A two‐sided P‐value of less than 0.05 was considered to be statistically significant.
Results
A total of 996 patients with ATL were registered from 126 participating hospitals (see Appendix 1 for details). Of those, 74 patients were excluded from the central review process because of the following reasons: insufficient information available to make a diagnosis (n = 21), HTLV‐1 carrier state (n = 32), relapsed ATL with unknown date of first diagnosis (n = 1), date of diagnosis outside the study period (n = 15), and insufficient information on sex and/or age (n = 5). Consequently, a total of 922 patients were included in the analysis (Fig. 1a).
Figure 1.
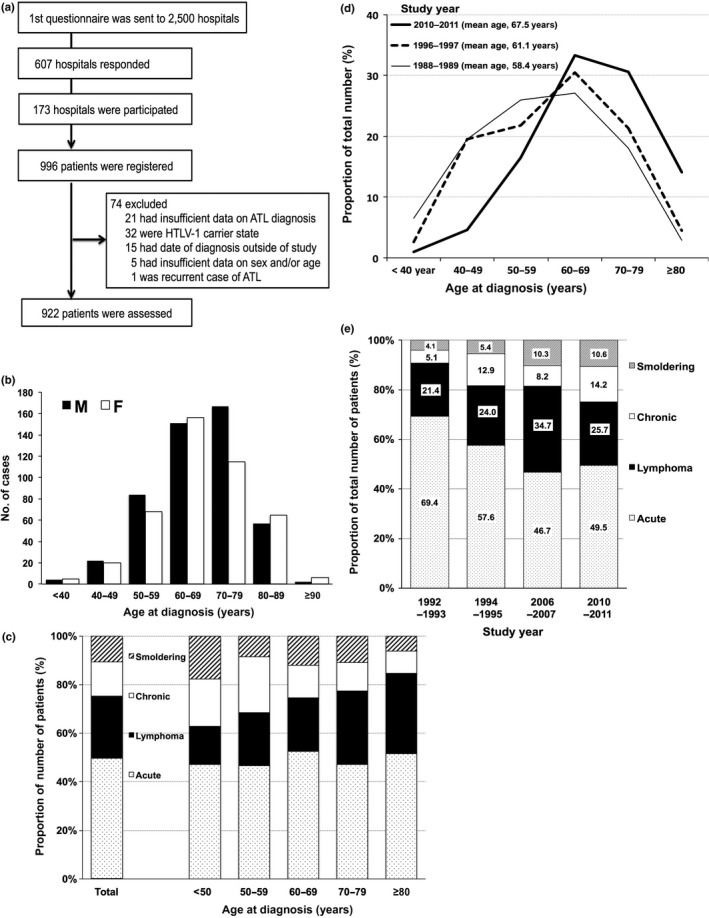
(a) Flowchart of the study of epidemiological and clinical features of adult T‐cell leukemia–lymphoma (ATL) in Japan, 2010–2011. HTLV‐1, human T‐cell leukemia virus type 1. (b) Distribution of age at diagnosis by sex. F, female; M, male. (c) Distribution of age at diagnosis by ATL subtype based on the central reviewers’ classification based on the Shimoyama classification.19 (d) Changes in the proportion of patients in each age category from years 1988–1989,14 1996–1997,18 to 2010–2011 (current study). (e) Changes in the proportion of ATL subtype from years 1992‐1993,16 1994‐1995,17 2006–2007,20 to 2010–2011 (current study).
The patients’ epidemiological characteristics are summarized in Tables 1 and 2. Overall, 487 (52.6%) patients were male. The median age at diagnosis was 68 years (interquartile range, 60–75 years) for all, with those aged 60–69 years and 70–79 years accounting for two‐thirds of all patients. The median age at diagnosis was not significantly different by sex, but the most frequent age group was different: the 70–79 years group for men, but the 60–69 years group for women (Table 1, Fig. 1b).
Table 1.
Epidemiological characteristics of Japanese patients with adult T‐cell leukemia–lymphoma, grouped by sex (n = 922)
| Characteristic | Total | Male | Female | M / F ratio | P‐value |
|---|---|---|---|---|---|
| Evaluable patients, n | 922 | 487 | 435 | 1.12 | |
| Year of diagnosis, n (%) | |||||
| 2010 | 468 (50.8) | 253 (51.9) | 215 (49.4) | 1.05 | 0.440 |
| 2011 | 454 (49.2) | 234 (48.1) | 220 (50.6) | 0.95 | |
| Age, years | |||||
| Median (min, IQR, max) | 68 (34, 60–75, 100) | 68 (34, 60–75, 100) | 67 (34, 61–76, 94) | 0.730 | |
| Mean (SD) | 67.5 (10.8) | 67.4 (10.5) | 67.7 (11.2) | 0.720 | |
| Age category, years, n (%) | |||||
| <40 | 9 (1.0) | 4 (0.8) | 5 (1.2) | 0.67 | 0.080 |
| 40–49 | 42 (4.6) | 22 (4.5) | 20 (4.6) | 0.98 | |
| 50–59 | 152 (16.5) | 84 (17.3) | 68 (15.6) | 1.11 | |
| 60–69 | 307 (33.3) | 151 (31.0) | 156 (35.9) | 0.86 | |
| 70–79 | 282 (30.6) | 167 (34.3) | 115 (26.4) | 1.30 | |
| 80–89 | 122 (13.2) | 57 (11.7) | 65 (14.9) | 0.79 | |
| ≥90 | 8 (0.9) | 2 (0.4) | 6 (1.4) | 0.29 | |
| Geographical area of hospital, n (%) | |||||
| Hokkaido–Tohoku | 50 (5.4) | 28 (5.8) | 22 (5.1) | 1.14 | 0.790 |
| Kanto | 59 (6.4) | 28 (5.8) | 31 (7.1) | 0.82 | |
| Chubu–Hokuriku | 55 (6.0) | 28 (5.8) | 27 (6.2) | 0.94 | |
| Kinki | 88 (9.5) | 44 (9.0) | 44 (10.1) | 0.89 | |
| Chugoku–Shikoku | 48 (5.2) | 29 (5.9) | 19 (4.4) | 1.34 | |
| Kyushu–Okinawa | 622 (67.5) | 330 (67.7) | 292 (67.1) | 1.01 | |
| Geographical areas of birth, n (%) | |||||
| Missing | 316 | 157 | 159 | ||
| Hokkaido–Tohoku | 45 (7.4) | 25 (7.6) | 20 (7.2) | 1.06 | 0.130 |
| Kanto | 10 (1.7) | 2 (0.6) | 8 (2.9) | 0.21 | |
| Chubu–Hokuriku | 17 (2.8) | 13 (3.9) | 4 (1.4) | 2.79 | |
| Kinki | 22 (3.6) | 11 (3.3) | 11 (4.0) | 0.83 | |
| Chugoku–Shikoku | 35 (5.8) | 18 (5.5) | 17 (6.2) | 0.89 | |
| Kyushu–Okinawa | 477 (78.7) | 261 (79.1) | 216 (78.3) | 1.01 | |
| Medical history, n (%) | |||||
| Transfusion before 1986, yes | 15 (1.7) | 5 (1.0) | 10 (2.3) | 0.43 | 0.130 |
| Skin diseases, yes | 43 (4.8) | 23 (4.7) | 20 (4.6) | 1.02 | 0.920 |
| Infectious diseases, yes | 98 (10.9) | 57 (11.7) | 41 (9.4) | 1.24 | 0.260 |
| Malignancies, yes | 108 (12.0) | 63 (12.9) | 45 (10.3) | 1.25 | 0.220 |
| Autoimmune diseases, yes | 36 (4.0) | 8 (1.6) | 28 (6.4) | 0.25 | <0.001 |
| Subtype by central review, n (%) | |||||
| Acute | 456 (49.5) | 241 (49.5) | 215 (49.4) | 1.00 | 0.007 |
| Lymphoma | 237 (25.7) | 141 (28.9) | 96 (22.1) | 1.31 | |
| Chronic | 131 (14.2) | 53 (10.9) | 78 (17.9) | 0.61 | |
| Smoldering | 98 (10.6) | 52 (10.7) | 46 (10.6) | 1.01 | |
ATL, adult T‐cell leukemia–lymphoma; F, female; IQR, interquartile range; M, male; max, maximum; min, minimum.
Table 2.
Epidemiological characteristics of Japanese patients with adult T‐cell leukemia–lymphoma (ATL) (n = 922) by area of participating hospitals
| Characteristic | Total | Hokkaido–Tohoku | Kanto | Chubu–Hokuriku | Kinki | Chugoku–Shikoku | Total of areas other than Kyusyu/Okinawa | Kyushu–Okinawa | P‐value |
|---|---|---|---|---|---|---|---|---|---|
| Evaluable patients, n | 922 | 50 | 59 | 55 | 88 | 48 | 300 | 622 | |
| Male sex, n (%) | 487 (52.8) | 28 (56.0) | 28 (47.5) | 28 (50.9) | 44 (50.0) | 29 (60.4) | 157 (52.3) | 330 (53.1) | 0.800 |
| Age at diagnosis, years, median (IQR) | 68 (60–75) | 67.5 (61–77) | 63 (60–71) | 66 (58–73) | 66 (61–71) | 66 (57.5–76) | 66 (60–73) | 69 (61–77) | 0.002 |
| Transfusion history before 1986, n (%) | 15 (1.7) | 0 (0.0) | 2 (3.4) | 0 (0.0) | 2 (2.3) | 1 (2.1) | 5 (1.7) | 10 (1.6) | 0.950 |
| Disease history before ATL diagnosis, n (%) | |||||||||
| Skin diseases | 43 (4.8) | 4 (8.0) | 6 (10.2) | 6 (10.9) | 2 (2.3) | 0 (0.0) | 18 (6.0) | 25 (4.0) | 0.180 |
| Infectious diseases | 98 (10.9) | 8 (16.0) | 6 (10.2) | 11 (20.0) | 8 (9.1) | 1 (2.1) | 34 (11.3) | 64 (10.3) | 0.620 |
| Malignancies | 108 (12.0) | 9 (18.0) | 6 (10.2) | 6 (10.9) | 7 (8.0) | 5 (10.4) | 33 (11.0) | 75 (12.1) | 0.640 |
| Autoimmune diseases | 36 (4.0) | 4 (8.0) | 5 (8.5) | 3 (5.5) | 6 (6.8) | 0 (0.0) | 18 (6.0) | 18 (2.9) | 0.020 |
| Familial history of ATL, n (%) | |||||||||
| Mother | 22 (2.4) | 0 (0.0) | 2 (3.4) | 1 (1.8) | 0 (0.0) | 2 (4.2) | 5 (1.7) | 17 (2.7) | 0.320 |
| Father | 7 (0.8) | 0 (0.0) | 1 (1.7) | 1 (1.8) | 1 (2.1) | 0 (0.0) | 3 (1.0) | 4 (0.6) | 0.560 |
| Sibling | 55 (6.2) | 2 (4.0) | 4 (6.8) | 8 (14.6) | 5 (5.7) | 7 (14.6) | 26 (8.7) | 29 (4.7) | 0.020 |
| Children | 8 (0.9) | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.5) | 5 (1.7) | 3 (0.5) | 0.070 |
| Spouse | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0.490 |
| Familial history of HTLV‐1 carrier, n (%) | |||||||||
| Mother | 19 (2.1) | 1 (2.0) | 1 (1.7) | 2 (3.6) | 0 (0.0) | 4 (8.3) | 8 (2.7) | 11 (1.8) | 0.370 |
| Father | 10 (1.1) | 0 (0.0) | 1 (1.7) | 1 (1.8) | 0 (0.0) | 5 (10.4) | 7 (2.3) | 3 (0.5) | 0.010 |
| Sibling | 50 (5.5) | 5 (10.0) | 5 (8.5) | 4 (7.3) | 5 (5.7) | 8 (16.7) | 27 (9.0) | 23 (3.7) | <0.001 |
| Children | 28 (3.1) | 0 (0.0) | 2 (3.4) | 3 (5.5) | 0 (0.0) | 7 (14.6) | 12 (4.0) | 16 (2.6) | 0.240 |
| Spouse | 12 (1.3) | 0 (0.0) | 1 (1.7) | 1 (1.8) | 4 (4.6) | 1 (2.1) | 7 (2.3) | 5 (0.8) | 0.050 |
HTLV‐1, human T‐cell leukemia virus type 1; IQR, interquartile range.
Of all the patients, 67.5% were diagnosed at participating hospitals in Kyushu–Okinawa, where HTLV‐1 is endemic, followed by 9.5% in Kinki and 6.4% in Kanto (Table 1). The distribution by patients’ place of residence was similar to the distribution by hospital (data not shown). Focusing on distribution by place of birth, 78.8% of patients were born in Kyushu–Okinawa, whereas the proportion of patients was smaller in three metropolitan areas (Kanto, Chubu–Hokuriku, and Kinki). Patients in Kyushu–Okinawa were significantly older than those in the other areas (P = 0.002) (Table 2).
The transfusion history before 1986 was reported for 15 (1.7%) patients. Of those, 10 were female in Kyushu–Okinawa (Table 1). Patients with a past history of any skin diseases, infectious diseases, malignancies, or autoimmune diseases were reported in 43 (4.8%), 98 (10.9%), 108 (12%), and 36 (4%) patients, respectively. Autoimmune diseases were significantly higher in female than male patients (P < 0.001) (Table 1) and higher in areas other than Kyushu–Okinawa (P = 0.02) (Table 2).
A family history of ATL was reported in 22 (2.4%) patients’ mothers, 7 (0.8%) fathers, 55 (6.1%) siblings, and 8 (0.9%) children (Table 2). The frequency of sibling history of ATL was significantly lower in Kyushu–Okinawa than other areas (P = 0.02). There was no sex difference in the frequencies of family history of ATL. A family history of HTLV‐1 carriers was reported in 19 (2.1%) patients’ mothers, 10 (1.1%) fathers, 50 (5.5%) siblings and 28 (3.1%) children. As with the family history of ATL, the frequency of sibling history of HTLV‐1 carriers was significantly lower in Kyushu–Okinawa than other areas (P < 0.001); whereas the frequency of fathers being HTLV‐1 carriers was significantly lower in Kyushu–Okinawa than other areas (P < 0.001). [Correction added on 09 November 2017, after first online publication: In the sentence, “The frequency of sibling history of ATL was significantly higher in Kyushu‐Okinawa than other areas (P = 0.02)”, “higher” was changed to “lower”. In the sentence, “A family history of HTLV‐1 carriers was reported in 19 (2.1%) patients’ mothers, 10 (1.1%) fathers, 50 (5.5%) siblings and 8 (3.1%) children”, “8 (3.1%)” was changed to “28 (3.1%)”. In the sentence, “As with the family history of ATL, the frequency of sibling history of HTLV‐1 carriers was significantly higher in Kyushu‐Okinawa than other areas (P < 0.001)”, “higher” was changed to “lower”.]
The prevalence of subtypes diagnosed by central reviewers showed that acute was the most prevalent (n = 456; 49.5%), followed by lymphoma (n = 237; 25.7%), chronic (n = 131; 14.2%), and smoldering (n = 98; 10.6%) (Tables 1 and 3). The prevalence pattern of the subtypes by participating hospitals was similar to the those of central reviewers, except for the “other” type (n = 14; 1.5%) (Table S1). All the other types were diagnosed as “cutaneous type of ATL” based on the tumor cell‐infiltrating skin histopathology and the absence of other organ lesions.
Table 3.
Epidemiological characteristics and comorbidities of Japanese patients with adult T‐cell leukemia–lymphoma, by disease subtype (n = 922)
| Characteristic | Total | Acute | Lymphoma | Chronic | Smoldering | P‐value |
|---|---|---|---|---|---|---|
| Total patients, n | 922 | 456 | 237 | 131 | 98 | |
| Male sex, n (% of each subtype) | 487 | 241 (52.9) | 141 (59.5) | 53 (40.5) | 52 (53.1) | 0.007 |
| Geographic area of hospitals, n (% of each subtype) | ||||||
| Hokkaido–Tohoku | 50 | 19 (38.0) | 15 (30.0) | 10 (20.0) | 6 (12.0) | 0.380 |
| Kanto | 59 | 28 (47.5) | 15 (25.4) | 8 (13.6) | 8 (13.6) | |
| Chubu–Hokuriku | 55 | 25 (45.4) | 22 (40.0) | 5 (9.1) | 3 (5.5) | |
| Kinki | 88 | 36 (40.9) | 25 (24.4) | 16 (18.2) | 11 (12.5) | |
| Chugoku–Shikoku | 48 | 24 (50.0) | 11 (22.9) | 6 (12.5) | 7 (14.6) | |
| Kyushu–Okinawa | 622 | 324 (52.1) | 149 (24.0) | 86 (13.8) | 63 (10.1) | |
| Age at diagnosis, years | ||||||
| Median (IQR) | 922 | 68 (61–75) | 70 (63–77) | 65 (57–73) | 67.5 (60–74) | <0.001 |
| Median (min, max) | 922 | 68 (34, 100) | 70 (37, 90) | 65 (36, 85) | 67.5 (40, 89) | <0.001 |
| Mean (SD) | 922 | 67.7 (11.1) | 69.6 (10.1) | 64.5 (11.0) | 66.0 (10.6) | <0.001 |
| Age category, years, n (% of each subtype) | ||||||
| <50 | 51 | 24 (5.2) | 8 (3.4) | 10 (7.6) | 9 (9.2) | 0.003 |
| 50–59 | 152 | 71 (15.6) | 33 (13.9) | 35 (26.7) | 13 (13.3) | |
| 60–69 | 307 | 161 (35.3) | 68 (28.7) | 41 (31.3) | 37 (37.7) | |
| 70–79 | 282 | 133 (29.2) | 85 (35.9) | 33 (25.2) | 31 (31.6) | |
| ≥80 | 130 | 67 (14.7) | 43 (16.1) | 12 (9.2) | 8 (8.2) | |
| Comorbidities, n (% of each subtype) | ||||||
| Any disease, yes | 297 | 145 (32.2) | 67 (28.5) | 43 (33.6) | 42 (43.8) | 0.060 |
| Hematological malignancies, yes | 8 | 4 (0.9) | 2 (0.8) | 0 (0.0) | 2 (2.0) | 0.440 |
| Non‐hematological malignancies, yes | 113 | 53 (11.6) | 21 (8.9) | 17 (13.0) | 22 (22.4) | 0.007 |
| Infectious diseases, yes | 93 | 53 (11.6) | 16 (6.8) | 14 (10.7) | 10 (10.2) | 0.250 |
| Neurologic diseases, yes | 21 | 13 (2.9) | 4 (1.7) | 3 (2.3) | 1 (1.0) | 0.630 |
| Autoimmune diseases, yes | 9 | 3 (0.7) | 1 (0.4) | 3 (2.3) | 2 (2.0) | 0.190 |
IQR, interquartile range; max, maximum; min, minimum.
Approximately 80% of the subtype classification at participating hospitals was consistent with the central reviewers’ classification, indicating substantial agreement (weighted kappa coefficient, 0.72; 95% confidence interval, 0.68–0.76; P < 0.0001) (Table S1). The major disagreements were as follows: 19.1% of lymphoma in participating hospitals were reclassified to acute; 14.6% of chronic were reclassified to acute; 17.5% of smoldering were reclassified to chronic; and 71.4% of other types were reclassified to smoldering. Most of the disagreement was within the aggressive or indolent category of ATL, which did not affect the treatment choice.
The clinical characteristics by subtype are summarized in Tables 3 and S2. Among chronic subtypes, 83 (63.4%) had an unfavorable factor (abnormal albumin, blood urea nitrogen, or lactate dehydrogenase levels). There was no significant difference in the frequency of subtype by hospital area. There was a significant difference in sex in the distribution of subtype (P = 0.007); the frequency of chronic type was higher in female compared with male patients; conversely, other types were higher in male than female patients (the highest was lymphoma type in male patients, 60%). There was a significant difference in age at diagnosis by subtype (P < 0.001) (Fig. 1c). Patients with chronic subtypes tended to be diagnosed at a younger age, whereas those with lymphoma subtypes were diagnosed at an older age (Fig. 1c, Table 3).
A total of 297 (32.2%) patients were comorbid with one or more diseases, including hematological malignancies (n = 8), non‐hematological malignancies (n = 113), infectious diseases (n = 93), neurologic diseases (n = 21), autoimmune diseases (n = 9), and other diseases (n = 167) (Table 3). In patients comorbid for malignancies, colorectal cancer was the most common (n = 26); in patients comorbid for infectious diseases, cytomegalovirus infection was the most common (n = 14).
To elucidate changes in the characteristics of patients over time, we reviewed previous nationwide studies of ATL in Japan.10, 11, 12, 13, 14, 15, 16, 17, 18, 20 Of these, we excluded three earlier surveys,8, 9, 10 because they only accumulated data from a few selected hospitals. From the remaining seven surveys and the present study (Table S3), we found that the mean age at diagnosis of ATL increased with study year from 58.4 years in 1988–1989 to 67.5 years in the present study (Fig. 1d), and that the proportion of lymphoma subtype increased from 21.4% in 1992–1993 to 34.7% in 2006–2007 and 25.7% in 2010–2011 (Fig. 1e).
Discussion
The present study provides the most up‐to‐date data on epidemiological and clinical features of ATL in Japan. The main features were: (i) a significant shift toward older age at diagnosis, with a median of 68 years; (ii) an increase in the proportion of lymphoma subtype; and (iii) age at diagnosis and male to female patient ratio differed by subtype.
Patients with ATL were diagnosed throughout Japan. However, 67.5% of patients were diagnosed in Kyushu–Okinawa. This was greater than those observed in previous studies (Table S3). This was unexpected because the most recent data of nationwide HTLV‐1 carriers in Japan indicated that the number of HTLV‐1 carriers in Kyushu–Okinawa decreased between 1988 and 2006–2007.4
The median age at diagnosis of 68 years indicates that more than half of current ATL patients are unable to receive intensive cytotoxic chemotherapies followed by allogeneic hematopoietic stem‐cell transplantation, which is considered to be a promising treatment for young patients with aggressive ATL.26, 27 Given the rapid aging of patients with ATL, new, less toxic therapeutic options are urgently needed in Japan. Recently, for aggressive ATL, several promising therapeutic antibodies, such as anti‐CC chemokine receptor 4 mAb28 and an anti‐CD52 antibody,29 have been introduced and have achieved significant reduction of ATL cells. Furthermore, lenalidomide, an immune‐modulatory agent, has been introduced for relapsed aggressive ATL patients.30 In Europe and America, an antiviral therapy with a combination of zidovudine (AZT) and interferon‐α has been introduced for ATL patients with leukemic manifestation.31 The long‐term effectiveness of these new agents is greatly anticipated for older patients with ATL.
The increase in lymphoma subtype was first reported in a simplified ATL survey in 2006–2007 (Table S3),20 in which lymphoma subtype accounted for 34.8% of all ATL, in contrast to 21%–24% in the 1980s–1990s studies.12, 16, 17 The proportion of lymphoma subtype in the present study (27.8%) was smaller than the 2006–2007 study, but greater than the 1980s–1990s studies (Fig. 1e, Table S3). The reason for the recent increase in lymphoma subtype of ATL remains unknown. However, considering that the median age of lymphoma subtype patients (70 years) was significantly older than chronic and smoldering subtypes (Fig. 1c, Table 3), any age‐related genetic factors may be involved in the development of the “lymphoma subtype of ATL” in HTLV‐1 carriers. Recently, Kataoka et al.32 comprehensively analyzed genetic alterations in ATL, and age‐related single‐nucleotide variants were predominantly recognized, although they did not mention whether or not the age‐related single‐nucleotide variants are high in the lymphoma subtype.
The proportion of smoldering subtype (10.6%) in the present study was similar to the 2006–2007 study (10.3%),20 but was greater than the 1980s–1990s studies, in which the proportion was less than 10%12, 16, 17 (Fig. 1e, Table S3). This may be partly influenced by participation of the dermatology departments for the first time in the present study, because the early phase of ATL localized to skin is frequently diagnosed by a dermatologist.
The overall male to female patient ratio in the present study was similar to previous studies10, 11, 12, 13, 14, 15, 16, 17, 18, 20 and there was no statistical difference in age at diagnosis by sex. However, in the present study, the most frequent age group was 10 years younger in women (60–69 years) compared with men (70–79 years), both of which shifted 10 years older than the previous 2006–2007 study.20 This also supports the theory that most current ATL patients are getting older.
The present study showed, for the first time, that chronic ATL patients were younger than those with acute and lymphoma type ATL (Fig. 1c, Table 3), although the reason is unknown. The age difference may be plausible as patients with chronic ATL frequently transform into acute type within a few years, and may suggest multistep leukemogenesis of ATL. More detailed analyses of the clinical data, including long‐term prognosis, are currently being undertaken. Further studies are needed on the treatment and prognosis of each subtype of ATL, especially the chronic and smoldering types.
In conclusion, this study highlights that some characteristics of ATL patients have changed over recent years in Japan. In particular, the increasing number of elderly patients with aggressive ATL is a public concern. For the elderly patients, the development of new, less toxic agents such as mAbs or small molecule agents, rather than intensive cytotoxic therapies, are urgently needed. Furthermore, any strategies for preventing the development of ATL in HTLV‐1 carriers by identifying definite markers for high‐risk HTLV‐1 carriers using, for example, multicolor flow cytometry is also needed.33
Disclosure Statement
Takashi Ishida declares research funding from Kyowa Hakko Kirin Co., Ltd., Bayer Pharma AG, Celgene K.K., and J‐Pharma Co., Ltd., and honoraria from Kyowa Hakko Kirin Co., Ltd. The other authors have no conflict of interest.
Supporting information
Fig. S1. Geographical areas and the name and code of the 47 prefectures in Japan.
Table S1. Agreement of diagnosis of subtype between participating hospitals and the central review.
Table S2. Summary of clinical characteristics of adult T‐cell leukemia–lymphoma patients by subtype.
Table S3. Summary of previous nationwide studies and the present study of adult T‐cell leukemia–lymphoma in Japan.
Acknowledgments
We thank Dr. Masanori Shimoyama, Dr. Keiji Iwatsuki, and Dr. Shimeru Kamihira for their expert opinions on this study. This work is dedicated to the memory of Dr. Kazunari Yamaguchi, who devoted a great part of his professional life to the researchers of HTLV‐1 in Japan and gave valuable suggestions on this study, and who passed away in April 2016. This work was partially supported by Grants‐in‐Aid from the Ministry of Health, Labour and Welfare of Japan (grant nos. H23‐GanRinsho‐Ippan‐020 and H26‐GanSeisaku‐Ippan‐006) to MI, UK, TW, and KT, and by the Japan Agency for Medical Research and Development (grant no. 17ck0106338h0001) to KN, MI, YI, KI, AU, and KT. We thank Edanz Group for editing a draft of this manuscript.
Appendix 1.
The authors would like to thank all the investigators at 126 Japanese hospitals who participated in this study.
Akira Kitanaka, Masahiro Amano (Miyazaki University);
Asahi Ito, Takashi Ishida (Nagoya City University);
Atae Utsunomiya, Kentaro Yonekura (Imamura Bun‐in Hospital);
Atsuko Mugitani (Fuchu Hospital);
Atsushi Wake (Toranomon Hospital Kajigaya);
Chiaki Kato (Meitetsu Hospital);
Daisuke Ogawa (Nagasaki Prefecture Shimabara Hospital);
Daisuke Tsuruta (Osaka City University);
Eizaburo Sueoka (Saga University);
Eriko Sato (Juntendo University Nerima Hospital);
Fujio Matsubara (Shin‐Kokura Hospital);
Fumihiko Nakamura (Tokyo University);
Hajime Kobayashi (Obihiro Kosei Hospital);
Hideho Heizan (Hamanomachi Hospital);
Hiroe Fuse (Matsudo City Hospital);
Hirofumi Kobayashi (Saitama Cancer Center);
Hirohiko Shibayama (Osaka University);
Hiroki Yamaguchi (Nippon Medical School);
Hiromasa Harada (Yao Tokusyukai General Hospital);
Hironori Ueno (Tokyo Medical Center);
Hiroshi Iwasaki (Sapporo‐Kosei General Hospital);
Hiroshi Kawano (Koga General Hospital);
Hiroshi Kazama, Junji Tanaka (Tokyo Women's Medical University);
Hiroshi Kojima (Daido Hospital);
Ichiro Hanamura (Aichi Medical University);
Jun Konishi (Okayama Medical Center);
Junichi Miyatake (Kinki University);
Junko Tanaka (Shimane University);
Kaname Miyashita (Saiseikai Fukuoka General Hospital);
Kaoru Uchimaru (The University of Tokyo, The Institute of Medical Science);
Katsuhiro Hitomi (Saitama Medical Center);
Katsuyasu Saigo (Kobe‐kyodo Hospital);
Kazuei Ogawa (Fukushima Medical University);
Kazuiku Ohshiro (Nanbu Medical Center/Nanbu Child Medical Center);
Kazunori Ohnishi, Yoshiki Tokura (Hamamatsu University School of Medicine);
Keiji Sugimoto (Juntendo University Urayasu Hospital);
Keita Kirito, Takashi Inozume (University of Yamanashi);
Ken Ohmachi (Tokai University);
Kenichiro Etoh (Kumamoto General Hospital);
Kenji Ishitsuka, Monji Koga (Fukuoka University);
Ki‐Ryang Koh (Osaka General Hospital of West Japan Railway Company);
Kimiharu Uozumi (Kagoshima Medical Center);
Kisato Nosaka (Kumamoto University);
Koichi Nagai (Niigata Prefectural Central Hospital);
Koji Izutsu (Toranomon Hospital);
Koji Kato (Kyushu University);
Koji Oka (Suzuka Kaisei Hospital);
Komei Kubo (Aomori Prefectural Central Hospital);
Kunihiro Tsukasaki (National Cancer Center Hospital East);
Makoto Yoshimitsu (Imamura Hospital);
Makoto Yoshimitsu, Youhei Uchida, Kazuhiro Kawai, Takuro Kanekura (Kagoshima University);
Masaharu Miyahara (Karatsu Red Cross Hospital);
Masakatsu Hishizawa, Akifumi Takaori (Kyoto University);
Masakazu Higuchi (Kyushu Hospital);
Masaki Hayashi (Nakagami Hospital);
Masaki Iino (Yamanashi Prefectural Central Hospital);
Masanari Kodera (Social Insurance Chukyo Hospital);
Masao Hagihara (Eiju General Hospital);
Masaru Shibano (Sakai City Medical Center);
Masato Saito (Nikko Memorial Hospital);
Michiaki Koike (Juntendo University Shizuoka Hospital);
Michihiro Hidaka (Kumamoto Medical Center);
Michiko Yamada (Steel Memorial Muroran Hospital);
Miki Takeuchi (Kohka Public Hospital)
Misato Kikuchi (Saitama Citizens Medical Center);
Mitsuhiro Matsuda (PL Hospital);
Mitsutoshi Kurosawa (Hokkaido Cancer Center);
Motohiro Shindo (Asahikawa Medical University);
Motonobu Nakamura (University of Occupational and Environmental Health);
Naoki Kobayashi (Sapporo Hokuyu Hospital);
Naokuni Uike (Kyushu Cancer Center);
Naoyuki Anzai (Takatsuki Red Cross Hospital);
Natsuko Daikoku (Nara Medical University);
Nobuharu Kosugi (Numazu City Hospital);
Nobuhiko Uoshima (Matsushita Memorial Hospital);
Nobuhiro Kanemura (Gifu University);
Nobuyoshi Hanaoka (Wakayama Medical University);
Noriko Fukuhara, Kenichi Ishizawa (Tohoku University);
Rika Sakai (Kanagawa Cancer Center);
Ryohei Nawata (Shimonoseki Medical Center);
Seiichi Okamura (National Kyushu Medical Center);
Sen Ushida (Ichinomiya Muncipal Hospital);
Shigetoshi Sano (Kochi University);
Shinichiro Yoshida, Hiroshi Ishikawa (Nagasaki Medical Center);
Shuji Ueda (Hyogo Prefectural Nishinomiya Hospital);
Taizo Shimomura (Kumamoto Shinto General Hospital);
Takae Minami (Sapporo Dohto Hospital);
Takafumi Okuno (Shiga University of Medical Science);
Takahiro Nagashima (Japanese Red Cross Kitami Hospital);
Takahiro Tsuji (Kumamoto City Hospital);
Takako Morita, Shinya Inoue (Suita Municipal Hospital);
Takamasa Hayashi (Hyogo Prefectural Amagasaki General Medical Center);
Takashi Okamura (Kurume University);
Takayoshi Ito (JA Toride Medical Cencer);
Takeshi Fujimoto (Nagasaki National Hospital);
Takuo Ito (Kure Medical Center);
Takuya Miyagi, Takeaki Tomoyose (Ryukyu University);
Tatsuro Jo (Japanese Red Cross Nagasaki Genbaku Hospital
Tatsuya Kaji (Okayama University);
Tetsuo Nagatani (Tokyo Medical University Hachioji Medical Center);
Tohru Murayama (Hyogo Cancer Center);
Tomohiro Myojo (Edogawa Hospital);
Toru Motokura, Koji Adachi (Tottori University);
Toru Takahashi (Tenshi Hospital);
Toru Takahashi (Yamaguchi Prefectural Grand Medical Center);
Toshiaki Sai (Iwaki Kyoritsu General Hospital);
Toshiaki Yujiri (Yamaguchi University);
Toshiharu Tamaki (Rinku General Medical Center);
Toshimi Mitsuishi (Saku Central Hospital Advanced Care Center);
Toshinori Kondo (Kawasaki Medical School);
Toshiyuki Nakayama (Tsurumi Hospital);
Tsutomu Sato (Sapporo Medical University);
Wataru Izumida (Iwate Prefectural Ofunato Hospital);
Yasuhiro Ishizuka (Funabashi Central Hospital);
Yoji Ishida (Iwate Medical University);
Yoko Adachi (Kobe Central Hospital);
Yoshio Saburi (Oita Prefectural Hospital);
Yoshitaka Asakura (Okinawa Red Cross Hospital);
Yoshitaka Imaizumi, Motoi Takenaka (Nagasaki University);
Yoshiyasu Kato (Kagoshima Prefectural Satsunan Hospital);
Yuichi Hasegawa (University of Tsukuba);
Yukimi Moriuchi (Sasebo City General Hospital);
Yukio Kobayashi, Kensei Tobinai (National Cancer Center Hospital)
Yuko Ogata (Nishibeppu National Hospital);
Yuta Katayama (Hiroshima Red Cross Hospital and Atomic‐bomb Survivors Hospital);
Yutaka Tsutsumi (Nikko Memorial Hospital).
Cancer Sci 108 (2017) 2478–2486
Funding Information
Ministry of Health, Labour and Welfare of Japan; Japan Agency for Medical Research and Development.
Contributor Information
Kisato Nosaka, Email: knosaka@kumamoto-u.ac.jp.
Masako Iwanaga, Email: masakoiwng@nagasaki-u.ac.jp.
Kunihiro Tsukasaki, Email: tsukasak@saitama-med.ac.jp.
References
- 1. Uchiyama T, Yodoi J, Sagawa K et al Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood 1977; 50: 481–92. [PubMed] [Google Scholar]
- 2. Ohshima K, Jaffe ES, Kikuchi M. Adult T‐cell leukaemia/lymphoma In: Swerdllow SH, Campo E, Harris NL, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. IARC: Lyon, 2008; 281–4. [Google Scholar]
- 3. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV‐1 infection. Front Microbiol 2012; 3: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV‐1 in Japan as determined by screening of blood donors. J Med Virol 2012; 84: 327–35. [DOI] [PubMed] [Google Scholar]
- 5. Iwanaga M, Watanabe T, Yamaguchi K. Adult T‐cell leukemia: a review of epidemiological evidence. Front Microbiol 2012; 10: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ikeda N, Inoue M, Iso H et al Adult mortality attributable to preventable risk factors for non‐communicable diseases and injuries in Japan: a comparative risk assessment. PLoS Med 2012; 9: e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osame M, Usuku K, Izumo S et al HTLV‐I associated myelopathy, a new clinical entity. Lancet 1986; 1: 1031–2. [DOI] [PubMed] [Google Scholar]
- 8. Mochizuki M, Watanabe T, Yamaguchi K et al HTLV‐I uveitis: a distinct clinical entity caused by HTLV‐I. Jpn J Cancer Res 1992; 83: 236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tokudome S, Tokunaga O, Shimamoto Y et al Incidence of adult T‐cell leukemia/lymphoma among human T‐lymphotropic virus type I carriers in Saga. Japan. Cancer Res 1989; 49: 226–8. [PubMed] [Google Scholar]
- 10. The T‐ and B‐cell Malignancy Study Group . Statistical analysis of immunologic, clinical and histopathologic data of lymphoid malignancies in Japan. Jpn J Clin Oncol 1981; 11: 15–38. [Google Scholar]
- 11. The T‐ and B‐cell Malignancy Study Group . Statistical analyses of clinico‐pathological, virological and epidemiological data on lymphoid malignancies with special reference to adult T‐cell leukemia/lymphoma: a report of the second nationwide study of Japan. Jpn J Clin Oncol 1985; 15: 517–35. [PubMed] [Google Scholar]
- 12. The T‐ and B‐cell Malignancy Study Group . The third nationwide study of adult T‐cell leukemia/lymphoma (ATL) in Japan: Characteristic patterns of HLA antigen and HTLV‐I infection in ATL patients and their relatives. Int J Cancer 1988; 41: 505–12. [DOI] [PubMed] [Google Scholar]
- 13. Tajima K, the T‐ and B‐cell Malignancy Study Group, co‐authors . The 4th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan: estimates of risk of ATL and its geographical and clinical features. Int J Cancer 1990; 45: 237–43. [DOI] [PubMed] [Google Scholar]
- 14. The T‐ and B‐cell Malignancy Study Group . The 5th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan. Gan No Rinsho 1992; 38: 405–12. [Japanese] [PubMed] [Google Scholar]
- 15. The T‐ and B‐cell Malignancy Study Group . The 6th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan. Gan No Rinsho 1994; 40: 229–46. [Japanese] [PubMed] [Google Scholar]
- 16. The T‐ and B‐cell Malignancy Study Group . The 7th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan. Gan No Rinsho 1996; 42: 231–47. [Japanese] [PubMed] [Google Scholar]
- 17. The T‐ and B‐cell Malignancy Study Group . The 8th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan]. Gan No Rinsho 1998; 44: 381–97. [Japanese] [PubMed] [Google Scholar]
- 18. The T‐ and B‐cell Malignancy Study Group . The 9th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan. Gan No Rinsho 2001; 47: 341–57. [Japanese] [PubMed] [Google Scholar]
- 19. Shimoyama M, members of The Lymphoma Study Group . Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. A report from the Lymphoma Study Group (1984‐87). Br J Haematol 1991; 79: 428–37. [DOI] [PubMed] [Google Scholar]
- 20. Yamada Y, Atogami S, Hasegawa H et al Nationwide survey of adult T‐cell leukemia/lymphoma (ATL) in Japan. Rinsho Ketsueki 2011; 52: 1765–71. [Japanese] [PubMed] [Google Scholar]
- 21. Watanabe T. Anti‐HTLV‐1 task force by the Prime Minister of Japan and his cabinet: new government policy against HTLV‐1/ATL. Rinsho Ketsueki 2011; 52: 1439–47. [Japanese] [PubMed] [Google Scholar]
- 22. Satake M, Iwanaga M, Sagara Y et al Incidence of human T‐lymphotropic virus 1 infection in adolescent and adult blood donors in Japan: a nationwide retrospective cohort analysis. Lancet Infect Dis 2016; 16: 1246–54. [DOI] [PubMed] [Google Scholar]
- 23. Gorczyca W, Weisberger J, Liu Z et al An approach to diagnosis of T‐cell lymphoproliferative disorders by flow cytometry. Cytometry 2002; 50: 177–90. [DOI] [PubMed] [Google Scholar]
- 24. Karube K, Aoki R, Nomura Y et al Usefulness of flow cytometry for differential diagnosis of precursor and peripheral T‐cell and NK‐cell lymphomas: analysis of 490 cases. Pathol Int 2008; 58: 89–97. [DOI] [PubMed] [Google Scholar]
- 25. Sawada Y, Hino R, Hama K et al Type of skin eruption is an independent prognostic indicator for adult T‐cell leukemia/lymphoma. Blood 2011; 117: 3961–7. [DOI] [PubMed] [Google Scholar]
- 26. Tsukasaki K, Hermine O, Bazarbachi A et al Definition, prognostic factors, treatment, and response criteria of adult T‐cell leukemia–lymphoma: a proposal from an international consensus meeting. J Clin Oncol 2009; 27: 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Utsunomiya A, Choi I, Chihara D, Seto M. Recent advances in the treatment of adult T‐cell leukemia‐lymphomas. Cancer Sci 2015; 106: 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishida T, Joh T, Uike N et al Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase II study. J Clin Oncol 2012; 30: 837–42. [DOI] [PubMed] [Google Scholar]
- 29. Sharma K, Janik JE, O'Mahony D et al Phase II Study of Alemtuzumab (CAMPATH‐1) in Patients with HTLV‐1‐Associated Adult T‐cell Leukemia/lymphoma. Clin Cancer Res 2017; 23: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishida T, Fujiwara H, Nosaka K et al Multicenter Phase II Study of Lenalidomide in Relapsed or Recurrent Adult T‐Cell Leukemia/Lymphoma: ATLL‐002. J Clin Oncol 2016; 34: 4086–93. [DOI] [PubMed] [Google Scholar]
- 31. Bazarbachi A, Plumelle Y, Ramos JC et al Meta‐analysis on the use of zidovudine and interferon‐alfa in adult T‐cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol 2010; 28: 4177–83. [DOI] [PubMed] [Google Scholar]
- 32. Kataoka K, Nagata Y, Kitanaka A et al Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet 2015; 47: 1304–15. [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi S, Watanabe E, Ishigaki T et al Advanced human T‐cell leukemia virus type 1 carriers and early‐stage indolent adult T‐cell leukemia‐lymphoma are indistinguishable based on CADM1 positivity in flow cytometry. Cancer Sci 2015; 106: 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Geographical areas and the name and code of the 47 prefectures in Japan.
Table S1. Agreement of diagnosis of subtype between participating hospitals and the central review.
Table S2. Summary of clinical characteristics of adult T‐cell leukemia–lymphoma patients by subtype.
Table S3. Summary of previous nationwide studies and the present study of adult T‐cell leukemia–lymphoma in Japan.


