Abstract
Introduction
High rectal doses are associated with increased toxicity. A rectal displacement device (RDD) reduces rectal dose in prostate stereotactic body radiation therapy (SBRT). This study investigates any dosimetric difference between two methods of rectal displacement (Rectafix and SpaceOAR) for prostate SBRT.
Methods
Rectal dosimetry of 45 men who received SBRT within the PROMETHEUS trial was retrospectively examined, across two radiation therapy centres using the two RDD's. Men received a total dose (TD) of 19 or 20 Gy in two fractions followed by 46 Gy in 23 fractions. Centre 1 contributed 16 Rectafix and 10 SpaceOAR patients. Centre 2 contributed 19 Rectafix patients. Rectal dose volume histogram (DVH) data were recorded as a TD percentage at the following volume intervals; V1%, V2%, V5%, V10% and then 10% increments to V80%. As only one centre employed both RDD's, three sequential rectal dosimetry comparisons were performed; (1) centre 1 Rectafix versus centre 1 SpaceOAR; (2) centre 1 Rectafix versus centre 2 Rectafix and (3) centre 1+ centre 2 Rectafix versus centre 1 SpaceOAR.
Results
In comparison (1) Rectafix demonstrated lower mean doses at 9 out of 11 measured intervals (P = 0.0012). Comparison (2) demonstrated a moderate difference with centre 2 plans producing slightly lower rectal doses (P = 0.013). Comparison (3) further demonstrated that Rectafix returned lower mean doses than SpaceOAR (P < 0.001). Although all dose levels were in favour of Rectafix, in absolute terms differences were small (2.6–9.0%).
Conclusions
In well‐selected prostate SBRT patients, Rectafix and SpaceOAR RDD's provide approximately equivalent rectal sparing.
Keywords: Prostatic neoplasm, rectafix, rectal displacement device, spaceOAR, stereotactic body radiotherapy
Introduction
The rectum is a radiosensitive organ, and the ability to reduce rectal dose during prostate radiation therapy has been associated with lower rates of late rectal toxicity.1
Hypofractionated radiotherapy schedules have been explored in a series of recent randomised controlled trials.2, 3, 4, 5 These have shown that 20–28 days fractionation schedules result in approximately equivalent biochemical control and rectal toxicities compared with 37–39 days conventional fractionation regimens. Stereotactic body radiation therapy (SBRT) has been increasingly explored for prostate treatment, often on a platform including some degree of real‐time imaging to reduce the impact of intrafraction motion.6 The need for such specialised equipment may impede the wider application of prostate SBRT in the community setting.
An alternative approach is to immobilise and/or displace the rectum from the prostate using a rectal displacement device (RDD). A RDD can be used to allow larger doses of radiation to be delivered safely to the prostate and to facilitate a reduction in radiation dose to the rectum.7 There are two main different strategies used to achieve this. SpaceOAR (Augmenix, Waltham, USA) is a hydrogel which is surgically inserted between the rectum and prostate. The Rectafix (Scanflex Medical AB, Tumstocksvägen, Sweden) is a plastic rod which is temporarily inserted into the rectum for each treatment allowing the rectum to be moved posteriorly from the prostate. Both approaches reduce radiation dose to the rectum.8, 9 For the SpaceOAR, a randomised trial has shown that this dosimetric benefit translates to subsequent reductions in late rectal toxicity and improvements in patient‐related quality of life.10
The PROMETHEUS (PROstate Multicentre External beam radioTHErapy Using Stereotactic boost) clinical trial is a phase 2 multicentre study where men receive ‘Virtual High Dose Rate Brachytherapy’ with two SBRT fractions prior to a 46 Gy in 23 fraction course of conventionally fractionated radiotherapy. For the SBRT component, a RDD is mandated for study participants, with subsets having use of either the Rectafix or SpaceOAR. We investigated if there is any significant difference in rectal dosimetry for prostate SBRT between the two methods of rectal displacement.
Methods
Patient recruitment and selection
Participant data were sourced retrospectively from patients treated on the PROMETHEUS trial. Ethics approval for the PROMETHEUS study was granted by the South Western Sydney Local Health District Human Research Ethics Committee on the 2/12/2013 reference number HREC/13/LPOOL/311. The PROMETHEUS study is registered on the Australian and New Zealand Clinical Trials Registry (ACTRN 126150002235380). Patients provided informed consent to participate in this trial, and this substudy assessed data from two participating Australian centres.
PROMETHEUS trial
The PROMETHEUS trial is a Phase 2 multicentre clinical trial exploring a SBRT boost to the prostate with fractionated external beam radiation therapy for men with non‐metastatic intermediate or high‐risk prostate cancer.
All participants received two SBRT fractions with a RDD in situ totalling either 19 or 20 Gy, using two volumetric modulated arc therapy (VMAT) partial arcs. The current patient subset consists of men managed with either a Rectafix or SpaceOAR. The SBRT fractions were treated a week apart followed by a 2 weeks break. A subsequent phase of standard fractionated IMRT treatment was then delivered to a total dose of 46 Gy in 23 fractions for all patients. This approach was chosen to mimic high‐dose rate (HDR) brachytherapy boost regimens.11
Rectal displacement devices (RDD)
Both the SpaceOAR and the Rectafix are approved for use in Australia and registered on the Australian Register of Therapeutic Goods (SpaceOAR, ARTG No. 179172; Rectafix, ARTG No. 201889).
SpaceOAR
The SpaceOAR is a hydrogel that is surgically inserted under transrectal ultrasound (TRUS) guidance. This hydrogel is injected through the perineum into the transrectal space between the posterior prostate and the anterior rectal wall creating a physical gap. The SpaceOAR remains in situ for the duration of the patient's treatment and is slowly resorbed over time. The patient receives a computed tomography (CT) scan, magnetic resonance imaging (MRI) and both SBRT VMAT treatments with this SpaceOAR in situ, as well as the conventionally fractionated radiotherapy treatment.
There is evidence that the use of hydrogel SpaceOAR is beneficial to reduce the anterior rectal wall dose when treating prostate cancer.12 The injected hydrogel creates a physical gap between the posterior prostate and the anterior rectal wall, as demonstrated in Figure 1. This allows for a high‐dose gradient to be created between the two structures, helping to minimise the dose to the rectum.9, 13
Figure 1.
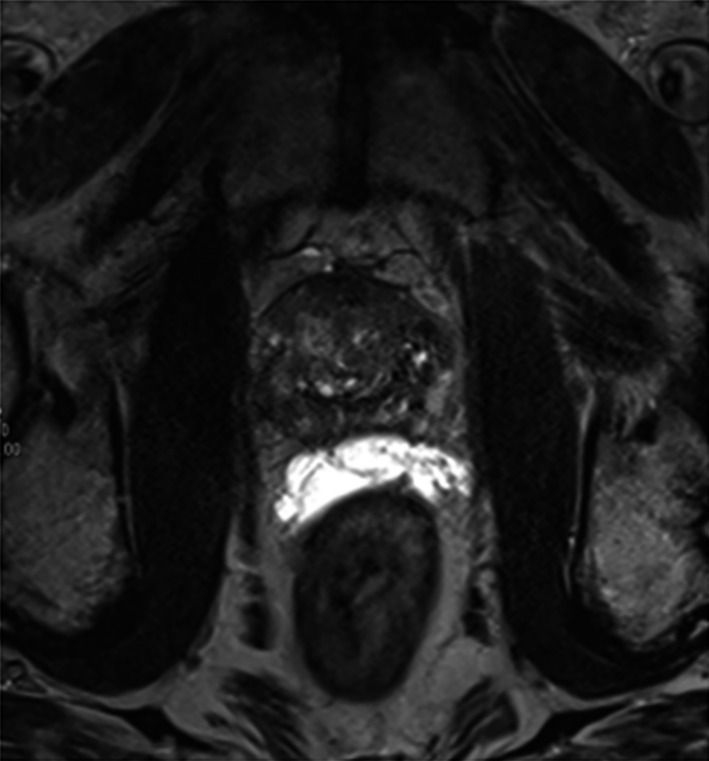
MRI image of SpaceOAR in situ. SpaceOAR appears bright on a T2‐weighted sequence. Note the separation between the posterior prostate and anterior rectal wall.
Rectafix
The Rectafix system involves a plastic rod that is inserted into the anus to extend beyond the superior limit of the prostate gland. The Rectafix is then connected to a vertical locking column attached to the treatment couch. The rectal rod is gently depressed to a distance guided by the patient's comfort and tolerability in order to gradually displace the rectum posteriorly away from the prostate gland.14 This vertical depression is indexed at CT, and then reproduced for the planning MRI and both fractions of the SBRT VMAT treatments. The rectal rod is visualised on both days of SBRT via cone beam CT to ensure that it is at the same depth and depression as attained at the time of treatment planning (Fig. 2).
Figure 2.
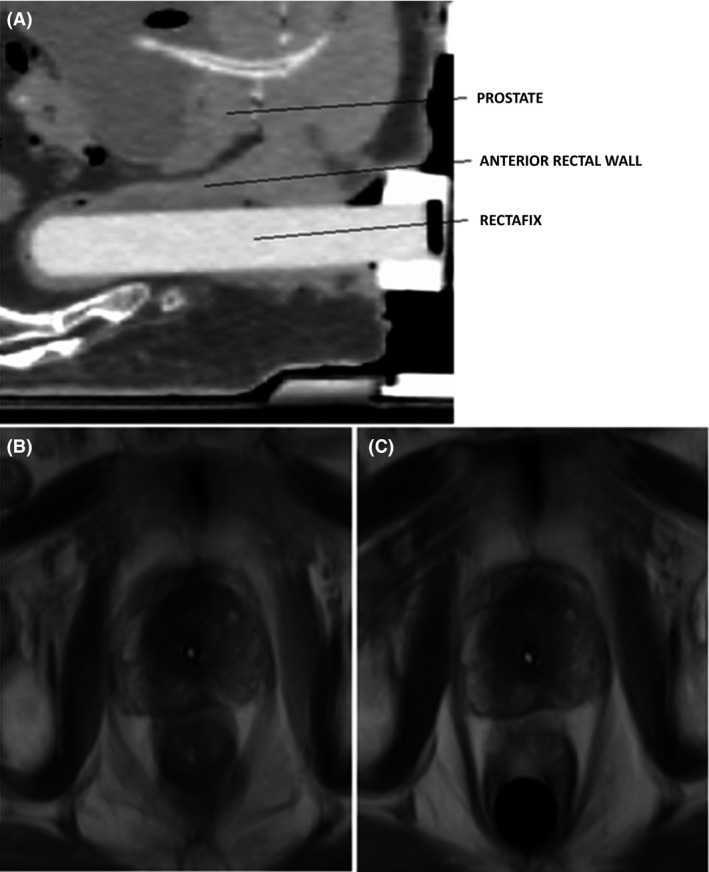
(A) Sagittal CT image of Rectafix RDD in situ with relevant structures identified, (B) MRI images of patient without Rectafix in situ and (C) with Rectafix in situ. Note the posterior displacement of the rectum.
Radiation therapy planning
All patients were on a protocol aiming to achieve an empty rectum for planning and treatment. An in‐dwelling catheter (IDC) was inserted by the clinician at the CT planning scan, and remained in situ until the MRI planning scan was completed. The IDC was clamped for the procedure to control bladder filling, and used to determine the urethral path through the prostate so that a planning risk volume (PRV) for dose calculation could be identified. The CT and MRI scan were fused using the prostatic fiducial markers. The clinical target volume (CTV) was the prostate gland, and planning target volume (PTV) involved a 5 mm expansion in all directions, but only 3 mm posteriorly. In both SpaceOAR and Rectafix scenarios, the registered planning MRI was used to guide the delineation of the total rectal volume. The rectal wall structure was then created from the outermost 3 mm of the rectal volume as an annular structure from the anorectal junction to the recto‐sigmoid junction using the Eclipse or Pinnacle ‘wall extraction’ planning software tool. VMAT SBRT planning was performed for all patients. Plans were optimised to ensure that trial dosimetric constraints were met as per trial guidelines (Table 1). Additionally, the rectal mucosa, posterior rectal wall, bladder, urethra, penile bulb and intermediate dose spillage were also contoured and optimised against predetermined PROMETHEUS trial dose constraints.
Table 1.
Prometheus PTV and rectal wall dose constraint guidelines. Total Dose (TD) was either 19 or 20 Gy
| PROMETHEUS trial dose constraints | |||
|---|---|---|---|
| Structure | Per‐protocol | Minor variation | Major variation |
| CTV D98 | >100% TD | 95–100% TD | <95% TD |
| PTV D50 | <105% TD | 105–110% TD | >110% TD |
| PTV D90 | >100% TD | 95–100% TD | <95% TD |
| PTV D95 | >95% TD | 90–95% TD | <90% TD |
| PTV D99 | >16 Gy | 15–16 Gy | <15 Gy |
| PTV Dmax to 0.1 cc | <110% TD | 110–120% TD | >120% TD |
| PTV Dmax | Not in OAR | In OAR | |
| Rectal Wall Dmax to 0.1 cc | <17 Gy | 17–17.5 Gy | >17.5 Gy |
| Rectal Wall V16 Gy | <0.5 cc | 0.5–1 cc | >1 cc |
| Rectal Wall V14 Gy | <3 cc | 3–5 cc | >5 cc |
| Rectal wall V12 Gy | <30% TD | 30–40% TD | >40% TD |
| Rectal wall V10 Gy | <40% TD | 40–50% TD | >50% TD |
| Rectal wall V8 Gy | <60% TD | 60–70% TD | >70% TD |
CTV, clinical target volume; PTV, planning target volume; TD, total dose; Gy, dose in grey; V, structure volume.
Plans were optimised to have a steep dose gradient posterior to the prostate to minimise the dose to the rectum but still achieve PTV, CTV and rectal dose volume histogram (DVH) constraints as listed in Table 1. Centre 1 used the Pinnacle planning system (Philips Healthcare, Andover), and centre 2 used the Eclipse planning system (Varian Medical Systems, Palo Alto).
Treatment
A pre‐treatment lateral kV image was acquired to assess for bowel gas and accurate rectal rod depth (for Rectafix patients). A cone beam computed tomography (CBCT) was then performed to primarily match to the three intra‐prostatic fiducials. Further assessment of CTV and critical structures was then performed to verify agreement with the planning CT. Treatment was then delivered, with repeat imaging between arcs to assess and correct for any intrafraction motion. Centre 1 used Elekta linear accelerators, and centre 2 used Varian (both Clinac and Truebeam platforms).
Multiple dose levels
The PROMETHEUS trial has a dose escalation component. As a result, there is a mix of patients treated at both 19 and 20 Gy. This has been managed in this analysis by assessing rectal DVH data as a percentage of target dose (TD), rather than absolute dose, to normalise the data for comparison. All plans satisfied all CTV and PTV trial constraints described in Table 1.
The first 22 patients were treated to 19 Gy in two fractions. The remaining 23 patients were treated to 20 Gy in two fractions.
Data collection
Data were extracted from the planning DVH's for all participants. Centre 1 treated 10 participants with SpaceOAR and 16 patients with the Rectafix; whereas 19 participants were treated with the Rectafix at centre 2.
The rectal dose data were measured at the following dose levels: Dmax, V1%, V2%, V5%, V10%, V20%, V30%, V40%, V50%, V60%, V70% and V80%. All the percentages relate to the relevant TD, so the V50% was the volume of rectum receiving 9.5 or 10 Gy depending on the prescribed dose. Note that due to the steep dose gradient at the interface between the PTV and rectum, the high‐dose increments (ie ≤V10%) were non‐linear.
Data analysis
A random effects model with robust standard errors was used to estimate whether the distribution of percent of total dose to the rectum differed for the two RDDs. The categorical effects of device and percent volume of rectum were modelled, as was their interaction which gauged whether the difference in outcome for the devices changed with increasing volume percentage. This process was repeated for the pooled hospital populations.
Three sequential rectal dosimetry comparisons were performed;
centre 1 Rectafix versus centre 1 SpaceOAR
centre 1 Rectafix versus centre 2 Rectafix
centre 1+ centre 2 Rectafix versus centre 1 SpaceOAR
Comparing the performance of the Rectafix device at the two hospitals was done using a random effects model. The percentage total dose was used as the outcome measure, and hospital and percentage rectum volume were included as fixed effects. P‐values for these trends are presented, as are the estimated differences in the outcome. For each hospital, estimates of the mean percentage total dose at each volume are plotted with 95% confidence intervals.
Results
Centre 1 Rectafix versus Centre 1 SpaceOAR
The dosimetry data for 16 Rectafix and 10 SpaceOAR men treated at centre 1 were compared and the estimates of the mean percentages of total dose received at each volume for each device. The overall P‐value for the volume by device effect was 0.0012 indicating that the difference between RDDs was not constant over the range of volumes. Figure 3A depicts the estimates for each RDD, suggesting a reduction in low to intermediate dose with the Rectafix. This was expected due to the stretching and displacement of the posterior rectal wall away from the lower isodose distribution.
Figure 3.
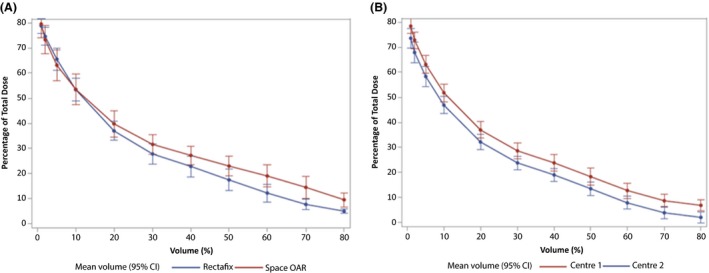
(A) Comparison of mean percentage of total dose received by percentage volumes of rectum, for each device at Centre 1. (B) Comparison of the hospital estimates of mean TD (%) received at each volume for the Rectafix device.
Centre 1 Rectafix versus Centre 2 Rectafix
The measurements of volumetric doses to the rectum for the Rectafix device at centre 1 and centre 2 were compared. There was a significant difference in volumetric dose measures between hospitals (P = 0.013). For the 19 patients treated at centre 2, for all volumes, the percentage total dose received was consistently lower than for the ten men managed at centre 1 by an average of 4.9%. The estimates of the mean TD (%) received at each volume are presented in Figure 3B.
Centre 1 and Centre 2 Rectafix versus Centre 1 SpaceOAR
The patients from both hospitals were pooled and the volumetric dose measurements for Rectafix (35 patients) and SpaceOAR (10 patients) devices were compared in Table 2.
Table 2.
Mean percentage of total dose received for percentage volumes of rectum for each device, and the difference between the device estimates for the pooled hospital populations
| Mean estimates of total dose | |||||||
|---|---|---|---|---|---|---|---|
| Space OAR | Rectafix | Difference | |||||
| Volume of rectum (%) | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | P |
| 1 | 79.5 | 74, 85 | 75.8 | 72.8, 78.9 | −3.7 | 10, 2.6 | 0.249 |
| 2 | 73.3 | 67.7, 79 | 70.2 | 66.7, 73.7 | −3.1 | −9.7, 3.5 | 0.359 |
| 5 | 63.1 | 57.1, 69.1 | 60.5 | 57, 64 | −2.6 | −9.6, 4.4 | 0.462 |
| 10 | 53.5 | 47.4, 59.6 | 49.2 | 45.9, 52.4 | −4.3 | −11.2, 2.5 | 0.216 |
| 20 | 39.8 | 34.6, 44.9 | 34.4 | 31.7, 37.1 | −5.4 | −11.1, 0.4 | 0.069 |
| 30 | 31.6 | 27.7, 35.6 | 26.0 | 23.7, 28.3 | −5.6 | −10.2, −1.1 | 0.016 |
| 40 | 27.2 | 23.4, 30.9 | 21.2 | 18.9, 23.5 | −6.0 | −10.3, −1.6 | 0.008 |
| 50 | 23.0 | 19.1, 26.9 | 15.7 | 13.3, 18.1 | −7.3 | −11.9, −2.7 | 0.002 |
| 60 | 19.1 | 14.8, 23.3 | 10.1 | 8, 12.2 | −9.0 | −13.8, −4.2 | <0.001 |
| 70 | 14.4 | 10, 18.9 | 6.1 | 4.7, 7.5 | −8.4 | −13, −3.7 | <0.001 |
| 80 | 9.4 | 6.6, 12.3 | 4.2 | 3.3, 5.1 | −5.3 | 8.2, −2.3 | <0.001 |
The results for the pooled population differed slightly from those obtained for centre 1 only. The volume‐device effect strengthened (P < 0.0001) and the Rectafix mean percentage TD was always lower than that of SpaceOAR (P = 0.018). In absolute terms at each level the difference in the means between the two devices was in the vicinity of 1–2 Gy (Fig. 4).
Figure 4.
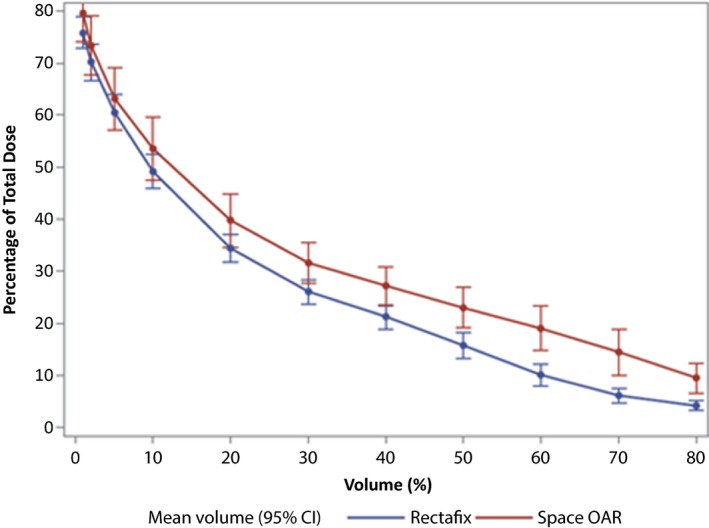
Comparison of mean percentage of total dose received by percentage volumes of rectum for each device over the pooled hospital populations.
Discussion
The Rectafix and the SpaceOAR are both effective RDD's, able to decrease volumes of high radiation dose to the rectum during prostate SBRT. In this exploratory study, the Rectafix, in well‐selected patients who tolerate the procedure and achieve adequate displacement, appears to be equivalent to the SpaceOAR at sparing the anterior rectal wall from high doses, and may have the potential to further reduce the volume of rectum receiving low and intermediate dose than the SpaceOAR. This is consistent and not unexpected with the increased posterior displacement of the posterior rectal wall offered by the Rectafix during SBRT. Whether this reduction is of clinical significance is yet to be determined for the Rectafix, although the recently reported results of a randomised trial of over 200 men exploring the utility of the SpaceOAR verified that the dosimetric benefits seen at planning did indeed translate into clinical benefit at later follow‐up.10
All plans were assessed retrospectively from the PROMETHEUS trial data. Plans were optimised to the strict trial constraints which in itself required meticulous sparing of the rectal structures. However, nearly all of these constraints focused on high and intermediate doses. A future prospective analysis presents the opportunity to explore the clinical impact of decreasing the volume of rectum receiving low doses in both RDD scenarios. Within the confines of the PROMETHEUS trial, the potential benefit offered by the Rectafix in the low‐dose spectrum may be countered by the lack of rectal displacement during the conventionally fractionated phase of the treatment, when compared with the SpaceOAR patients who maintain rectal displacement for both phases of their radiation therapy. The SBRT component which has been assessed in our data shows that the Rectafix potentially offers improved rectal dosimetry in the low‐ and intermediate‐dose regions. For future definitive stand‐alone SBRT options this may have clinical relevance.
Historically, higher levels of radiation proctitis have been reduced through intensification in treatment delivery.15 Evidence supports a reduction in rectal toxicity in IMRT and other modern techniques.16 There is also a suggestion of improved patient symptoms after the introduction of soft tissue image‐guided radiation therapy.17 The next frontier is the management of intrafraction motion, with solutions available, such as real‐time imaging using Kilovoltage Intrafraction Monitoring.18 At the same time, the ability to remedy radiation‐induced rectal injury has improved with interventions such as argon photocoagulation.19, 20 Despite this, for potentially higher risk regimens the use of RDD's will minimise rectal dose for such SBRT regimens.
The dosimetric similarity of the RDD's presents several interesting discussion points. The Rectafix RDD has the potential to eliminate the need for an additional surgical procedure to insert a SpaceOAR Hydrogel for prostate SBRT patients. This has the potential to reduce the associated costs and surgical risks to both the patient and the healthcare system. The Rectafix device is a reusable system requiring sterilisation of the rod and clamp mechanism between uses. Economically, the Rectafix is a one‐off departmental purchase whereas the insertion of individual SpaceOAR's is cumulatively more costly. There is, however, some discomfort associated with Rectafix insertion, which requires some expertise to be managed by both patients and attending staff.
Limitations
The main limitation of this study is that the total sample population was relatively small. Being retrospective in nature, there is a discrepancy in the size of the two RDD groups, with the SpaceOAR cohort being the smaller of the samples. This could influence the differences observed. In regards to the Rectafix cohort, at centre 1 three patients were excluded from the analysis as inadequate rectal displacement was achieved by the Rectafix such that planning constraints could not be met. A further two patients attempted, but could not tolerate the insertion of the Rectafix. All five were treated off‐trial with the departmental standard 78 Gy fractionation VMAT approach. Patients were also not offered entry into the trial if their radiological anatomy suggested a high chance that the Rectafix would not achieve adequate rectal displacement. Had these patients been included in the analysis then the impact of the Rectafix may well have been found to be lessened. Not being a randomised study, these results cannot be generalised to all patients. However, all centre 2 patients were managed with the Rectafix, suggesting that with appropriate processes this RDD provides a viable solution for many patients.
There is literature to suggest that a learning curve exists in regard to the uniformity and symmetry of SpaceOAR insertion.21, 22 The SpaceOAR cohort examined in our study were all reviewed and deemed to be of acceptable quality by the treating physician. No SpaceOAR patients were excluded from the trial due to poor insertion quality or adverse events.
There may be differences in the hospital patient populations that account for the observed differences, and as such this is purely an exploratory analysis. Accordingly, the magnitude of the differences between devices in Table 2 may not reflect true differences, and could be biased because centre 2 only contributed data for the Rectafix device.
Further limitations of note include hardware and software discrepancies between the centres, for example differences in specifications of the linear accelerators or the known difference in the dosimetric coverage achieved by alternative planning software.23
We feel that these concerns were at least partially managed through the sequential analysis approach where broader comparisons were only made after dosimetric differences between the RDD's had been noted between patient groups solely managed at centre 1, with the data from centre 2 adding further support to the analysis.
It is also important to note that the SpaceOAR remains in situ for the phase two EBRT component of the PROMETHEUS trial which will likely result in a dosimetric benefit compared with no rectal displacement.12 There may be an additional intrafraction motion benefit offered by the Rectafix over SpaceOAR due to the mechanics of the device fixating the posterior rectum and therefore reducing prostatic motion. This has been identified as an avenue of potential further study.
Conclusion
This work suggests that in well‐selected patients, the Rectafix and SpaceOAR RDD's provide approximately equivalent rectal sparing for hypofractionated prostate SBRT treatment. The Rectafix may have the potential to further reduce the volume of rectum receiving low and intermediate dose than the SpaceOAR in the SBRT setting, however, further validation with a larger subset is recommended.
J Med Radiat Sci 64 (2017) 266–273
References
- 1. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate‐cancer survivors. N Engl J Med 2008; 358: 1250–61. [DOI] [PubMed] [Google Scholar]
- 2. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (hypro): Late toxicity results from a randomised, non‐inferiority, phase 3 trial. Lancet Oncol 2016; 16: 274–83. [DOI] [PubMed] [Google Scholar]
- 3. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate‐risk localised prostate cancer: 2‐year patient‐reported outcomes of the randomised, non‐inferiority, phase 3 chhip trial. Lancet Oncol 2015; 16: 1605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase iii noninferiority study comparing two radiotherapy fractionation schedules in patients with low‐risk prostate cancer. J Clin Oncol 2016; 34: 2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high‐dose intensity‐modulated radiotherapy for prostate cancer: 5‐year outcomes of the randomised, non‐inferiority, phase 3 chhip trial. Lancet Oncol 2016; 17: 1047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi‐institutional consortium of prospective phase ii trials. Radiother Oncol 2013; 109: 217–21. [DOI] [PubMed] [Google Scholar]
- 7. Nicolae A, Davidson M, Easton H, et al. Clinical evaluation of an endorectal immobilization system for use in prostate hypofractionated stereotactic ablative body radiotherapy (sabr). Radiat Oncol 2015; 10: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wachter S, Gerstner N, Dorner D, et al. The influence of a rectal balloon tube as internal immobilization device on variations of volumes and dose‐volume histograms during treatment course of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2002; 52: 91–100. [DOI] [PubMed] [Google Scholar]
- 9. Song DY, Herfarth KK, Uhl M, et al. A multi‐institutional clinical trial of rectal dose reduction via injected polyethylene‐glycol hydrogel during intensity modulated radiation therapy for prostate cancer: Analysis of dosimetric outcomes. Int J Radiat Oncol Biol Phys 2013; 87: 81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamstra DA, Mariados NF, Shah DK, et al. Continued benefit to rectal separation for prostate RT: Final results of a phase III trial. Int J Radiat Oncol Biol Phys 2016; 96: 939. [DOI] [PubMed] [Google Scholar]
- 11. Spratt DE, Scala LM, Folkert M, et al. A comparative dosimetric analysis of virtual stereotactic body radiotherapy to high‐dose‐rate monotherapy for intermediate‐risk prostate cancer. Brachytherapy 2013; 12: 428–33. [DOI] [PubMed] [Google Scholar]
- 12. van Gysen K, Kneebone A, Alfieri F, Guo L, Eade T. Feasibility of and rectal dosimetry improvement with the use of spaceoar(r) hydrogel for dose‐escalated prostate cancer radiotherapy. J Med Imaging Radiat Oncol 2014; 58: 511–6. [DOI] [PubMed] [Google Scholar]
- 13. Susil RC, McNutt TR, DeWeese TL, Song D. Effects of prostate‐rectum separation on rectal dose from external beam radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nilsson K, Johansson AK, Montelius A, et al. Decreasing the dose to the rectal wall by using a rectal retractor during radiotherapy of prostate cancer: A comparative treatment planning study. J Radiother 2014; 2014: 7. [Google Scholar]
- 15. Andreyev HJ, Muls AC, Norton C, et al. Guidance: The practical management of the gastrointestinal symptoms of pelvic radiation disease. Frontline Gastroenterol 2015; 6: 53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ratnayake G, Martin J, Plank A, Wong W. Incremental changes verses a technological quantum leap: The additional value of intensity‐modulated radiotherapy beyond image‐guided radiotherapy for prostate irradiation. J Med Imaging Radiat Oncol 2014; 58: 503–10. [PubMed] [Google Scholar]
- 17. Singh J, Greer P, White M, et al. Treatment‐related morbidity in prostate cancer: A comparison of 3‐dimensional conformal radiation therapy with and without image guidance using implanted fiducial markers. Int J Radiat Oncol Biol Phys 2013; 85: 1018–23. [DOI] [PubMed] [Google Scholar]
- 18. Keall PJ, Ng JA, Juneja P, et al. Real‐time 3d image guidance using a standard linac: Measured motion, accuracy, and precision of the first prospective clinical trial of kilovoltage intrafraction monitoring‐guided gating for prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys 2016; 94: 1015–21. [DOI] [PubMed] [Google Scholar]
- 19. de la Serna Higuera C, Martin Arribas M, Rodriguez Gomez S, et al. Efficacy and safety of argon plasma coagulation for the treatment of hemorrhagic radiation proctitis. Rev Esp Enferm Dig 2004; 96: 758–64. [DOI] [PubMed] [Google Scholar]
- 20. Yeoh E, Tam W, Schoeman M, et al. Argon plasma coagulation therapy versus topical formalin for intractable rectal bleeding and anorectal dysfunction after radiation therapy for prostate carcinoma. Int J Radiat Oncol Biol Phys 2013; 87: 954–9. [DOI] [PubMed] [Google Scholar]
- 21. Pinkawa M, Klotz J, Djukic V, et al. Learning curve in the application of a hydrogel spacer to protect the rectal wall during radiation therapy of localized prostate cancer. Int J Radiat Oncol Biol Phys 2013; 87: S348. [DOI] [PubMed] [Google Scholar]
- 22. Fischer‐Valuck BW, Chundury A, Gay H, Bosch W, Michalski J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: Impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract Radiat Oncol 2016; 7: 195–202. [DOI] [PubMed] [Google Scholar]
- 23. Kumar SAS, Holla R, Sukumar P, Padmanaban S, Vivekanandan N. Treatment planning and dosimetric comparison study on two different volumetric modulated arc therapy delivery techniques. Rep Pract Oncol Radiother 2013; 18: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


