Abstract
This randomized phase II study investigated the immunological efficacy of herbal medicines (HM) using Hochu‐ekki‐to and Keishi‐bukuryo‐gan in combination with personalized peptide vaccination (PPV) for castration‐resistant prostate cancer (CRPC). Seventy patients with CRPC were assigned to two arms; PPV plus HM or PPV alone. Two to four peptides were chosen from 31 peptides derived from cancer antigens for a s.c. injection of PPV given eight times according to the patient's human leukocyte antigen type and levels of antigen‐specific IgG titer before PPV treatment. Peptide‐specific CTL, IgG, regulatory T cells (Treg), monocytic myeloid‐derived suppressor cells (Mo‐MDSC), and interleukin‐6 (IL‐6) responses were measured before and at the eighth vaccination. Clinical outcomes were also analyzed. Combination therapy of PPV with HM was well tolerated without severe adverse events. There was no significant change in antigen‐specific IgG, CTL, Treg or clinical outcomes. Combination therapy of PPV with HM stabilized the frequency of Mo‐MDSC (1.91%–1.92%, P = 0.96) and serum levels of IL‐6 (19.2 pg/mL to 16.1 pg/mL, P = 0.63) during the treatment. In contrast, the frequency of Mo‐MDSC and levels of IL‐6 in the PPV‐alone group were significantly increased (0.91%–1.49% for Mo‐MDSC and 9.2 pg/mL to 19.4 pg/mL for IL‐6, respectively). These results suggest that the combined use of HM has the potential to prevent the immunosuppression induced by Mo‐MDSC or IL‐6 during immunotherapy. More research is needed to validate the findings of the present study.
Keywords: Herbal medicine, immunotherapy, personalized peptide vaccination, phase II study, prostate cancer
Although several new drugs for CRPC have recently been approved, prostate cancer remains the second leading cause of cancer‐related death in men in Western countries. Each of these new drugs has prolonged survival by only a few months. Consequently, new therapies with longer survival benefits are greatly needed.1 Recently, immunotherapy has become an important part of treating some malignant tumors. Newer types of immune strategies are now being studied which will impact how we treat cancer in the future. One novel strategy of cancer immunotherapy is PPV, in which up to four peptides are selected for vaccination from 31 candidate peptides derived from cancer antigens based on HLA type and pre‐existing host immunity.2, 3 Recent clinical studies of PPV have demonstrated safety, immunological efficacy and survival benefits in patients with CRPC.4, 5, 6
The emergence of immunotherapy provides opportunities for HM to contribute beyond reducing the side‐effects of toxic chemicals. They now have a role in enhancing the body's natural immunity in support of the cancer vaccines and targeted immune responses of cancer immunotherapy.7 Hochu‐ekki‐to (TJ‐41) and Keishi‐bukuryo‐gan (TJ‐25) are Japanese traditional HM. Combined use of these two HM are prescribed to improve immunity and general conditions of cancer patients. Several studies have suggested that TJ‐41 can affect the immune system in experiments on mice.8, 9, 10 TJ‐25 also has anti‐inflammatory, analgesic, and anti‐pyretic effects.11 These functions lead to the hypothesis that TJ‐25 improves the TME for cancer immunotherapy to attack cancer cells.
Although there is some evidence for immune reinforcement effects of TJ‐41 in animal models, the roles of TJ‐41 and TJ‐25 in combination with immunotherapy for CRPC patients have not been defined. Thus, a randomized phase II study of PPV in combination with TJ‐41 and TJ‐25 was conducted to assess the immune‐enhancing effects in CRPC patients.
Materials and Methods
Patients
Eligibility criteria included a diagnosis of CRPC. CRPC was defined by at least two consecutive increases in PSA or clinically apparent disease progression, including diagnosis of PD, on cross‐sectional imaging despite appropriate androgen ablative therapy. At least two positive responses were also required on the pretreatment peptide‐specific IgG titer test for HLA matched peptides in 31 candidate peptides. Safety and immunological effects of the 31 candidate peptides were previously reported.5, 6, 12 All patients were required to be 20 years of age or older, have a performance status of 0 or 1 in accordance with EOCG, have a life expectancy of 12 weeks or more, an HLA haplotype of HLA‐A2, ‐A24, ‐A26, ‐A3, ‐A11, ‐A31, or ‐A33, and no hepatic or renal dysfunction. Exclusion criteria included an acute infection, a history of severe allergic reactions, or pulmonary, cardiac, or other inappropriate conditions for enrolment as judged by clinicians. The Kurume University Ethical Committee approved the protocol and this study was registered in the UMIN Clinical Trials Registry (UMIN 0000010290). After a full explanation of the protocol, all patients provided written informed consent before participating in the study.
Study design and treatment
In this open‐label, randomized phase II trial of PPV plus HM (TJ‐41 and TJ‐25) for CRPC patients, enrolled patients were assigned to two arms: PPV plus HM treatment or PPV‐alone treatment. They were randomized in a 1:1 ratio using the minimization technique with the following stratification factors: age (<70 or ≥70 years old), PS (0 or 1), and baseline PSA levels (<100 or ≥100 ng/mL). Primary endpoint was immune response in combination with HM in PPV treatment. OS, PFS, and safety were secondary endpoints.
Thirty‐one candidate peptides were prepared for PPV treatment: 12 peptides matched for HLA‐A2; 14 peptides matched for HLA‐A24; four peptides matched for HLA‐A26; nine peptides matched for HLA‐A3 super type (HLA‐A3, HLA‐A11, HLA‐A31 and HLA‐A33). Two to four peptides were selected for each patient according to HLA‐typing and peptide‐specific IgG titer. Each selected peptide with Montanide ISA 51 Incomplete Freund's Adjuvant (Seppic, Paris, France) in 1.5 mL emulsion (3 mg/peptide) was injected s.c.
Patients received vaccinations a total of eight times at 1‐week intervals in the first course of PPV in both groups. In the PPV plus HM arm, 2.5 g of both TJ‐41 and TJ‐25 was taken before each meal (total 7.5 g/day each) for 50 days during the 8 weeks of PPV treatment. TJ‐41 is manufactured as a spray‐dried powder extract made from 10 medical herbs in the following ratio: Ginseng radix (40%), Astragli radix (4%), Atractylodes lancea rhizoma (4%), Angelicae radix (3%), Aurantii nobilis pericarpium (2%), Zizyphi fructus (2%), Bupleuri radix (2%), Glycyrrhizae radix (1.5%), Cimicifugae rhizoma (1%), and Gingiberis rhizoma (0.5%) (Tsumura Co. Ltd, Tokyo, Japan).13 TJ‐25 is also manufactured as a spray‐dried powder extract and composed of equal volumes of Cinnamomum cortex, Hoelen, Moutan cortex, Paeoniae radix and Persicae semen (Tsumura Co. Ltd).14 After the first course, HM were not continued. In the PPV‐alone group, patients received the vaccination only.
Immune responses
The patient's peripheral blood was collected for measuring peptide‐specific CTL pressure in PBMC and IgG in plasma at pretreatment and at 8 weeks. CTL response to the vaccinated peptide epitopes was measured using the IFN‐γ ELISPOT assay based on previously reported methods.12 Briefly, PBMC were incubated for 2 days with each vaccinated peptide in culture medium containing IL‐2. Then, the cells were cultured with each peptide or control peptides and HLA‐matched C1R‐cells in an ELISPOT plate coated with anti IFN‐γ antibody. Eighteen hours later, the plates were washed and color developed, and then the spots were read using an ELISPOT reader (CTL‐ImmunoSpot S5 Series; Cellular Technology Ltd, Shaker Heights, OH, USA). Peptide‐specific IgG titers were measured using a Luminex system as reported previously.15 When the total of vaccinated peptide‐specific IgG titer at 8 weeks was higher than that at pre‐vaccination, it was considered to be a positive response. Positive CTL responses were defined as a more than 50‐spot increase in the total IFN‐γ spots at 8 weeks. Total IFN‐γ spots was the sum of spots for each vaccinated peptide minus the spots for control peptides.
Treg, Mo‐MDSC and IL‐6
Treg, Mo‐MDSC, and IL‐6 were examined at pretreatment and at 8 weeks. Treg were defined as CD4 + CD25 + FoxP3 + cells in lymphocytes (Fig. 1a). They were stained using the One Step Staining Human Treg Flow™ Kit (Biolegend, San Diego, CA, USA) in accordance with the manufacturer's instructions. MDSC were identified as CD33 + 11b+ cells from the lineage markers (CD3, CD19, CD56, and CD16)‐ and HLA‐DR‐cells. The monocytic subset was identified as CD14 + (Fig. 1b). For the analysis, anti‐CD3‐FITC, anti‐CD56‐FITC, anti‐CD19‐FITC, anti‐CD16‐FITC, anti‐CD33‐APC, anti‐HLA‐DR‐PE/Cy7, and anti‐CD14‐APC/Cy7 were used. Stained PBMC were run on a FACSCanto II (BD Biosciences, San Diego, CA, USA), and data were analyzed using the Diva software package (BD Biosciences). Frequencies of Treg and MDSC in the lymphocytes were calculated. Serum IL‐6 concentration was determined by ELISA.
Figure 1.
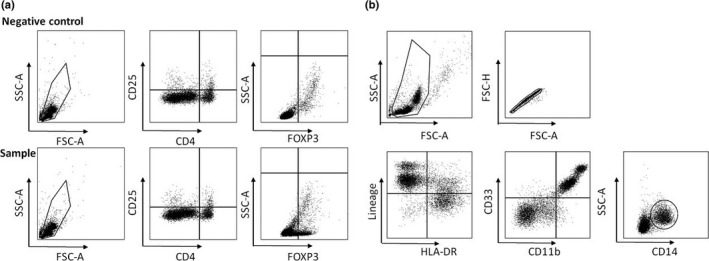
Gating strategy of regulatory T cells (Treg) and monocytic myeloid‐derived suppressor cells (Mo‐MDSC). (a) Treg were defined as CD4 + CD25 + FoxP3 + cells in lymphocytes. (b) MDSC were identified as CD33 + 11b+ cells from the lineage markers (CD3, CD19, CD56, and CD16)‐ and HLA‐DR‐cells. The monocytic subset was identified as CD14 + .
Assessment of toxicity and clinical activity
Patient's symptoms and physical examination data were checked at each visit. Toxicity was assessed in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI‐CTCAE Ver. 4). General biochemical examinations, including serum PSA test, were done every eight vaccinations. CT and bone scans were carried out every 6 months or at progression of symptoms. PD was defined as two consecutive increases in PSA from baseline or nadir, and CT images were evaluated in accordance with RECIST criteria or death.
Statistical methods
Student's t‐test and the chi‐squared test were used to compare quantitative and categorical variables among safety and immune responses to the treatment, respectively. Analyses of PFS and OS were based on population of a full analysis set that included all randomly assigned and treated patients. PFS and OS were defined as the time in months from study enrolment to event: death for OS and diagnosis of PD for PFS. PFS and OS data for each arm were analyzed using the Kaplan–Meier method. The log–rank test was used for comparison of the survival curves and Cox proportional hazards analysis for estimation of HR. CI reported are 95%. Statistical analyses were carried out using SAS software version 9.1 (SAS Institute, Cary, NC, USA) with a two‐sided significance level of 5%.
Results
Patients
Between June 2013 and March 2014, a total of 70 patients with CRPC were enrolled in this randomized phase II trial at the Cancer Vaccine Center of Kurume University in Japan. They were assigned to PPV plus HM (n = 35) or PPV alone (n = 35). Four patients withdrew consent before initiation of treatment in the PPV plus HM arm. Three patients in the PPV plus HM arm and four patients in the PPV‐alone arm did not complete the treatment for 8 weeks because of disease progression or death. Two patients in the PPV plus HM arm did not have immunological evaluation as a result of sample availability. The study flowchart is shown in Figure 2. Demographic and baseline disease characteristics were well balanced between the treatment arms (Table 1).
Figure 2.
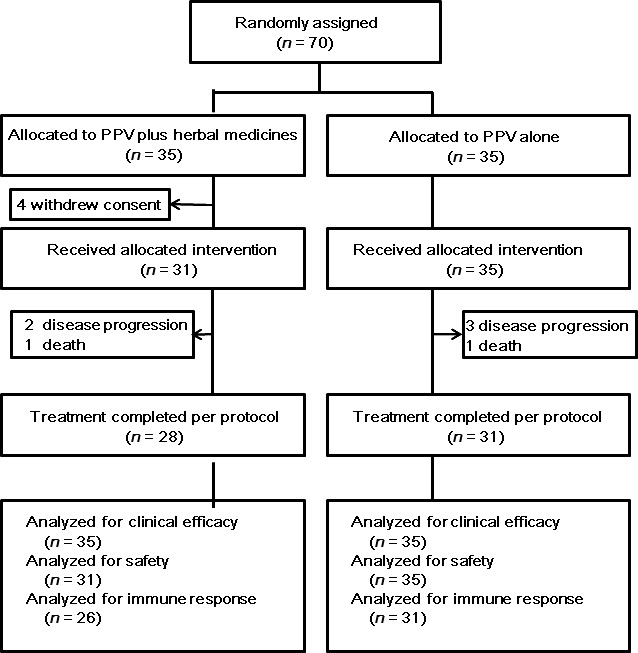
Study flowchart. PPV, personalized peptide vaccination.
Table 1.
Patient characteristics at baseline
| Characteristics | PPV plus herbal medicines | PPV alone | P‐value | ||
|---|---|---|---|---|---|
| (n = 35) | (n = 35) | ||||
| No. | % | No. | % | ||
| Age, years | 0.75 | ||||
| Median | 68 | 69 | |||
| Range | 48–85 | 56–81 | |||
| ECOG PS | 0.8 | ||||
| 0 | 23 | 66 | 24 | 69 | |
| 1 | 12 | 34 | 11 | 31 | |
| PSA, ng/mL | 0.4 | ||||
| Mean ± SD | 146.1 ± 55.3 | 417.1 ± 310.4 | |||
| Median (range) | 34.8 (1.4–1490) | 16.2 (0.4–1089) | |||
| Gleason score | 0.45 | ||||
| ≤7 | 3 | 9 | 5 | 14 | |
| ≥8 | 32 | 91 | 30 | 86 | |
| HLA typing of vaccinated peptide | 0.1 | ||||
| HLA‐A24 | 18 | 51 | 23 | 66 | |
| HLA‐A2 | 13 | 37 | 9 | 26 | |
| HLA‐A3 super‐type† | 4 | 12 | 3 | 8 | |
| Metastatic sites | 0.9 | ||||
| Bone only | 12 | 34 | 11 | 31 | |
| Bone with nodal/nodal only | 11 | 32 | 11 | 32 | |
| Others | 12 | 34 | 13 | 37 | |
| Prior additional treatments to ADT | 0.2 | ||||
| Radical prostatectomy | 1 | 3 | 1 | 3 | |
| Docetaxel | 22 | 63 | 15 | 43 | |
| Radiation | 5 | 14 | 3 | 9 | |
| Enzalutamide/Abiraterone | 9 | 26 | 3 | 9 | |
| None | 8 | 23 | 8 | 23 | |
χ2 test and t‐test were used for categorical and continuous variables, respectively. †HLA‐A3 super‐type included HLA‐A3, ‐A11, ‐A31 and ‐A33. ADT, androgen‐deprivation therapy; ECOG PS, Eastern Cooperative Oncology Group performance status; HLA, human leukocyte antigen; PSA, prostate‐specific antigen; PPV, personalized peptide vaccination.
Toxicity
Overall AE as a result of any cause are listed in Table 2. Treatment‐related deaths did not occur in either arm. Grade 1 or 2 injection site reactions were the most frequent AE in both arms (84% in the PPV plus HM arm and 71% in the PPV‐alone arm). Appetite loss was less frequent with the PPV plus HM treatment than with PPV‐alone treatment.
Table 2.
Adverse events
| Adverse event | PPV plus herbal medicines (n = 31) | PPV alone (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (%) | G1 | G2 | G3 | Total (%) | G1 | G2 | G3 | |
| Systemic symptoms | ||||||||
| Injection site reaction | 26 (84) | 24 | 2 | 0 | 25 (71) | 24 | 1 | 0 |
| Tumor pain | 3 (10) | 3 | 0 | 0 | 5 (14) | 2 | 3 | 0 |
| Appetite loss | 1 (3) | 1 | 0 | 0 | 8 (23) | 3 | 5 | 0 |
| Fever | 1 (3) | 1 | 0 | 0 | 4 (11) | 2 | 2 | 0 |
| Fatigue | 2 (6) | 2 | 0 | 0 | 3 (9) | 3 | 0 | 0 |
| Edema peripheral | 5 (16) | 3 | 2 | 0 | 1 (3) | 1 | 0 | 0 |
| Hematuria | 3 (10) | 1 | 2 | 0 | 3 (9) | 2 | 1 | 0 |
| Blood/bone marrow | ||||||||
| Lymphocytopenia | 7 (23) | 4 | 3 | 0 | 10 (29) | 6 | 4 | 0 |
| Anemia | 8 (26) | 1 | 4 | 3 | 7 (20) | 2 | 4 | 1 |
| Laboratory | ||||||||
| ALP increased | 10 (32) | 7 | 2 | 1 | 8 (23) | 4 | 1 | 3 |
| AST increased | 4 (13) | 2 | 2 | 0 | 2 (6) | 0 | 1 | 1 |
| ALT increased | 3 (10) | 2 | 1 | 0 | 5 (14) | 1 | 3 | 1 |
| Hypo‐albuminemia | 8 (26) | 8 | 0 | 0 | 11 (31) | 9 | 2 | 0 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PPV, personalized peptide vaccination.
Immune responses
Number of lymphocytes during the treatment was not changed in either arm (Fig. 3a). Peptide‐specific immune responses in each patient are shown in Table S1 (Supporting Information). Peptide‐specific IgG responses were measured in 15 of 26 patients (58%) in the PPV plus HM arm and in 14 of 31 patients (45%) in the PPV‐alone arm. Peptide‐specific CTL responses were observed in nine of 26 patients (35%) in the PPV plus HM arm and in nine of 31 patients (29%) in the PPV‐alone arm. IgG responses in both arms significantly increased after the treatment (Fig. 3b). Although CTL responses in both arms exhibited an increase after treatment, the results did not differ (Fig. 3c). Rate of patients with positivity for IgG or CTL responses was assessed. There were no significant differences in the two arms. Average frequency of Mo‐MDSC was compared between the two arms. Before treatment, Mo‐MDSC frequency was lower in the PPV‐alone arm than in the PPV plus HM arm (0.91% vs 1.91%, P = 0.024). Average frequency of Mo‐MDSC in the PPV plus HM arm was not changed after the treatment (1.91% at pre‐therapy and 1.92% at the eighth vaccination, P = 0.96). In the PPV‐alone arm, frequency of the average Mo‐MDSC significantly increased after the treatment (0.91% at pre‐therapy and 1.49% at the eighth vaccination, P = 0.012) (Fig. 3e). Regarding IL‐6, average IL‐6 level at pre‐therapy was also lower in the PPV‐alone arm than in the PPV plus HM arm (9.2 pg/mL vs 19.2 pg/mL, P = 0.021). Average IL‐6 level in the PPV plus HM arm showed a decreasing trend after the treatment, but this result did not differ significantly (19.2 pg/mL at pre‐therapy and 16.1 pg/mL at the eighth vaccination, P = 0.63). In contrast, average IL‐6 level in the PPV‐alone arm significantly increased after the treatment (9.2 pg/mL at pre‐therapy and 19.4 pg/mL at the eighth vaccination, P = 0.04) (Fig. 3f).
Figure 3.
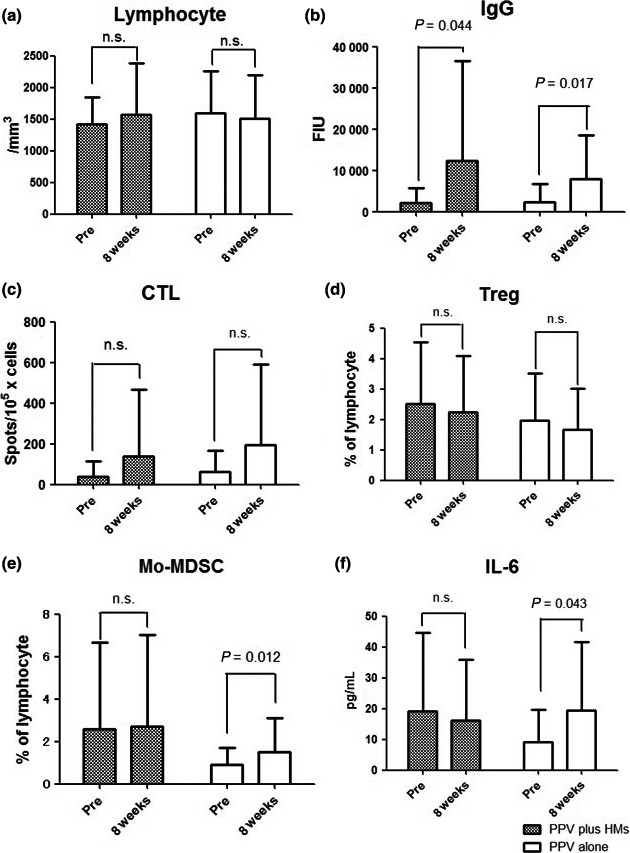
(a) Changes in lymphocytes, (b) IgG, (c) CTL, (d) regulatory T cells (Treg), (e) monocytic myeloid‐derived suppressor cells (Mo‐MDSC), and (f) interleukin‐6 (IL‐6) during treatment in both arms. HM, herbal medicines; PPV, personalized peptide vaccination; Pre, pretreatment.
Clinical responses
At the end of follow up, 45 of 65 patients (69%) had progressed or died: 19 of 30 patients (63%) in the PPV plus HM arm and 26 of 35 patients (74%) in the PPV‐alone arm. Median duration of follow up was 14.3 months (IQR, 7.4–30.7 months): 14.9 months in the PPV plus HM arm and 13.6 months in the PPV‐alone arm. Median PFS in both arms was not significantly different (HR, 1.0; 95% CI, 0.6–1.7; P = 0.98): 4.6 months (95% CI, 3–7.1 months) in the PPV plus HM arm and 4.6 months (95% CI, 10.8–26.3 months) in the PPV‐alone arm (Fig. 4a). Median OS in the PPV plus HM arm was 15.5 months (95% CI, 7.2–33.6 months) and that in the PPV‐alone arm was 13.6 months (95% CI, 10.8–26.3 months). However, there was no significant difference (HR, 0.9; 95% CI, 0.5–1.6; P = 0.68) (Fig. 4b)
Figure 4.
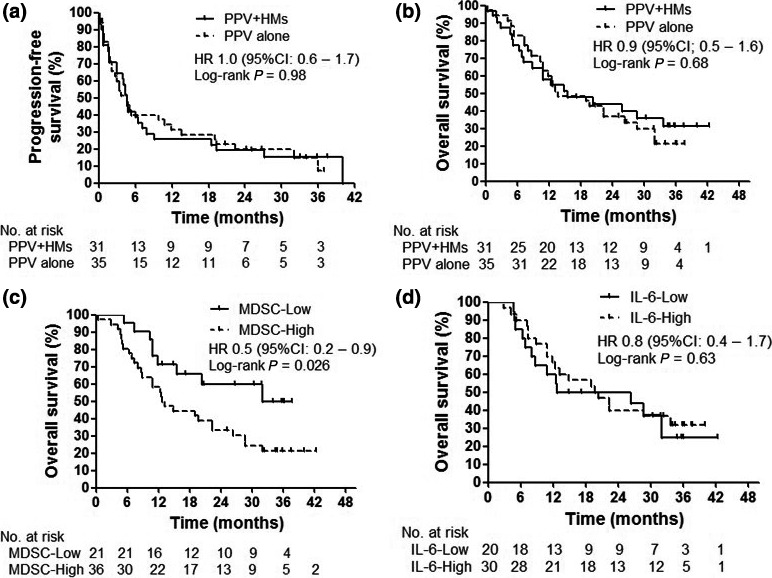
Survival of patients. (a) Progression‐free survival, (b) overall survival, (c) overall survival stratified by increase of monocytic myeloid‐derived suppressor cells (MDSC), and (d) overall survival stratified by increase in interleukin‐6 (IL‐6). HM, herbal medicines; PPV, personalized peptide vaccination.
Mo‐MDSC and IL‐6 in relation to survival
To clarify the clinical meaning of the frequency of Mo‐MDSC and levels of IL‐6, we defined the subgroups according to the increase of Mo‐MDSC (>0.8%) and IL‐6 (>6.5 pg/mL) at eight vaccinations from each median value at pretreatment, and carried out an OS subgroup analysis. Median OS times for patients with an increase of Mo‐MDSC (Mo‐MDSC‐High) and without an increase of Mo‐MDSC (Mo‐MDSC‐Low) were 12.9 months (95% CI, 8.7–22.4) and 32.6 months (95% CI, 11.9 to not reached), respectively. Patients without an increase of Mo‐MDSC demonstrated a significantly longer survival than patients with an increase of Mo‐MDSC (HR, 0.48; 95% CI, 0.2–0.9; P = 0.026) (Fig. 4c). In contrast, median OS times for patients with an increase in IL‐6 (IL‐6‐High) and without an increase in IL‐6 (IL‐6‐Low) were 20 months (95% CI, 11.9–34) and 19.5 months (95% CI, 6.8–32), respectively. There was no significant difference in OS between the two groups stratified by the increase in IL‐6 (HR, 0.8; 95% CI, 0.4–1.7, P = 0.63) (Fig. 4d).
Discussion
With the emergence of a new class of immune‐based cancer therapies, HM are expected as enhancers of the body's immunity.7 TJ‐41 was originally used to enhance digestive function and maintain homeostasis.16 Recently, the action mechanisms of TJ‐41 were reported in some studies, and its immunostimulative ability came to attract attention. Several reports demonstrated that TJ‐41 enhances NK cell activity, activates anticancer immunity, and defends against infections in animal models.8, 9, 10, 13, 17 TJ‐25 has been known as a HM that improves impaired microcirculation and congestive conditions. Based on these functions, we investigated the immunological and clinical efficacy of HM using TJ‐41 and TJ‐25 in combination therapy with PPV for CRPC patients in this randomized phase II study.
In the present study, the combined use of HM with PPV was well tolerated without severe AE, and reduced anorexia, but it did not improve indices of immune function such as antigen‐specific IgG or CTL. PFS and OS were not significantly extended. In contrast, this study demonstrated that the combination therapy of PPV with HM stabilized the frequency of Mo‐MDSC and serum levels of IL‐6 after the treatment. Mo‐MDSC is one of the major groups of MDSC and has immunosuppressive activity. Mo‐MDSC were previously detected in the serum of patients with prostate cancer and were correlated with levels of PSA, which is used as a marker of tumor burden.18 The immune inhibition caused by MDSC in the cancer‐bearing patients was reported in a number of studies. It was also reported that MDSC correlated with the clinical cancer stage in patients with solid tumors.19 Our results demonstrated that patients with increased Mo‐MDSC after the treatment had shorter survival than those without increased Mo‐MDSC, and the frequency of Mo‐MDSC was stabilized during the combination therapy of PPV with HM. Hoelen (Poria cocos) is one of the main components of TJ‐25. It is a type of mushroom and is reported to have antitumor effects.20 β‐Glucan levels are high in mushrooms and were reported to enhance antitumor immune responses by regulating differentiation and function of Mo‐MDSC.21 Mushroom powder was also reported to decrease MDSC and PSA levels in patients with biochemically recurrent prostate cancer.22 These findings suggest that HM inhibit the increase of Mo‐MDSC under immunotherapy with survival benefits. However, more research is needed to validate the findings of the present study.
It should also be noted that the increase in IL‐6 was stabilized by combined usage of TJ‐41 and TJ‐25. TJ‐41 showed a preventive effect on surgical stress‐induced immunosuppression by inhibiting the elevation of IL‐6.17 IL‐6 is a representative inflammatory cytokine. IL‐6 affects the biological activity of the cancer cell as well as inflammation.23, 24, 25 Chronic inflammation may promote the development, progression, and immunosuppression of cancers.25, 26 Constitutional activation of STAT3 is associated with the transition to castration‐resistance of prostate cancer and IL‐6 is a major activator of JAK/STAT3 signaling.23, 24 STAT3 is considered to be the main transcription regulator in the expansion of MDSC.27 These results suggest that IL‐6 is crucial for aggressive tumor growth and the transition to castration‐resistance of prostate cancer by the dual effects of IL‐6 on tumor cells and MDSC expansion.28 A recent report showed that IL‐6 and MDSC have positive correlation.29 We previously reported that increased IL‐6 levels before PPV was an unfavorable factor for OS in docetaxel‐based chemotherapy‐resistant CRPC patients (HR, 0.024; 95% CI, 0.001–0.499; P = 0.0161).6 Therefore, the stabilization effects of TJ‐41 and TJ‐25 on increased IL‐6 levels may be beneficial for controlling prostate cancer progression.
In conclusion, this study demonstrated that combination therapy of PPV with HM is well tolerated and, although the combination with HM had no significant impact on antigen‐specific IgG, CTL, PFS, or OS, PPV with combined use of HM stabilized the increase of Mo‐MDSC and IL‐6 in patients with CRPC during PPV treatment. These results suggest that the combined use of HM has the potential to prevent immunosuppression induced by Mo‐MDSC or IL‐6 during immunotherapy.
Conflicts of Interest
Noguchi M has served as an advisory board consultant for Green Peptide Co. Ltd. Itoh K has served as a consultant and received research funding from Taiho Pharmaceutical Company. Yamada A is a part‐time executive of Green Peptide Co. Ltd and has stock in this company. Koga N, Moriya F and Waki K declare no competing interests.
Abbreviations
- ADT
androgen‐deprivation therapy
- AE
adverse event
- CI
confidence interval
- CRPC
castration‐resistant prostate cancer
- CT
computed tomography
- ELISPOT
Enzyme‐Linked ImmunoSpot
- FIU
fluorescence intensity units
- HLA
human leukocyte antigen
- HM
herbal medicine
- HR
hazard ratio
- IFN‐γ
interferon‐γ
- IL
interleukin
- IQR
interquartile range
- MDSC
myeloid‐derived suppressor cells
- Mo‐MDSC
monocytic myeloid‐derived suppressor cells
- NK
natural killer
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PPV
personalized peptide vaccine
- PS
performance status
- PSA
prostate‐specific antigen
- TAA
tumor‐associated antigen
- TME
tumor microenvironment
- Treg
regulatory T cells
Supporting information
Table S1. Peptide‐specific immune responses in each patient.
Cancer Sci 108 (2017) 2326–2332
Funding information
None.
References
- 1. Rekoske BT, McNeel DG. Immunotherapy for prostate cancer: false promises or true hope. Cancer 2016; 122: 3598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sasada T, Yamada A, Noguchi M, Itoh K. Personalized peptide vaccine for treatment of advanced cancer. Curr Med Chem 2014; 21: 2332–45. [DOI] [PubMed] [Google Scholar]
- 3. Noguchi M, Sasada T, Itoh K. Personalized peptide vaccination: a new approach for advanced cancer as therapeutic cancer vaccine. Cancer Immunol Immunother 2013; 62: 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noguchi M, Kakuma T, Uemura H et al A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother 2010; 59: 1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noguchi M, Uemura H, Naito S et al A phase I study of personalized peptide vaccination using 14 kinds of vaccine in combination with low‐dose estramustine in HLA‐A24‐positive patients with castration‐resistant prostate cancer. Prostate 2011; 71: 470–9. [DOI] [PubMed] [Google Scholar]
- 6. Noguchi M, Moriya F, Suekane S et al Phase II study of personalized peptide vaccination for castration‐resistant prostate cancer patients who failed in docetaxel‐based chemotherapy. Prostate 2012; 72: 834–45. [DOI] [PubMed] [Google Scholar]
- 7. Bodeker G. Integrative oncology meets immunotherapy: new prospects for combination therapy grounded in eastern medical knowledge. Chin J Integr Med 2012; 18: 652–62. [DOI] [PubMed] [Google Scholar]
- 8. Utsuyama M, Seidlar H, Kitagawa M et al Immunological restoration and anti‐tumor effect by Japanese herbal medicine in aged mice. Mech Ageing Dev 2001; 122: 341–52. [DOI] [PubMed] [Google Scholar]
- 9. Abe S, Tansho S, Ishibashi H et al Protection of immunosuppressed mice from lethal Candida infection by oral administration of a Kampo medicine. Hochu‐ekki‐to. Immunopharmacol Immunotoxicol 1999; 21: 331–42. [DOI] [PubMed] [Google Scholar]
- 10. Cho JM, Sato N, Kikuchi K. Prophylactic anti‐tumor effect of Hochu‐ekki‐to (TJ41) by enhancing natural killer cell activity. In Vivo 1991; 5: 389–91. [PubMed] [Google Scholar]
- 11. Yasui H. Guide for Clinical Application of Kampo Prescription, ver. 1. Tokyo: Korin, 2004; 92–3. [Google Scholar]
- 12. Noguchi M, Moriya F, Koga N et al A randomized phase II clinical trial of personalized peptide vaccination with metronomic low‐dose cyclophosphamide in patients with metastatic castration‐resistant prostate cancer. Cancer Immunol Immunother 2016; 65: 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mori K, Kido T, Daikuhara H et al Effect of Hochu‐ekki‐to (TJ‐41): a Japanese herbal medicine, on the survival of mice infected with influenza virus. Anti‐viral Res 1999; 44: 103–11. [DOI] [PubMed] [Google Scholar]
- 14. Noguchi M, Ikarashi Y, Yuzurihara M et al Effects of the Japanese herbal medicine Keishi‐bukuryo‐gan and 17β‐estradiol on calcitonin gene‐related peptide‐induced elevation of skin temperature in ovariectomized rats. J Endocrinol 2003; 176: 359–66. [DOI] [PubMed] [Google Scholar]
- 15. Komatsu N, Shichijo S, Nakagawa M et al New multiplexed flow cytometric assay to measure antipeptide antibody: a novel tool for monitoring immune responses to peptides used for immunization. Scand J Clin Lab Invest 2004; 64: 535–45. [DOI] [PubMed] [Google Scholar]
- 16. Kuroiwa A. Liou. Hochu‐ekki‐to. J Integr Med 2005; 15: 79–81. [Google Scholar]
- 17. Kimura M, Sasada T, Kanai M et al Preventive effect of a traditional herbal medicine, hochu‐ekki‐to, on immunosuppression induced surgical stress. Surg Today 2008; 38: 316–22. [DOI] [PubMed] [Google Scholar]
- 18. Vuk‐Pavlovic S, Bulur PA, Lin Y et al Immunosuppressive CD14 + HLA‐DRlow/‐ monocytes in prostate cancer. Prostate 2010; 70: 443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diaz‐Montero CM, Salem ML, Nishimura MI et al Increased circulating myeloid‐derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin‐cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009; 58: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qi F, Zhao L, Zhou A et al The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Bioscience Trends 2015; 9: 16–34. [DOI] [PubMed] [Google Scholar]
- 21. Tian J, Ma J, Ma K et al β‐Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid‐derived suppressor cells. Eur J Immunol 2013; 43: 1220–30. [DOI] [PubMed] [Google Scholar]
- 22. Twardowski P, Kanaya N, Frankel P et al A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: roles of cytokines and myeloid‐derived suppressor cells for Agaricus bisporus‐induced prostate‐specific antigen responses. Cancer 2015; 12: 2942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kishimoto T. Interleukin‐6: from basic science to medicine‐40 years in immunology. Annu Rev Immunol 2005; 23: 1–21. [DOI] [PubMed] [Google Scholar]
- 24. Schafer ZT, Brugge JS. IL‐6 involvement in epithelial cancer. J Clin Invest 2007; 117: 3660–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357: 539–45. [DOI] [PubMed] [Google Scholar]
- 27. Gabriovich DI, Nagaraj S. Myeloid‐derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9: 162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu CT, Hsieh CC, Lin CC et al Significance of IL‐6 in the transition of hormone‐resistant prostate cancer and the induction of myeloid‐derived suppressor cells. J Mol Med 2012; 90: 1343–55. [DOI] [PubMed] [Google Scholar]
- 29. Gonda K, Shibata M, Ohtake T et al Myeloid‐derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncol Lett 2017; 14: 1766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Peptide‐specific immune responses in each patient.


