Abstract
There is increasing evidence that cytoskeleton remodeling is involved in cancer progression. Wiskott‐Aldrich syndrome protein (WASP) family represents a key regulator of actin cytoskeleton remodeling. However, the underlying mechanism of the WASP family in cancer progression remains elusive. Here, we studied the role of WASP and SCAR Homolog (WASH), a recently identified WASP family member, in human esophageal squamous cell carcinoma (ESCC). Using three human ESCC cell lines, we found that WASH expression was significantly elevated in cancer stem‐like cells enriched by sphere formation assay. WASH knockdown decreased the sphere‐forming capacity of esophageal cancer cells whereas WASH over‐expression exhibited the opposite effect. Mechanistically, we identified interleukin‐8 (IL‐8) as a key downstream target of WASH. IL‐8 knockdown completely attenuated tumor sphere formation induced by WASH overexpression. WASH knockdown also delayed the growth of human ESCC xenografts in BALB/c nude mice. Importantly, high WASH levels were associated with poor clinical prognosis in a total of 145 human ESCC tissues. Collectively, our results suggest an essential role of the WASH/IL‐8 pathway in human ESCC by maintaining the stemness of cancer cells. Hence, targeting this pathway might represent a promising strategy to control human esophageal carcinoma.
Keywords: Cancer stem‐like cell, esophageal squamous cell carcinoma, interleukin‐8, prognosis, Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH)
Esophageal carcinoma is one of the leading causes of cancer mortality worldwide, with approximately half of all cases occurring in China. Esophageal squamous cell carcinoma (ESCC) is the most common pathological type of the disease in central and south‐eastern Asia whereas esophageal adenocarcinoma (EAC) is more common in Western countries.1 The poor prognosis of esophageal cancer is largely as a result of local invasion and distant metastasis. Cancer stem‐like cells (CSC) are a small subpopulation of cancer cells with self‐renewal and differentiation potential, providing one explanation for cancer relapse, metastasis, and drug resistance.2 With various methods for identification, CSC have been confirmed in many types of solid tumors, including cancer of the brain, breast, lung, pancreas, liver, esophagus, stomach, colon and rectum.3 Thus, it is of great importance to elucidate the driving mechanism of CSC activity in esophageal cancer for developing novel therapeutics.
Cancer stem‐like cells often have increased motility that allow them to invade the surrounding environment prior to metastasis. Reorganization of the cytoskeleton is crucial for the conformation changes and invasive properties of cancer cells.4 Wiskott‐Aldrich syndrome protein (WASP) family members, including WASP, N‐WASP, WAVE1‐3 and WHAMM, are structurally related and responsible for actin remodeling through activation of the actin‐related protein 2/3 (Arp2/3) complex.5 Thus, WASP family proteins may promote the aggressiveness of certain malignancies by regulation of cytoskeletal dynamics. For example, strong WAVE2 expression was found in hepatocellular carcinoma and correlated with a poorer clinical outcome.6 In glioma, N‐WASP has exhibited an association with low‐oxygen‐induced brain invasion.7 However, the roles of the WASP family proteins in cancer progression are still far from elucidated.
Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH), a more recently identified member of the WASP family, is highly conserved and essential for development.8, 9 Similar to other WASP family members, WASH acts as the downstream target of Rho family GTPase and activates actin filament nucleation through the Arp2/3 complex.10 Numerous studies have demonstrated that WASH has many important functions in physiological conditions, such as endosomal sorting, autophagy, hematopoiesis and natural killer (NK) cell cytotoxicity.11, 12, 13 However, very little is known about the precise function of WASH in cancer cells. In the present study, we identify a novel important role of WASH in maintaining stem cell‐like properties of human esophageal cancer cells by the interleukin‐8 (IL‐8) pathway. High levels of WASH expression are closely associated with poor prognosis of ESCC patients. Our data suggest that WASH drives IL‐8 secretion to promote malignant progression, likely serving as a potential therapeutic target for esophageal carcinoma.
Materials and Methods
Cell lines and cell cultures
Human ESCC cell lines were obtained from the Cancer Hospital of the Peking Union Medical College (Beijing, China) and human embryonic kidney epithelial 293T cells were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). All cell lines were maintained in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (HyClone, Logan, UT, USA), 100 units/mL penicillin and 100 μg/mL streptomycin.
RNA extraction and real‐time PCR
Total RNA was extracted from cancer cell lines or tumor tissue using TRIzol® reagent (Takara Bio, Kusatsu, Japan) according to the manufacturer's instructions. Total RNA was quantified by a NanoDrop spectrophotometer (Thermo Fisher Scientific, Rockford, IL, USA), and 1 μg total RNA was used to generate cDNA using PrimeScript RT reagent Kit with gDNA Eraser (Takara Bio). For detection of mRNA, quantitative PCR (qPCR) reactions were carried out using specific primers and SYBR Green qPCR Master Mix (Roche, Mannheim, Germany) on Stratagene Mx3005P qPCR System (Agilent Technologies, Santa Clara, CA, USA). The sequence of PCR primers used in this study was as follows: WASH forward 5׳‐ GGTGCCTGAGATCCATGTTCC‐3′, reverse 5′‐CCCATCTTGGTCTACCTTGA‐3′; IL‐8 forward 5′‐GTTGTAGGGTTGCCAGATGC‐3′, reverse 5′‐TTCTCCCGTGCAATATCTAGG‐3′; Nanog forward 5′‐TTTGTGGGCCTGAAGAAAACT‐3′, reverse 5′‐AGGGCTGTCCTGAATAAGCAG‐3′; Oct‐4 forward 5′‐CTTGAATCCCGAATGGAAAGGG‐3′, reverse 5′‐GTGTATATCCCAGGGTGATCCTC‐3′; c‐Myc forward 5′‐GGCTCCTGGCAAAAGGTCA ‐3′, reverse 5′‐CTGCGTAGTTGTGCTGATGT‐3′; Sox‐2 forward 5′‐TGAGCGCCCTGCAGTACAA‐3′, reverse 5′‐GCTGCGAGTAGGACATGCTGTAG‐3′; KLF4 5′‐ACACAAAGAGTTCCCATCTCAAG‐3′, reverse 5′‐GGTAGTGCCTGGTCAGTTCATC‐3′; CD133 forward 5′‐AGTCGGAAACTGGCAGATAGC‐3′, reverse 5′‐GGTAGTGTTGTACTGGGCCAAT‐3′; GAPDH forward 5′‐GCACCGTCAAGGCTGAGAAC‐3′, reverse 5′‐TGGTGAAGACGCCAGTGGA‐3′.
Western blotting
Cells were lysed in ice‐cold lysis buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris, 1% NP‐40, 0.5% sodium deoxycholate, 0.1% SDS) plus protease inhibitor cocktail (Sigma‐Aldrich, St Louis, MO, USA). An equal amount of total protein from each sample (50 μg) was resolved on 10% SDS‐PAGE gel and then transferred to nitrocellulose membrane. The membrane was blocked with 5% non‐fat milk and then incubated with commercially available primary antibodies for the detection of WASH (Abcam, Cambridge, UK) and β‐actin (Cell Signaling Technology, Beverly, MA, USA), followed by the appropriate secondary antibodies (Cell Signaling Technology). Protein expression was detected using enhanced chemiluminescent HRP substrate (Thermo Fisher Scientific) and photographed with a Chemiluminescence Imaging System (Bio‐Rad Laboratories, Hercules, CA, USA).
RNA interference
For transient knockdown, siRNA designed and purchased from Genepharma Company (Shanghai, China) were transfected into target cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). Stable knockdown experiments were carried out using the lentiviral vector pSIN‐EF2‐IRES‐GFP. Lentiviral vectors containing scrambled shRNA or another WASH‐specific shRNA were packaged in 293T cells. For selection of stable transfection, GFP‐expressing cells were isolated by MoFlo XDP flow cytometer (Beckman Coulter, Danvers, MA, USA).
Sphere formation assay
Cancer cells were seeded into ultra‐low attachment culture plates (Corning, Bedford, MA, USA) at a density of 2000 cells/mL and cultured in serum‐free DMEM/F12 medium, containing 20 ng/mL epidermal growth factor (Sigma‐Aldrich), 20 ng/mL basic fibroblast growth factor (Sigma‐Aldrich) and 5 μg/mL insulin (Sigma‐Aldrich). After 5–7 days of culture, these cells can form stem‐like cell aggregates and the number of tumor spheres formed was counted under the microscope.
Plasmids and transfection
cDNA of full‐length human WASH was amplified by PCR and cloned into the mammalian expression vector pFlag‐cmv‐2. Successful cloning was verified by DNA sequencing. Transfection of plasmids was done using Lipofectamine 3000 reagent according to the manufacturer's instructions.
Human cytokine array
Cells were plated at a density of 5 × 103/well and cultured in a 24‐well plate overnight. The plate was replaced with fresh medium and incubated for 48 h. Supernatants were collected by centrifugation (1000 g, 10 min) and infiltration (0.22 μm). Secretion of various cytokines and chemokines was evaluated using human LEGENDplex array kits (BioLegend, San Diego, CA, USA) according to the manufacturer's protocol.
ELISA
Level of IL‐8 from cell culture supernatants was measured using the ELISA kilt (BioLegend) according to the manufacturer's instructions.
Tumor xenografts
All animal studies were carried out in accordance with the institutional guidelines for the care and use of experimental animals. Female 7‐week‐old BALB/c nude mice (Vital River Laboratory Animal Technology, Beijing, China) were randomly divided into two groups (n = 6 per group) and injected with the indicated tumor cells (5 × 106 per mouse). Tumor sizes were measured by calipers and tumor volumes were calculated using the formula: (length × width2)/2. At the end of the experiments, all animals were killed and the tumors were excised for examination.
Collection of samples from patients
All human ESCC tissue samples were obtained from the First Affiliated Hospital of Zhengzhou University, China. Protocols for collection of human specimens with informed consent were approved by the Institutional Ethical Committee of the First Affiliated Hospital of Zhengzhou University, China. We collected 80 fresh ESCC tissues with paired adjacent normal tissues to examine WASH expression at mRNA level, and 65 formalin‐fixed paraffin‐embedded ESCC tissue samples to examine WASH expression at protein level.
Immunohistochemistry (IHC)
Detection of WASH expression was carried out on human ESCC sections by IHC staining as previously described.14 Anti‐WASH rabbit monoclonal antibody (Abcam) was used at a dilution of 1:100. Results of IHC staining were evaluated and scored by two individuals. Proportion of stained tumor cells was graded as follows: 0 (no positive tumor cells), 1 (<10% positive tumor cells), 2 (10–50% positive tumor cells), and 3 (>50% positive tumor cells). Intensity of WASH expression was evaluated at four levels: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). Staining index (SI) was calculated as staining intensity × proportion of positive tumor cells, resulting in scores of 0, 1, 2, 3, 4, 6 and 9. An SI score ≥4 was considered high expression, and an SI score ≤3 was considered low expression.
Statistical analysis
Student's t‐test was used for comparison between two groups. WASH expression with overall survival was tested suing log–rank and Cox proportional hazards regression analysis. All tests were two‐sided and differences were considered significant when P < 0.05. Data are presented as the mean ± SD from at least two to three independent experiments.
Results
WASH expression is significantly elevated in human esophageal CSC
To examine whether cancer cells and CSC differ in the expression of WASH, we first established a method to enrich human esophageal CSC. Using sphere formation assays, we screened six human ESCC cell lines (EC1, EC109, KYSE450, KYSE70, TE1, and TE7) and found three of them (KYSE70, KYSE450 and TE1) with the ability to form typical tumor spheres in vitro (Fig. 1a). The stem‐like properties of tumor spheres were validated by increased expression of stemness genes, such as Sox‐2, Nanog and KLF4 (Fig. 1b). Interestingly, WASH mRNA expression was significantly higher in spheroid cells as compared to adherent monolayer counterparts (Fig. 1c). In addition, elevated WASH expression in these esophageal tumor spheres was further validated at protein level by Western blotting (Fig. 1d). The results suggest a potential role of WASH in CSC derived from human ESCC cell lines.
Figure 1.
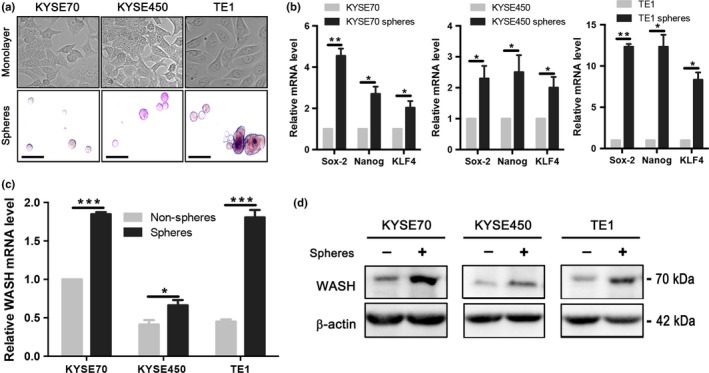
Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) is highly expressed in spheres derived from human esophageal squamous cell carcinoma (ESCC) cell lines. (a) Sphere formation of three human ESCC cell lines (KYSE70, KYSE450 and TE1). Scale bars, 500 μm. (b) Validation of stemness gene expression (Sox‐2, Nanog and KLF4) in ESCC spheres by qPCR. (c) qPCR was used to detect mRNA expression of WASH in three ESCC cell lines and their derived spheres. (d) Western blotting was used to detect protein expression of WASH. Mean ± SD of relative fold changes from triplicate experiments was plotted. *P < 0.05, **P < 0.01, ***P < 0.001.
WASH promotes the sphere formation of esophageal cancer cells in vitro
To test whether WASH, indeed, regulates esophageal CSC, KYSE70 cells that had the highest expression of WASH were transfected with specific siRNA targeting WASH (siWASH) or control siRNA (siCTRL). Knockdown efficiency of WASH expression was confirmed by qPCR (Fig. 2a) and Western blotting (Fig. 2b). Silencing of WASH expression had little impact on proliferation and survival of esophageal cancer cells in vitro (2Fig. S1). However, inhibition of WASH expression by siRNA interference significantly impaired the sphere formation of KYSE70 cells (Fig. 2c). We next evaluated the impact of WASH knockdown on transcription of stemness‐related genes and markers. As expected, WASH knockdown repressed the transcription of stemness genes, such as Nanog, OCT‐4, c‐Myc and Sox‐2, as well as surface markers of CSC, such as CD133 (Fig. 2d).
Figure 2.
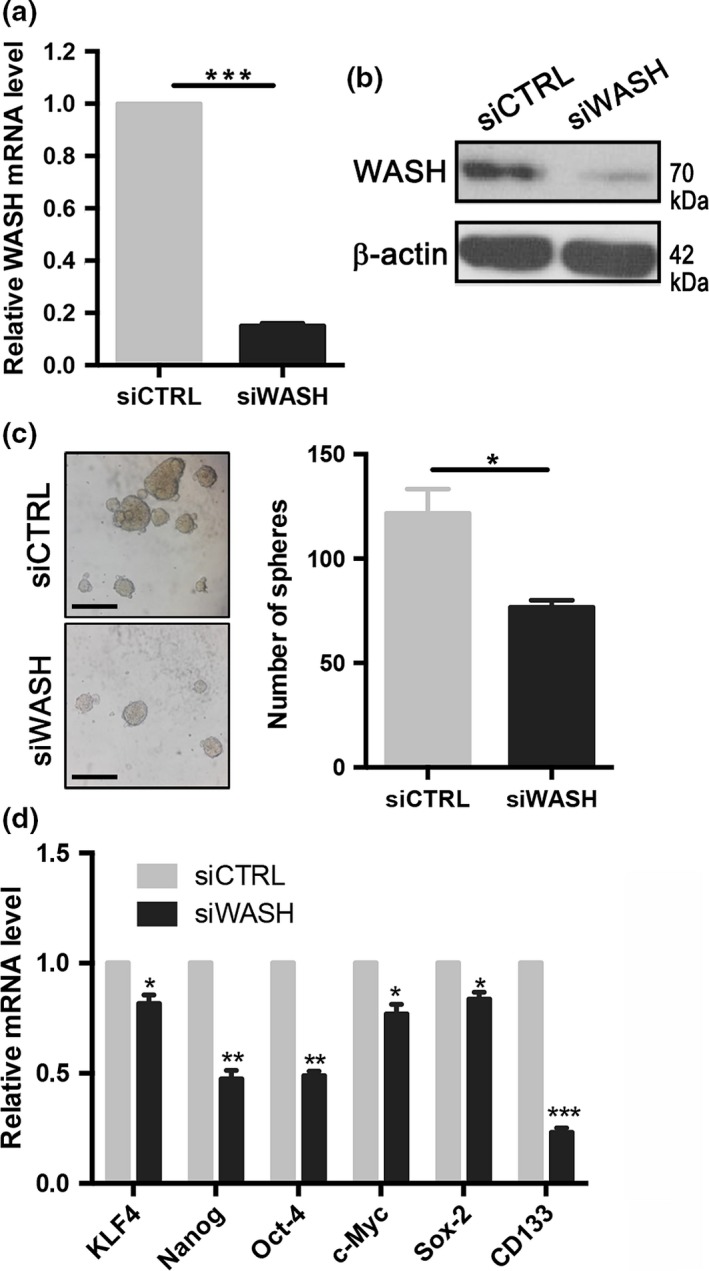
Knockdown of Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) impairs stem cell‐like characteristics of human esophageal squamous cell carcinoma (ESCC) cell line. KYSE70 cells were transfected with control siRNA (siCTRL) or WASH‐specific siRNA (siWASH) for 48–72 h. (a) qPCR was used to detect mRNA expression of WASH. (b) Western blotting was used for detection of WASH protein. β‐actin as a loading control. (c) Sphere formation assay in KYSE70 cells transfected with siCTRL or siWASH. Scale bars, 500 μm. (d) Stemness gene expression was examined by qPCR in KYSE70 cells transfected with siCTRL or siWASH. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
To further confirm the effect of WASH overexpression on esophageal CSC, two human ESCC cell lines (KYSE450 and TE1) that had low endogenous WASH expression were transfected with Flag‐tagged WASH‐expressing (Flag‐WASH) or control (Flag‐CTRL) plasmids. After 48 h of transient transfection, WASH overexpression was confirmed in KYSE450 cells and TE1 cells by qPCR (Fig. 3a) and Western blotting (Fig. 3b), respectively. Consistently, overexpression of WASH promoted sphere formation in both ESCC cell lines (Fig. 3c). Also, WASH overexpression resulted in upregulation of stemness‐related genes in both KYSE450 and TE1 cells (Fig. 3d).
Figure 3.
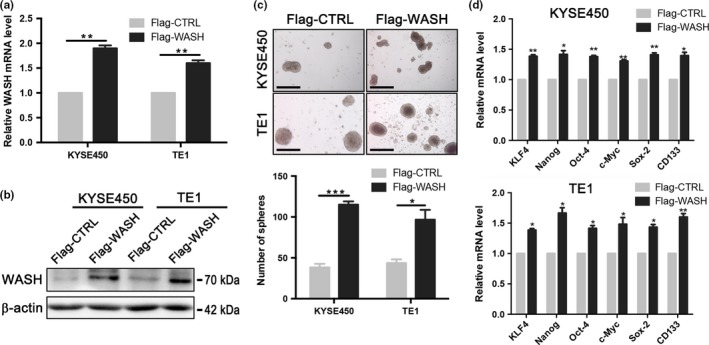
Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) overexpression promotes sphere formation of human esophageal squamous cell carcinoma (ESCC) cell lines. KYSE450 and TE1 cells were transfected with Flag‐tagged WASH‐expressing vector (Flag‐WASH) or control vector (Flag‐CTRL) for 48 h. (a) qPCR was used to detect mRNA expression of WASH. (b) Western blotting was used for detection of WASH protein. β‐actin as a loading control. (c) Sphere formation assay in KYSE70 cells transfected with Flag‐CTRL or Flag‐WASH. Scale bars, 500 μm. (d) Stemness gene expression in KYSE70 cells transfected with Flag‐CTRL or Flag‐WASH. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Similarly, knockdown of WASH expression in KYSE450 cells or TE1 cells impairs cancer stemness properties (Fig. S2a) whereas upregulation of WASH expression in KYSE70 cells promotes the capability of sphere formation (Fig. S2b). Taken together, these results support a functional role of WASH expression in maintenance of esophageal CSC.
Inhibition of WASH expression affects sphere formation of esophageal cancer cells through the IL‐8 pathway
To explore the underlying mechanism, we assessed the profiling of chemokines and cytokines in esophageal cancer cells after silencing of WASH expression. Using multiplex ELISA assays, we found that CXCL8 (also called IL‐8) was the highest level of cytokine produced in KYSE70 cells and stable WASH knockdown remarkably blocked the secretion of IL‐8 (Fig. 4a). This finding was further confirmed by qPCR (Fig. 4b) and ELISA assays (Fig. 4c), which showed the essential role of WASH in IL‐8 secretion. However, inhibition of IL‐8 expression had little impact on WASH expression (data not shown). The data suggest that IL‐8 is a downstream target of the WASH signaling pathway.
Figure 4.
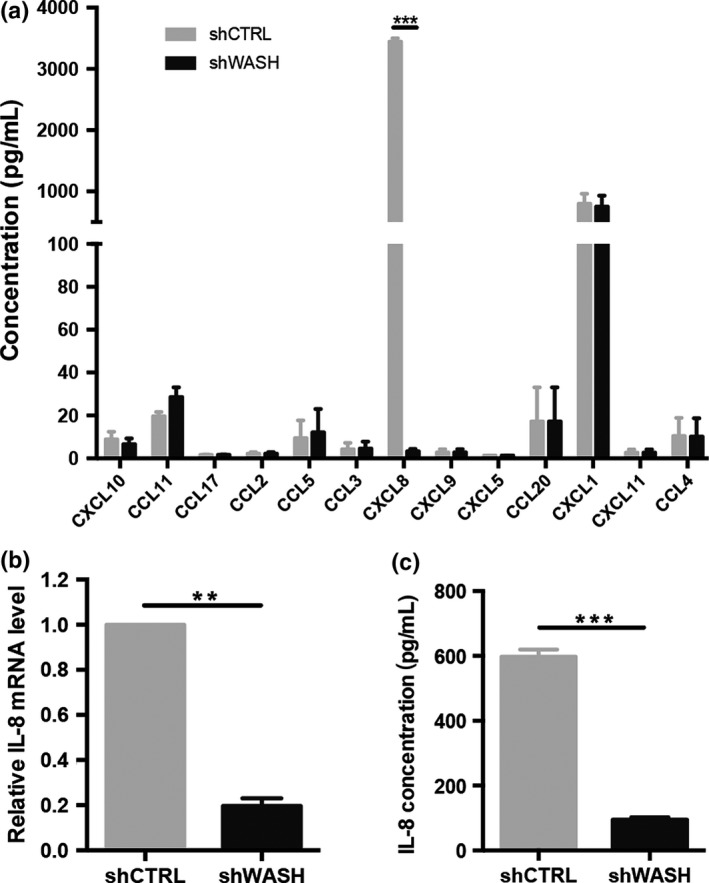
Suppression of Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) blocks interleukin (IL)‐8 expression. (a) Supernatants were collected from KYSE70 cells stably transduced with control shRNA (shCTRL) or another WASH‐specific shRNA (shWASH), and then screened by human chemokine assay. (b) IL‐8 expression in KYSE70 cells stably transduced with shCTRL or shWASH was evaluated by qPCR. (c) IL‐8 secretion by KYSE70 cells stably transduced with shCTRL or shWASH was confirmed by ELISA. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001.
We subsequently investigated the effect of IL‐8 on sphere formation in KYSE450 and TE1 cells, especially under the condition of WASH overexpression. As shown in Figure 5, blockade of IL‐8 expression by siRNA treatment significantly decreased sphere formation in both ESCC cell lines. More importantly, loss of IL‐8 expression completely blocked the sphere formation induced by WASH over‐expression. IL‐8 knockdown also affects the stemness of KYSE70 cells (Fig. S3a). In contrast, exogenous IL‐8 enhances sphere formation of ESCC cell lines (Fig. S3b). These data demonstrate that WASH regulate human esophageal CSC through the IL‐8 pathway.
Figure 5.
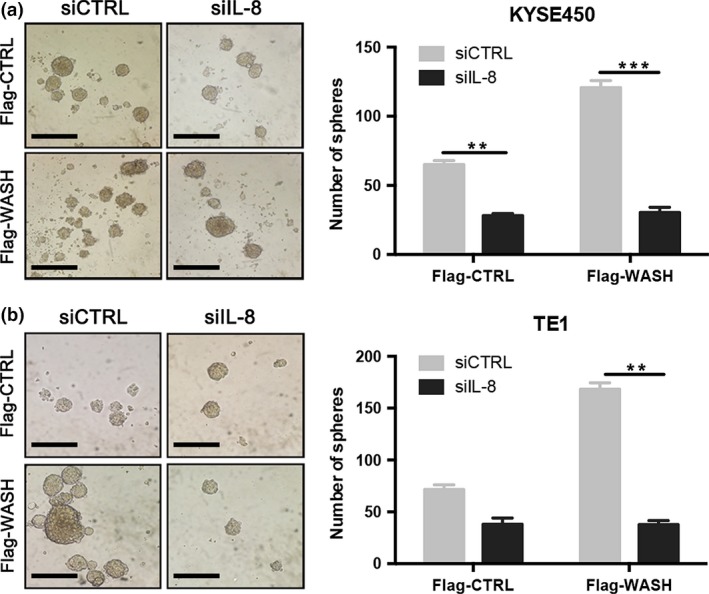
Knockdown of Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) affects sphere formation of esophageal cancer cells by the interleukin (IL)‐8 pathway. (a) KYSE450 cells or (b) TE1 cells were transfected with Flag‐tagged WASH‐expressing vector (Flag‐WASH) or control vector (Flag‐CTRL) in the presence of control siRNA (siCTRL) or IL‐8 siRNA (siIL‐8), and then used for sphere formation assay. Spheres were photographed and counted. Scale bars, 500 μm. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001.
Stable WASH knockdown decreases human esophageal cancer growth in vivo
To directly assess the role of WASH in tumor growth, KYSE70 cells stably transduced with control shRNA (shCTRL) or another WASH‐specific shRNA (shWASH) were s.c. inoculated into BALB/c nude mice. As shown in Figure 6(a), knockdown of WASH expression significantly delayed tumor growth in vivo. By 28 days of tumor challenge, KYSE70 cells with stable WASH knockdown developed much smaller tumors compared with control cells (Fig. 6b). Loss of WASH expression was confirmed in tumors isolated from the shWASH group of mice (Fig. 6c). Consistent with the in vitro results, knockdown of WASH expression inhibited tumor expression of IL‐8 (Fig. 6d) and several stemness genes (Fig. 6e). Together, these findings indicated that WASH inhibition reduced human esophageal cancer progression in vivo.
Figure 6.

Suppression of Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) expression decreases esophageal tumor growth in vivo. Nude mice were injected with KYSE70 cells stably transduced with control shRNA (shCTRL) or WASH‐specific shRNA (shWASH). (a) Average sizes of xenograft tumors were measured every 4 days (n = 6). (b) Tumor weight of xenografts harvested at day 28 after tumor challenge. (c) Validation of WASH, (d) IL‐8 and (e) stemness gene expression in xenograft tumor tissues by qPCR. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.
WASH overexpression is a poor prognostic factor for patients with esophageal carcinoma
To study the clinical relevance, we first examined WASH mRNA expression in 80 fresh human ESCC samples with paired adjacent normal tissues using qPCR. As shown in Figure 7(a), expression levels of WASH mRNA were significantly higher in human ESCC tissues as compared to adjacent normal tissues (P < 0.001). We next analyzed the differences of WASH mRNA expression according to pathological parameters. WASH mRNA expression was associated with tumor stage (P < 0.01, Fig. 7b). However, there were no significant correlations between WASH mRNA levels and other clinical parameters, including age, gender, grade and metastasis (data not shown).
Figure 7.
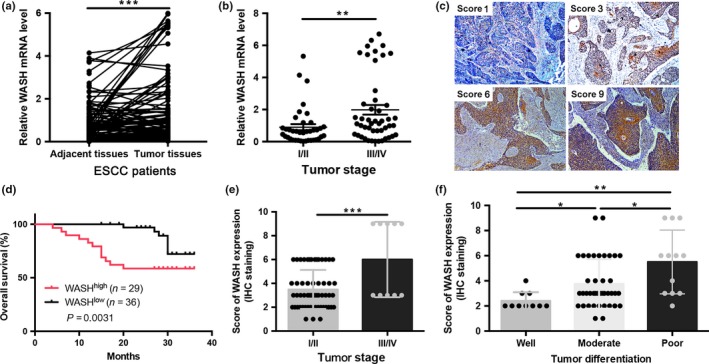
High levels of Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) expression in primary esophageal squamous cell carcinoma (ESCC) predicts poor clinical outcome. (a) Relative mRNA levels of WASH expression in human ESCC tissues and adjacent normal tissues. (b) Comparison of WASH mRNA expression between ESCC patients at stage I/II and stage III/IV. (c) Immunohistochemistry (IHC) staining of ESCC specimens with antibody specific for WASH. Original magnification, ×200. (d) Kaplan–Meier analysis of overall survival was stratified by expression levels of WASH protein expression, with high (red) or low (black) WASH expression. (e) Comparison of WASH protein expression detected by IHC between patients at stage I/II or stage III/IV. (f) Comparison of WASH protein expression detected by IHC among patients with well to poor tumor cell differentiation. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
More specifically, IHC analysis confirmed that WASH protein is highly expressed in primary tumor cells of ESCC patients (Fig. 7c). In a set of 65 ESCC patients for whom overall survival data were available, patients with higher WASH protein expression had shorter overall survival than those expressing lower levels of WASH protein (log–rank test, P = 0.0031; Fig. 7d). In parallel, WASH expression detected by IHC staining was associated with tumor stage (Fig. 7e) and cancer cell differentiation (Fig. 7f). These data demonstrate that WASH is markedly upregulated in human ESCC and correlates with overall survival.
Discussion
Dynamic remodeling of actin cytoskeleton contributes to a wide range of important cellular processes, including cell migration and invasion.15 It has been shown that aberrant expression of WASP family members such as WAVE2, N‐WASP and WASF2 is sufficient to drive the metastasis of various cancers.16, 17 WASH is a novel WASP family member and also has an essential role in actin assembly. However, the role of WASH in cancer progression has not been characterized previously. Our work has discovered that WASH overexpression promotes the sphere formation of esophageal cancer cells by IL‐8 signaling. We also found that high WASH overexpression correlates with worse clinical outcome of ESCC patients. These data provide a rationale to target the WASH/IL‐8 pathway as a novel therapeutic strategy for esophageal cancer.
Wiskott‐Aldrich syndrome protein and SCAR Homolog has previously been described as a scaffold to coordinate signals from Rho GTPase and actin nucleators for actin and microtubule cytoskeleton dynamics.18 Malignant cells form aberrant actin‐based protrusions such as invadopodia to initiate invasion and metastasis.19 The hypothesis of CSC is attractive because it may explain treatment resistance and distant metastasis of cancer patients.20, 21 Thus, we first characterized the expression of WASH in CSC of esophageal cancer. CSC can be enriched by various approaches, including FACS with antibodies against specific cell‐surface markers or side population assay. In this study, tumor spheres were prepared from human ESCC cell lines in a serum‐free medium commonly used to support the growth of CSC. The tumor spheres exhibited higher levels of stem‐cell gene expression, confirming the characteristics of CSC.22 We observed upregulation of WASH expression at both RNA and protein levels in esophageal tumor spheres. Loss‐ and gain‐of‐function studies further demonstrated an important role of WASH in maintenance of esophageal CSC. In fact, recent data show that WASP‐interacting protein (WIP), an actin polymerization regulator, is responsible for mutant p53 oncogenic properties in CSC.23 Further studies are needed to elucidate the link between WASH‐mediated cytoskeleton remodeling and maintenance of CSC.
Besides an important regulator of actin assembly, WASP also serves as an adaptor to affect the activation of various downstream signaling.24 In the current study, we found that knockdown of WASH significantly repressed the expression of IL‐8 in ESCC cell lines, suggesting an essential role of WASH in regulating the IL‐8 pathway. IL‐8, also known as CXCL8, is a proinflammatory chemokine originally identified as a leukocyte chemoattractant, contributing to neutrophil chemotaxis.25 However, independent studies have shown the effect of IL‐8 on CSC phenotype and function. For example, IL‐8 was found to be overexpressed in CSC isolated from liver cancer cell lines or clinical samples.26 Also, recombinant IL‐8 was reported to enhance ex vivo sphere formation of breast cancer.27 Moreover, a preclinical study showed that blockade of IL‐8 receptor was capable of targeting breast cancer stem cells in xenograft models.28 Recent studies have shown that IL‐8 could induce CSC activity through activation of Akt/Slug pathways.29 Our study raises the possibility that IL‐8 is a downstream target of WASH, a relevant pathway of WASH‐mediated tumorigenesis. The mechanism through which WASH regulates the production of IL‐8 remains unknown.
Our esophageal tumor xenograft experiments indicated that WASH has a profound impact on tumor growth. Given little effect of WASH knockdown on tumor cell growth in vitro, it is more likely that loss of WASH expression affects tumor growth in vivo as a result of dysfunction of IL‐8 signaling and stemness maintenance in the tumor microenvironment. Numerous studies have revealed that IL‐8 can facilitate tumor initiation, maintenance and metastasis by promotion of CSC properties.30 Our observations in both cancer cells and immunocompromised mice support a direct function of IL‐18 on tumor cells in an autocrine way. However, it should be noted that IL‐8 is an inflammatory chemokine and can also recruit suppressive immune cells to inhibit antitumor response.31 In addition, a recent study showed that WASP has a role in promoting breast cancer metastasis through a leukocyte‐dependent method.32 Thus, the involvement of immune cells in WASH‐induced esophageal cancer progression remains to be determined.
In line with previous reports of other WASP family proteins,33, 34 we observed high WASH expression in esophageal cancer specimens and its association with poor prognosis. It is possible that distinct WASP family proteins contribute to disease progression in various types of cancers. Consistently, high levels of IL‐8 are associated with poor clinical outcome in many types of human cancer.35 In addition to cancer cells, IL‐8 can be produced by other cell types in the tumor microenvironment, such as macrophage and endothelial cells. It needs further study to define the precise role of WASH‐mediated production of IL‐8 from cancer cells. Our results highlight an important role of WASH in the maintenance of CSC to promote aggressiveness of esophageal carcinoma. Thus, WASH expression has potential value in predicting clinical outcome of esophageal cancer patients.
In summary, our findings indicate that overexpression of WASH may promote the progression of esophageal cancer by the IL‐8 pathway. In light of clinical relevance, this pathway may become a potential therapeutic target for treatment of esophageal cancer.
Disclosure Statement
Authors declare no conflicts of interest for this article.
Supporting information
Fig. S1. Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) expression does not affect cell growth and survival of esophageal cancer cells. KYSE70 cells were transfected with control siRNA (siCTRL) or WASH‐specific siRNA (siWASH) for 72 h. (a) Cell viability was measured by CCK8 assay. (b) Annexin V‐PI staining was used to detect cell apoptosis. Data are presented as mean ± SD. NS, no significance by two‐tailed Student's t‐test.
Fig. S2. Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) expression regulates cancer stemness properties in esophageal squamous cell carcinoma (ESCC) cell lines. (a) Sphere formation assay in KYSE450 cells or TE1 cells transfected with control siRNA (siCTRL) or WASH‐specific siRNA (siWASH), respectively. (b) Sphere formation assay in KYSE70 cells transfected with Flag‐tagged control vector (Flag‐CTRL) or WASH‐expressing vector (Flag‐WASH). Scale bars, 500 μm. Data are presented as mean ± SD. **P < 0.01.
Fig. S3. Interleukin (IL)‐8 promotes cancer stemness in esophageal squamous cell carcinoma (ESCC) cell lines. (a) Sphere formation assay in KYSE70 cells transfected with control siRNA (siCTRL) or IL‐8‐specific siRNA (siIL‐8). (b) Sphere formation assay in KYSE450 cells or TE1 cells treated with 100 ng/mL IL‐8. Scale bars, 500 μm. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 31270946, 31100641) and the Science and Technology Department of Henan Province, China (No. 142300410387). We appreciate Dr Pingping Zhu (Institute of Biophysics, Chinese Academy of Sciences, China) for his technical expertise. We thank our laboratory members for their fruitful discussion.
Cancer Sci 108 (2017) 2358–2365
Funding Information
Science and Technology Department of Henan Province, China, (Grant/Award Number: ‘142300410387’) National Natural Science Foundation of China, (Grant/Award Number: ‘31100641’,’31270946’).
Contributor Information
Lan Huang, Email: lanhuang@zzu.edu.cn.
Yi Zhang, Email: yizhang@zzu.edu.cn.
References
- 1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013; 381: 400–12. [DOI] [PubMed] [Google Scholar]
- 2. Takebe N, Miele L, Harris PJ et al Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015; 12: 445–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawson DA, Bhakta NR, Kessenbrock K et al Single‐cell analysis reveals a stem‐cell program in human metastatic breast cancer cells. Nature 2015; 526: 131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neradil J, Veselska R. Nestin as a marker of cancer stem cells. Cancer Sci 2015; 106: 803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem 2010; 79: 707–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang LY, Tao YM, Ou DP, Wang W, Chang ZG, Wu F. Increased expression of Wiskott‐Aldrich syndrome protein family verprolin‐homologous protein 2 correlated with poor prognosis of hepatocellular carcinoma. Clin Cancer Res 2006; 12: 5673–9. [DOI] [PubMed] [Google Scholar]
- 7. Tang Z, Araysi LM, Fathallah‐Shaykh HM. c‐Src and neural Wiskott‐Aldrich syndrome protein (N‐WASP) promote low oxygen‐induced accelerated brain invasion by gliomas. PLoS One 2013; 8: e75436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Linardopoulou EV, Parghi SS, Friedman C, Osborn GE, Parkhurst SM, Trask BJ. Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet 2007; 3: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verboon JM, Rincon‐Arano H, Werwie TR et al Wash interacts with lamin and affects global nuclear organization. Curr Biol 2015; 25: 804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jia D, Gomez TS, Metlagel Z et al WASH and WAVE actin regulators of the Wiskott‐Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci USA 2010; 107: 10442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell 2009; 17: 712–23. [DOI] [PubMed] [Google Scholar]
- 12. Xia P, Wang S, Du Y et al WASH inhibits autophagy through suppression of Beclin 1 ubiquitination. EMBO J 2013; 32: 2685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang L, Zhu P, Xia P, Fan Z. WASH has a critical role in NK cell cytotoxicity through Lck‐mediated phosphorylation. Cell Death Dis 2016; 7: e2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu JY, Li F, Wang LP et al CTL‐ vs Treg lymphocyte‐attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinoma. Br J Cancer 2015; 113: 747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mouneimne G, Hansen SD, Selfors LM et al Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell 2012; 22: 615–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Semba S, Iwaya K, Matsubayashi J et al Coexpression of actin‐related protein 2 and Wiskott‐Aldrich syndrome family verproline‐homologous protein 2 in adenocarcinoma of the lung. Clin Cancer Res 2006; 12: 2449–54. [DOI] [PubMed] [Google Scholar]
- 17. Ziv‐Av A, Giladi ND, Lee HK et al RTVP‐1 regulates glioma cell migration and invasion via interaction with N‐WASP and hnRNPK. Oncotarget 2015; 6: 19826–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verboon JM, Rahe TK, Rodriguez‐Mesa E, Parkhurst SM. Wash functions downstream of Rho1 GTPase in a subset of Drosophila immune cell developmental migrations. Mol Biol Cell 2015; 26: 1665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paz H, Pathak N, Yang J. Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene 2014; 33: 4193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iinuma H, Watanabe T, Mimori K et al Clinical significance of circulating tumor cells, including cancer stem‐like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol 2011; 29: 1547–55. [DOI] [PubMed] [Google Scholar]
- 21. Yong KJ, Gao C, Lim JS et al Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N Engl J Med 2013; 368: 2266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K. Tumor‐derived spheroids: relevance to cancer stem cells and clinical applications. Cancer Sci 2017; 108: 283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Escoll M, Gargini R, Cuadrado A, Anton IM, Wandosell F. Mutant p53 oncogenic functions in cancer stem cells are regulated by WIP through YAP/TAZ. Oncogene 2017; 36: 3515–27. [DOI] [PubMed] [Google Scholar]
- 24. Huang W, Ochs HD, Dupont B, Vyas YM. The Wiskott‐Aldrich syndrome protein regulates nuclear translocation of NFAT2 and NF‐kappa B (RelA) independently of its role in filamentous actin polymerization and actin cytoskeletal rearrangement. J Immunol 2005; 174: 2602–11. [DOI] [PubMed] [Google Scholar]
- 25. Yoshimura T. Discovery of IL‐8/CXCL8 (The Story from Frederick). Front Immunol 2015; 6: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang KH, Ma S, Lee TK et al CD133(+) liver tumor‐initiating cells promote tumor angiogenesis, growth, and self‐renewal through neurotensin/interleukin‐8/CXCL1 signaling. Hepatology 2012; 55: 807–20. [DOI] [PubMed] [Google Scholar]
- 27. Charafe‐Jauffret E, Ginestier C, Iovino F et al Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 2009; 69: 1302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ginestier C, Liu S, Diebel ME et al CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest 2010; 120: 485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Visciano C, Liotti F, Prevete N et al Mast cells induce epithelial‐to‐mesenchymal transition and stem cell features in human thyroid cancer cells through an IL‐8‐Akt‐Slug pathway. Oncogene 2015; 34: 5175–86. [DOI] [PubMed] [Google Scholar]
- 30. Hwang WL, Yang MH, Tsai ML et al SNAIL regulates interleukin‐8 expression, stem cell‐like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology 2011; 141: 279–91. [DOI] [PubMed] [Google Scholar]
- 31. Asfaha S, Dubeykovskiy AN, Tomita H et al Mice that express human interleukin‐8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology 2013; 144: 155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishihara D, Dovas A, Hernandez L et al Wiskott‐Aldrich syndrome protein regulates leukocyte‐dependent breast cancer metastasis. Cell Rep 2013; 4: 429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo JC, Li J, Zhao YP et al N‐wasp in pancreatic ductal adenocarcinoma: associations with perineural invasion and poor prognosis. World J Surg 2014; 38: 2126–31. [DOI] [PubMed] [Google Scholar]
- 34. Kulkarni S, Augoff K, Rivera L et al Increased expression levels of WAVE3 are associated with the progression and metastasis of triple negative breast cancer. PLoS One 2012; 7: e42895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Q, Li A, Tian Y et al The CXCL8‐CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev 2016; 31: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) expression does not affect cell growth and survival of esophageal cancer cells. KYSE70 cells were transfected with control siRNA (siCTRL) or WASH‐specific siRNA (siWASH) for 72 h. (a) Cell viability was measured by CCK8 assay. (b) Annexin V‐PI staining was used to detect cell apoptosis. Data are presented as mean ± SD. NS, no significance by two‐tailed Student's t‐test.
Fig. S2. Wiskott‐Aldrich syndrome protein and SCAR Homolog (WASH) expression regulates cancer stemness properties in esophageal squamous cell carcinoma (ESCC) cell lines. (a) Sphere formation assay in KYSE450 cells or TE1 cells transfected with control siRNA (siCTRL) or WASH‐specific siRNA (siWASH), respectively. (b) Sphere formation assay in KYSE70 cells transfected with Flag‐tagged control vector (Flag‐CTRL) or WASH‐expressing vector (Flag‐WASH). Scale bars, 500 μm. Data are presented as mean ± SD. **P < 0.01.
Fig. S3. Interleukin (IL)‐8 promotes cancer stemness in esophageal squamous cell carcinoma (ESCC) cell lines. (a) Sphere formation assay in KYSE70 cells transfected with control siRNA (siCTRL) or IL‐8‐specific siRNA (siIL‐8). (b) Sphere formation assay in KYSE450 cells or TE1 cells treated with 100 ng/mL IL‐8. Scale bars, 500 μm. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.


