Abstract
Peritumoral microenvironment affects cancer development and chemoresistance, and visceral adipose tissue may play a critical role. We aimed to identify depot‐specific adipose characteristics associated with carcinogenesis and resistance to neoadjuvant therapy in esophageal adenocarcinoma (EAC). We analyzed: (i) the peritumoral adipose tissue of rats following the induction of esophageal carcinogenesis; (ii) the peritumoral and distal (omental) adipose tissue of patients affected by EAC; (iii) adipose‐derived stem cells (ADSC) isolated from healthy patients and treated with conditioned medium (CM), collected from tumoral and adipose tissue of patients with EAC. In peritumoral adipose tissue of rats, CD34, CD31 and vascular endothelial growth factor (VEGF) expression increased progressively during EAC development. In patients with EAC, expression of CD34, CD45, CD90 and nucleostemin (NSTM) was higher in peritumoral than in distal adipose tissue and decreased in the presence of neoadjuvant therapy. Moreover, expression of NSTM, octamer‐binding transcription factor 4 (OCT‐4) and VEGF was higher in peritumoral (but not in distal) adipose tissue of chemoresistant patients. In ADSC, treatment with peritumoral adipose tissue CM increased the adipogenic potential and the expression of CD34, CD90, NSTM and OCT‐4. These effects were similar to those induced by cancer‐derived CM, but were not observed in ADSC treated with distal adipose tissue CM and were partially reduced by a leptin antagonist. Last, ADSC treated with peritumoral CM of chemoresistant patients displayed increased expression of NSTM, OCT‐4, leptin, leptin receptor, alpha‐smooth muscle actin (α‐SMA), CD34 and VEGF. These results suggest that peritumoral adipose tissue may promote, by paracrine signaling, the expression of depot‐specific factors associated with therapeutic resistance.
Keywords: adipose tissue, esophageal adenocarcinoma, neoadjuvant therapy, peritumoral microenvironment, stem cell
Adipose tissue distribution can affect overall and disease‐free survival in gastric cancer1 and fat‐mass loss after neoadjuvant therapy seems to be associated with progression‐free survival in pancreatic cancer.2 Moreover, an excess of visceral adipose tissue is related to a poorer response to neoadjuvant therapy in breast cancer.3 It has been hypothesized that the pathophysiology of adipose tissue might influence the clinical outcome of patients, but no clear consensus exists on the pathways directly involved in this process.
We previously observed that peritumoral adipose tissue plays a role in lymph node involvement in esophageal adenocarcinoma (EAC)4 and some reports suggest that adipose derived stem cells (ADSC) may have a role in the interplay between obesity and cancer.5, 6 ADSC can differentiate into cancer‐associated fibroblasts under the influence of specific tumor‐derived factors7 and cumulative evidence supports the hypothesis that cancer‐associated adipose tissue represents a key component in carcinogenesis.8 On the basis of these reports, several studies investigated the relationship between the expression of specific genes in the tumor microenvironment and their potential association with chemotherapy.
CD34, a mesenchymal stem cell marker usually used to identify microvessel density in the tumor microenvironment, is reduced in breast cancer9 and prostate cancer10 after neoadjuvant treatment and is usually related to vascular endothelial growth factor (VEGF) expression, which negatively correlates with the response to neoadjuvant therapy in esophageal cancer tissue.11 CD90, another mesenchymal stem cell marker implicated in the regulation of fibrosis, is expressed by ADSC and is upregulated in cancer‐associated fibroblasts, and it has been shown to be related to recurrence in hepatocellular carcinoma12 and to survival in neuroblastoma.13 Nucleostemin (NSTM), a stemness marker expressed by cancer stem cells, is upregulated in recurrent esophageal carcinoma,14 in advanced malignant phenotype of oral squamous cell carcinoma (SCC)15 and in human breast cancer cells resistant to chemotherapy.16 Octamer‐binding transcription factor 4 (OCT‐4), expressed in adult stem cells and mesenchymal adipose tissue, has been considered essential for anti‐apoptotic behavior of chemoresistant cell lines.17 Moreover, OCT‐4 expression is increased in chemoresistant stem‐like cells with low reactive oxygen species and in tumor treated with neoadjuvant therapy.18 All these data suggest that specific cellular cross‐talk may be involved in tumor biology such as invasiveness and/or recurrence. However, although the expression of CD34, CD90, NSTM and OCT‐4 has been widely investigated in cancer cells, no data exist on their expression in the adipose tissue microenvironment of EAC.
Our hypotheses are that peritumoral adipose tissue plays a crucial role in tumor development through progressive involvement in neo‐angiogenesis throughout the carcinogenesis process, and through a continuous cross‐talk between cancer tissue and adipose stem cells surrounding EAC. Interaction between cancer cells and adipose cells may influence cancer behavior and its resistance to chemotherapy. Therefore, the aim of the present study was to investigate the cross‐talk between cancer tissue and peritumoral adipose tissue and to correlate this to the response to neoadjuvant therapy in patients with EAC.
Materials and Methods
Study design
The first step of the present study aimed to assess angiogenesis in periesophageal adipose tissue during esophageal carcinogenesis. A surgical rat model of gastrointestinal reflux was chosen to reproduce the different steps of esophageal carcinogenesis. Procedures involving animals and their care were conducted according the Ethical Committee of Animal Experimentation of University of Padua (Comitato Etico di Ateneo sulla Sperimentazione Animale – CEASA).
In the second step, we aimed to analyze peritumoral adipose tissue in patients treated or not treated with neoadjuvant therapy, in order to assess the relationship between adipose tissue‐specific gene expression and the response to neoadjuvant therapy. Adipose tissue from 60 consecutive patients with EAC and 20 with esophageal squamous cell carcinoma (SCC) who underwent esophagectomy at the Esophageal and Digestive Tract Surgical Unit of the Veneto Institute of Oncology (IOV‐IRCSS) were collected and analyzed. This prospective study obtained the approval of the Ethical Committee of the Veneto Institute of Oncology (IOV‐IRCSS). The study was carried out in accordance with the principles of the Helsinki Declaration and all potential patients were asked to give written consent to have their data (including age, sex, tumor location and size, treatment protocol, histology, TNM stage, and outcome) collected. Finally, in the third step, we aimed to study the possible cross‐talk between cancer tissue and the peritumoral adipose tissue surrounding the EAC and its influence on the response to neoadjuvant therapy. Thus, we carried out several in vitro analyses of adipose tissue stem cells, using visceral adipose tissue of 18 patients who underwent abdominal surgery for benign conditions to isolate ADSC, then treated the cells with conditioned medium isolated from both tumoral and adipose tissue of patients affected by EAC.
Surgical procedure to induce esophageal cancer in animals
In this step of the study, 10 male Sprague Dawley rats (Charles River, Lecco, Italy) were consecutively submitted to a side‐to‐side surgical esophagogastric‐jejunal anastomosis. The animals were kept under standard laboratory conditions and acclimatized for a week before the procedure. Water and standard chow food were given ad libitum before surgery. Water was permitted 2 h after surgery, and rat chow was provided on the following day. Postoperatively, the animals were housed individually in a conventional cage. Five male non‐operated rats of the same age were used as control animals.
The operation was carried out according to the microsurgical procedure previously described.19 Briefly, a 1.5 cm side‐to‐side surgical esophagogastric‐jejunal anastomosis was created between the first jejunal loop and the gastroesophageal junction, approximately 3 cm distal to Treitz's ligament, with accurate mucosa‐to‐mucosa opposition, allowing jejunal and gastric contents to flow back into the esophagus. The surviving animals were killed at 28 ± 2 weeks after surgery.
Pathology and immunohistochemistry of animal specimens
Immediately after death, the thoracic and abdominal cavities were examined and the esophagus, stomach, and jejunum were excised en bloc. Gross specimens were fixed in 10% neutral‐buffered formalin for 24 h. All specimens were cut serially (2–3‐mm thick coronal sections). Tissue sections (4 μm thick) were obtained from paraffin blocks and stained with hematoxylin & eosin or with anti‐CD34 (monoclonal rabbit EP373Y; Abcam, Cambridge, UK), aAnti‐CD31 (mouse monoclonal cloneJc70A; DakoDenmark A/S, Glostrup, Denmark), or anti‐VEGF (rabbit polyclonal to VEGFA; Abcam) and then counterstained with hematoxylin. Immunocomplexes were detected using the Dako Real Envision System Peroxidase/DAB (Dako, Glostrup, Denmark). CD34, CD31 and VEGF positive cells per mm2 were counted in 10 different microscopic areas for each section by a single pathologist in a blinded way.
Preoperative staging and neoadjuvant therapy in patients
Based on preoperative staging and according to the recommendations of the multidisciplinary team work‐up, patients with tumors staged above T3N0 or any T N1 were considered suitable for neoadjuvant therapy. Patients were considered resectable when staged below T3N0 or, after the termination of neoadjuvant treatment, when there was no evidence of distant metastases or locally advanced tumor with gross periesophageal involvement at restaging.
Preoperative chemotherapy regimen for patients who were prescribed combined modality therapy consisted of 5‐fluorouracil (5‐FU) and a platinum agent (standard regimen for treatment was DDP 100 mg/m2 on day 1, and 5‐FU 1000 mg/m2 per day in continuous infusion from day 1 to day 5 for 3–4 cycles). Chemotherapy was usually given concurrently with radiation therapy: radiation was usually given in daily fractions of 1.8 Gy for a total dose of 45–50 Gy. For carcinoma of the lower third esophagus, the field was extended to include both the perigastric and celiac nodes. This involved an initial phase using anteroposterior/posteroanterior fields with a total dose up to 30.6 Gy in 1.8 fractions. Patients prescribed chemotherapy only underwent two cycles of cisplatin in combination with 5‐FU before surgery. After the second cycle of chemotherapy, the tumor was restaged and patients with stable or progressive disease at that point underwent surgery without further delay. A third cycle of chemotherapy was begun in those patients who responded to the first two cycles. In all cases, surgery was carried out 3–4 weeks after the last cycle of chemotherapy.20
Surgical resection and tissue sample collection
Details concerning surgical techniques have been published elsewhere.21 Briefly, esophagectomy was carried out using an Ivor Lewis procedure, by laparotomy or laparoscopy, and right thoracotomy for tumors of the midlower esophagus and the gastric cardia. During surgical procedures, two biopsies of visceral adipose tissue were collected for each patient from different fat depots. In particular, one sample from a periesophageal depot (2 cm close to cardia) and one sample from a distal omental depot (at least 15 cm from the esophagogastric junction). Thus, in patients with EAC, the periesophageal depot was close (within 2 cm) to the tumor site, whereas in patients with SCC, the periesophageal depot was at least 6–8 cm from the tumor site. One sample of tumoral tissue from the esophageal mucosa was also isolated from nine patients. Fresh specimens were rapidly divided and prepared for in vitro culture, or immediately frozen in liquid nitrogen, or fixed in formalin for subsequent analysis.
Immunohistochemistry of patient specimens
Formalin‐fixed adipose tissue samples were paraffin‐embedded and serial sections were immunostained with anti‐human CD34 (clone QBEnd‐10; DakoDenmark A/S), anti‐human CD45 and anti‐human CD31 (both from Abcam) according to the manufacturer's instructions. Double staining for CD34 and C/EBPα was done using an automated staining system (BOND‐MAX; Leica Biosystems, Wetzlar, Germany). Heat‐induced antigen retrieval was carried out using ER1 solution (pH6; Leica Biosystems) for 30 min. Anti‐CD34 antibody (clone QBEnd‐10; Dako) was incubated, followed by 15 min of AP‐labeled polymer (Bond Polymer Refine Red detection system; (Leica Biosystems). Red substrate was incubated for 15 min. Anti‐C/EBPalpha (D56F10) (Cell Signaling Technology, Danvers, MA, USA) immunostaining was carried out subsequently using the Bond Polymer Refine Detection kit (Leica Biosystems). Staining was developed using 3,3′diaminobenzidine (DAB) chromogen (Leica) for 10 min. Slides were counterstained with hematoxylin, dehydrated, and coverslipped. Ten areas (1 mm2) for each section were analyzed and all the parameters were measured by computer‐assisted morphometric analysis (Image‐Pro Plus version 5).
ADSC isolation and treatment
Adipose‐derived stem cells were isolated from omental adipose tissue of 15 patients undergoing abdominal surgery for non‐neoplastic conditions. Briefly, freshly collected adipose tissue was collected from a distal omental adipose depot (peritoneal) and separated from major vessels and fibers, minced and digested with 1 mg/mL collagenase type II (Sigma‐Aldrich, St Louis, MO, USA) in DMEM/F12 at 37°C for 1 h. Cell suspension containing stromal vascular fraction was centrifuged (350 g, 8 min), pelleted and resuspended in erythrocyte‐lysing buffer, washed in DMEM/F12, filtered with a 100‐μm cell strainer, centrifuged (350 g, 8 min), washed again in DMEM/F12 and seeded in DMEM/F12 supplemented with 10% FBS (0.7 × 106 cells/well in 24‐well plates). After 24 h for cell attachment, cells were washed twice in warm sterile PBS and medium was replaced by simple culture medium (control cells) or conditioned media from cultured tissue biopsies from EAC patients (CM‐treated cells), collected as previously described.4 Leptin antagonist treatment was carried out by adding 0.7 ng/mL super human leptin antagonist (SHLA; Protein Laboratories Rehovot Ltd) to culture medium. After 48 h of treatment, cells were washed twice with warm PBS and harvested for subsequent analysis. For adipocyte differentiation, after 48 h of CM treatment, cells were washed twice in warm PBS and incubated with adipogenic medium: DMEM/F12 supplemented with 33 μM biotin, 17 μM pantothenate, 10 μg/mL human transferrin (Sigma‐Aldrich), 66 μM insulin (Lilly Research, Indianapolis, IN, USA), 100 μM dexamethasone, 1 μM T3, 0.25 μM 3‐isobutyl‐1‐methylxanthine (IBMX; Sigma‐Aldrich) and 10 μM rosiglitazone. Rosiglitazone and IBMX were removed after 3 days, and the medium was changed three times per week until complete differentiation was obtained (after 10 days). Preadipocytes maintained in DMEM/F12 alone were used as controls (undifferentiated cells).
Cytofluorimetric analysis
Surface expression of CD34, CD31 and CD45 cells was assessed by cytofluorimetric analysis. Briefly, 200 000 freshly isolated ADSC were washed twice in warm PBS, pelleted and stained with 10 μL of the following antibodies: anti‐human CD34 PerCP‐Cy™ 5.5 (clone 8G12), anti‐human CD45 FITC (clone 2D1) and Phycoerythrin mouse anti‐human CD31 (clone WM59) (all from BD Biosciences, Franklin Lakes, NJ, USA). Samples were washed in ice‐cold FACS buffer (2% BSA in PBS), pelleted and resuspended in ice‐cold FACS buffer. BD FACSCalibur™ flow cytometer (BD Biosciences) was used for acquisition and CellQuest software was used for analysis. Fluorescence of cells treated with fluorescent isotype monoclonal antibody was evaluated in each experiment to determine the level of background fluorescence of negative cells.
RNA isolation and real‐time quantitative PCR
Total RNA was isolated from frozen visceral adipose tissues and ADSC using Rneasy Lipid Tissue Mini Kit and Rneasy Mini Kit (Qiagen, Hilden, Germany) respectively, treated with DNase (TURBO‐DNase‐free; Life Technologies, Carlsbad, CA, USA) and reverse transcribed using random primers (Promega, Madison, WI, USA) and M‐MLV reverse transcriptase (Promega). mRNA levels were measured by real‐time PCR (DNA Engine Opticon 2; Bio‐Rad, Hercules, CA, USA) using SYBR Green PCR Master Mix (Life Technologies) and specific intron‐spanning human primers, according to the manufacturer's instructions. Values were calculated as the mean of triplicate measurements and levels were normalized to hydroxymethylbilane synthase (HMBS) mRNA expression.
Statistical analysis
All statistical analyses were carried out using GraphPad Prism software version 6.0 (GraphPad Software, San Diego, CA, USA). Parametric one‐way anova and non‐parametric Mann–Whitney U‐test (or Kruskal–Wallis test when comparing more than two groups) were carried out to assess differences between continuous variables with Bonferroni correction for multiple comparison when appropriate. Results are expressed as mean ± SEM and in vitro experiments were carried out in triplicate. All tests were two‐sided and a P‐value <0.05 was considered significant.
Results
Angiogenesis in periesophageal adipose tissue of a rat model for EAC
Barrett's mucosa and, subsequently, a spontaneous form of EAC were obtained in the esophagus of operated rats, promoted by a surgically induced gastric reflux chronic exposure (Fig. 1a, H&E). In order to evaluate angiogenesis in periesophageal adipose tissue depot of animals, we measured the immunohistochemical expression of CD34‐, CD31‐ and VEGF‐positive cells in non‐operated rats (Normal), rats with Barrett's mucosa (Surgery‐BM) and rats that had fully developed EAC (Surgery‐EAC). We observed a significant increase in CD34‐positive adipocytes in periesophageal adipose tissue of operated rats in comparison with non‐operated control rats (Fig. 1a: CD34). In particular, CD34 was significantly more abundant in the peritumoral adipose tissue of rats that developed EAC compared to rats with simple Barrett's mucosa (Fig. 1b). Similar results were obtained for CD31 (Fig. 1a: CD31) and VEGF (Fig. 1a: VEGF) expression, which significantly increased in peritumoral adipose tissue throughout cancer development (Fig. 1b).
Figure 1.
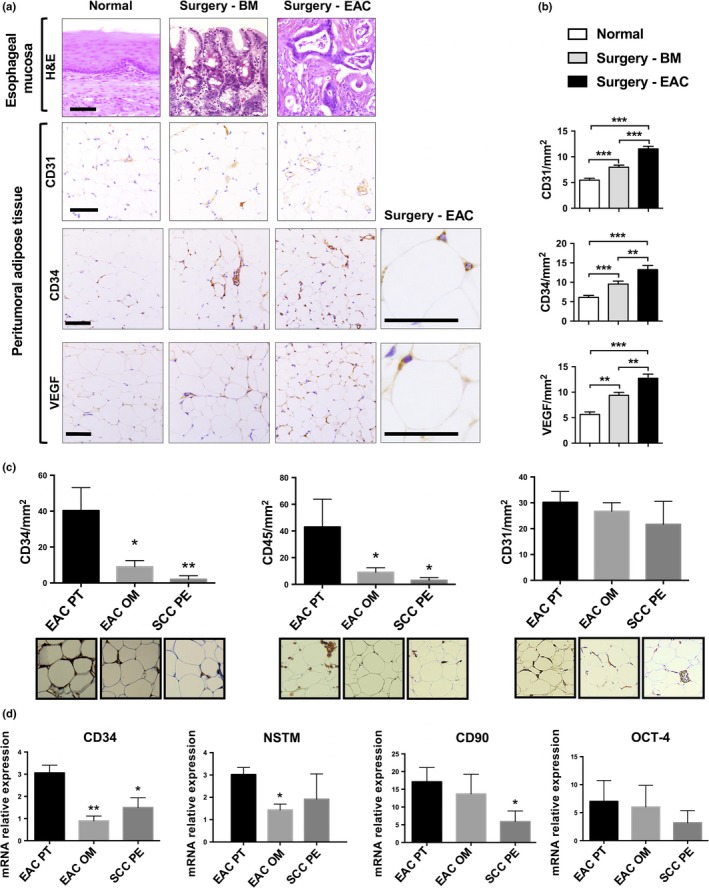
Immunohistochemistry and mRNA expression in a rat model for esophageal adenocarcinoma (EAC) and in patients not treated with neoadjuvant therapy. (a) Representative images of immunohistochemical staining in esophageal mucosa (H&E) and peritumoral adipose tissue (CD31, CD34 and vascular endothelial growth factor [VEGF]) of non‐operated rats (Normal), operated rats with Barrett's esophagus mucosa (Surgery‐BM) or operated rats that had fully developed adenocarcinoma (Surgery‐EAC). Scale bars, 100 μm. (b) CD31, CD34 and VEGF distribution in three different peritumoral adipose tissue depots. (c) Immunohistochemical expression of CD34, CD45 and CD31 in sections of peritumoral (EAC PT) and omental (EAC OM) adipose tissue of EAC patients, and periesophageal adipose tissue of SCC patients (SCC PE). (d) Total RNA was isolated from the same visceral adipose tissue samples as in part (c) and CD34, nucleostemin (NSTM), CD90 and octamer‐binding transcription factor 4 (OCT‐4) mRNA expression was measured by means of qRT‐PCR using HMBS as internal control. Parametric and non‐parametric statistics were used. *P < 0.05; **P < 0.01 and ***P < 0.001.
Peritumoral adipose tissue in patients not treated with neoadjuvant therapy
Mean age of patients was 62 (54.5–70) years for EAC and 64.5 (58.5–70.5) years for SCC patients. All adenocarcinomas were located in the esophagogastric junction and all SCC were located in the upper thoracic esophagus. Fourteen EAC patients and eight SCC patients underwent surgery alone, whereas 46 EAC patients and 12 SCC patients also underwent neoadjuvant therapy. Twenty‐eight EAC patients and 17 SCC patients did not have lymph node metastases, whereas 32 EAC patients and three SCC patients had positive lymph node involvement. Additional clinical characteristics of patients and tumor staging data are summarized in Table S1. We first measured immunohistochemical expression of CD34, CD45 and CD31 in sections of periesophageal (<2 cm from cardia) adipose tissue of EAC patients not treated with neoadjuvant therapy (EAC PT [peritumoral]; Fig. 1c). As control tissues, we used a fat depot distant from the tumor: the omental (OM) adipose tissue of EAC patients (EAC OM) and the periesophageal (<2 cm from cardia) adipose tissue of SCC patients (SCC PE) who developed a tumor in the upper portion of the esophagus, distant from the cardia. Expression of CD34 and CD45 was increased in peritumoral adipose tissue of EAC patients (EAC PT), in comparison with both omental adipose tissue of EAC patients (EAC OM) and periesophageal adipose tissue of SCC patients (SCC PE). Also, CD31 expression showed a slight increase in EAC peritumoral adipose tissue, although no significant differences were detected among the three different groups (Fig. 1c). CD34 and NSTM mRNA expression was increased in peritumoral adipose tissue of EAC patients, in comparison with both omental adipose tissue of EAC patients and periesophageal adipose tissue of SCC patients. Moreover, CD90 expression was increased in peritumoral adipose tissue of EAC patients compared to periesophageal adipose tissue of SCC patients (Fig. 1d).
Peritumoral adipose tissue in patients treated with neoadjuvant therapy
We measured the expression of CD34, NSTM, CD90 and OCT‐4 in peritumoral adipose tissue of EAC patients and we observed a significantly decreased mRNA expression of CD34, CD90 and NSTM in patients treated with neoadjuvant therapy, in comparison with patients who did not receive neoadjuvant treatment (Fig. 2a). The lower expression of CD34 was also confirmed by immunohistochemical analysis (Fig. 2b) and adipocyte‐specific expression of CD34 in peritumoral adipose tissue was confirmed by double staining for CD34 and the adipose‐specific marker C/EBPα22 (Fig. 2c). In order to evaluate a possible relationship between the pathological response to radiochemotherapy and peritumoral adipose tissue gene expression, we measured mRNA levels of CD34, NSTM, CD90 and OCT‐4 in peritumoral adipose tissue of EAC patients who had undergone neoadjuvant therapy. CD90 expression was decreased, whereas OCT‐4 was increased in patients with poor response to treatment (Mandard Index from 3 to 5) in comparison with peritumoral adipose tissue isolated from patients with a better response to therapy (Mandard Index from 1 to 2) (Fig. 2d). We investigated gene expression of VEGF in omental and peritumoral adipose tissue of EAC patients. VEGF mRNA levels were increased in peritumoral adipose tissue of patients with poor response to treatment (Mandard Index from 3 to 5) in comparison with peritumoral adipose tissue isolated from patients with a better response to therapy (Mandard Index from 1 to 2) (Fig. 2e). Omental adipose tissue did not show significant differences in terms of VEGF mRNA expression in patients with poor response to treatment compared with patients with a better response to therapy; however, VEGF levels were significantly lower in comparison with peritumoral depot in patients who did not receive neoadjuvant treatment (Fig. 2e). Circulating leptin and adiponectin were also measured, but there were no significant differences in their levels between patients with positive or negative lymph node involvement, different tumor sizes or different responses to neoadjuvant therapy (Fig. S1). Moreover, adiponectin mRNA levels were measured in peritumoral and distal adipose tissue of EAC patients and we did not observe significant correlations with angiogenesis (CD31 and CD34 expression) or chemoresistance (Mandard Index) in EAC patients (Fig. S2).
Figure 2.
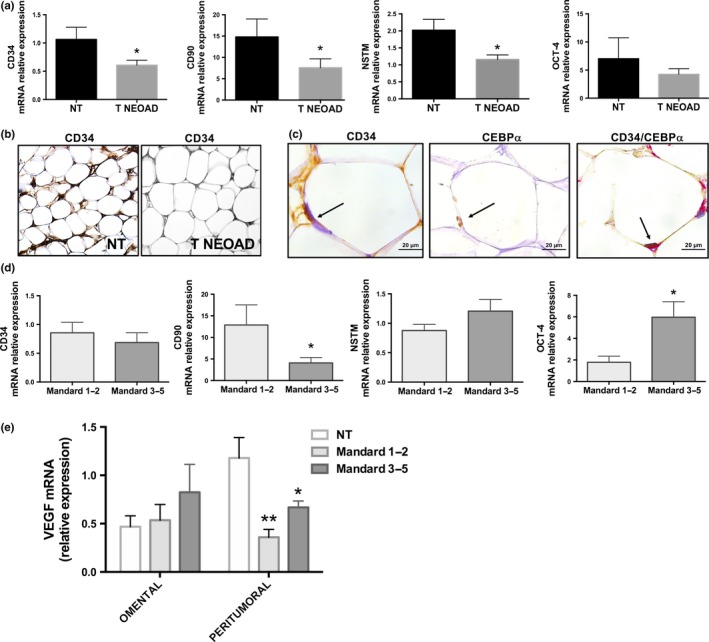
mRNA expression and immunohistochemistry in patients affected by esophageal adenocarcinoma (EAC) and treated with neoadjuvant therapy. (a) mRNA expression of CD34, nucleostemin (NSTM), CD90 and octamer‐binding transcription factor 4 (OCT‐4) in peritumoral adipose tissue of patients with EAC who did not receive (NT) or received (T NEOAD) neoadjuvant treatment. (b) Representative images of immunohistochemical expression of CD34 in peritumoral adipose tissue of the same patients as in part (a). (c) Representative images of immunohistochemical expression of CD34 and C/EBPα (in consecutive or same tissue section) in peritumoral adipocytes of patients affected by EAC. Black arrows indicate adipocyte nuclei. (d) mRNA expression of CD34, NSTM, CD90 and OCT‐4 in peritumoral adipose tissue of patients with EAC treated with neoadjuvant therapy who had a positive tumor regression grade (Mandard 1‐2) or had a poor response to chemotherapy (Mandard 3‐5). (e) mRNA expression of vascular endothelial growth factor (VEGF) in omental and peritumoral adipose tissue of EAC patients who did not receive any treatment (NT) and patients treated with neoadjuvant therapy who had a positive tumor regression grade (Mandard 1‐2) or a poor response to chemotherapy (Mandard 3‐5). mRNA expression was measured by means of qRT‐PCR using HMBS as internal control. Non‐parametric statistics were used. *P < 0.05 and **P < 0.01.
Effects of cancer‐derived conditioned medium on ADSC gene expression
To investigate whether EAC could influence ADSC gene expression, we analyzed the effects of a cancer‐derived conditioned medium (CD‐CM) collected from tumoral esophageal mucosa of patients affected by EAC on primary ADSC isolated from healthy visceral adipose tissue of patients undergoing abdominal surgery for benign diseases. Collection of ADSC from non‐cancer patients allowed us to reproduce the effect of cancer‐derived secreted factors on a healthy cell population to avoid misleading results from experiments on tumor‐derived ADSC. After 48 h of treatment with CD‐CM, we analyzed the expression of CD34, NSTM, CD90, OCT‐4, α‐SMA, E‐cadherin, VEGF and VEGF‐C and we observed that NSTM, α‐SMA, VEGF and VEGF‐C levels were increased, whereas E‐cadherin levels were decreased in ADSC treated with CD‐CM compared to untreated ADSC (Fig. 3a).
Figure 3.
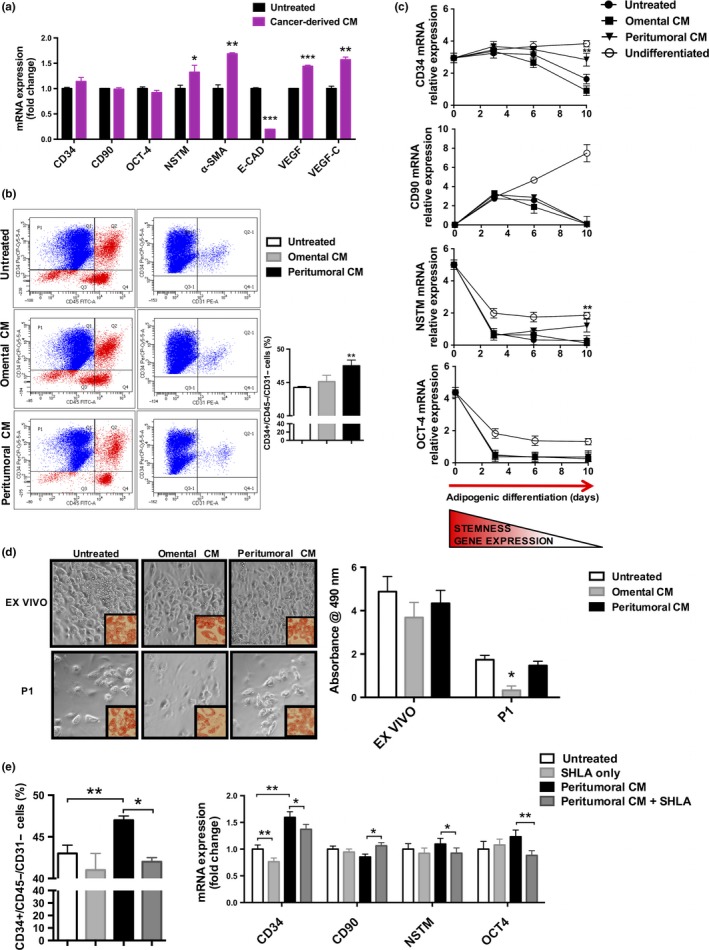
Gene expression and adipocyte differentiation of adipose‐derived stem cells (ADSC) treated with cancer‐derived and adipose tissue‐derived conditioned medium (CM). (a) mRNA expression of CD34, CD90, octamer‐binding transcription factor 4 (OCT‐4), nucleostemin (NSTM), alpha‐smooth muscle actin (α‐SMA), E‐Cadherin (E‐CAD), vascular endothelial growth factor (VEGF) and VEGF‐C in ADSC isolated from healthy patients and treated with cancer‐derived conditioned medium (CM), collected from esophageal tumoral tissue of patients with esophageal adenocarcinoma (EAC). Untreated cells were used as control. (b) Representative images of cytofluorimetric analysis and percentage of CD34+/CD45–/CD31– cells in ADSC isolated from visceral adipose tissue of healthy patients and cultured with conditioned medium derived from omental (Omental CM) or peritumoral (Peritumoral CM) fat depots of EAC patients. (c) mRNA expression of CD34, CD90, NSTM, and OCT‐4 in ADSC at different time points during the differentiation process, as indicated. (d) Representative images of adipogenic differentiation at the end of the differentiation process (day 10) of freshly isolated ADSC (ex vivo) and after a subculture passage (P1). The differentiation protocol started after 48 h of treatment with conditioned medium derived from omental (Omental CM) or peritumoral (Peritumoral CM) fat depots of EAC patients. Bright field microscopy (20× magnification) and after Oil‐Red‐O staining (40× magnification, smaller frames). Differentiation capacity was quantified as Oil‐Red‐O absorbance at 490 nm. (e) Cytofluorimetric analysis of CD34+/CD45–/CD31– cells (expressed as percentage) and mRNA expression of CD34, NSTM, CD90 and OCT‐4 (expressed as fold change compared to control untreated cells) in ADSC treated with peritumoral adipose tissue CM in the presence of a Leptin antagonist (SHLA). Untreated cells or cells treated only with SHLA (SHLA only) were used as negative controls. mRNA expression was measured by means of qRT‐PCR using HMBS as internal control. Non‐parametric statistics were used. *P < 0.05, **P < 0.01 and ***P < 0.001.
Effects of peritumoral and distal adipose tissue conditioned medium on ADSC gene expression and adipocyte differentiation
In order to investigate the potential mechanism by which adipose tissue may influence ADSC expression and whether peritumoral adipose tissue is able to induce depot‐specific effects on ADSC, we treated healthy visceral ADSC with adipose tissue conditioned medium (AT‐CM) collected from peritumoral and distal adipose tissue biopsies. After 48 h of treatment with CM, the population of CD34+/CD45–/CD31– cells was significantly higher in cells treated with peritumoral adipose tissue CM in comparison with cells treated with omental adipose tissue CM or untreated control cells (Fig. 3b). We then analyzed the expression of CD34, CD90, NSTM and OCT‐4 from day zero and during a 10‐day adipogenic differentiation of ADSC. mRNA expression of CD34 and NSTM at the end of the differentiation protocol remained higher in cells treated with peritumoral adipose tissue CM in comparison with cells treated with omental adipose tissue CM, or untreated control cells (Fig. 3c). Similar results were observed in undifferentiated cells, which were maintained in culture without any adipogenic stimulus added to the medium and tended to maintain the mRNA expression of all the stemness genes during the differentiation process (Fig. 3c).
Quantification of stemness capacity was carried out at the end of the adipogenic differentiation protocol in both an ex vivo cell fraction (passage 0, P0) and a P1 cell fraction (after one subculture passage). Oil Red O staining showed that, despite a general well‐known loss of differentiation capacity in P1 cells compared to the ex vivo fraction, ADSC treated with peritumoral adipose tissue CM maintained a higher differentiation capacity after a P1 subculture, whereas cells treated with omental adipose tissue CM substantially decreased their adipogenetic potential (Fig. 3d). Also, we measured the percentages of CD34+/CD45–/CD31– cells and the mRNA expression of CD34, NSTM, CD90 and OCT‐4 in ADSC treated with peritumoral adipose tissue CM in the presence of a leptin antagonist (SHLA). Our results demonstrated that the leptin antagonist partially inhibited the effect of peritumoral adipose tissue CM treatment (Fig. 3e).
Effects of peritumoral and distal AT‐CM associated with neoadjuvant therapy and chemoresistance
CD34, NSTM, CD90 and OCT‐4 expression was analyzed in ADSC treated with AT‐CM. ADSC treated with AT‐CM derived from omental adipose tissue showed dramatically lower mRNA levels compared to ADSC treated with AT‐CM from peritumoral adipose samples and did not show any significant difference in mRNA expression between patients treated or not treated with neoadjuvant therapy (Fig. 4a). In ADSC treated with peritumoral AT‐CM, mRNA expression of CD34 and OCT‐4 was significantly decreased in cells treated with AT‐CM of patients who underwent neoadjuvant therapy compared to cells treated with AT‐CM derived from untreated patients (Fig. 4a).
Figure 4.
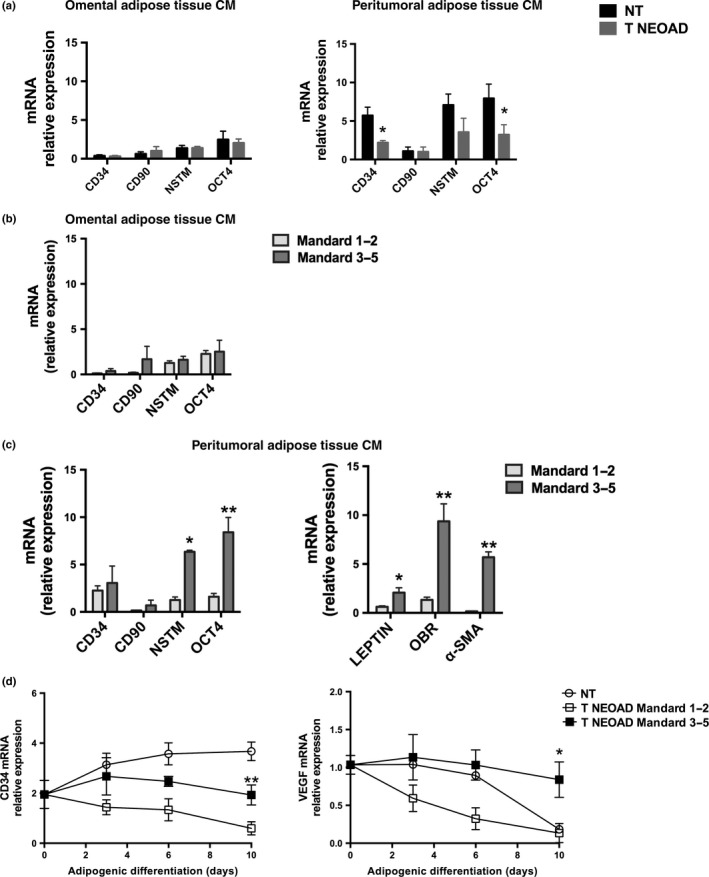
Gene expression of adipose‐derived stem cells (ADSC) treated with adipose tissue conditioned medium (CM) associated with neoadjuvant treatment and chemoresistance. (a) mRNA expression of CD34, CD90, nucleostemin (NSTM), and octamer‐binding transcription factor 4 (OCT‐4) in ADSC isolated from healthy patients and treated with omental or peritumoral adipose tissue CM derived from patients with esophageal adenocarcinoma (EAC) who did not receive (NT) or received (T NEOAD) neoadjuvant treatment. (b) mRNA expression of CD34, CD90, NSTM, and OCT‐4 in ADSC isolated from healthy patients and treated with omental adipose tissue CM derived from patients with EAC treated with neoadjuvant therapy who had a positive tumor regression grade (Mandard 1‐2) or poor response to chemotherapy (Mandard 3‐5) (c) mRNA expression of CD34, CD90, NSTM, OCT‐4, leptin, leptin receptor (OBR) and alpha‐smooth muscle actin (α‐SMA) measured in ADSC isolated from healthy visceral adipose tissue and treated with peritumoral adipose tissue CM derived from patients with EAC treated with neoadjuvant therapy who had a positive tumor regression grade (Mandard 1‐2) or poor response to chemotherapy (Mandard 3‐5). (d) CD34 and vascular endothelial growth factor (VEGF) mRNA expression at different time points in ADSC isolated from healthy visceral adipose tissue treated with peritumoral adipose tissue CM isolated from the same patients as in part (c) and then differentiated into mature adipocytes. mRNA expression was measured by means of qRT‐PCR using HMBS as internal control. Non‐parametric statistics were used. *P < 0.05 and **P < 0.01.
Adipose‐derived stem cells treated with AT‐CM derived from omental adipose tissue samples did not show any significant difference in mRNA expression between patients with good or poor response to neoadjuvant therapy (Fig. 4b). Interestingly, ADSC treated with AT‐CM isolated from peritumoral adipose tissue of patients with bad response showed a significantly increased expression of NSTM and OCT‐4 mRNA compared to cells treated with CM from patients with a good response (Fig. 4c). In the same ADSC, we measured the mRNA expression of Leptin, Leptin receptor (ObR) and α‐SMA and we observed that these genes were all upregulated in cells treated with peritumoral adipose tissue CM from patients with a bad response compared to CM derived from patients with a good response to neoadjuvant therapy (Fig. 4c).
Finally, we measured ADSC gene expression during a 10‐day adipogenic differentiation protocol, after a 48‐h treatment with CM from peritumoral adipose of EAC patients. At the end of adipogenic differentiation, CD34 and VEGF mRNA levels were higher in ADSC treated with AT‐CM of biopsies isolated from tissue of patients with a bad response compared to cells treated with AT‐CM derived from patients with a good response to neoadjuvant therapy (Fig. 4d).
Discussion
Several studies suggest that adipose tissue and stem cells play an important role in resistance to therapy23 and tumor relapse.24, 25, 26 The relentless growth of epithelial carcinomas, compounded with resistance to therapy, still represents a major clinical challenge that might be correlated with the involvement of other cell types.
In order to assess the role of adipose tissue vascularization in esophageal carcinogenesis, we investigated vessel distribution and angiogenesis expression in periesophageal adipose tissue of a surgical rat model where reflux induced by surgical esophagogastric‐jejunal anastomosis promoted a spontaneous form of EAC in Barrett's esophagus, as reported in a previous study.27 These data could not have been observed in humans as the analysis of periesophageal tissue in healthy subjects and in patients with Barrett's esophagus would have implied an unnecessary invasive operation. We measured the expression of CD34 and VEGF in peritumoral adipose tissue of rats and we observed a progressive increase in rats with Barrett's mucosa and rats that developed EAC in comparison with controls. Moreover, we verified in tissue sections that a component of this increase was adipocyte‐derived, confirming a direct involvement of adipose cells in these alterations. These results suggest that peritumoral adipose tissue is enriched with factors able to stimulate and enhance the formation of new vessels and whose expression is increased during EAC development. In fact, we observed that the abundance of CD31‐positive vessels in the peritumoral adipose tissue directly correlated with the progression of EAC development, from normal mucosa of non‐operated rats, through an intermediate stage of Barrett's mucosa, to a fully developed EAC. As rats were killed at the same time, we assume that the difference in terms of vascularization could not be attributed to a different exposure to gastric reflux and to chronic inflammation; therefore, during carcinogenesis of EAC, peritumoral adipose tissue actively contributes to neovascularization, possibly favoring tumor progression.
In order to evaluate a possible relationship between the pathological response to neoadjuvant therapy and the characteristics of peritumoral adipose tissue, we first characterized the expression profile of different fat depots in EAC patients not treated with neoadjuvant therapy in terms of angiogenesis, inflammation and stemness. We used adipose tissue of a depot distant from the tumor (omental in case of EAC or pericardial in case of SCC) as control tissue, given the impossibility of safely sampling a periesophageal specimen from healthy subjects. Immunohistochemical and gene expression analysis showed an increase of CD34, CD45 NSTM and CD90 in peritumoral adipose tissue of patients, in agreement with the results observed in other tumors.28, 29 Moreover, we did not observe any significant correlation between clinicopathological data and circulating levels of leptin and adiponectin, further suggesting a local action of microenvironment‐specific factors. Thus, we hypothesized that even in humans, EAC might be surrounded by a microenvironment that promotes adipose tissue vascularization, inflammation and fibrogenesis.
To verify whether these findings may represent a direct action of EAC on peritumoral adipose tissue, we first analyzed the effects of a CD‐CM on primary ADSC isolated from healthy patients. With this experimental approach, we aimed to recreate the cross‐talk between tumor‐derived secreted factors and normal ADSC. In ADSC treated with CD‐CM we observed an increase in the expression of genes involved in stemness (NSTM), epithelial‐mesenchymal transition (EMT) (α‐SMA and E‐cadherin), neoangiogenesis (VEGF) and neo‐lymphangiogenesis (VEGF‐C), suggesting that EAC may exert a paracrine effect on peritumoral adipose stem cells.
To further explore whether adipose tissue itself may affect the expression of ADSC, we treated adipose stem cells with AT‐CM isolated from two different fat depots. Treatment of ADSC with peritumoral (but not distal) AT‐CM led to an enrichment of the CD34+/CD45–/CD31– subpopulation, which represents the more adipose‐specific expression profile, characterized by a major adipogenic potential as a result of the exclusion of pericytes (CD34–), endothelial (CD31+) and hematopoietic (CD45+) components, as shown in different studies (30, 31) and induced ADSC to maintain a higher expression of stemness genes during their differentiation towards mature adipocytes. These effects were reflected by the differentiation capacity of peritumoral AT‐CM‐treated ADSC observed after the first subculture passage and were partially inhibited by a leptin antagonist, indicating that leptin may be a potential player in this cross‐talk, as already suggested by our previous study.4 We also measured the expression of adiponectin in order to investigate a possible involvement of this adipokine in the observed effects, but we did not observe any significant correlation with angiogenesis or chemoresistance of patients. Therefore, these findings, associated with those obtained in distal (omental) adipose tissue, indicate that there is a paracrine effect of EAC on adipose stem cells that may promote vascularization and lymphangiogenesis, and a depot‐specific effect of adipose tissue on the same cells that may increase their differentiation capacity and their expression of factors involved in cell stemness.
Decreased mRNA expression of CD34, CD90 and NSTM was detected in peritumoral tissue of patients who underwent neoadjuvant treatment and was partially reflected in the expression profile of ADSC treated with peritumoral CM of these patients. These results were not reflected in the omental depot of adipose tissue and suggest that neoadjuvant therapy can directly influence peritumoral adipocytes, decreasing the expression of key genes and, probably, inhibiting peritumoral vascularization and fibrogenesis. To further investigate this, we evaluated the potential relationship between the pathological response to neoadjuvant therapy and the expression profile of peritumoral adipose tissue. We observed that CD90 expression was decreased, whereas VEGF and OCT‐4 were upregulated in peritumoral adipose tissue of patients who poorly responded to treatment. Moreover, in vitro, ADSC treated with peritumoral AT‐CM obtained from these patients showed a significantly increased expression of NSTM, OCT‐4, leptin, leptin receptor and α‐SMA. Furthermore, CD34 and VEGF levels were also increased during their differentiation process in comparison with ADSC treated with CM of patients with a good response to treatment. These data suggest that an expression profile of adipose cells, characterized by an enhanced capacity to express factors involved in EMT (such as α‐SMA) or in the stimulation of angiogenesis (such as VEGF), may be associated with the lack of response to neoadjuvant treatment in EAC patients. Interestingly, other aggressive tumors showing solid morphology with poor chemotherapy response demonstrated higher levels of these molecules.32, 33, 34 These characteristics may contribute to create a permissive microenvironment that allows the tumor to be more resistant to the effects of chemotherapy, as we observed in patients with poor response. The periesophageal (within 2 cm from the gastric cardia) adipose tissue analyzed in this study must be considered a peritumoral microenvironment for adenocarcinoma located in the esophagogastric junction and not for SCC that was located in the upper portion of esophagus. In fact, the correlation between adipose tissue‐specific markers expression and angiogenesis or chemoresistance that we observed in patients affected by EAC were not significant in patients affected by SCC.
In conclusion, our results indicate that adipose tissue surrounding EAC is rich in factors promoting tissue stemness, inflammation and neo‐angiogenesis, as we observed in both the animal model and in patients, and these processes may be induced by tumor through paracrine signaling (Fig. 5). Furthermore, neoadjuvant therapy can directly influence peritumoral adipocytes, which, in turn, are able to induce the expression of key genes in adipose stem cells and enhance their capacity to express factors involved in extracellular matrix remodeling and neo‐angiogenesis. These effects were significantly increased in chemoresistant patients with EAC, reinforcing the concept that depot‐specific adipose tissue characteristics may represent a potential prognostic index after surgery for EAC patients undergoing neoadjuvant therapy.
Figure 5.
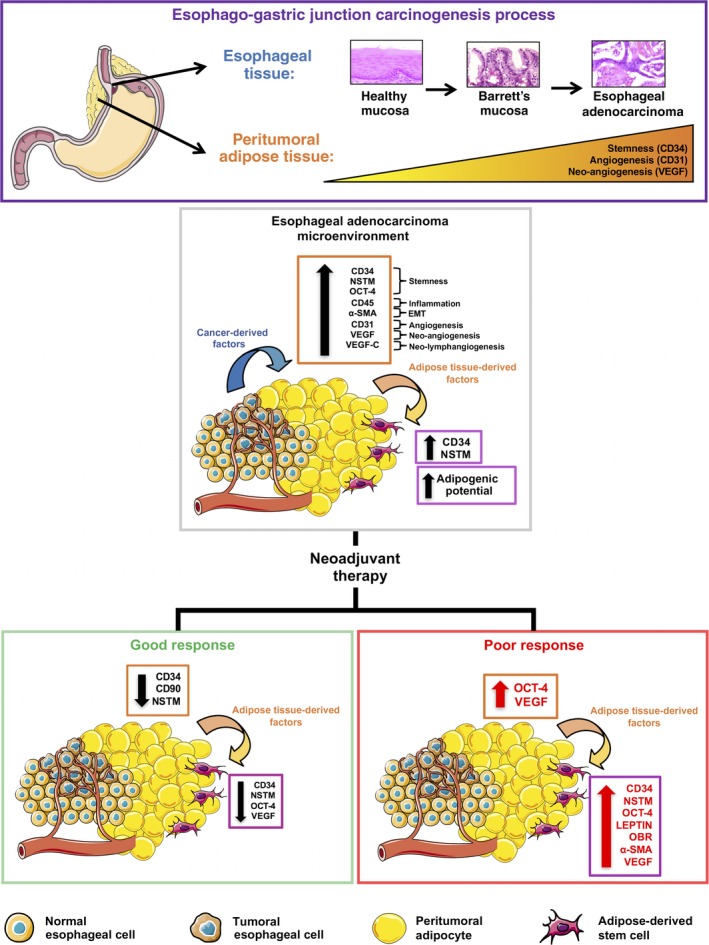
Possible cross‐talk between esophageal adenocarcinoma (EAC), peritumoral adipose tissue and adipose‐derived stem cells (ADSC) in chemoresistance. During the EAC carcinogenesis process, peritumoral adipose tissue progressively increases the expression of factors involved in stemness (CD34, nucleostemin [NSTM], octamer‐binding transcription factor 4 [OCT‐4]), angiogenesis (CD34, CD31), neo‐angiogenesis (vascular endothelial growth factor [VEGF]), inflammation (CD45), epithelial‐mesenchymal transition (EMT) (alpha‐smooth muscle actin [α‐SMA]) and neo‐lymphangiogenesis (VEGF‐C), under the stimulation of cancer‐derived factors secreted by tumor or adipokines secreted by adipose tissue itself (e.g. leptin). These effects are depot‐specific and are not observed in distal fat. In the presence of neoadjuvant therapy, some of these effects decrease in both peritumoral adipose tissue and ADSC), but in patients with poor response to therapy, the expression of factors involved in stemness, EMT, neo‐angiogenesis and leptin signaling remains higher in comparison with patients with good response, suggesting that peritumoral adipose tissue may promote microenvironment characteristics that sustain the progression and chemoresistance of EAC. Figure created using Servier Medical Art (http://www.servier.com/Powerpoint-image-bank) by Servier, under CC BY 3.0.
Disclosure Statement
Authors declare no conflicts of interest for this article.
Supporting information
Table S1. Clinical and pathological characteristics of patients.
Fig. S1. Circulating adipokines and clinicopathological characteristics of esophageal adenocarcinoma (EAC) patients. Circulating levels of leptin and adiponectin were measured in serum of EAC patients. (a) Lymph node negative (N–) or positive (N+) involvement was estimated by TNM values for each patient. (b) Tumor size (T) was estimated by TNM values for each patient. (c) EAC patients treated with neoadjuvant therapy who had a positive tumor regression grade (Mandard 1‐2) were compared with those who had a poor response to chemotherapy (Mandard 3‐5).
Fig. S2. Adiponectin expression in peritumoral and distal adipose tissue of esophageal adenocarcinoma (EAC) patients. Adiponectin mRNA levels were measured in adipose tissue of EAC patients. (a) Immunohistochemical expression of CD31 in sections of omental and peritumoral adipose tissue was expressed as positive cells/mm2. (b) CD34 mRNA expression was measured by means of qRT‐PCR using HMBS as internal control. (C) EAC patients treated with neoadjuvant therapy who had a positive tumor regression grade (Mandard 1‐2) were compared with those who had a poor response to chemotherapy (Mandard 3‐5).
Acknowledgments
This work was supported by Italian Ministry of Education, University and Research (grant for junior researcher no. CPDR128205/12 to Roberto Vettor) and Italian Ministry of Health (Current Research Funds to Carlo Castoro). We thank Anna Cabrelle for support during cytofluorimetric analysis, Francesco Cavallin for statistical consulting and Christina A. Drace for final language editing of the manuscript.
Cancer Sci 108 (2017) 2393–2404
Funding Information
Ministero della Salute, (Grant/Award Number: ‘Current research fund to Carlo Castoro’) Ministero dell'Istruzione, dell'Università e della Ricerca, (Grant/Award Number: ‘CPDR128205/12’).
References
- 1. Li XT, Tang L, Chen Y, Li YL, Zhang XP, Sun YS. Visceral and subcutaneous fat as new independent predictive factors of survival in locally advanced gastric carcinoma patients treated with neo‐adjuvant chemotherapy. J Cancer Res Clin Oncol 2015; 141: 1237–47. [DOI] [PubMed] [Google Scholar]
- 2. Cooper AB, Slack R, Fogelman D et al Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol 2015; 22: 2416–23. [DOI] [PubMed] [Google Scholar]
- 3. Del Fabbro E, Parsons H, Warneke CL et al The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 2012; 17: 1240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trevellin E, Scarpa M, Carraro A et al Esophageal adenocarcinoma and obesity: peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget 2015; 6: 11203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Bellows CF, Kolonin MG. Adipose tissue‐derived progenitor cells and cancer. World J Stem Cells 2010; 2: 103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freese KE, Kokai L, Edwards RP et al Adipose‐derived stems cells and their role in human cancer development, growth, progression, and metastasis: a systematic review. Cancer Res 2015; 75: 1161–8. [DOI] [PubMed] [Google Scholar]
- 7. Jotzu C, Alt E, Welte G et al Adipose tissue‐derived stem cells differentiate into carcinoma‐associated fibroblast‐like cells under the influence of tumor‐derived factors. Anal Cell Pathol (Amst) 2010; 33: 61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev 2013; 32: 303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Issa A, Gill JW, Heideman MR et al Combinatorial targeting of FGF and ErbB receptors blocks growth and metastatic spread of breast cancer models. Breast Cancer Res 2013; 15(1): R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyata Y, Mitsunari K, Asai A, Takehara K, Mochizuki Y, Sakai H. Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate 2015; 75: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imdahl A, Bognar G, Schulte‐Monting J, Schoffel U, Farthmann EH, Ihling C. Predictive factors for response to neoadjuvant therapy in patients with oesophageal cancer. Eur J Cardiothorac Surg 2002; 21: 657–63. [DOI] [PubMed] [Google Scholar]
- 12. Guo Z, Li LQ, Jiang JH, Ou C, Zeng LX, Xiang BD. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J Gastroenterol 2014; 20: 2098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiegel HC, Kaifi JT, Quaas A et al Lack of Thy1 (CD90) expression in neuroblastomas is correlated with impaired survival. Pediatr Surg Int 2008; 24: 101–5. [DOI] [PubMed] [Google Scholar]
- 14. Nakajima TE, Yoshida H, Okamoto N et al Nucleostemin and TWIST as predictive markers for recurrence after neoadjuvant chemotherapy for esophageal carcinoma. Cancer Sci 2012; 103: 233–8. [DOI] [PubMed] [Google Scholar]
- 15. Yoshida R, Nakayama H, Nagata M et al Overexpression of nucleostemin contributes to an advanced malignant phenotype and a poor prognosis in oral squamous cell carcinoma. Br J Cancer 2014; 111: 2308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tin AS, Park AH, Sundar SN, Firestone GL. Essential role of the cancer stem/progenitor cell marker nucleostemin for indole‐3‐carbinol anti‐proliferative responsiveness in human breast cancer cells. BMC Biol 2014; 12: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wen K, Fu Z, Wu X, Feng J, Chen W, Qian J. Oct‐4 is required for an antiapoptotic behavior of chemoresistant colorectal cancer cells enriched for cancer stem cells: effects associated with STAT3/Survivin. Cancer Lett 2013; 333: 56–65. [DOI] [PubMed] [Google Scholar]
- 18. Achuthan S, Santhoshkumar TR, Prabhakar J, Nair SA, Pillai MR. Drug‐induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J Biol Chem 2011; 286: 37813–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dall'Olmo L, Fassan M, Dassie E et al Role of proton pump inhibitor on esophageal carcinogenesis and pancreatic acinar cell metaplasia development: an experimental in vivo study. PLoS ONE 2014; 9: e112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ancona E, Ruol A, Santi S et al Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 2001; 91: 2165–74. [PubMed] [Google Scholar]
- 21. Ruol A, Castoro C, Portale G et al Trends in management and prognosis for esophageal cancer surgery: twenty‐five years of experience at a single institution. Arch Surg 2009; 144: 247–54; discussion 54. [DOI] [PubMed] [Google Scholar]
- 22. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab 2006; 4: 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shafee N, Smith CR, Wei S et al Cancer stem cells contribute to cisplatin resistance in Brca1/p53‐mediated mouse mammary tumors. Cancer Res 2008; 68: 3243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prantl L, Muehlberg F, Navone NM et al Adipose tissue‐derived stem cells promote prostate tumor growth. Prostate 2010; 70: 1709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karnoub AE, Dash AB, Vo AP et al Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007; 449: 557–63. [DOI] [PubMed] [Google Scholar]
- 26. Park YM, Yoo SH, Kim SH. Adipose‐derived stem cells induced EMT‐like changes in H358 lung cancer cells. Anticancer Res 2013; 33: 4421–30. [PubMed] [Google Scholar]
- 27. Realdon S, Dassie E, Fassan M et al In vivo molecular imaging of HER2 expression in a rat model of Barrett's esophagus adenocarcinoma. Dis Esophagus 2015; 28: 394–403. [DOI] [PubMed] [Google Scholar]
- 28. Wagner M, Bjerkvig R, Wiig H et al Inflamed tumor‐associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis. Angiogenesis 2012; 15: 481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amor S, Iglesias‐de la Cruz MC, Ferrero E et al Peritumoral adipose tissue as a source of inflammatory and angiogenic factors in colorectal cancer. Int J Colorectal Dis 2016; 31: 365–75. [DOI] [PubMed] [Google Scholar]
- 30. Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic potential of adipose stem cell subpopulations. Plast Reconstr Surg 2011; 128: 663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A 2013; 83: 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan H, Wang R, Yu J et al Predictive value of Smac, VEGF and Ki‐67 in rectal cancer treated with neoadjuvant therapy. Oncol Lett 2010; 1: 641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi CH, Song SY, Choi JJ et al Prognostic significance of VEGF expression in patients with bulky cervical carcinoma undergoing neoadjuvant chemotherapy. BMC Cancer 2008; 8: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang T, Srivastava S, Hartman M et al High expression of intratumoral stromal proteins is associated with chemotherapy resistance in breast cancer. Oncotarget 2016; 7: 55155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical and pathological characteristics of patients.
Fig. S1. Circulating adipokines and clinicopathological characteristics of esophageal adenocarcinoma (EAC) patients. Circulating levels of leptin and adiponectin were measured in serum of EAC patients. (a) Lymph node negative (N–) or positive (N+) involvement was estimated by TNM values for each patient. (b) Tumor size (T) was estimated by TNM values for each patient. (c) EAC patients treated with neoadjuvant therapy who had a positive tumor regression grade (Mandard 1‐2) were compared with those who had a poor response to chemotherapy (Mandard 3‐5).
Fig. S2. Adiponectin expression in peritumoral and distal adipose tissue of esophageal adenocarcinoma (EAC) patients. Adiponectin mRNA levels were measured in adipose tissue of EAC patients. (a) Immunohistochemical expression of CD31 in sections of omental and peritumoral adipose tissue was expressed as positive cells/mm2. (b) CD34 mRNA expression was measured by means of qRT‐PCR using HMBS as internal control. (C) EAC patients treated with neoadjuvant therapy who had a positive tumor regression grade (Mandard 1‐2) were compared with those who had a poor response to chemotherapy (Mandard 3‐5).


