Abstract
Millions of patients take antidepressant medications in the United States for the treatment of depression or anxiety disorders. Some antidepressants are prescribed off-label to treat problems such as chronic pain, low energy, and menstrual symptoms. Antidepressants are a broad and expansive group of medications, but the more common drug classes include tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors. A miscellaneous or “atypical” category covers other agents. Some herbal supplements that claim to have antidepressant activity will also be discussed. In Part I of this review, antidepressant pharmacology, adverse effects, and drug interactions with adrenergic agonists will be discussed. In part II, drug interactions with sedation and general anesthetics will be reviewed. Bleeding effects and serotonin syndrome implications in anesthetic practice will also be highlighted.
Key Words: Antidepressants, Selective serotonin reuptake inhibitors, Serotonin norepinephrine reuptake inhibitors, Tricyclic antidepressants, Monoamine oxidase inhibitors, St John's Wort, Adrenergic, Pharmacology, Drug interactions, SSRI, SNRI, TCA, MAOI
Millions of patients take antidepressant medications in the United States. The 2013 National Ambulatory Medical Care Survey revealed that antidepressants represented the third most frequently prescribed class of medications, following analgesics and lipid-lowering agents, in outpatient office visits.1 Antidepressants are indicated for the treatment of depression or anxiety disorders. Anxiety disorders include conditions such as generalized anxiety disorder, obsessive-compulsive disorder, panic disorders, phobias, and posttraumatic stress disorder. Although analgesic mechanisms are not fully understood, some antidepressants are prescribed off-label to treat problems such as chronic pain, low energy, and menstrual symptoms. Antidepressant use in chronic pain is especially promising for providing nonopioid analgesia in the face of a nationwide opioid crisis.2–4
A survey of dental patients indicated that 1 in 5 had a history of psychiatric disease, such as depression, anxiety, attention deficit–hyperactivity disorder, bipolar disorder, and/or seasonal affective disorder. Many of the patients surveyed had treatment that included administration of antidepressants.5 Antidepressants are a broad and expansive group of medications, but the more common drug classes include tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and monoamine oxidase inhibitors (MAOIs). A miscellaneous or “atypical” category covers other agents.
The administration of sedation or general anesthesia may mitigate acute situational anxiety as it relates to dental procedures. It is important for the provider to understand the pharmacology of antidepressant medications and herbal supplements with alleged antidepressant/antianxiety properties, their adverse effects, and potential perioperative drug-drug interactions, effects on bleeding, and interactions with various adrenergic agonists used in local anesthetics and anesthesiology practice. In part I of this series, antidepressant pharmacology and interactions with adrenergic agonists will be discussed. In part II, other anesthetic drug interactions and serotonin syndrome will be discussed.
ANTIDEPRESSANT PHARMACOLOGY
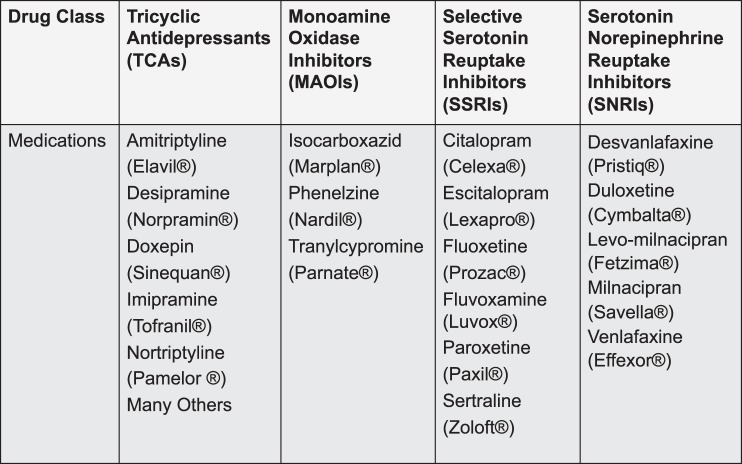
The antidepressants to be discussed belong to the following therapeutic classes: SSRIs, SNRIs, TCAs, and MAOIs (Figure 1). Their respective names reflect their structure or describe their effects on enzyme or reuptake inhibition. Although the exact mechanism of action is not known, it is thought that antidepressants increase the amount of serotonin and/or norepinephrine at the synapse. There is a delay of 2–4 weeks for the onset of significant clinical improvement. It is postulated that this latent period correlates with the downregulation of postsynaptic central nervous system (CNS) receptors.6
Figure 1. .
Antidepressant medications within the 4 therapeutic drug classes.
For each drug class, these articles review the mechanism of action and adverse effects relevant to the perioperative period. St John's wort is an herbal supplement that is included in this discussion because of its wide consumer use and potential for perioperative drug-drug interactions.
Tricyclic Antidepressants
TCAs are a group of medications that carry the name tricyclic because of their 3-ring structure. The tetracyclic antidepressant maprotiline is included here as it acts comparably to the tricyclic group. Similar to local anesthetics, TCAs contain a hydrophobic portion, which is connected to an intermediate group that connects to a secondary or tertiary amide. Therapeutic effects are mediated by limiting the reuptake of neurotransmitters by inhibiting the presynaptic reuptake transporter of the affected neurotransmitter. Tertiary amines, such as amitriptyline and imipramine, predominantly inhibit serotonin reuptake, whereas secondary amines, such as nortriptyline and desipramine, predominantly inhibit norepinephrine reuptake.3,6,7 However, it should be noted that amitriptyline is converted to nortriptyline and imipramine is converted to desipramine, such that these more serotonergic TCAs also have significant norepinephrine reuptake inhibition effects by virtue of their metabolites. There is a latent period of 2–4 weeks before therapeutic antidepressant effects become manifest. This is thought to be related to an alteration in receptor density and/or sensitivities, including increased sensitivity of postsynaptic alpha-1 receptors and decreased sensitivity of presynaptic alpha-2, postsynaptic beta-1, and postsynaptic serotonin-2 (5-HT2) receptors.6,8 Chronic administration may also lead to depletion of norepinephrine.7 TCAs also have alpha-1 antagonist, anticholinergic, and antihistaminic properties.3,6,7,9,10 These effects are variable among the drugs in the class and account for many of the adverse effects, such as orthostatic hypotension, dry mouth, constipation, and sedation. These adverse effects are, however, dose dependent. Doses for chronic pain management, a more common use for TCAs today, are much lower than those required for treatment of depression.
TCAs have long played a role in the treatment of chronic pain.3,6,8 TCAs have demonstrated analgesic efficacy in placebo-controlled trials for various chronic pain states, including postherpetic neuralgia and chronic lower back pain in patients with and without depression. Analgesic effects with TCAs occur at lower doses (eg, 10–25 mg/d amitriptyline for chronic pain vs 100–200 mg/d for depression) and with more rapid onset (days vs weeks) compared to typical antidepressant therapy. This supports the likelihood that analgesia is mediated by a different mechanism than depression, likely involving augmenting the descending opioid pain modulation system.8 It is thought that inhibition of norepinephrine reuptake is important in the analgesic effect of TCAs, as the SSRIs exhibit minimal analgesia. TCAs, because of their structural similarity to local anesthetics, may attenuate the inflammatory component of certain chronic pain syndromes.6
The most common adverse effects of TCAs include anticholinergic, CNS, and cardiovascular effects.3,6,7,9 Anticholinergic effects occur because of the drug's antagonism of muscarinic cholinergic receptors, causing blurry vision, constipation, tachycardia, urinary retention, and xerostomia.6,7 Desipramine has the lowest reported occurrence of these adverse drug reactions and amitriptyline has the highest. Concomitant administration of centrally acting anticholinergic medications may result in postoperative delirium, especially in the elderly.6 Centrally acting anticholinergic medications, such as scopolamine and to a lesser extent atropine, are more likely to increase the risk of postoperative delirium than glycopyrrolate, as this quaternary ammonium does not cross the blood-brain barrier.6,11
Notable CNS effects of TCAs include sedation and a reduction in the seizure threshold.3,6,9 Sedation is most pronounced with amitriptyline and doxepin, such that these medications are typically taken at bedtime. This sedative effect may augment sedatives used in sedation or general anesthesia practice. Weight gain can occur. Sexual dysfunction is also common and associated with serotonin and anticholinergic effects. A reduction in the seizure threshold may place patients at risk for seizures or cause epileptic patients to lose good seizure control, especially with clomipramine and maprotiline.6 Caution is warranted if large doses of local anesthetics are planned for dental procedures, especially in children taking TCAs.12
Cardiovascular effects of TCAs include orthostatic hypotension, tachycardia, and changes in cardiac conduction.3,6,9 It is likely that the orthostatic or postural hypotension is attributable to postsynaptic alpha adrenergic blockade and the tachycardia to norepinephrine reuptake inhibition and antimuscarinic effects.6,10 It is also possible that hypotension may occur intraoperatively during general anesthesia in patients taking TCAs.6 TCAs also delay cardiac impulse conduction by blocking cardiac sodium channels.3,10 Changes in cardiac electrophysiology include delayed conduction through the atrioventricular node as well as prolongation of the cardiac action potential and the refractory period. These effects will be reflected on the electrocardiogram as atrioventricular block, prolongation of the PR and QT intervals, widened QRS complex, and flat or inverted T waves.6,10 Atropine is the initial drug of choice in treating any bradycardia that may occur secondary to atrioventricular block, although minimal effectiveness is expected in third-degree heart block.6 Sodium bicarbonate can be used initially for wide QRS complex or heart block to reverse potential metabolic acidosis. Conventional antidysrhythmic drugs can also be used. Class I antiarrhythmic drugs are generally not appropriate because of the sodium-blocking effects of TCAs.13
Patients on chronic TCA therapy commonly develop some tolerance to certain adverse effects such as orthostatic hypotension, xerostomia, tachycardia, and blurred vision. Sudden termination of TCA therapy may result in physiologic withdrawal. Withdrawal of a medication is generally characterized by symptoms that are the opposite of the drug's actions. Patients in TCA withdrawal may experience runny nose; sore, achy skeletal muscle; chills; and malaise. Death may occur as a result of TCA overdose resulting in seizures, coma, respiratory depression, and cardiovascular depression (hypotension, arrhythmias, heart block, and myocardial depression). These signs of overdose require electrocardiographic monitoring for potentially fatal arrhythmias, possibly for as long as 10 days.6
Selective Serotonin Reuptake Inhibitors
SSRIs are the most widely prescribed antidepressants for the treatment of mild to moderate depression, obsessive-compulsive syndrome, panic disorder, social phobias, and posttraumatic stress disorder.6,7 SSRIs include citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline. The mechanism of action of SSRIs involves inhibition of almost exclusively serotonin reuptake with an eventual downregulation of postsynaptic serotonergic receptors. A reduction of CNS proinflammatory cytokines may have implications in CNS inflammation, which has been implicated in depressive illness. The SSRIs are inhibitors of various CYP-450 enzymes and the P-glycoprotein transporter. However, CYP inhibition is minimal with citalopram and escitalopram; of more clinical significance with fluoxetine, fluvoxamine, and paroxetine; and intermediate with sertraline. The significance of P-glycoprotein interactions is not yet clear. Adverse effects include agitation, headache, insomnia, nausea, diarrhea, and sexual dysfunction.3,6 Unlike TCAs, SSRIs do not affect the seizure threshold or delay cardiac conduction, nor do they exhibit any analgesic effects. When compared to other antidepressants, SSRIs have relatively minimal anticholinergic properties (paroxetine being the exception), sedative effects, and less orthostatic hypotension.3,6,7 Weight loss can occur with some SSRIs. The US Food and Drug Administration (FDA) issued a “black box” warning describing increased risks of suicidal tendencies in pediatric, adolescent, and young adult patients taking SSRIs for the first few months and/or during dosing increases or decreases.14 On discontinuation for all patients, it is recommended to taper SSRIs that have a short half-life. Rapidly discontinuing especially short-acting SSRIs can precipitate withdrawal symptoms such as hyperarousal, irritability, insomnia, flulike symptoms, nausea, myalgias, paresthesias, dizziness, and visual disturbances.6 If an overdose of SSRIs occurs, it is rarely fatal, and serotonin syndrome (discussed in part II) may occur.6,15 SSRIs are usually continued during the perioperative period and do not have the same hemodynamic concerns as TCAs, SNRIs, or MAOIs with respect to the concomitant use of direct- or indirect-acting sympathomimetics.7
Serotonin-Norepinephrine Reuptake Inhibitors
The SNRIs, desvenlafaxine, duloxetine, levomilnacipran, milnacipran, and venlafaxine, inhibit the reuptake of both serotonin and norepinephrine in the synaptic cleft. Unlike the TCAs, which also inhibit serotonin and norepinephrine reuptake, there are minimal direct effects on other neurotransmitters or receptors.3,6 Decrease in CNS proinflammatory cytokines has also been documented. The side effect profile of SNRIs includes sedation, nausea, insomnia, tachycardia, sexual dysfunction, and anticholinergic effects, including xerostomia and constipation.6,8 Elevations in heart rate and blood pressure are possible because of norepinephrine reuptake inhibition. Venlafaxine is an inhibitor of CYP-450 enzymes.6 Its active metabolite, desvenlafaxine, was developed to overcome this concern. Venlafaxine and duloxetine have also been used for the treatment of chronic neuropathic pain.3 In addition to FDA indications for major depression and generalized anxiety disorder, duloxetine is also FDA indicated for the treatment of painful diabetic neuropathy, postherpetic neuralgia, and chronic musculoskeletal pain. Milnacipran is FDA approved for treatment of fibromyalgia. Two randomized, placebo-controlled trials for the treatment of fibromyalgia and diabetic neuropathy with duloxetine demonstrated that analgesic onset occurred at lower doses with a more rapid onset as compared to dosing and onset for antidepressant therapy. Additionally, the analgesic effects occurred independently of whether the patients had been diagnosed with depression.8
Monoamine Oxidase Inhibitors
MAOIs are an older class of antidepressants that are reserved for treatment of patients who are not responsive to other classes of medications because of significant adverse effects and dietary restrictions. MAOIs inhibit presynaptic monoamine oxidase (MAO) enzymes, which increases presynaptic neuronal cytoplasmic concentrations of MAO substrates, especially norepinephrine, serotonin, and dopamine.3,6,7 It is thought that the improvement in patients' symptomatology is related to the subsequent increased release of these neurotransmitters into the synaptic cleft, again leading to a downregulation of postsynaptic receptors.6
MAO enzymes metabolize monoamine neurotransmitters by oxidative deamination and are located on the outer mitochondrial membranes inside the presynaptic nerve terminals, both centrally and peripherally. There are 2 subtypes of MAO: MAO-A and MAO-B. The substrates for MAO-A include dopamine, epinephrine, norepinephrine, serotonin, and tyramine. MAO-B substrates include mainly dopamine and phenylethylamine. Tyramine is a monoamine found in foods such as cheese, avocado, fava beans, liver, and wine, which must be avoided in patients taking MAO-A inhibitors as the combination can lead to acute high blood pressure. Phenylethylamine is found in some of the same foods but is present in such trace amounts in the CNS as to not be of clinical significance. Both MAO subtypes are found in the CNS. In the periphery, MAO-A is found mainly in the gastrointestinal tract, kidneys, liver, and lungs. MAO-B is found mostly in platelets. Isocarboxazid, phenelzine, and tranylcypromine are all nonselective, irreversible inhibitors of MAO and are indicated for the treatment of depression, especially if depression is refractory to other classes of antidepressant drugs. Selegiline, a selective, irreversible MAO-B inhibitor, is used for treatment of early-stage Parkinson disease at a maximum dose of 10 mg/d. At doses over 30 mg/d, it nonselectively inhibits MAO.6
The side effect profile of MAOIs can be attributed to alterations in neurotransmitter concentrations as a result of MAO inhibition as well as to the anticholinergic properties of the drugs. Orthostatic hypotension is common. MAOIs can cause sexual dysfunction and significant weight gain. Unlike TCAs, MAOIs do not directly alter cardiac conduction. MAOI overdose will demonstrate signs and symptoms of excessive sympathetic activity, including mydriasis, hypertension, tachycardia, and hyperthermia, as well as seizures and potentially coma or death.3 Supportive care includes treatment of seizures, hemodynamic and thermal control, and gastric lavage.6
St John's Wort
St John's wort is an herbal supplement that claims to promote positivity and good overall mood. Although the FDA does not evaluate claims of efficacy made by manufacturers of herbal supplements, St John's wort causes notable drug interactions. St John's wort can inhibit the reuptake of dopamine, norepinephrine, and serotonin. Therefore, there is a potential to have synergistic effects of dangerously elevating these neurotransmitter levels regardless of whether patients are taking other antidepressants. Ingesting St John's wort may also interfere with medications administered perioperatively, as it is also an inducer of CYP 3A4, 1A2, and 2C9. Induction of CYP 3A4 will increase the metabolism and reduce the efficacy of orally administered midazolam. Intravenous agents may show increased metabolism affecting residual sedative and analgesic effects. Induction of CYP 2C9 will also induce the metabolism and diminish the therapeutic effect of drugs such as warfarin and some nonsteroidal anti-inflammatory drugs.6,16–18
ANTIDEPRESSANTS AND ADRENERGIC AGONIST INTERACTIONS
Drugs affecting CNS norepinephrine presynaptic stores (MAOIs) or increasing activity of synaptic norepinephrine (SNRIs and TCAs) may affect adrenergic neurotransmission in the periphery. Because the activity of epinephrine that reaches the peripheral synaptic cleft is also principally terminated by presynaptic norepinephrine transporter reuptake, drugs that inhibit this process (SNRIs and TCAs) also may prolong and exaggerate epinephrine activity. Levonordefrin (alpha-methyl norepinephrine) has predominantly alpha-2 effects with mild beta-1 effects. Presynapatic reuptake is also responsible for termination of activity at the synapse. Although prolonged activity can also be expected, the effects may be predominantly related to blood pressure.
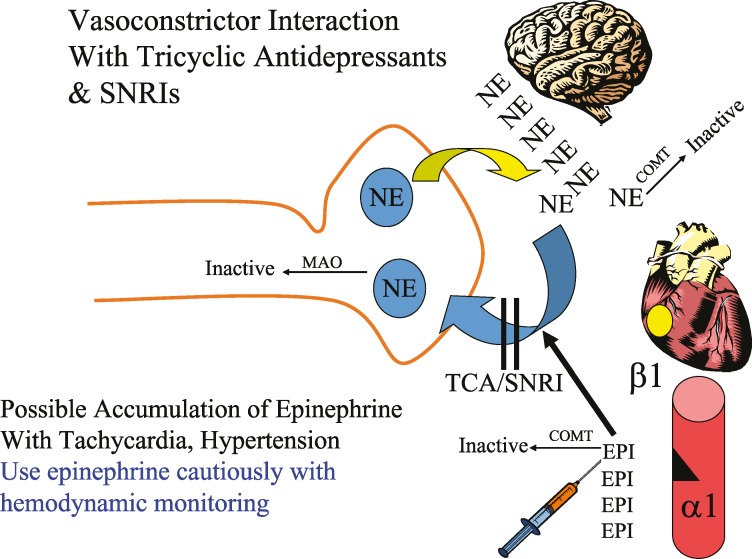
In patients taking TCAs and SNRIs, direct-acting catecholamines, such as epinephrine in local anesthetic solutions, may therefore have exaggerated effects on blood pressure and heart rate for that portion of the drug that reaches the heart and peripheral vasculature (Figure 2). If possible, injected epinephrine in local anesthetic solutions should be minimized or injections given over time to minimize systemic absorption. Slow injection may also help to limit systemic absorption (Figure 3). Particularly with the TCAs, these effects are likely dose dependent, such that low doses (10–25 mg), as used for chronic pain management, may not display significant interaction. It should be remembered that both these classes of drugs can cause resting tachycardia as an adverse effect. Animal studies have demonstrated that the actions of epinephrine and levonordefrin have been enhanced in dogs receiving TCAs and local anesthetic with these vasoconstrictors. It has been recommended to limit the dose of vasoconstrictor to 0.05 mg.19–21 However, these studies were performed on dogs who received TCAs for 4–5 days, which is insufficient time for downregulation of the adrenergic receptors. It is possible that on chronic doses, the interaction between TCAs and local anesthetics containing vasoconstrictors does not occur.23 Although this is still highlighted in local anesthetic texts, additional human research is needed to determine if therapeutic doses of local anesthetics containing vasoconstrictors really cause untoward events in patients taking TCAs.24 For sedation and general anesthesia–trained dentists, continual cardiovascular monitoring should guide appropriate vasoconstrictor use.
Figure 2. .
Vasoconstrictor interaction with tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors.
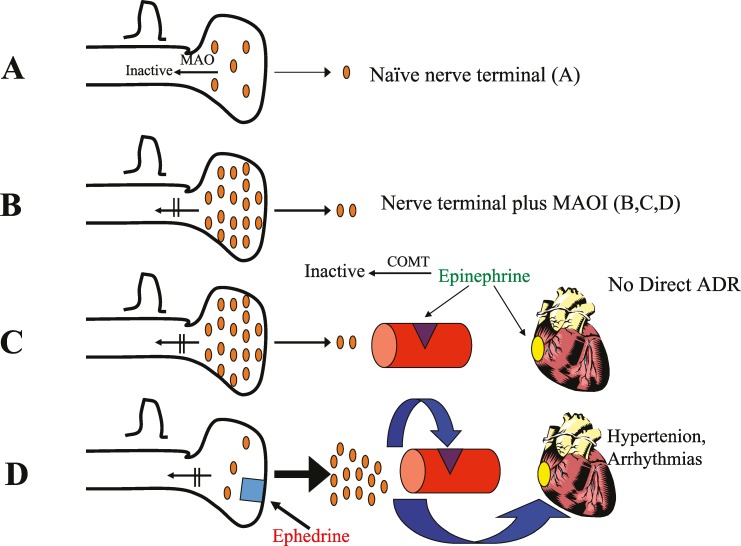
Figure 3. .
(A) Naïve nerve terminal. (B) Nerve terminal plus a monoamine oxidase inhibitor. (C) Nerve terminal with epinephrine. (D) Nerve terminal with ephedrine.
MAOIs, which increase presynaptic stores of serotonin, norepinephrine, and dopamine, leading to postsynaptic downregulation, would, from a strictly pharmacological standpoint, not interact with direct-acting adrenergic agonists (Figure 3). In decades past, extreme caution was recommended when dentists used epinephrine in local anesthetics in patients taking MAOIs. Although pharmacologically this interaction should not occur, it is possible that pain and/or anxiety from local anesthetic injection might activate the sympathetic nervous system with exaggerated effects from MAOIs on blood pressure and heart rate, accounting for numerous reports of this occurrence. It is also possible that adrenergic receptors become sensitized to the effects of sympathomimetics in patients taking MAOIs. Therefore, it seems prudent that systemic direct-acting intravenous sympathomimetics should be titrated to the desired hemodynamic effect, starting with lower than usual doses to avoid exaggerated tachycardia and/or pressor responses. Likewise, vasoconstrictors in local anesthetic solutions should be used cautiously, monitoring hemodynamics.3,6,9 Regardless of these interactions, adequate local anesthesia, as part of a multimodal anesthetic regimen, will reduce the need for intraoperative general anesthetics and postoperative opioid consumption.6,24 Therefore, careful titration with appropriate cardiovascular monitoring of local anesthetics utilizing the appropriate minimum amount of vasoconstrictors is recommended.
Indirect-acting agents, such as ephedrine and pseudoephedrine, can cause a hypertensive crisis in patients taking MAOIs, and their use in contraindicated for these patients (Figure 3). Because ephedrine in part releases stores of presynaptic neurotransmitters, large amounts of stored norepinephrine may be released.3,6,7,9
Likewise, because of prolongation of action of norepinephrine/epinephrine by reuptake inhibitors like TCAs and SNRIs, exaggerated cardiovascular effects may be seen with indirect-acting agents. In the early stages of TCA and SNRI therapy, patients are particularly prone to exaggerated response to pressors, especially indirect-acting sympathomimetic like ephedrine.6,7 If TCAs have been administered chronically for a period longer than 4–6 weeks, there may be a downregulation of adrenergic receptors and potentially a depletion of catecholamine stores, resulting in a diminution of sympathetic activity. In this scenario, as with direct-acting agents, it is advisable to treat hypotension by titrating smaller doses of indirect-acting sympathomimetics to assess initial efficacy and determine future dose modifications.
Because of these concerns over unstable hemodynamics in patients taking MAOIs, the need for perioperative discontinuation of MAOIs has always been controversial. It was previously recommended to taper MAOIs over the course of 2–3 weeks preceding elective surgery to avoid CNS and cardiovascular lability. However, MAOIs should be discontinued only pending consultation with the patient's psychiatrist, for concerns that an interruption of MAOI therapy may result in a relapse of psychiatric symptoms. If the drug is discontinued, a plan for restarting treatment postoperatively is important. Anesthetic care for elective dental procedures should avoid hypotension and excessive sympathetic stimulation. Avoidance of triggering agents for serotonin syndrome, which will be discussed in part II, is critical.6,9 If the patient needs urgent or emergent treatment, and it is not possible to taper the medications, then indirect-acting sympathomimetics should be avoided. Hypotension should be addressed ideally by fluid management and carefully titrating a direct-acting vasoconstrictor, such as phenylephrine, a direct-acting alpha-1 agonist that does not undergo presynaptic reuptake.7 Therefore, its use is generally preferred for treatment of hypotension if the heart rate is acceptable. Because of unpredictable postsynaptic receptivity, careful titration is recommended.7
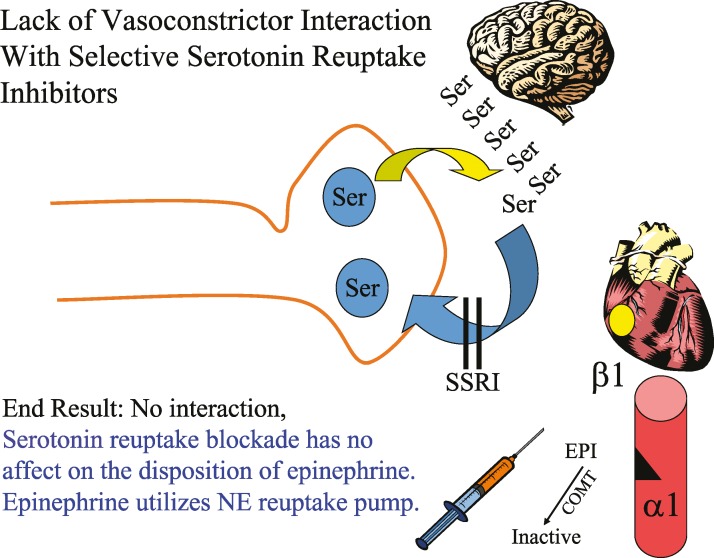
Although not antidepressants per se, other drugs that exhibit norepinephrine reuptake inhibition, such as the amphetamines (eg, Adderall) and atomoxetine (Strattera) used for attention deficit–hyperactivity disorders, can exhibit the same drug interaction concerns noted above. The SSRIs and other atypical antidepressants (eg, trazadone, mirtazapine, bupropion) do not have significant epinephrine or ephedrine interactions (Figure 4).
Figure 4. .
Lack of vasoconstrictor interaction with selective serotonin reuptake inhibitors.
CONCLUSION
Part I of this series has reviewed antidepressant pharmacology. Important considerations regarding the use of adrenergic agonists have also been discussed. Part II of this series will highlight other anesthetic drug interactions as well as serotonin syndrome.
CONTINUING EDUCATION QUESTIONS
This continuing education (CE) program is designed for dentists who desire to advance their understanding of pain and anxiety control in clinical practice. After reading the designated article, the participant should be able to evaluate and utilize the information appropriately in providing patient care.
The American Dental Society of Anesthesiology (ADSA) is accredited by the American Dental Association and Academy of General Dentistry to sponsor CE for dentists and will award CE credit for each article completed. You must answer 3 of the 4 questions correctly to receive credit.
Submit your answers online at www.adsahome.org. Click on “On Demand CE.”
-
1.
Tricyclic antidepressants possess all of the following properties except:
-
A.
Alpha-1 antagonist
-
B.
Anticholinergic
-
C.
Antihistaminic
-
D.
Beta-1 antagonist
-
A.
-
2.
With respect to the administration of direct- or indirect-acting sympathomimetics in the perioperative period, which drug class has the least concern for cardiovascular instability?
-
A.
Monoamine oxidase inhibitors
-
B.
Selective serotonin reuptake inhibitors
-
C.
Serotonin-norepinephrine reuptake inhibitors
-
D.
Tricyclic antidepressants
-
A.
-
3.
Which statement is true with respect to the use of St John's Wort?
-
A.
St John's wort has minimal potential side effects affecting sedation/anesthesia.
-
B.
St John's wort inhibits the reuptake of serotonin, epinephrine, and norepinephrine.
-
C.
St John's wort is an inhibitor of CYP enzymes.
-
D.
The US Food and Drug Administration has granted St John's wort an indication to treat depression.
-
A.
-
4.
Which statement describes the best initial medication management for the urgent treatment of hypotension with mild tachycardia for a patient taking monoamine oxidase inhibitors under sedation?
-
A.
Careful titration of the anticholinergic atropine.
-
B.
Careful titration of the direct-acting sympathomimetic epinephrine.
-
C.
Careful titration of the direct-acting sympathomimetic phenylephrine.
-
D.
Careful titration of the indirect-acting sympathomimetic ephedrine.
-
A.
REFERENCES
- 1 . National Center for Health Statistics, Centers for Disease Control and Prevention. Therapeutic drug use. Available at: https://www.cdc.gov/nchs/fastats/drug-use-therapeutic.htm. Accessed June 30, 2017.
- 2 . Insel T. . Antidepressants: a complicated picture. National Institute of Mental Health, National Institutes of Health: Available at: https://www.nimh.nih.gov/about/directors/thomas-insel/blog/2011/antidepressants-a-complicated-picture.shtml. Accessed June 30, 2017. [Google Scholar]
- 3 . Catalani B, Hamilton CS, Herron EW, et al. Psychiatric agents and implications for perioperative analgesia. Best Pract Res Clin Anaesthesiol. 2014; 28: 167– 181. [DOI] [PubMed] [Google Scholar]
- 4 . Hendley TM, Hersh EV, Moore PA, et al. Treatment of opioid overdose: a brief review of naloxone pharmacology and delivery. Gen Dent. 2017; 65: 18– 21. [PubMed] [Google Scholar]
- 5 . Giglio JA, Laskin DM. . Prevalence of psychiatric disorders in a group of adult patients seeking general dental care. Quintessence Int. 2010; 41: 433– 437. [PubMed] [Google Scholar]
- 6 . Stoelting RK, Hillier SC. . Pharmacology & Physiology in Anesthetic Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 7 . Attri JP, Bala N, Chatrath V. . Psychiatric patient and anaesthesia. Indian J Anaesth. 2012; 56: 8– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 . Hersh EV, Balasubramaniam R, Pinto A. . Pharmacologic management of temporomandibular disorders. Oral Maxillofac Surg Clin North Am. 2008; 20: 197– 210. [DOI] [PubMed] [Google Scholar]
- 9 . Huyse FJ, Touw DJ, van Schijndel RS, et al. Psychotropic drugs and the perioperative period: a proposal for a guideline in elective surgery. Psychosomatics. 2006; 47: 8– 22. [DOI] [PubMed] [Google Scholar]
- 10 . Thanacoody HK, Thomas SH. . Tricyclic antidepressant poisoning: cardiovascular toxicity. Toxicol Rev. 2005; 24: 205– 214. [DOI] [PubMed] [Google Scholar]
- 11 . Morgan GE, Mikhail MS, Murray MJ. . Clinical Anesthesiology. 4th ed. New York, NY: The McGraw-Hill Companies; 2006. [Google Scholar]
- 12 . Saraghi M, Moore PA, Hersh EV. . Local anesthetic calculations: avoiding trouble with pediatric patients. Gen Dent. 2015; 63: 48– 52. [PubMed] [Google Scholar]
- 13 . Bradberry S, Thanacoody H, Watt B, Thomas S, Vale J. . Management of the cardiovascular complications of tricyclic antidepressant poisoning: role of sodium bicarbonate. Toxicol Rev. 2005; 24: 195– 204. [DOI] [PubMed] [Google Scholar]
- 14 . FDA warning regarding antidepressants. Available at: https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm096273.htm. Accessed October 7, 2017.
- 15 . Barbey JT, Roose SP. . SSRI safety in overdose. J Clin Psychiatry. 1998; 59 suppl 15: 42– 48. [PubMed] [Google Scholar]
- 16 . De Baerdemaeker L, Audenaert K, Peremans K. . Anaesthesia for patients with mood disorders. Curr Opin Anaesthesiol. 2005; 18: 333– 338. [DOI] [PubMed] [Google Scholar]
- 17 . Haller CA, Benowitz NL. . Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med. 2000; 343: 1833– 1838. [DOI] [PubMed] [Google Scholar]
- 18 . Sukhminder JSB, Aparajita P. . Alternative medicine and anesthesia: implications and considerations in daily practice. Ayu. 2012; 33: 475– 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19 . Sisk AL. . Vasoconstrictors in local anesthesia for dentistry. Anesth Prog. 1992; 39: 187– 193. [PMC free article] [PubMed] [Google Scholar]
- 20 . Yagiela JA, Duffin SR, Hunt LM. . Drug interactions and vasoconstrictors used in local anesthetic solutions. Oral Surg Oral Med Oral Pathol. 1985; 59: 565– 571. [DOI] [PubMed] [Google Scholar]
- 21 . Naftalin LW, Yagiela JA. . Vasoconstrictors: indications and precautions. Dent Clin North Am. 2002; 46: 733– 746, ix. [DOI] [PubMed] [Google Scholar]
- 22 . Brown RS, Rhodus NL. . Epinephrine and local anesthesia revisited. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 100: 401– 408. [DOI] [PubMed] [Google Scholar]
- 23 . Hersh EV. . Local anesthetics. : Fonseca R. J. . Oral and Maxillofacial Surgery. Vol 1. 1st ed. Philadelphia, PA: WB Saunders Company; 2000: 58– 78. [Google Scholar]
- 24 . Saraghi M, Hersh EV. . Three newly approved analgesics: an update. Anesth Prog. 2013; 60: 178– 187. [DOI] [PMC free article] [PubMed] [Google Scholar]