Abstract
An aberrantly elevated expression of DNA polymerase ι (Pol ι) is significantly associated with poor prognosis of patients with esophageal squamous cell carcinoma (ESCC), yet the mechanisms behind this phenomenon remain obscure. Based on the RNA‐Seq transcriptome and real‐time PCR analysis, we identified ETS‐1 as a candidate gene involved in Pol ι‐mediated progression of ESCC. Wound‐healing and transwell assay indicated that downregulation of ETS‐1 attenuates Pol ι‐mediated invasiveness of ESCC. Signaling pathway analysis showed that Pol ι enhances ETS‐1 phosphorylation at threonine‐38 through the Erk signaling pathway in ESCC cells. Kaplan–Meier analysis, based on 93 clinical tissue samples, revealed that ETS‐1 phosphorylation at threonine‐38 is associated with poor prognosis of ESCC patients. The present study thus demonstrates that phosphorylation of ETS‐1 is a critical event in the Pol ι‐induced invasion and metastasis of ESCC.
Keywords: DNA polymerase iota, esophageal squamous cell carcinoma, ETS‐1 phosphorylation, metastasis, tumor invasion
Esophageal squamous cell carcinoma (ESCC) is one of the most aggressive cancers. ESCC causes approximately 300 000 deaths each year worldwide, with 70% of cases occurring in China.1 Despite the improvement in treatment throughout the past decades, the prognosis of ESCC patients remains poor, with a 5‐year survival rate of approximately 20–30%.2 The high mortality rate is largely attributed to aggressive invasion and metastasis of ESCC cells.3 Hence, a better understanding of the molecular mechanisms relating to cancer invasion and metastasis is critical for improving the prognosis of patients with ESCC.
Translesion DNA synthesis (TLS) is an important biological pathway that allows continuous DNA replication in the presence of DNA lesions.4 The process is usually associated with widespread mutagenesis and genomic instability, because it is undertaken by some error‐prone DNA polymerases.5 Recent studies have demonstrated that error‐prone TLS polymerases are involved in carcinogenesis, cancer metastasis and tumor progression.6, 7, 8 DNA polymerase ι (Pol ι), the product of the RAD30B gene, is well known to participate in the TLS pathway with extremely low fidelity.9 During the TLS process, Pol ι preferentially incorporates G opposite a template T in an undamaged DNA strand, which results in the accumulation of DNA mutation and genetic instability.10 Accumulation of DNA mutation and genetic instability are predisposed to cancer initiation. Some studies have revealed that the expression pattern of Pol ι appears to be tissue‐specific in cancer. Pol ι is overexpressed in human bladder cancer, uveal melanoma and breast cancer,10, 11, 12 while it is downregulated in human lung, stomach and colorectal cancers.13 Hence, Pol ι is considered a double‐edged sword in regulating cancer progression.
Our previous work demonstrated that the expression of Pol ι is upregulated in ESCC tissues, and overexpression of Pol ι is positively correlated with lymph node metastasis and poor prognosis of ESCC patients.14, 15 We also found that Pol ι promotes invasiveness and migration of ESCC cells in vitro.15 However, the process of Pol ι‐mediated invasion and metastasis of ESCC cells is not well understood. To explore the potential mechanisms responsible for Pol ι‐mediated metastasis, RNA‐Seq transcriptome analysis was performed using RNA isolated from Pol ι‐knocking down and control ESCC cells. We found that, among Pol ι‐affected genes, expression of ETS‐1, a key regulator in cancer cell invasion and metastasis, is highly correlated with expression of Pol ι. We further examined the role of ETS‐1 in Pol ι‐mediated invasion and metastasis of ESCC cells in the present study.
Materials and Methods
Tissue samples and cell lines
Human ESCC tissues and adjacent tissues used in this study were obtained from Nanjing Medical University Affiliated Suzhou Hospital (Jiangsu, China). The tissue samples were immediately snap‐frozen and stored at −80°C for real‐time PCR analysis and histological examination. All of the samples were obtained with informed consent and the study was approved by the Institutional Ethics Committee of Nanjing Medical University. Human ESCC cell lines, including ECA‐109 and KYSE‐150, were obtained from the Shanghai Cell Bank (Shanghai, China). ECA‐109 cells were cultured in DMEM medium and KYSE‐150 cells were cultured in RPMI‐1640 medium. All of the media (Hyclone, Logan, UT, USA) were supplemented with 10% FBS (Hyclone). The cells were incubated in a humidified atmosphere, with 5% CO2 at 37°C.
RNA extraction and quantitative RT‐PCR
Total RNA was isolated using TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) following the manufacturer's instructions. The concentrations of RNA were determined using a NanoDrop2000 (Thermo Scientific, Rochester, NY, USA). For reverse transcription, 1 μg of RNA per sample was reverse transcribed using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). Quantitative PCR analyses were conducted to quantitate mRNA expression using a QuantiNova SYBR Green PCR Kit (QIAGEN, Hilden, Germany) and TransStart Tip Green qPCR Supermix (Transgen, Beijing, China) with β‐actin mRNA level as an internal control. The primers are listed in Table S2. Relative expression levels were calculated by using the method. PCR was performed using a StepOne Plus instrument (Applied Biosystems, Rochester, NY, USA).
Stable cell line generation
The human Pol ι coding region was amplified by RT‐PCR. The amplified fragment of Pol ι was cloned into the lentivirus vector LV5 (Shanghai GenePharma, Shanghai, China) to produce infection viruses. The cell line ECA‐109 NC/Pol ι was infected with the lentivirus containing control vector or Pol ι cDNA. Pol ι or ETS‐1 shRNA and control shRNA were obtained from Guangzhou RIBOBIO (Guangzhou, China) and cloned into the lentivirus vector LV16 (Shanghai GenePharma). ECA‐109 and KYSE‐150 cells were infected with indicated lentivirus. All transfected cells were selected by the medium containing 1 μg/mL Puromycin (Sigma‐Aldrich, St. Louis, MO, USA) for 7 days. Pol ι and ETS‐1 expression levels in the cells were verified using quantitative RT‐PCR (qRT‐PCR) and western blot analysis.
RNA‐Seq transcriptome analysis
Total RNA from KYSE‐150 shNC/shPol ι was prepared and kept at −80°C. The RNA quality was determined using a Bioanalyzer 2200 (Agilent, Santa Clara, CA, USA). RNA with RIN (RNA integrity number) >8.0 was considered acceptable for cDNA library construction. Sequencing and bioinformatic analysis were performed by Shanghai Novelbio. Genes were considered to be significantly differentially expressed between groups when the P‐value was <0.05 and the fold change of expression was more than 1.5.
Western blot analysis
Cells were harvested and lysed in RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) containing protease inhibitors for 20 min at 4°C. Equal amounts of the proteins were separated by 10% SDS‐PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). After blocking with 5% nonfat milk, the membranes were incubated with primary antibodies against β‐actin (Beyotime Biotechnology), Pol ι (Proteintech, Rosemont, IL, USA), ETS‐1, p‐ETS‐1, E‐cadherin, Erk1/2, p‐Erk1/2 (Abcam, Cambridge, MA, USA) and N‐cadherin (Multi Sciences, Hangzhou, China). The membranes were then incubated with an HRP‐conjugated anti‐mouse or anti‐rabbit secondary antibody (Beyotime Biotechnology). The protein bands were visualized using High‐sig ECL Western Blotting Substrate (Tanon, Shanghai, China). Images were collected using the Tanon‐5200 Chemiluminescent Imaging System (Tanon). Endogenous β‐actin protein expression was detected as the internal control for each sample.
Immunohistochemical analyses
The expression pattern of Pol ι and ETS‐1 in human tissue samples was analyzed using immunohistochemistry. Tumor tissue sections were deparaffinized and heat‐treated with citrate buffer, pH 6.0, for 5 min as an epitope retrieval protocol. The tissue sections were then exposed to 0.03% hydrogen peroxide for 5 min to block endogenous peroxidase activity followed by incubation with Pol ι antibody (Proteintech) and ETS‐1 antibody (Abcam) diluted at 1:100 for 2 h at 37°C. HRP‐conjugated anti‐mouse/rabbit antibody was then added for 1 h and the color was developed using 3‐3′‐diaminobenzidine. Following washing, the sections were counterstained with hematoxylin, washed and dipped briefly in a water bath containing drops of ammonia, prior to dehydration and mounting in Diatex. The stained sections were analyzed and scored using a Leica Microscope (Leica, Wetzlar, Germany). Immunohistochemical scoring was based on the intensity and average percentage of positive cells. The staining intensity was scored with “1” (negative or weakly positive), “2” (moderately positive) and “3” (strongly positive). The average percentage of positive cells was scored as: 1 (<25%), 2 (25–50%), 3 (50–75%) and 4 (>75%).
Wound‐healing and transwell assays
ECA‐109 and Kyse‐150 cells were seeded in six‐well plates and reached confluence in 24 h. A thin mark was drawn vertically with a pipette tip in the six‐well plate. Cells were then washed three times with PBS to remove the floating and detached cells. Fresh serum‐free medium was added, and photos were taken at an appropriate time to assess cell migration using a light microscope (Leica Corporation).
Cell invasion was assessed using Matrigel‐coated Transwell chambers (Corning, NY, USA). A total of 1 × 105 cells were plated in medium without FBS on 24‐well Transwell inserts pre‐coated with Matrigel at 1:8 dilution (BD Bioscience, Billerica, MA, USA). The lower chambers were filled with medium that contained 20% FBS. After incubation for 24 or 48 h, the cells remaining in the upper chambers were scraped off, and the invading cells were stained with Wright‐Giemsa solution (Nanjing Jiancheng Bioengineering Technology, Nanjing, China). The penetration of cells through the membrane was photographed under a microscope.
Statistical analysis
The quantitative results are presented as the mean values ± SEM. Statistical analyses were performed using SPSS 19.0 software (IBM, Chicago, IL, USA). Statistical significance was considered at a P‐value <0.05. Differences between groups were estimated using the χ2‐test and Student's t‐test. The overall survival rate was calculated actuarially according to the Kaplan–Meier method and analyzed by the log‐rank test. Relationships of variables were explored using Pearson's correlation.
Results
Differential gene expression in Pol ι knocking down KYSE‐150 cells
Pol ι was constitutively overexpressed in KYSE‐150 cells. To explore the potential mechanisms of Pol ι‐mediated motility and invasiveness of ESCC cells, RNA‐Seq was performed to profile the transcriptome in Pol ι‐knocking down KYSE‐150 cells (KYSE‐150 shPol ι) versus control KYSE‐150 cells. A total of 718 genes were identified to be differentially expressed (Table S1, shPol ι/NC ratio ≥1.50‐fold or ≤0.67‐fold, P < 0.05). All differentially expressed genes between Pol ι‐knocking down KYSE‐150 cells and control cells were analyzed to characterize potential pathways or biological processes. The pathway analysis revealed the following pathways in which those differentially expressed genes are involved: oxidative phosphorylation, metabolic pathways and the p53 signaling pathway. The gene ontology analysis revealed the biological processes in which those differentially expressed genes are involved: the respiratory electron transport chain, the cellular metabolic process and the mitochondrial electron transport process (Fig. 1).
Figure 1.
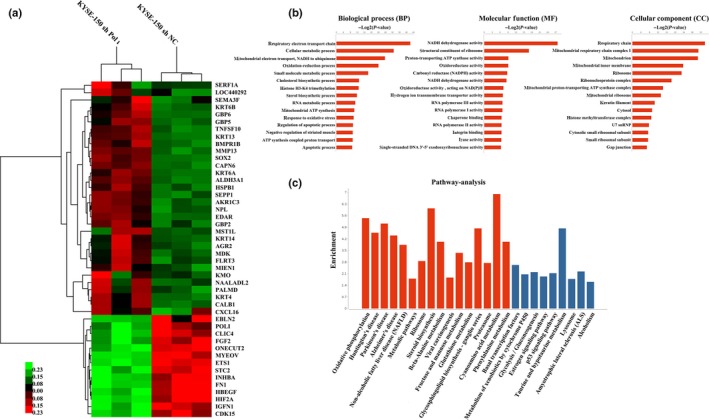
Informatics of identified transcripts. RNA isolated from KYSE‐150 shPol ι cells and control KYSE‐150 cells were analyzed by RNA‐Seq analysis. (a) Heat map of differentially expressed transcripts. (b) Gene ontology (GO) analysis and (c) pathway analysis, based on all identified transcripts. P‐value of GO analysis and enrichment of pathway analysis are listed for each category. P‐value of pathway is colored in red (P < 0.05) and blue (0.05< P <0.1).
The protein–protein interaction network was predicted by STRING (Fig. S1). As seen in the network, some growth factors, including TGFA, TGFBI, FGF2 and HBEGF, in company with FN1 are located in the key nudes and their expressions are downregulated in KYSE‐150 shPol ι cells. In addition, expression of some transcriptional factors was found to be downregulated, including Hif‐2α, ONECUT2, SOX9 and members of the ETS family (ETS‐1, ETV4, ETV5 and ELK3).
ETS‐1 expression is positively correlated with Pol ι expression
Twelve genes involved in cancer cell invasion or metastasis were selected and validated by qRT‐PCR in KYSE‐150 shPol ι and KYSE‐150 shNC cells (Fig. 2a). Expression of FGF2, HBEGF, ETS‐1, STC2, ONECUT2, CLIC4 and Hif‐2α was downregulated, while expression of MIEN1, HSPB1, MDK, SEMA3F and CXCL16 was upregulated in KYSE‐150 shPol ι cell. These observations are consistent with the results from our transcriptome analysis. To further establish the relationship between Pol ι and the selected candidate genes, expression of ETS‐1 and Hif‐2α was validated in 75 ESCC tissue samples, and expression of other selected genes in 40 ESCC tissue samples by qRT‐PCR analysis. As shown in Fig. 2b, Pol ι expression is positively correlated to that of ETS‐1, CLIC4, CXCL16 and Hif‐2α. Surprisingly, CXCL16 was upregulated in KYSE‐150 shPol ι cells, but was positively correlated with Pol ι in tissue samples. Generally, esophageal cancer tissues also include fibroblasts16 that express high level of CXCL16.17, 18 Whether other types of cells with tumor tissues contribute to the high expression of CXCL16 merits further investigation. We focused on ETS‐1 for further exploration because of the higher correlation of Pol ι and ETS‐1 expression and the established nature of ETS‐1 as a proto‐oncogene.19
Figure 2.
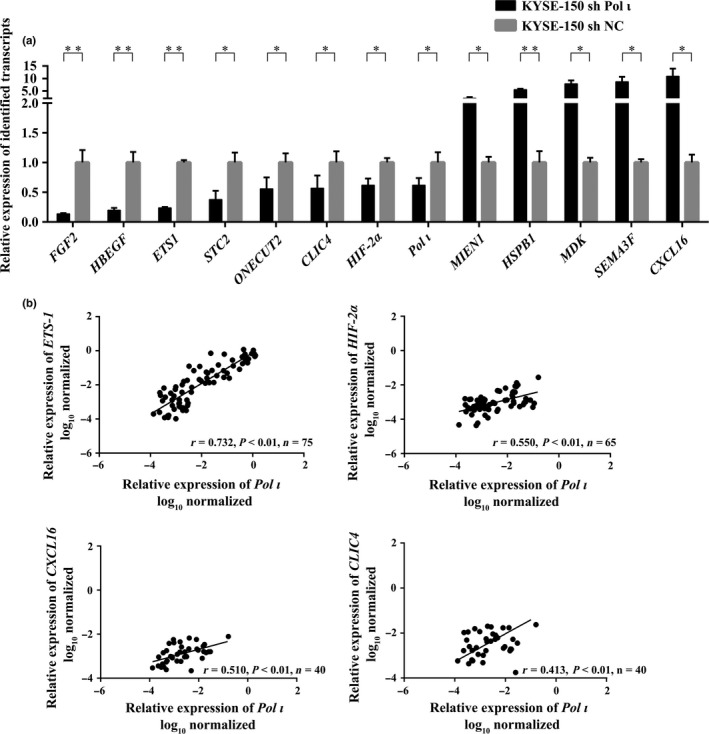
Validation of differentially expressed transcripts in Pol ι downregulation cells. (a) Thirteen differentially expressed transcripts were validated by quantitative RT‐PCR (qRT‐PCR) in Pol ι downregulation KYSE‐150 cells (n = 3, mean ± SD). Student's t‐test was applied for statistical analyses, *P < 0.05, **P < 0.01. (b) The Pearson's correlation between expression of Pol ι and that of ETS‐1, Hif‐2α, CXCL16 and CLIC4 was evaluated, based on qRT‐PCR results using patient tissue samples.
Downregulation of ETS‐1 attenuates Pol ι‐induced esophageal squamous cell carcinoma cell invasion and migration in vitro
To explore the role of ETS‐1 in Pol ι‐mediated invasion and migration of ESCC cells, ETS‐1 expression was downregulated using the RNA interference technology. ETS‐1‐shRNA was cloned into the lentivirus LV16 plasmid and infectious lentivirus was established to infect ECA‐109 Pol ι (Pol ι overexpression cell line) and KYSE‐150 cells. After puromycin selection, the knockdown efficiency was confirmed by western blot. ETS‐1 expression was successfully downregulated (data not shown). Transwell and wound‐healing assays were then employed to assess the ability of invasion and migration of the ETS‐1 knockdown cells. As shown in Fig. 3a, the ECA‐109 Pol ι cells are better able to invade through the Matrigel coated membrane than the control cells, and knockdown of ETS‐1 significantly decreases the invasive potential of ECA‐109 Pol ι and KYSE‐150 cells. The wound‐healing assay indicated that the ECA‐109 Pol ι cells narrow the wound area more effectively as compared to the control cells; the gap in the ETS‐1 knockdown cell monolayer is more slowly closed as compared with that of control ECA‐109 and KYSE‐150 cells (Fig. 3b). Thus, the expression of ETS‐1 in ECA‐109 Pol ι cells was closely correlated to cell migration ability. Collectively, these data demonstrated that Pol ι‐mediated invasion and migration in vitro is attenuated by downregulation of ETS‐1 expression.
Figure 3.
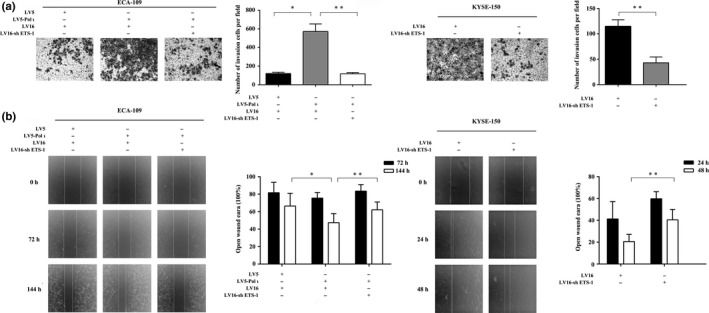
Downregulation of ETS‐1 inhibits esophageal squamous cell carcinoma cell invasion and migration. (a) The invasion of ECA‐109 and KYSE‐150 cells was measured using a Transwell assay (n = 3). 1 × 105 cells were seeded per chamber and after culture for 24 or 48 h, the cells were stained with Wright‐Giemsa solution and photographed at ×200. (b) The migration of ECA‐109 and KYSE‐150 cells was measured with the wound healing assay (n = 5, mean ± SD). 4 × 105 cells were seeded per well in a six‐well plate and photographed at ×40. The results were compared using Student's t‐test, *P < 0.05, **P < 0.01.
Pol ι activates ETS‐1 through the Erk signaling pathway
To examine the potential interaction of Pol ι and ETS‐1, western blot was performed using cell lysates from ESCC cells. As shown in Fig. 4, in ECA‐109 Pol ι cells, phosphorylation of ETS‐1 at threonine‐38 is dramatically enhanced as compared with that in control cells. Conversely, downregulation of Pol ι expression significantly reduced the phosphorylation of ETS‐1 in ESCC cells. Previous reports indicate that phosphorylation of threonine‐38 by Erk1/2 activates Ets1.19 Thus, Pol ι could induce phosphorylation of ETS‐1 through the Erk signaling pathway. As expected, we found that overexpression of Pol ι augments Erk1/2 phosphorylation, whereas downregulation of Pol ι decreases Erk1/2 phosphorylation (Fig. 4). Because ETS‐1 is capable of activating its own transcription,20 the altered phosphorylation at threonine‐38 will lead to altered expression of ETS‐1. These results indicated that Pol ι activates ETS‐1 through the Erk signaling pathway.
Figure 4.
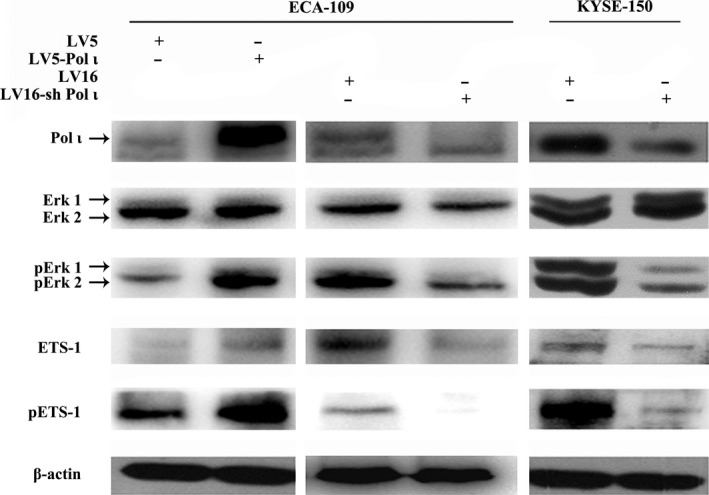
Western blot analysis of ETS‐1, pETS‐1, Erk1/2 and pErk1/2 in esophageal squamous cell carcinoma cells. Expressions of ETS‐1, pETS‐1, Erk1/2 and pErk1/2 were validated by western blot in ECA‐109 and KYSE‐150 cells with Pol ι overexpression or downregulation.
Epithelial to mesenchymal transition (EMT) contributes to cancer cell invasion, metastasis and acquisition of therapeutic resistance, and is marked by loss of E‐cadherin and gain of N‐cadherin.21 Hence, EMT markers, E‐cadherin and N‐cadherin, were examined by western blot in our model systems. Our results demonstrated that Pol ι is able to initiate the EMT process in ESCC cells (Fig. S2).
Phosphorylation of ETS‐1 is positively correlated with Pol ι expression and poor prognosis in human esophageal squamous cell carcinoma
Our previous work demonstrated that the expression level of Pol ι is closely associated with tumor progression and poor prognosis of ESCC.15 To confirm the correlated expression among Pol ι, ETS‐1 and ETS‐1 phosphorylation at threonine‐38, immunohistochemical staining was performed in 93 ESCC tissues. A significant positive correlation was observed between phosphorylation of ETS‐1 and expression of Pol ι in these ESCC tissues, yet no correlation was evident between ETS‐1 expression and Pol ι expression (Fig. 5a and Tables S3 and S4). One possible explanation is that the positive correlation of Pol ι and ETS‐1 is due to the phosphorylation of ETS‐1, which is capable of activating its own transcription,20 while the ETS‐1 protein is regulated by posttranslational modification, such as ubiquitination, resulting in variation of stability and exhibiting no correlation with Pol ι in ESCC tissues. Kaplan–Meier survival analysis indicated that the phosphorylation of ETS‐1 is closely correlated with poor prognosis, while the expression of ETS‐1 is not related to overall survival (Fig. 5b). These results indicated that ETS‐1 phosphorylation at the threonine‐38 participates in Pol ι‐mediated invasion and metastasis in ESCC, and is highly correlated with poor prognosis in ESCC patients.
Figure 5.
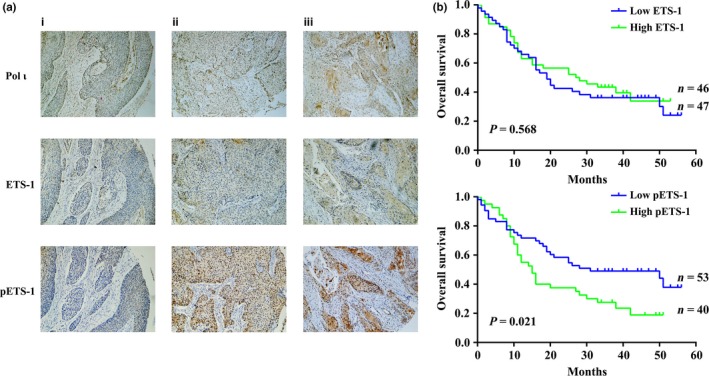
Phosphorylation of ETS‐1 is positively correlated with Pol ι expression and poor prognosis in clinical esophageal squamous cell carcinoma samples. (a) Immunohistochemical staining of Pol ι, ETS‐1 and p‐ETS‐1 in paraffin‐embedded tissues: (i) low expression of Pol ι; (ii) moderate expression of Pol ι; and (iii) high expression of Pol ι. (b) Kaplan–Meier survival analysis based on expression of ETS‐1 and p‐ETS‐1. All samples were grouped according to the staining intensity.
Discussion
Due to its error‐prone characters, ectopic expression of Pol ι may contribute to the accumulation of genomic mutation, carcinogenesis and tumor progression. Previously, we found that overexpression of Pol ι is associated with a metastatic and aggressive tumor phenotype in ESCC.15 However, the underlying mechanisms remain to be clarified. In this study, using RNA‐Seq transcriptome analysis, 718 genes were identified to be differentially expressed between Pol ι‐knocking down and control ESCC cells. Among the identified genes, expression of 12 cancer metastasis‐related genes was found to be significantly altered and these genes may be involved in the process of Pol ι‐mediated metastasis of ESCC.
Of these cancer metastasis‐related genes, ETS‐1 was selected as a key candidate gene because its expression was highly correlated with Pol ι expression in ESCC tissues and it is a known proto‐oncogene.19 ETS‐1, belonging to the family of ETS transcriptional factors, is a key regulator in cancer cell invasion, metastasis and EMT.20 A number of studies indicated that ETS‐1 overexpression is associated with lymph node and distant metastasis, and poor prognosis in most cancer types.22, 23, 24 Importantly, we demonstrated in the present study that Pol ι‐mediated invasion and migration of ESCC cells is attenuated by specific knockdown of ETS‐1. These findings indicate that ETS‐1 acts as a mediator of the Pol ι‐induced invasion and migration of ESCC cells. Interestingly, we found that phosphorylation of ETS‐1 at Threonine‐38 is significantly enhanced in Pol ι‐overexpression ESCC cells as compared to control cells. Moreover, Pol ι expression was not correlated with the level of ETS‐1 protein in ESCC tissues. Hence, the ETS‐1 phosphorylation at Threonine‐38 plays an important role in Pol ι‐mediated invasion and migration of ESCC cells.
Phosphorylation at threonine‐38 residue is an established event that regulates ETS‐1 activity.25 A large number of studies have reported that threonine‐38 is phosphorylated by the RAS/ERK1/2 signaling pathway, resulting in the recruitment of CBP (CAMP‐responsive element binding protein‐CREB‐binding protein) and P300 to the pointed domain of ETS‐1.26, 27, 28 An interaction of CBP/P300 with ETS‐1 transcriptionally activates ETS‐1 target genes, such as MMP and EMT markers,29, 30 to promote cancer invasion and metastasis. In ESCC cells lines, we observed that elevated Pol ι expression enhances Erk phosphorylation and inhibition of Pol ι attenuates Erk phosphorylation. It has been well established that Pol ι increases the mutagenesis11, 31 and consequent DNA damage repair.10, 32 Some DNA damage repair is Erk1/2‐dependent or accelerated by Erk1/2 activation.33, 34 It has also been reported that Pol ι can interact with p53,35 which can activate Erk1/2.36 Therefore, we hypothesize that Erk1/2 is activated by Pol ι via the DNA damage repair system. Meanwhile, the expression of EMT markers, N‐cadherin and E‐cadherin, was also altered in Pol ι‐overexpression or Pol ι‐knocking down ESCC cells. These results indicated that Pol ι induces ETS‐1 phosphorylation at threonine‐38 through the Erk signaling pathway. In addition, our clinical data demonstrated that phosphorylation of ETS‐1 at threonine‐38 is closely correlated with poor prognosis of ESCC patients, whereas there is no correlation between ETS expression and overall survival of ESCC patients. Thus, phosphorylation of ETS‐1 is critical to Pol ι‐mediated invasion and migration of ESCC cells.
In conclusion, the present study demonstrates that Pol ι‐induced invasion and metastasis of ESCC cells is, at least in part, mediated by phosphorylation of ETS‐1 at threonine‐38. These findings provide new insight that is valuable for our understanding of the mechanisms of Pol ι‐induced metastasis and progression in ESCC, and that further indicates that Pol ι is a potential biomarker and therapeutic target for ESCC.
Disclosure
The authors have no conflicts of interest to disclose.
Supporting information
Fig. S1. Protein–protein interactions network of differentially expressed transcripts.
Fig. S2. E‐cadherin and N‐cadherin expression were validated by western blot in Pol ι upregulation and downregulation cells.
Table S1. All identified transcripts in Pol ι downregulation KYSE‐150 cells.
Table S2. Primers for quantitative RT‐PCR analysis.
Table S3. The correlation between Pol ι and ETS‐1.
Table S4. The correlation between Pol ι and pETS‐1.
Cancer Sci 108 (2017) 2503–2510
Funding Information
The present study was supported by the National Natural Science Foundation of China (81372433 and 81672975), the Six Talent Peaks Project of Jiangsu Province of China (WSN095), the “333” Project of Jiangsu Province of China (BRA2016071), the Suzhou Administration of Science & Technology (SYS201430) and the Suzhou Key Medical Center (SZZX201506).
Contributor Information
Jinchang Wu, Email: wjinchang@sina.com.
Li Yang, Email: pwkyangli@163.com.
Jundong Zhou, Email: zhoujundong330@163.com.
References
- 1. Chen W, Zheng R, Zhang S et al The incidences and mortalities of major cancers in China, 2009. Chin J Cancer 2013; 32: 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li SH, Chen CH, Lu HI et al Phosphorylated p70S6K expression is an independent prognosticator for patients with esophageal squamous cell carcinoma. Surgery 2015; 157: 570–80. [DOI] [PubMed] [Google Scholar]
- 3. Tanaka M, Kijima H, Shimada H et al Expression of podoplanin and vimentin is correlated with prognosis in esophageal squamous cell carcinoma. Mol Med Rep 2015; 12: 4029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shachar S, Ziv O, Avkin S et al Two‐polymerase mechanisms dictate error‐free and error‐prone translesion DNA synthesis in mammals. EMBO J 2009; 28: 383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedberg EC, Wagner R, Radman M. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science 2002; 296: 1627–30. [DOI] [PubMed] [Google Scholar]
- 6. Zhu X, Zou S, Zhou J et al REV3L, the catalytic subunit of DNA polymerase zeta, is involved in the progression and chemoresistance of esophageal squamous cell carcinoma. Oncol Rep 2016; 35: 1664–70. [DOI] [PubMed] [Google Scholar]
- 7. Knobel PA, Kotov IN, Felley‐Bosco E et al Inhibition of REV3 expression induces persistent DNA damage and growth arrest in cancer cells. Neoplasia 2011; 13: 961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stallons LJ, McGregor WG. Translesion synthesis polymerases in the prevention and promotion of carcinogenesis. J Nucleic Acids 2010; 2010: 64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tissier A, McDonald JP, Frank EG et al Poliota, a remarkably error‐prone human DNA polymerase. Genes Dev 2000; 14: 1642–50. [PMC free article] [PubMed] [Google Scholar]
- 10. Yang J, Chen Z, Liu Y et al Altered DNA polymerase iota expression in breast cancer cells leads to a reduction in DNA replication fidelity and a higher rate of mutagenesis. Cancer Res 2004; 64: 5597–607. [DOI] [PubMed] [Google Scholar]
- 11. Yuan F, Xu Z, Yang M et al Overexpressed DNA polymerase iota regulated by JNK/c‐Jun contributes to hypermutagenesis in bladder cancer. PLoS One 2013; 8: e69317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gening LV, Grishina EE, Petrochenkov AN et al Association between high activity of DNA polymerase iota and the development of human uveal melanoma. Genetika 2006; 42: 98–103. [PubMed] [Google Scholar]
- 13. Pan Q, Fang Y, Xu Y et al Down‐regulation of DNA polymerases kappa, eta, iota, and zeta in human lung, stomach, and colorectal cancers. Cancer Lett 2005; 217: 139–47. [DOI] [PubMed] [Google Scholar]
- 14. Zhou J, Zhang S, Xie L et al Overexpression of DNA polymerase iota (Pol iota) in esophageal squamous cell carcinoma. Cancer Sci 2012; 103: 1574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zou S, Shang ZF, Liu B et al DNA polymerase iota (Pol iota) promotes invasion and metastasis of esophageal squamous cell carcinoma. Oncotarget 2016; 7: 32274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okawa T, Michaylira CZ, Kalabis J et al The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev 2007; 21: 2788–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allaoui R, Bergenfelz C, Mohlin S et al Cancer‐associated fibroblast‐secreted CXCL16 attracts monocytes to promote stroma activation in triple‐negative breast cancers. Nat Commun 2016; 7: 13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Chen S, Wang W et al Cancer‐associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine‐activated hedgehog and TGF‐beta pathways. Cancer Lett 2016; 379: 49–59. [DOI] [PubMed] [Google Scholar]
- 19. Dittmer J. The biology of the Ets1 proto‐oncogene. Mol Cancer 2003; 2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dittmer J. The role of the transcription factor Ets1 in carcinoma. Semin Cancer Biol 2015; 35: 20–38. [DOI] [PubMed] [Google Scholar]
- 21. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9: 265–73. [DOI] [PubMed] [Google Scholar]
- 22. Peng C, Gao H, Niu Z et al Integrin alphavbeta6 and transcriptional factor Ets‐1 act as prognostic indicators in colorectal cancer. Cell Biosci 2014; 4: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakayama T, Ito M, Ohtsuru A et al Expression of the ets‐1 proto‐oncogene in human colorectal carcinoma. Mod Pathol 2001; 14: 415–22. [DOI] [PubMed] [Google Scholar]
- 24. Nakayama T, Ito M, Ohtsuru A et al Expression of the Ets‐1 proto‐oncogene in human gastric carcinoma: correlation with tumor invasion. Am J Pathol 1996; 149: 1931–9. [PMC free article] [PubMed] [Google Scholar]
- 25. Slupsky CM, Gentile LN, Donaldson LW et al Structure of the Ets‐1 pointed domain and mitogen‐activated protein kinase phosphorylation site. Proc Natl Acad Sci USA 1998; 95: 12129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hollenhorst PC, McIntosh LP, Graves BJ. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu Rev Biochem 2011; 80: 437–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmqvist PH, Mannervik M. Genomic occupancy of the transcriptional co‐activators p300 and CBP. Transcription 2013; 4: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foulds CE, Nelson ML, Blaszczak AG et al Ras/mitogen‐activated protein kinase signaling activates Ets‐1 and Ets‐2 by CBP/p300 recruitment. Mol Cell Biol 2004; 24: 10954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vetter M, Blumenthal SG, Lindemann RK et al Ets1 is an effector of protein kinase Calpha in cancer cells. Oncogene 2005; 24: 650–61. [DOI] [PubMed] [Google Scholar]
- 30. Li C, Wang Z, Chen Y et al Transcriptional silencing of ETS‐1 abrogates epithelial–mesenchymal transition resulting in reduced motility of pancreatic cancer cells. Oncol Rep 2015; 33: 559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Faili A, Aoufouchi S, Flatter E et al Induction of somatic hypermutation in immunoglobulin genes is dependent on DNA polymerase iota. Nature 2002; 419: 944–7. [DOI] [PubMed] [Google Scholar]
- 32. Choi JY, Lim S, Kim EJ et al Translesion synthesis across abasic lesions by human B‐family and Y‐family DNA polymerases alpha, delta, eta, iota, kappa, and REV1. J Mol Biol 2010; 404: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yadavilli S, Hegde V, Deutsch WA. Translocation of human ribosomal protein S3 to sites of DNA damage is dependant on ERK‐mediated phosphorylation following genotoxic stress. DNA Repair 2007; 6: 1453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin YW, Chuang SM, Yang JL. Persistent activation of ERK1/2 by lead acetate increases nucleotide excision repair synthesis and confers anti‐cytotoxicity and anti‐mutagenicity. Carcinogenesis 2003; 24: 53–61. [DOI] [PubMed] [Google Scholar]
- 35. Hampp S, Kiessling T, Buechle K et al DNA damage tolerance pathway involving DNA polymerase iota and the tumor suppressor p53 regulates DNA replication fork progression. Proc Natl Acad Sci USA 2016; 113: E4311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee SY, Choi HC, Choe YJ et al Nutlin‐3 induces BCL2A1 expression by activating ELK1 through the mitochondrial p53‐ROS‐ERK1/2 pathway. Int J Oncol 2014; 45: 675–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Protein–protein interactions network of differentially expressed transcripts.
Fig. S2. E‐cadherin and N‐cadherin expression were validated by western blot in Pol ι upregulation and downregulation cells.
Table S1. All identified transcripts in Pol ι downregulation KYSE‐150 cells.
Table S2. Primers for quantitative RT‐PCR analysis.
Table S3. The correlation between Pol ι and ETS‐1.
Table S4. The correlation between Pol ι and pETS‐1.


