Abstract
The treatment outcomes of patients with high‐risk localized prostate cancer (PC) after carbon‐ion radiotherapy (CIRT) combined with long‐term androgen deprivation therapy (LTADT) were analyzed, and compared with those of other treatment modalities, focusing on PC‐specific mortality (PCSM). A total of 1247 patients were enrolled in three phase II clinical trials of fixed‐dose CIRT between 2000 and 2013. Excluding patients with T4 disease, 608 patients with high‐risk or very‐high‐risk PC, according to the National Comprehensive Cancer Network classification system, who received CIRT with LTADT were evaluated. The median follow‐up time was 88.4 months, and the 5‐/10‐year PCSM rates were 1.5%/4.3%, respectively. T3b disease, Gleason score of 9–10 and percentage of positive biopsy cores >75% were associated with significantly higher PCSM on univariate and multivariate analyses. The 10‐year PCSM rates of patients having all three (n = 16), two (n = 74) or one of these risk factors (n = 217) were 27.1, 11.6 and 5.7%, respectively. Of the 301 patients with none of these factors, only 1 PCSM occurred over the 10‐year follow‐up (10‐year PCSM rate, 0.3%), and significant differences were observed among the four stratified groups (P <0.001). CIRT combined with LTADT yielded relatively favorable treatment outcomes in patients with high‐risk PC and very favorable results in patients without any of the three abovementioned factors for PCSM. Because a significant difference in PCSM among the high‐risk PC patient groups was observed, new categorization and treatment intensity adjustment may be required for high‐risk PC patients treated with CIRT.
Keywords: Adverse effect, carbon‐ion radiotherapy, high‐risk prostate cancer, mortality, risk factor
Radical treatments for patients with localized high‐risk prostate cancer (PC) include radical prostatectomy (RP) or radiotherapy (RT).1, 2 In an X‐ray external beam RT (EBRT) series, several recent reports revealed that addition of androgen deprivation therapy (ADT), especially long‐term ADT (LTADT), has contributed to an improvement in the survival of patients with high‐risk PC.3, 4, 5 However, no prospective study has reported an improvement in survival after dose escalation to the target volume, although this was recently conveyed in one retrospective study.6 In contrast, favorable treatment outcomes after RP have been demonstrated,7, 8, 9, 10, 11 and the superiority of RP compared with EBRT, including intensity modulated RT (IMRT) with or without ADT, has been suggested by several authors.12, 13, 14, 15, 16 In addition, high‐dose or low‐dose‐rate brachytherapy combined with EBRT plus ADT is sometimes selected for patients with high‐risk PC.17, 18 However, to the best of our knowledge, the best treatment for high‐risk PC is still unknown, because no adequate comparisons of treatment modalities for high‐risk PC have been performed.
The National Institute of Radiological Science (NIRS) started carbon‐ion RT (CIRT) for localized PC in 1995. After two phase I/II dose‐escalation clinical trials,19 three phase II studies using fixed‐dose fractionations (66 and 63 gray [Gy] relative biological effectiveness [RBE] in 20 fractions and 57.6 Gy [RBE] in 16 fractions) were performed between April 2000 and March 2013. Theoretically, CIRT has physical and biological advantages over other forms of EBRT, such as a high linear energy transfer (LET) coupled with a sharp dose distribution with minimal penumbra.20, 21, 22, 23 For high‐risk PC, a dose of over 80 Gy was prescribed and delivered in 2 Gy fractions, yielding an α/β ratio of 1.5–2.0 Gy. This enabled a relatively hypofractionated treatment of 16–20 total fractions. Both high‐dose radiation and hypofractionation are generally considered biologically advantageous in treating high‐risk PC.24 Moreover, because of evidence that it prolongs survival, LTADT was generally given for 2 years. However, few studies have been conducted on high‐risk PC patients after CIRT combined with LTADT. We recently showed a significant impact of biochemical recurrence (BR) on mortality, especially PC‐specific mortality (PCSM) with a high hazard ratio (HR), in the first two of the three phase II trials.25 However, the study population of those trials included patients who received short‐term ADT for <6 months, and population size was limited; to overcome these limitations, the present study excluded patients with an ADT duration of <12 months and pooled the results from all three phase II trials.
The aims of the present study were to retrospectively analyze the treatment outcomes of more than 600 high‐risk and very high‐risk PC patients after CIRT combined with LTADT, and to identify factors considered unfavorable for PCSM, to help determine new treatment strategies for high‐risk PC patients.
Materials and Methods
Between April 2000 and March 2013, three phase II trials (protocol 9904: 66 Gy [RBE] in 20 fractions, from April 2000 to July 2005; 9904‐(2): 63 Gy [RBE] in 20 fractions, from September 2005 to August 2007; 9904‐(3): 57.6 Gy [RBE] in 16 fractions, from September 2007 to March 2013) involving a total of 1247 patients with clinically localized PC at our institution were carried out. The eligibility criteria for the three protocols were reported previously.20, 21, 22, 23 In brief, eligible patients had biopsy‐proven adenocarcinoma and T1–T3N0M0 disease. Patients with T4 disease did not meet the eligibility criteria for protocol 9904–9904(3), because they were considered a separate group who tend to have micrometastases, such as those of the lymph node and bone. The T stage, determined by a digital rectal examination (DRE), ultrasonography, pelvic computed tomography (CT), magnetic resonance imaging (MRI) and isotope bone scanning, was categorized according to the TNM Classification of Malignant Tumours, 7th edition.26 Staging evaluation was determined by at least three specialists: a urologist, who performed DRE and transrectal ultrasonography; a radiologist, who performed CT, MRI and isotope bone scanning; and a radiation oncologist, who reconfirmed all data. The Gleason score (GS) and percentage of positive biopsy cores (PPC) were determined for all tumors by the central pathologist before starting treatment. The GS was evaluated according to the original Gleason grading system in protocols 9904 and 9904‐(2)27 and the 2005 International Society of Urological Pathology consensus conference in protocol 9904‐(3).28 Exclusion criteria were previous radiation therapy to the pelvis, a performance status of 3–4 and the presence of active double cancer. Furthermore, patients with previous PC treatments, other than ADT within the last 6 months, were excluded from the present study. All patients signed an informed consent form before initiating therapy, and the present study was approved by the National Institute of Radiological Sciences Ethics Committee of Human Clinical Research.
The definition of a high‐risk group at NIRS is modified from those of the standard risk classifications, including the National Comprehensive Cancer Network (NCCN) or D'Amico classification,2, 29 as follows: T3a/b disease, GS ≥8, or prostate‐specific antigen (PSA) level >20 ng/mL, which correspond to high‐risk and very‐high‐risk disease (excluding T4 disease) according to the NCCN classification.
Androgen deprivation therapy consisted of medical (luteinizing hormone‐releasing hormone analogue) or surgical castration with or without anti‐androgen therapy. All patients received neoadjuvant and concomitant ADT for 2–6 months. In the present study, LTADT was defined as ADT, including adjuvant ADT, administered for more than 1 year.
The irradiation dose was expressed in Gy (RBE) (physical carbon ion dose [Gy] × RBE).30 The RBE value for CIRT was estimated to be 3.0 at the distal part of the spread‐out Bragg peak based on previous experience at NIRS.31 For treatment planning, the clinical target volume (CTV) was defined as the whole prostate and the proximal third or half of the seminal vesicle (SV) for T1–T3a disease and as much of the SV as possible for T3b disease. The planning target volume (PTV) 1 was defined as the CTV plus 5‐mm margins in the cranial, caudal and posterior directions and 10‐mm margins in the right, left and anterior directions. The PTV 2 was created by adding 2–3‐mm margins from the dorsal aspect of the CTV and was identical to the CTV in the cranial and caudal directions; PTV 2 was used for the latter half of the treatment course. Patients were examined once every 3 months for the first 5 years after CIRT and every 3–6 months thereafter. The follow‐up interval was defined from the date of starting CIRT to the date of the last follow‐up. Clinical records were collected in January 2017. BR was defined using the Phoenix definition.32 PCSM was measured from the date of starting CIRT to that of death due to PC or the last day of follow‐up. The rate of PCSM, accounting for non‐PC‐specific mortality (NPCM) as a competing event, was calculated according to Gray's test.33 Late toxicity was evaluated according to the Common Terminology Criteria for Adverse Events, version 4.0.34
T stage and the GS for PCSM were compared with the reference factors, T1–T2c disease and GS ≤7, respectively. To determine the optimal cutoff values for PSA and PPC at which the Youden index was maximized,35 receiver operating characteristic (ROC) curves were used. As a result, the cutoff values for PSA and PPC for predicting PCSM were determined to be 30 ng/mL and 75%, respectively (Figs S1 and S2, respectively). ADT duration and age were used as continuous variables for the prognostic analyses. To evaluate factors associated with PCSM, Fine and Gray regression was performed, because NPCM was considered to be a competing risk.36 Curves for PCSM were calculated according to Gray's test. The Mann–Whitney U‐test was used to compare the median follow‐up duration. Fisher's exact test was used to compare the proportions of patients. Differences were considered significant at a P‐value <0.05. Gray's test, Fine and Gray regression, and ROC analyses were performed using EZR software.37 All other calculations were performed using IBM SPSS Statistics 20 (IBM Japan, Tokyo, Japan).
Results
Patient characteristics
Of the 1247 patients treated in the three phase II studies (9904, 9904‐(2) and 9904‐(3)), a total of 608 patients with high‐risk or very‐high‐risk PC according to the NCCN classification, treated with CIRT combined with LTADT, were evaluated in the present study. A flow chart of this study is shown in Figure 1. The median follow‐up interval was 88.4 months (interquartile range [IQR], 62.0–119.0 months) and the median duration of ADT was 27.0 months (IQR, 24.3–36.0 months). Of the 608 patients, 473 (78%) received combined androgen blockade over the duration of receiving ADT. Table 1 summarizes the characteristics of the patients, tumors and treatments.
Figure 1.
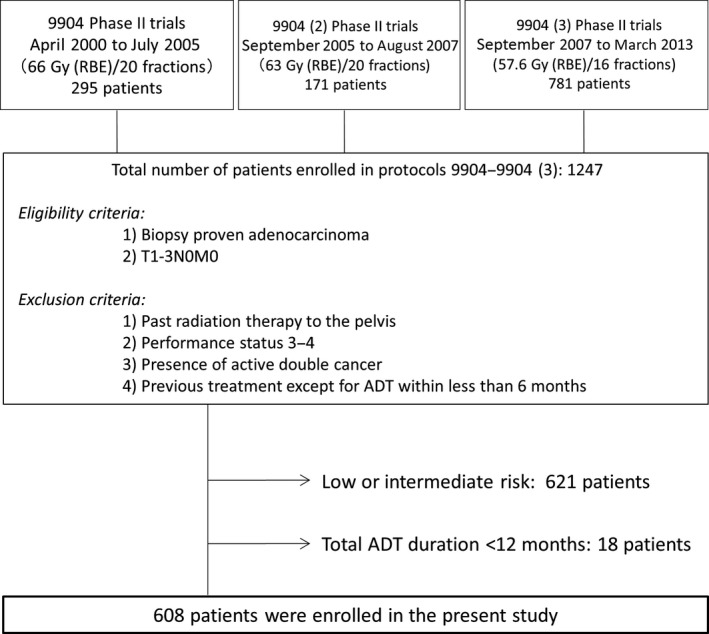
Study flow chart.
Table 1.
Tumor, patient and treatment characteristics
| (n = 608) | |
|---|---|
| Age, years | |
| Median | 69 |
| Range, 45–90 | |
| Follow‐up time, months | |
| Median | 88.4 |
| IQR | 62.0–119.0 |
| T stage, n (%) | |
| T1–T2c | 287 (47%) |
| T3a | 253 (42%) |
| T3b | 68 (11%) |
| PSA, ng/mL, n (%) | |
| Median | 21.2 |
| IQR | 9.6–37.0 |
| Gleason score, n (%) | |
| ≤7 | 271 (44%) |
| 8 | 137 (23%) |
| 9 or 10 | 200 (33%) |
| Percentage positive biopsy cores, % | |
| Median | 50 |
| IQR | 30–70 |
| Prescribed dose, n (%) | |
| 66 Gy (RBE)/20 fr. | 145 (24%) |
| 63 Gy (RBE)/20 fr. | 128 (21%) |
| 57.6 Gy (RBE)/16 fr. | 335 (55%) |
| Duration of ADT, months | |
| Median | 27.0 |
| IQR | 24.3–36.0 |
ADT, androgen deprivation therapy; fr., fractions; IQR, interquartail range; PSA, prostate specific antigen; RBE, relative biological effect.
Treatment outcomes
Biochemical recurrence was observed in 97 patients by the end of follow‐up, and salvage ADT (s‐ADT) was performed in 90 of these patients, who had a median PSA level of 2.8 ng/mL (IQR; 2.2–4.4 ng/mL) at initiation of s‐ADT. By the end of follow‐up, the PCSM and NPCM cases numbered 19 (3.1%) and 74 (12.2%), respectively. Of the 74 NPCM cases, there were 11 cardiovascular disease‐related deaths (4 acute myocardial infarctions, 3 acute cardiac failures and 4 brain infarctions). Of the remaining 63 deaths, 31 were attributed to other cancers, 11 to pneumonitis, 3 to aortic dissection, 2 to emphysema and liver failure, 1 each to renal failure, acute pancreatitis, pulmonary embolism, colon hemorrhage, death from natural causes or accidental death, and 8 to unknown causes. Of the 19 PCSM, first recurrence was observed in bone in 12, lung in 3, and a lymph node outside of the pelvis and within the pelvis in 2 each. The 5‐/10‐year rates of PCSM, accounting for NPCM as a competing risk, and overall mortality (PCSM and NPCM) adjusted by ADT duration were 1.5% (95% CI: 0.7–2.7)/4.3% (95% CI: 2.5–6.8) (Fig. 2) and 5.0% (95% CI: 3.5–7.0)/20.0% (95% CI: 16.0–24.7), respectively.
Figure 2.
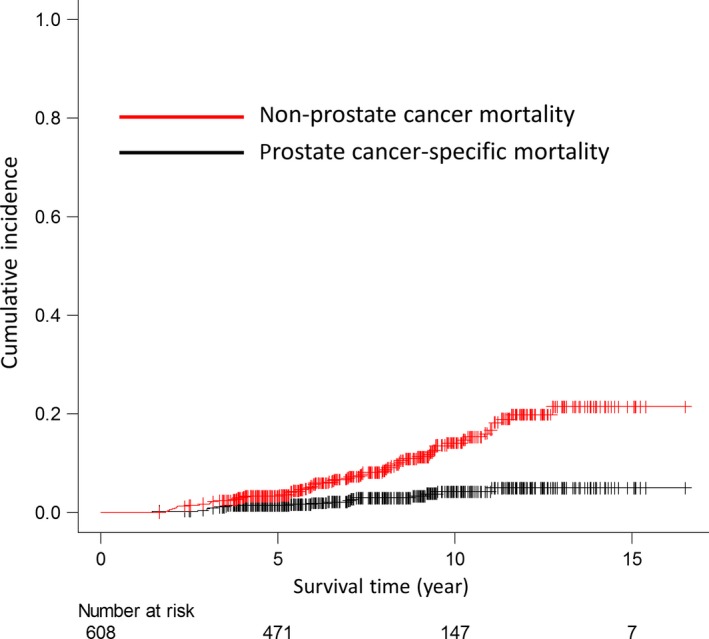
Prostate cancer‐specific mortality and non‐prostate cancer‐specific mortality of 608 high‐risk prostate cancer patients treated with carbon‐ion radiotherapy combined with long‐term androgen deprivation therapy.
Prognostic analyses
Table 2 shows the HR for PCSM compared with the reference values. T3b disease, GS of 9–10 and PPC >75% were associated with a significantly higher PCSM on both univariate and multivariate analyses, and thus, these were defined as unfavorable factors. Although 57.6 Gy (RBE)/16 fractions was associated with a significantly lower PCSM on both univariate and multivariate analyses, a remarkable difference in the follow‐up interval was observed between 66 Gy (RBE)/20 fractions (median 143.1 months; range, 13.3–198.1) and 57.6 Gy (RBE)/16 fractions (median 68.2 months; range 21.2–112.8) (P < 0.001). In contrast, the type or duration of ADT was not associated with PCSM after CIRT.
Table 2.
Univariate and multivariate analyses of factors influencing prostate cancer‐specific mortality in patients with high‐risk prostate cancer treated with carbon‐ion radiotherapy
| Factor | Univariate analyses | Multivariate analyses | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| T stage | ||||||
| T1–T2c | Reference | Reference | ||||
| T3a | 5.664 | 1.250–25.670 | 0.025 | 3.880 | 0.839–17.940 | 0.083 |
| T3b | 14.690 | 3.035–71.130 | <0.001 | 8.475 | 1.776–40.430 | 0.007 |
| PSA, ng/mL† | ||||||
| ≤30 | Reference | Reference | ||||
| >30 | 2.041 | 0.816–5.100 | 0.130 | 0.854 | 0.281–2.595 | 0.780 |
| Gleason score | ||||||
| ≤7 | Reference | Reference | ||||
| 8 | 0.372 | 0.045–3.075 | 0.360 | 0.705 | 0.095–5.216 | 0.730 |
| 9–10 | 3.301 | 1.231–8.856 | 0.018 | 4.106 | 1.562–10.800 | 0.004 |
| PPC, %† | ||||||
| <75 | Reference | Reference | ||||
| ≥75 | 6.625 | 2.519–17.420 | <0.001 | 4.180 | 1.324–13.200 | 0.015 |
| Dose prescription, Gy (RBE) | ||||||
| 66 | Reference | Reference | ||||
| 63 | 0.577 | 0.203–1.641 | 0.300 | 0.446 | 0.151–1.317 | 0.140 |
| 57.6 | 0.216 | 0.059–0.792 | 0.021 | 0.158 | 0.034–0.740 | 0.019 |
| Age, year | ||||||
| Continuous | 0.985 | 0.921–1.054 | 0.660 | 0.992 | 0.912–1.080 | 0.860 |
| ADT duration, month | ||||||
| Continuous | 1.007 | 0.994–1.019 | 0.290 | 0.989 | 0.968–1.011 | 0.320 |
| ADT drug used | ||||||
| CAB | Reference | Reference | ||||
| Other‡ | 0.596 | 0.169–2.105 | 0.420 | 0.503 | 0.130–1.948 | 0.320 |
†Cut‐off values of PSA and PPC were determined by receiver operating analyses for prostate cancer‐specific mortality (Figs S1 and S2). ‡Primarily luteinizing hormone releasing hormone analogue monotherapy. ADT, androgen deprivation therapy; CAB, combined androgen blockade; CI, confidence interval; fr., fractions; HR, hazard ratio; PPC, percentage of positive biopsy cores; PSA, prostate specific antigen; RBE, relative biological effect.
All 608 patients with high‐risk PC were stratified into four groups according to the number of unfavorable factors present (all three [n = 16], two [n = 74], one [n = 217] and none [n = 301]). Figure 3 shows the 5‐/10‐year PCSM rates for the four groups, respectively: 18.8% (95% CI, 4.3–41.1)/27.1% (95% CI, 7.4–51.9), 4.1% (95% CI, 1.1–10.4%)/11.6% (95% CI, 4.3–23.1), 0.9% (95% CI, 0.2–3.1%)/5.7% (95% CI, 2.3–11.3) and 0.3% (95% CI, 0.0–1.8)/0.3% (95% CI, 0.0–1.8), respectively, and significant differences were observed among the four groups (P <0.001).
Figure 3.
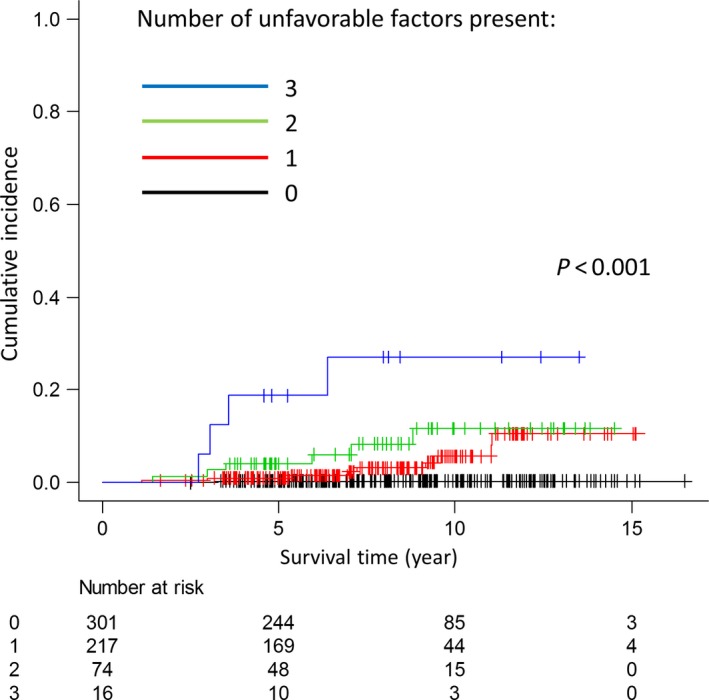
Comparison of prostate cancer‐specific mortality among four patient groups stratified according to the number of unfavorable factors present (T3b disease, Gleason score of 9–10 and percentage of positive cores ≥75%).
Adverse effects
Table 3 details the observed gastrointestinal (GI)/genitourinary (GU) adverse effects of grade (G) 2 or more severe, including the incidence rates and symptoms. The 5‐/10‐year cumulative incidence rates of GI G2 and GU G2–3 were 1.7% (95% CI, 0.9–3.1)/1.7% (95% CI, 0.9–3.1) and 6.2% (95% CI, 4.5–8.5)/11.7% (95% CI, 8.7–15.6), respectively. In addition, the rates of using anticoagulants or related drugs before CIRT for G2 rectal bleeding and G2–3 hematuria were significantly higher than those for G0–1 rectal bleeding and G0–1 hematuria, respectively, according to Fisher's exact test (4/10 [40%] vs 41/598 [7%], P = 0.004, and 9/47 (19%) vs 36/561 (6%), P = 0.005, respectively).
Table 3.
Adverse effect after carbon‐ion radiotherapy among patients with high‐risk prostate cancer (N = 608)
| Cumulative incidence rates of G2–3 | Crude incidence number (rates) with the maximum grade | Symptoms, n | Crude incidence number (rates) at end of follow‐up | |||
|---|---|---|---|---|---|---|
| 5‐year | 10‐year | |||||
| GI | G2 | 1.7% | 1.7% | 10 (1%) | Continuous rectal bleeding, 10 | 2 (0.3%) |
| GU | G2 | 51 (8%) | Hematuria requiring hemostasis, 44 | 20 (3%) | ||
| Urethral stricture requiring catheter insertion, 4 | ||||||
| 6.2% | 11.7% | Perineal pain, 2 | ||||
| Urodynia, 1 | ||||||
| G3 | 3 (0.5%) | Urinary diversion due to continuous hematuria, 2 | 3 (0.5%) | |||
| Frequent coagulation for vesical hematuria under general anesthesia, 1 | ||||||
G, grade; GI, gastrointestinal; GU, genitourinary; RBE, relative biological effect.
Discussion
Three phase II trials involving 608 patients with high‐risk or very‐high‐risk PC treated with CIRT combined with LTADT were conducted at NIRS over a 13‐year period, and the present study revealed 5‐/10‐year PCSM rates of 1.5%/4.3%, respectively.
Table 4 summarizes the PCSM rates for patients with high‐risk PC from previous reports.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 38, 39, 40 The 10‐year PCSM rates after RP and EBRT including IMRT were 5–9%7, 8, 9, 10, 11 and 8–12%,13, 39 respectively. The PCSM rate after CIRT combined with LTADT is comparable to that after RP, although phase III trials will be necessary to directly compare the different treatment modalities. In addition, there were few long‐term follow‐up results after IMRT at the time of writing. However, Mizowaki et al. demonstrated a favorable PCSM rate (8‐year PCSM rate, 3.4%) for T3–4 high‐risk PC patients who had undergone high‐dose IMRT (78 Gy) combined with neoadjuvant ADT (median duration, 6 months) plus s‐ADT if the PSA value was >4 ng/mL,40 which was comparable with the results of the present study (Table 4). There are several common points between their study and ours, including the early use of s‐ADT, a mainly Japanese study population, use of neoadjuvant ADT and a high irradiation dose. In fact, some studies have reported that starting s‐ADT early contributes to a low PCSM rate.41, 42 Moreover, other studies have suggested that Asians, including Japanese people, may intrinsically have good PCSM outcomes when ADT is used, although the reasons for this are unclear.43, 44 These common features may have affected the favorable results regarding PCSM seen in both studies.
Table 4.
Comparison of cancer‐specific mortality for high‐risk prostate cancer after radical prostatectomy or radiotherapy
| Author | Publication year | Definition of high risk | Treatment | n | Follow‐up time (year) | Total dose (Gy) | ADT duration (months, median) | PCSM rates | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| 5‐year | 10‐year | |||||||||
| Freedland et al. | 2007 | T3a | 58 | 10.3 | 0 | 2% | 9% | 7 | ||
| Boorjian et al. | 2008 | D'Amico | 1513 | NA | 0 | NA | 5% | 8 | ||
| Yossepowitch et al. | 2008 | NCCN | RP | 938 | 5.5 | NA | 2.8% | 8% | 9 | |
| Stephanson et al. | 2009 | D'Amico | 1962 | 4.0 | NA | NA | 8% | 10 | ||
| Loeb et al. | 2010 | D'Amico | 175 | 8 | 0 | NA | 8% | 11 | ||
| Zelfsky et al. | 2010 | NCCN | RP | NA | 5.1 | NA | 1% | 3.8% (8‐year) | 12 | |
| RT | NA | 5.1 | 81‐86.4 | 3–6 | 3.7% | 9.5% (8‐year) | ||||
| Boorjian et al. | 2011 | NCCN | RP | 1238 | 10.2 | NA | NA | 8% | 13 | |
| RT | 344 | 6.0 | 72 | 22.8 | NA | 8% | ||||
| RT | 265 | 7.3 | 72 | 0 | NA | 12% | ||||
| Merino et al. | 2013 | D'Amico | RP | 216 | 7.7 | 0 | NA | 7% (7‐year) | 14 | |
| RT | 78 | 7.7 | 76 | Various | NA | 15% (7‐year) | ||||
| Lee et al. | 2014 | NCCN | RP | 251 | 6.3 | NA | 3.5% | NA | 15 | |
| RT | 125 | 7.9 | 74–79 | 6.7 | 11.7% | NA | ||||
| Yamamoto et al. | 2014 | T3 | RP | 112 | 7.8 | <8 | NA | 6.2% | 16 | |
| RT | 119 | 7.1 | 70 | >9 | NA | 14.9% | ||||
| Spratt et al. | 2012 | NCCN | 344 | 5.5 | 86.4 | 6–24 | NA | 8.1% (7‐year) | 38 | |
| Dearnaley et al. | 2014 | NCCN | RT | 184 | 10.0 (overall) | 74 | <8 | NA | 11% | 39 |
| 178 | 64 | <8 | NA | 11% | ||||||
| Mizowaki et al. | 2016 | T3–T4 | 120 | 8.1 | 78 | 6 | NA | 3.4% (8‐year) | 40 | |
| Present study | NCCN | CIRT | 608 | 7.4 | 57.6–66 [Gy (RBE)] | 27 | 1.5% | 4.3% | ||
ADT, androgen deprivation therapy; NA, not available; NCCN, National Comprehensive Cancer Network; PCSM, prostate cancer‐specific mortality; RBE, relative biological effect; RP, radical prostatectomy; RT, radiotherapy.
Nonetheless, in the present study, three independent unfavorable factors for PCSM, including T3b disease, GS of 9–10 and PPC >75%, were identified by univariate and multivariate analyses, and patients with all three factors had a significantly higher 10‐year PCSM rate (27.1%). This result suggests that the overall PCSM rate in studies of high‐risk PC depends on the proportions of patients who have multiple unfavorable factors. In addition, most of the initial recurrence events in the patients who experienced PCSM in the present study were extra‐pelvic metastases (17/19, 89%). Hence, more aggressive systemic treatments may be necessary to improve the PCSM rate for patients with all three unfavorable factors. In a phase III study, Fizazi et al. demonstrated that ADT plus docetaxel‐based chemotherapy and estramustine significantly improved relapse‐free survival compared with ADT alone in patients with high‐risk PC (8‐year relapse‐free survival, 62 vs 50%; P = 0.017).45 Furthermore, in a phase I trial combining high‐dose IMRT (78 Gy) plus LTADT with dose‐escalated concurrent weekly docetaxel for high‐risk PC, weekly docetaxel 20 mg/m2 was determined to be safe, and no biochemical failure was observed over a median follow‐up of 2.2 years.46 These results suggest that CIRT with LTADT and chemotherapy may be a strategy for patients with all three unfavorable factors.
In contrast, of the 301 high‐risk PC patients without any of the aforementioned three factors, PCSM occurred in only 1 patient, who had T1cN0M0 disease, an initial PSA level of 9.93 ng/mL, GS of 4 + 4 = 8 and PPC of 20%. This patient developed castration‐resistant PC (CRPC) at 6 months and died at 39 months following CIRT; given his initial prognosis, it was difficult to know the progression of his disease prior to CIRT treatment. Nevertheless, the outcomes of high‐risk PC patients without any of the three unfavorable factors after CIRT combined with LTADT were very favorable (10‐year PCSM rate, 0.3%). Therefore, shortening the ADT duration, for example to 6 months, plus starting s‐ADT early, would cause less ADT‐related toxicity without increasing the PCSM rate after CIRT for patients without the three unfavorable factors identified in the present study.
With regard to adverse effects, the 5‐/10‐year rates of G2 or more severe GI and GU toxicities were 1.7%/1.7% and 6.2%/11.7%, respectively. The incidence of GI adverse effects after CIRT was lower than that after IMRT (5‐year rate G2+ GI toxicities, 4–6%).47, 48, 49 The low rates of GI adverse effects after CIRT seems to be attributed to the physical advantage of the carbon‐ion beam in reducing dose delivery to healthy tissue. In contrast, incidence rates of GU adverse effects after CIRT are considered to be comparable to those after IMRT (5‐year rate G2+ GU toxicities, 6–16%).47, 48, 49 The present study revealed that using anticoagulants or related drugs prior to CIRT was significantly associated with the incidence of G2–3 hematuria as well as that of G2 rectal bleeding. Nevertheless, the relatively high rate of G2+ GU (47/608, 7.7%) observed might have been because of the dose delivered to the urethra, which is difficult to reduce using the passive scattering beam irradiation technique. In contrast, the pencil‐beam scanning technique has been used at NIRS since 2011 for the purpose of reducing the dose to healthy tissue, including the bladder neck,50 which may help alleviate the GU adverse effects.
This study has some limitations. First, longer‐term follow‐up of the patients treated with 57.6 Gy (RBE)/16 fractions is warranted. The biologically effective dose of 57.6 Gy (RBE) for PC was slightly lower than that of 66 Gy (RBE) for PC with an α/β ratio of 1.5–2.0. Nevertheless, 57.6 Gy (RBE), compared with 66 Gy (RBE), afforded significantly better outcomes in terms of the PCSM rate in the present study, which is attributed mainly to the remarkable difference in follow‐up time between the groups. Second, the patients in this study were mainly Japanese; the unique interplay between ADT and patient ethnicity may be important.43, 44 Nevertheless, the 10‐year PCSM rate of the 301 patients without any of the unfavorable factors determined in the present study was 0.3%, although patients with not only T3a disease (n = 134, 45%) and a GS8 (n = 109, 36%), which are well‐known unfavorable factors, but also a median PSA level of 20 (range 4–150) ng/mL were included in the favorable high‐risk group. These surprising results might be expected under the unique conditions of the present study involving the combination of LTADT, high LET and/or a hypofractionated regimen. International and multinational studies are needed to compare and control for environmental and genetic factors. Third, salvage treatments after BR and treatments for CRPC were not fully determined, although ADT was generally restarted early after BR. Fourth, the results were retrospectively obtained from a single institution. Hence, a multicenter prospective study of fixed dose CIRT combined with ADT for 1–2 years in high‐risk PC patients was started in April 2017 in Japan to overcome some limitations of this study and to validate the efficacy of CIRT.
In conclusion, CIRT combined with LTADT for patients with high‐risk PC has yielded relatively favorable treatment outcomes similar to previous reports involving RP, with few severe adverse effects. Despite the low PCSM rate, patients with all three factors, T3b disease, GS of 9–10 and PPC >75%, had a significantly unfavorable PCSM rate compared with patients with none of these factors. Thus, a new categorization system taking into account the T stage, GS and PPC and adjustment of the treatment intensity, with inclusion of chemotherapy or a shortened ADT duration, should be considered for high‐risk PC patients treated with CIRT.
Disclosure Statement
None of the authors have any conflicts of interest to declare.
Supporting information
Fig. S1. Receiver operating characteristic curves for the prostate cancer‐specific mortality rate with respect to the prostate‐specific antigen level.
Fig. S2. Percentage of positive biopsy cores.
Acknowledgment
This work was supported by the Research Project with Heavy Ions at the National Institute of Radiological Sciences. We deeply appreciate the contribution of pathological diagnoses by Masaoki Harada.
Cancer Sci 108 (2017) 2422–2429
Funding Information
Research Project with Heavy Ions at the National Institute of Radiological Sciences, Japan.
References
- 1. Bolla M, Gonzalez D, Warde P et al Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997; 337: 295–300. [DOI] [PubMed] [Google Scholar]
- 2. Scherr D, Swindle PW, Scardino PT. National Comprehensive Cancer Network guidelines for the management of prostate cancer. Urology 2003; 61: 14–24. [DOI] [PubMed] [Google Scholar]
- 3. Bolla M, Van Tienhoven G, Warde P et al External irradiation with or without long‐term androgen suppression for prostate cancer with high metastatic risk: 10‐year results of an EORTC randomised study. Lancet Oncol 2010; 11: 1066–73. [DOI] [PubMed] [Google Scholar]
- 4. Zapatero A, Guerrero A, Maldonado X et al High‐dose radiotherapy with short‐term or long‐term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol 2015; 16: 320–7. [DOI] [PubMed] [Google Scholar]
- 5. Horwitz EM, Bae K, Hanks GE et al Ten‐year follow‐up of radiationtherapy oncology group protocol 92‐02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. Clin Oncol 2008; 26: 2497–504. [DOI] [PubMed] [Google Scholar]
- 6. Kalbasi A, Li J, Berman A, Swisher‐McClure S et al Dose‐escalated irradiation and overall survival in men with nonmetastatic prostate cancer. JAMA Oncol 2015; 1: 897–906. [DOI] [PubMed] [Google Scholar]
- 7. Freedland SJ, Partin AW, Humphreys EB, Mangold LA, Walsh PC. Radical prostatectomy for clinical stage T3a disease. Cancer 2007; 109: 1273–8. [DOI] [PubMed] [Google Scholar]
- 8. Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D'amico risk group classification for predicting survival following radical prostatectomy. J Urol 2008; 179: 1354–60. [DOI] [PubMed] [Google Scholar]
- 9. Yossepowitch O, Eggener SE, Serio AM et al Secondary therapy, metastatic progression, and cancer‐specific mortality in men with clinically high‐risk prostate cancer treated with radical prostatectomy. Eur Urol 2008; 53: 950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stephenson AJ, Kattan MW, Eastham JA et al Prostate cancer‐specific mortality after radical prostatectomy for patients treated in the prostate‐specific antigen era. J Clin Oncol 2009; 27: 4300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loeb S, Schaeffer EM, Trock BJ, Epstein JI, Humphreys EB, Walsh PC. What are the outcomes of radical prostatectomy for high‐risk prostate cancer? Urology 2010; 76: 710–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zelefsky MJ, Eastham JA, Cronin AM et al Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol 2010; 28: 1508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boorjian SA, Karnes RJ, Viterbo R et al Long‐term survival after radical prostatectomy versus external‐beam radiotherapy for patients with high‐risk prostate cancer. Cancer 2011; 117: 2883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merino T, San Francisco IF, Rojas PA, Bettoli P, Zúñiga A, Besa P. Intensity‐modulated radiotherapy versus radical prostatectomy in patients with localized prostate cancer: long‐term follow‐up. BMC Cancer 2013; 13: 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee JY, Cho KS, Kwon JK et al A competing risk analysis of cancer‐specific mortality of initial treatment with radical prostatectomy versus radiation therapy in clinically localized high‐risk prostate cancer. Ann Surg Oncol 2014; 21: 4026–33. [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto S, Kawakami S, Yonese J et al Long‐term oncological outcomes in men with T3 prostate cancer: radical prostatectomy versus external‐beam radiation therapy at a single institution. Int J Clin Oncol 2014; 19: 1085–91. [DOI] [PubMed] [Google Scholar]
- 17. Aoki M, Miki K, Kido M et al Analysis of prognostic factors in localized high‐risk prostate cancer patients treated with HDR brachytherapy, hypofractionated 3D‐CRT and neoadjuvant/adjuvant androgen deprivation therapy (trimodality therapy). J Radiat Res 2014; 55: 527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Konaka H, Egawa S, Saito S et al Tri‐Modality therapy with I‐125 brachytherapy, external beam radiation therapy, and short‐ or long‐term hormone therapy for high‐risk localized prostate cancer (TRIP): study protocol for a phase III, multicenter, randomized, controlled trial. BMC Cancer 2012; 12: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akakura K, Tsujii H, Morita S et al Phase I/II clinical trials of carbon ion therapy for prostate cancer. Prostate 2004; 58: 252–8. [DOI] [PubMed] [Google Scholar]
- 20. Ishikawa H, Tsuji H, Kamada T et al Carbon‐ion radiation therapy for prostate cancer. Int J Urol 2012; 19: 296–305. [DOI] [PubMed] [Google Scholar]
- 21. Tsuji H, Yanagi T, Ishikawa H et al Hypofractionated radiotherapy with carbon ion beams for prostate cancer. Int J Radiat Oncol Biol Phys 2005; 63: 1153–60. [DOI] [PubMed] [Google Scholar]
- 22. Ishikawa H, Tsuji H, Kamada T et al Carbon ion radiation therapy for prostate cancer: results of a prospective phase II study. Radiother Oncol 2006; 81: 57–64. [DOI] [PubMed] [Google Scholar]
- 23. Okada T, Tsuji H, Kamada T et al Carbon ion radiotherapy in advanced hypofractionated regimens for prostate cancer: from 20 to 16 fractions. Int J Radiat Oncol Biol Phys 2012; 84: 968–72. [DOI] [PubMed] [Google Scholar]
- 24. Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol 2005; 44: 265–76. [DOI] [PubMed] [Google Scholar]
- 25. Kasuya G, Ishikawa H, Tsuji H et al Significant impact of biochemical recurrence on overall mortality in patients with high‐risk prostate cancer after carbon‐ion radiotherapy combined with androgen deprivation therapy. Cancer 2016; 122: 3225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. International Union Against Cancer (UICC) , TNM Classification of Malignant Tumours, 5th edn New York: Wiley‐Liss, 1997. [Google Scholar]
- 27. Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep 1966; 50: 125–8. [PubMed] [Google Scholar]
- 28. Epstein JI, Allsbrook WC, Amin MB, Egevad LL, ISUP Grading Committee . The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2005; 29: 1228–42. [DOI] [PubMed] [Google Scholar]
- 29. D'Amico AV, Whittington R, Malkowicz SB et al Predicting prostate specific antigen outcome preoperatively in the prostate specific antigen era. J Urol 2001; 166: 2185–8. [PubMed] [Google Scholar]
- 30. Kanai T, Endo M, Minohara S et al Biophysical characteristics of HIMAC clinical irradiation system for heavy‐ion radiation therapy. Int J Radiat Oncol Biol Phys 1999; 44: 201–10. [DOI] [PubMed] [Google Scholar]
- 31. Kanai T, Matsufuji N, Miyamoto T et al Examination of GyE system for HIMAC carbon therapy. Int J Radiat Oncol Biol Phys 2006; 64: 650–6. [DOI] [PubMed] [Google Scholar]
- 32. Roach M III, Hanks G, Thames H Jr et al Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG‐ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006; 65: 965–74. [DOI] [PubMed] [Google Scholar]
- 33. Gray RJ. A class of k‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–54. [Google Scholar]
- 34. National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) v4.0 2010. [Cited 01 June 2017.] Available from URL: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 35. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32–5. [DOI] [PubMed] [Google Scholar]
- 36. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 37. Kanda Y. Investigation of the freely‐available easy‐to‐use software “EZR” (Eazy R) for medical statistics. Bone Marrow Transplant 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spratt DE, Pei X, Yamada J, Kollmeier MA, Cox B, Zelefsky MJ. Long‐term survival and toxicity in patients treated with high‐dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2013; 85: 686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dearnaley DP, Jovic G, Syndikus I et al Escalated‐dose versus control‐dose conformal radiotherapy for prostate cancer: long‐term results from the MRC RT01 randomised controlled trial. Lancet Oncol 2014; 15: 464–73. [DOI] [PubMed] [Google Scholar]
- 40. Mizowaki T, Norihisa Y, Takayama K et al Long‐term outcomes of intensity‐modulated radiation therapy combined with neoadjuvant androgen deprivation therapy under an early salvage policy for patients with T3‐T4N0M0 prostate cancer. Int J Clin Oncol 2016; 21: 148–55. [DOI] [PubMed] [Google Scholar]
- 41. Mydin AR, Dunne MT, Finn MA, Armstrong JG. Early salvage hormonal therapy for biochemical failure improved survival in prostate cancer patients after neoadjuvant hormonal therapy plus radiation therapy–A secondary analysis of Irish Clinical Oncology Research Group 97‐01. Int J Radiat Oncol Biol Phys 2013; 85: 101–8. [DOI] [PubMed] [Google Scholar]
- 42. Souhami L, Bae K, Pilepich M, Sandler H. Timing of salvage hormonal therapy in prostate cancer patients with unfavorable prognosis treated with radiotherapy: a secondary analysis of Radiation Therapy Oncology Group 85‐31. Int J Radiat Oncol Biol Phys 2010; 78: 1301–6. [DOI] [PubMed] [Google Scholar]
- 43. Trinh QD, Nguyen PL, Leow JJ et al Cancer‐specific mortality of Asian Americans diagnosed with cancer: a nationwide population‐based assessment. J Natl Cancer Inst 2015; 107: djv054. [DOI] [PubMed] [Google Scholar]
- 44. Cooperberg MR, Hinotsu S, Namiki M, Carroll PR, Akaza H. Trans‐Pacific variation in outcomes for men treated with primary androgen‐deprivation therapy (ADT) for prostate cancer. BJU Int 2016; 117: 102–9. [DOI] [PubMed] [Google Scholar]
- 45. Fizazi K, Faivre L, Lesaunier F et al Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high‐risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol 2015; 16: 787–94. [DOI] [PubMed] [Google Scholar]
- 46. Chen RC, Rosenman JG, Hoffman LG et al Phase I study of concurrentweekly docetaxel, high‐dose intensity‐modulated radiation therapy (IMRT) and androgen‐deprivation therapy (ADT) for high‐risk prostate cancer. BJU Int 2012; 110: E721–6. [DOI] [PubMed] [Google Scholar]
- 47. Cahlon O, Zelefsky MJ, Shippy A et al Ultra‐high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys 2008; 71: 330–7. [DOI] [PubMed] [Google Scholar]
- 48. Takeda K, Takai Y, Narazaki K et al Treatment outcome of high‐dose image‐guided intensity‐modulated radiotherapy using intra‐prostate fiducial markers for localized prostate cancer at a single institute in Japan. Radiat Oncol 2012; 7: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kobayashi M, Hatano K, Fukasawa S et al Therapeutic outcomes of neoadjuvant and concurrent androgen‐deprivation therapy and intensity‐modulated radiation therapy with gold marker implantation for intermediate‐risk and high‐risk prostate cancer. Int J Urol 2015; 22: 477–82. [DOI] [PubMed] [Google Scholar]
- 50. Ebner DK, Tsuji H, Yasuda S, Yamamoto N, Mori S, Kamada T. Respiration‐gated fast‐rescanning carbon‐ion radiotherapy. Jpn J Clin Oncol 2017; 47: 80–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Receiver operating characteristic curves for the prostate cancer‐specific mortality rate with respect to the prostate‐specific antigen level.
Fig. S2. Percentage of positive biopsy cores.


