Abstract
Nowadays, cancer immunotherapy is a promising strategy in solid tumour treatment. It has become a breakthrough in achieving long-term survival in many advanced cases. The essence of modern immunotherapy is to improve the host antitumour immune defence. Currently, it is critically important to determine the biomarkers that could be helpful in planning this type of individual therapy. It has turned out that an important prognostic factor is the evaluation of inflammatory infiltration of the tumour mass, including the characteristics of populations of lymphocytes and macrophages, and the expression of suppressive and regulatory molecules. For lung cancer, <30% of the tumours are resectable and available for a complete microscopic examination. In other cases, the material for the study of inflammatory infiltration may be a tumour biopsy, but this is of limited importance. A valuable way to evaluate the microenvironment of tumour growth is a bronchoalveolar lavage (BAL) fluid examination. In the BAL fluid, the cellular and noncellular components determine the specific type of inflammatory response in an environment of developing cancer. BAL fluid analysis may be a valuable addition to peripheral blood analysis during qualification for modern immunomodulatory therapy. Moreover, it is important material to seek biomarkers of clinical significance.
Short abstract
Bronchoalveolar lavage may be used to evaluate the immune status of lung cancer patients before immunotherapy http://ow.ly/lXRi30dzmGY
Introduction
In recent years, significant progress has been made in the development of new directions for the therapy of solid tumours, including lung cancer. For many patients, this affords the chance of achieving long-term survival.
In lung cancer, the treatment prognosis is generally poor. The number of cases continues to grow, reaching 1 900 000 cases per year (http://globocan.iarc.fr/Default.aspx). The mortality is high as well, reaching 1 800 000 deaths per year. It is the leading cause of death from malignant tumours in both sexes. Currently, the rate of complete cure does not exceed 15%. Such bad results from treatment are due to a high percentage of patients being diagnosed in advanced disease stages (∼70%). New therapies do not affect the result of treatment significantly but significantly contribute to prolonging survival and improving quality of life. The knowledge about molecular changes and introduction of molecularly targeted agents (e.g. small molecules targeted to EGFR (epidermal growth factor receptor) or ALK (anaplastic lymphoma kinase) were a major breakthrough in adenocarcinoma treatment [1]). Another achievement was to prove the effectiveness of immune therapy in squamous cell carcinoma, and then in nonsquamous cell carcinoma [2].
Cancer microenvironment
Tumour development and spread depend on the nature of tumour microenvironment. This is a separate and specific area wherein processes modulating cell-mediated and humoral immune responses occur. Its functional structure consists of:
cancer cells
fibroblasts
vascular endothelial cells
macrophages
dendritic cells
lymphocytes
extracellular matrix
Many substances that function as immune response mediators, such as cytokines, chemokines, enzymes and growth factors, are secreted by these cells [3]. The respiratory system, which is the environment of lung cancer growth, has a specific system of immune homeostasis. One of its main elements is the airway epithelium. The main function of airway epithelial cells is not only to be a protective barrier but also to secrete inflammatory mediators that attract lymphoid cells and stimulate antigen-presenting cells (APCs). The endothelium of pulmonary blood vessels has a similar function. In the functionally active interstitial tissue of the healthy lung, mesenchymal cells, extracellular matrix, macrophages and dendritic cells dominate [4]. These components of the microenvironment undergo different interactions that result in what is widely known as EMT (epithelial-to-mesenchymal transition). The cells that are present in the alveolar space are mainly lymphoid cells and macrophages. These cells have constant, specific phenotype. Macrophages can be divided into an alveolar population and an interstitial population, with a predominance of the former. Two types of macrophages exist: M1 and M2. It has been demonstrated by many studies that isolation of a clear macrophage population is very difficult. These cells are characterised by permanent dynamics and have a complex phenotype. Among lymphocytes, T-cells dominate, with a CD4+/CD8+ ratio of ∼1.5, over small number of B-cells and other populations [5].
Bronchoalveolar lavage (BAL) is the approved method of obtaining material from peripheral airways and allows identification of the type of local immune response. Introduction of BAL for diagnosis in the 1980s allowed the characterisation of the normal cellular composition of the pulmonary alveolar space and deviations resulting from different disease processes [6, 7]. During analysis of the lung immune system, the influence of external factors (constant influence of tobacco smoke, concomitant diseases such as chronic obstructive pulmonary disease, immunosuppressive treatment, and living and working environments) should be considered [8].
The premises of immunotherapy
Wider knowledge about disturbance of the antitumour immune response and mechanism of evasion the host antitumour immune defence has led to development of effective immunotherapy in lung cancer. The antitumour defence dominates in the first step of tumour progress. A population of cytotoxic cells (CD8+ and CD4+ lymphocytes, natural killer (NK) cells, and NKT-cells) participate in cytotoxic reaction. These cells are activated as a result of antigen presentation by APCs. Tumour cells, dendritic cells, macrophages and B-cells function as APCs. The attenuation of the effective antitumour immune response is rapid and is the result of a well-developed mechanism by which cancer cells are able to “hide” (e.g. through impairment of antigen presentation and mechanisms of attenuation of host immunity). Malignant cells are able to redirect the immune system, causing immunotolerance. Attenuation and modification of the immune response is connected to the suppression of cytotoxic cell receptor pathways, secretion of suppressive cytokines, and recruitment and generation of regulatory cells. An increased percentage of regulatory cells, especially regulatory T-cells (Tregs), has been reported in the tumour milieu. Increased expression of blocking pathways (programmed cell death protein (PD)-1 and its ligand PD-L1, and cytotoxic T-lymphocyte antigen 4 (CTLA-4)) plays an important role in the suppression process [9–12]. Inhibitory molecules (PD-1 and CLTA-4) are overexpressed on lymphocytes. This effectively inhibits their activation and cells become functionally inefficient. At the same time, apoptotic signalling of the receptor pathway of these cells has been observed: an elevated expression of the apoptosis receptor Fas on lymphocytes has been reported in lung cancer patients [13].
Over the years, much research on immunotherapy for lung cancer has been performed. Unfortunately, in majority of cases, the efficacy of these methods, including cancer vaccines, has not been proved. As the greatest contribution to the promotion of tumour development is attenuation of the immune system, the key objective of immunotherapy is on the one hand, the inhibition of attenuation mechanisms, and on the other hand, activation of self-defence. Drugs blocking inhibitory pathways of cytotoxic reactions by PD-1/PD-L1 have demonstrated the highest efficiency. This resulted in the introduction of anti-PD-L1 into non-small cell lung carcinoma treatment [2, 14, 15]. Anti-CTLA-4 treatment seems to be a promising strategy in lung cancer; to date, it has been demonstrated to be efficacious in malignant melanoma. Combination therapy, which connects conventional treatment with immunomodulating drugs, sometimes gives very good results [16]. This mode of treatment is confirmed by some data; for example, the result of combined therapy is cellular stress caused by cytostatic drugs and radiotherapy, which leads to exposure of tumour antigens and immune response activation. Furthermore, some chemotherapeutics (e.g. gemcitabine and vinorelbine) destroy MDSCs (myeloid-derived suppressor cells). It can be suspected that the population of suppressive and regulatory cells, which grow and multiply rapidly in the tumour milieu, is more susceptible to chemotherapeutics than the less numerous pool of depleted cytotoxic T-cells.
Detailed characteristics of infiltration around a tumour: the “immunogram”
The phenomenon of inflammatory inflammation around a tumour has been known for a long time. One of the first descriptions was proposed by Ioachim et al. [17]. The fundamental meaning of the antitumour response is related to lymphocytes. In the majority of earlier reports, the lymphocytic infiltrate was described as a positive factor. It was concluded that the greater the tumour-infiltrating lymphocyte (TIL) infiltration, the better the prognosis and the efficacy of treatment. However, it should be mentioned that population of TILs consist of different lymphocyte types and it is necessary to determine their phenotype. It has been proven that the presence of cytotoxic CD8+ lymphocytes in tumour infiltration has meaningful prognostic significance [18]. However, importantly, cytotoxic cells destroying tumour are in the minority, and TIL function is more connected with promoting tumour progression by the presence of cells inhibiting the antitumour response: Tregs, Bregs (regulatory B-cells) and lymphocytes with dominant expression of suppressive molecules. Predominance of Tregs and expression of the transcription factor forkhead box P3 (Foxp3) are associated with a markedly unfavourable prognosis [19]. In the population of macrophages surrounding the tumour (tumour-associated macrophages), M2 macrophages predominate vastly, and have a suppressive function and produce suppressive cytokines, especially transforming growth factor (TGF)-β and interleukin-10 [20, 21].
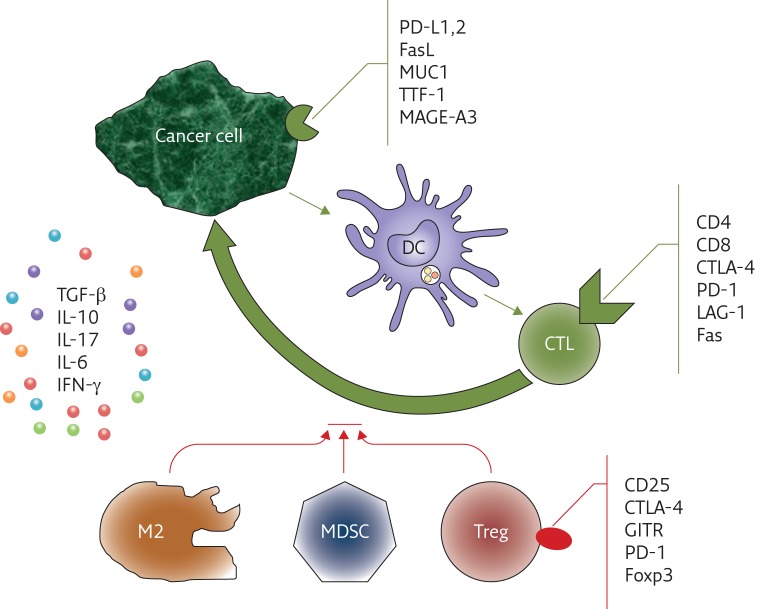
These observations led to the development of models of assessment of the immune response and prognostic significance estimation [22]. Based on the evaluation of inflammatory lesions in the tumour milieu, the scales defined as “immunoscoring” or an “immunogram” have been developed. However, the “states” of immune system progress or dysfunction are not defined or well known yet, and concerns have been expressed that immune status before treatment could have similar value to TNM staging system [23]. It should be mentioned that the listed factors were evaluated regardless of the method of treatment and without reference to immunomodulating treatment, so they were considered as prognostic factors. Nevertheless, due to the development of specific methods of immunotherapy, which may be personalised therapy, the evaluation of markers of immune response is a predictive factor. The importance of assessing the immune system state has an additional aspect: possible side-effects of the immunotherapy (e.g. autoimmune disorders) [24]. In the future, the necessity of earlier recognition of such complications in patients qualified for immunotherapy could be expected. O’Callagan et al. [25] demonstrated that evaluation of CD8+/Foxp3+ lymphocyte proportion could be useful in the prediction of overall survival in lung cancer. Thommen et al. [26] proved that evaluation of expression of the following factors on lymphocytes can be a crucial indicator of prognosis known as the “inhibitory receptor score”: PD-1, CTLA-4, LAG-3 (lymphocyte activation gene 3), Tim3 (mucin domain-containing molecule 3) and BTLA (B,T-lymphocyte attenuator). Other authors suggest additional analysis of lymphocyte activation (e.g. expression of CD69), maturity (CD45RA) and apoptosis (CD95 and Fas). Recently, Blank et al. [27] demonstrated the idea of connecting seven immunological features of a tumour (antigenicity of the tumour, expression of the MHC (major histocompatibility complex) by APCs, metabolic state of the tumour, the nature of lymphoid infiltration, anti- and pro-inflammatory cytokine balance and general state of immune system, and expression of immune control checkpoints) into a “cancer immunogram”. Another idea is elaboration of four states in TIME (Tumour Immunity in the Microenvironment) based on demonstration of PD-L1 expression and active lymphoid infiltration [28]. Particular degrees in that classification result from certain balances between PD-L1 in the tumour and TIL infiltration activity, and could be the basis for anti-PD-L1 treatment qualification. Figure 1 presents the main cells involved in anticancer immune responses and cellular markers of importance in immunoscoring.
Figure 1.
Markers of cancer cell and lymphocytes in inflammatory infiltration around a tumour as potential markers of immunomodulatory treatment response. Dendritic cells (DCs) recognise cancer cell antigens and display them to cytotoxic lymphocytes (CTLs), which destroy the tumour cell by apoptosis (long arrow). Interferon (IFN)-γ is capable of supporting this reaction. The process is inhibited by type-2 macrophages (M2), myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs). Suppression and regulation processes are enhanced by cytokines: transforming growth factor (TGF)-β, interleukin (IL)-10 and IL-17. The following antigens are expressed the most commonly: melanoma antigen MAGE-A3, glycoprotein antigen MUC-1, PD-1 ligand (PD-L1) and PD-L2, Fas ligand (FasL) and thyroid transcription factor (TTF)-1. CTLs express CD3, CD8, apoptotic factor (Fas), checkpoint molecules cytotoxic T-lymphocyte antigen (CTLA)-4 and programmed cell death protein (PD)-1, and LAG-3 (lymphocyte activation gene 3). The markers of Tregs are CD25, the Foxp3 transcription factor, PD-1, CTLA-4 and GITR (glucocorticoid-induced TNF receptor).
Analysis of the inflammatory, antitumour response status in solid tumours should concern a site of primary tumour development, possibly a reaction in the adjacent lymph nodes. Much research has emphasised the distinctness of the tumour environment as manifested by systemic changes assessed by means of peripheral blood analysis. It was even demonstrated that an inverse relationship exists between some cellular populations: the higher the percentage of a cell type in the lungs, the lower the percentage of the same cells in the peripheral blood [29, 30]. In lung cancer, the greatest obstacle to research is the poor accessibility of tumour mass. This is a result of the small percentage of resected tumours (<30%) because in the majority of non-small cell lung cancer cases, the tumours are in an advanced stage, and in small cell lung cancer cases, they are inaccessible. Histological biopsies or cytological samples are too small and not representative for evaluating inflammatory infiltration. The examination of infiltration in adjacent lymph nodes could be valuable in the evaluation of the inflammatory response. This is possible in sentinel nodes of melanoma, breast cancer and mediastinal nodes in lung cancer. Our own research demonstrated that lymphocyte phenotype in nodes containing metastatic cells of lung cancer is different than in metastasis-free nodes. In lung adenocarcinoma, the Foxp3+/CD8+ lymphocyte ratio was greater in metastatic lymph nodes than in intact ones [31]. These nodes were collected by a surgical method, TEMLA (transcervical extended mediastinal lymphadenectomy), and integrally examined [32]; however, this method is not commonly used. Recently, nonsurgical techniques, such as EBUS/TBNA (endobronchial ultrasound-guided transbronchial needle aspiration), have been used for assessment of metastatic lymph nodes. Unfortunately, that material is insufficient for complete evaluation of infiltration. However, it is potentially eligible for flow cytometry.
Usefulness of BAL fluid analysis in the assessment of the lung cancer microenvironment
Considering the limited possibilities of assessing tissue from the lung tumour microenvironment, BAL fluid analysis seems to be a perfect alternative. There are lymphoid cells in suitable numbers in the BAL material to gain information about their phenotype. Moreover, it is possible to check the cytokine profile and fully evaluate the immune response in the tumour milieu [30, 33, 34]. The reasons for using BAL fluid analysis before immunomodulatory treatment are listed in table 1.
Table 1.
Reasons for the use of BAL fluid analysis as a method of evaluation of immune status of lung cancer patients
| The material is available in each state of advancement and in different histological types of lung cancer, both non-small cell lung cancer and small cell lung cancer |
| BAL may be performed during diagnosis, before treatment, and may be used to monitoring of treatment and complications |
| The method is well standardised and reproducible, and the results can be compared between different centres |
| The different types of inflammatory cells and their mediators can be recognised in BAL material, and may be used for immunoscoring |
| Cancer cells may be identified in BAL fluid in the case of peripheral tumours |
| The results of many studies confirm the significance of that method |
We have previously reported that in the BAL obtained from a lung afflicted by cancer, the following changes may be observed:
increased number of neutrophils
predominance of T-cells and cytotoxic CD8+ lymphocytes
prominent percentage of CTLA-4+ Tregs
polarisation of macrophages to the M2 population
These alterations were significantly different than in the healthy individuals, peripheral blood and BAL material obtained from the healthy lung, symmetrically to tumour localisation in the lung afflicted by cancer. Based on these studies, it was concluded that the nature of immune response in the tumour environment in a lung afflicted by a primary tumour is different than in another, “healthy” lung. This proves that in the case of cancer, lungs are not a homogenous environment and changes caused by the tumour are so severe that they modify the integrity of immune system homeostasis. Planning BAL examination, these differences should be taken into consideration: the lung afflicted by tumour should be lavaged.
Evaluation of BAL fluid requires established rules for handling the material [6]. Through standardisation, results are reproducible, can be compared between different centres and are reliable. The classical description of basic cellular components gives little information about inflammatory response, except the neutrophil percentage, which increases nonspecifically in malignancy, whereas a precise examination of the phenotype of lymphocytes and macrophages gives valuable information. For lymphocyte phenotyping the best method is flow cytometry; to assess phenotypes of macrophages, immunocytochemistry seems to be the best choice [38]. In order to complete the information obtained from BAL, it is possible to measure the cytokine concentration in the extracellular fraction after BAL fluid centrifugation. During routine BAL assessment, a supernatant is frozen at −80°C and can be examined at any time. The techniques for concentration measurement are varied, from enzymatic and cytometric methods, to modern Luminex technology.
Conclusion
With the demonstration of PD-1/PD-L1 and CTLA-4 pathways blocker efficacy in lung cancer, many studies have been performed to validate biomarkers that as predictive factors. In the early studies and clinical trials of nivolumab, the drug efficacy was connected with PD-L1 expression on lung cancer cells [2, 39]. With further research and clinical observations, it was demonstrated that there is no unequivocal relationship between PD-L1 expression on tumour cells and response to treatment [40]. Reasons for this include the use of different reagents, discrepancies in interpretation, alteration in PD-L1 expression, mutations occurring during tumour development and treatment, and poor accessibility of tissue. The range of positive reactions for PD-L1 was very discordant and was approximately 13–70%. An effective response to treatment was observed in tumours without PD-L1 expression. The development of therapy focused on improvement of the patient’s own antitumour response contributes to the necessity to evaluate new parameters. Both the patient’s immune system status and the characteristics of the tumour are very individual, and require evaluation before treatment introduction. The biggest challenge is to evaluate methods and select appropriate factors that could act as biomarkers for immunomodulatory treatment. Difficulties encountered in much of the research on these issues result from the lack of methods that guarantee recurrence and reliability of the results (e.g. an immunogram), because of the use of subjective methods of evaluation of immunocytochemistry tests or using different reagents. It cannot be forgotten that the dynamics of immunological reactions results in alterations not only in time but also in different fragments of the tumour: a histological dedifferentiation and mutations in metastases are observed. Tumours have their own, individual pathways of cancer transformation. A way of treatment and exposure to external factors is not without importance. These observations result in the need to create multidisciplinary research teams that guarantee the cooperation of oncologists, pathologists, immunologists and molecular biologists. There is no doubt that the next years will bring new, valuable answers to these problems.
Disclosures
J. Domagala-Kulawik EDU-0019-2017_Domagala-Kulawik (1.2MB, pdf)
Footnotes
Conflict of interest Disclosures can be found alongside this article at breathe.ersjournals.com
References
- 1.Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer 2010; 67: 257–274. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer JR. PD-1-targeted immunotherapy: recent clinical findings. Clin Adv Hematol Oncol 2012; 10: 674–675. [PubMed] [Google Scholar]
- 3.van Kempen LC, Ruiter DJ, van Muijen GN, et al. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol 2003; 82: 539–548. [DOI] [PubMed] [Google Scholar]
- 4.Thurlbeck WM, Churg AM, eds. Pathology of the Lung. New York, Thieme, 1998. [Google Scholar]
- 5.Hoser G, Kawiak J, Domagała-Kulawik J, et al. Flow cytometric evaluation of lymphocyte subpopulations in BALF of healthy smokers and nonsmokers. Folia Histochem Cytobiol 1999; 37: 25–30. [PubMed] [Google Scholar]
- 6.Chciałowski A, Chorostkowska-Wynimko J, Fal A, et al. Wskazowki Polskiego Towarzystwa Chorob Pluc dotyczace metod pozyskiwania, opracowywania oraz oceny plynu z plukania oskrzelowo-pecherzykowego (BAL) [Recommendation of the Polish Respiratory Society for bronchoalveolar lavage (BAL) sampling, processing and analysis methods]. Pneumonol Alergol Pol 2011; 79: 75–98. [PubMed] [Google Scholar]
- 7.Costabel U. Atlas of Bronchoalveolar Lavage. Chapman and Hall Medical, 1998. [Google Scholar]
- 8.Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol 2008; 59: Suppl. 6: 19–34. [PubMed] [Google Scholar]
- 9.Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013; 73: 2381–2388. [DOI] [PubMed] [Google Scholar]
- 10.Burkholder B, Huang RY, Burgess R, et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta 2014; 1845: 182–201. [DOI] [PubMed] [Google Scholar]
- 11.Domagała-Kulawik J, Osińska I. Zaburzenia odpowiedzi immunologicznej w raku pluca - nowy cel terapii [Immune alterations in lung cancer – the new therapeutic approach]. Pneumonol Alergol Pol 2014; 82: 286–299. [DOI] [PubMed] [Google Scholar]
- 12.Domagala-Kulawik J, Osinska I, Hoser G. Mechanisms of immune response regulation in lung cancer. Transl Lung Cancer Res 2014; 3: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoser G, Wasilewska D, Domagała-Kulawik J. Expression of Fas receptor on peripheral blood lymphocytes from patients with non-small cell lung cancer. Folia Histochem Cytobiol 2004; 42: 249–252. [PubMed] [Google Scholar]
- 14.Tartour E, Zitvogel L. Lung cancer: potential targets for immunotherapy. Lancet Respir Med 2013; 1: 551–563. [DOI] [PubMed] [Google Scholar]
- 15.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 16.Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11: 215–233. [DOI] [PubMed] [Google Scholar]
- 17.Ioachim HL, Dorsett BH, Paluch E. The immune response at the tumor site in lung carcinoma. Cancer 1976; 38: 2296–2309. [DOI] [PubMed] [Google Scholar]
- 18.Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell density – a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res 2015; 21: 2635–2643. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 2006; 107: 2866–2872. [DOI] [PubMed] [Google Scholar]
- 20.Domagala-Kulawik J, Osinska I, Hoser G. Mechanisms of immune response regulation in lung cancer. Transl Lung Cancer Res 2014; 3: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domagala-Kulawik J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl Lung Cancer Res 2015; 4: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senovilla L, Vacchelli E, Galon J, et al. Trial watch: prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology 2012; 1: 1323–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galon J, Pages F, Marincola FM, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med 2012; 10: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danlos FX, Pages C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest 2016; 149: e133–e136. [DOI] [PubMed] [Google Scholar]
- 25.O’Callaghan DS, Rexhepaj E, Gately K, et al. Tumour islet Foxp3+ T-cell infiltration predicts poor outcome in nonsmall cell lung cancer. Eur Respir J 2015; 46: 1762–1772. [DOI] [PubMed] [Google Scholar]
- 26.Thommen DS, Schreiner J, Müller P, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res 2015; 3: 1344–1355. [DOI] [PubMed] [Google Scholar]
- 27.Blank CU, Haanen JB, Ribas A, et al. The “cancer immunogram”. Science 2016; 352: 658–660. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Chen L. Classification of advanced human cancers based on Tumor Immunity in the MicroEnvironment (TIME) for cancer immunotherapy. JAMA Oncol 2016; 2: 1403–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domagała-Kulawik J, Hoser G, Droszcz P, et al. T-cell subtypes in bronchoalveolar lavage fluid and in peripheral blood from patients with primary lung cancer. Diagn Cytopathol 2001; 25: 208–213. [DOI] [PubMed] [Google Scholar]
- 30.Hoser G, Domagała-Kulawik J, Droszcz P, et al. Lymphocyte subsets differences in smokers and nonsmokers with primary lung cancer: a flow cytometry analysis of bronchoalveolar lavage fluid cells. Med Sci Monit 2003; 9: BR310–BR315. [PubMed] [Google Scholar]
- 31.Domagala-Kulawik J, Kwiecien I, Pankowski J, et al. Elevated Foxp3/CD8 ratio in lung adenocarcinoma metastatic lymphnodes resected by transcervical extended mediastinal lymphadenectomy. Biomed Res Int 2017; 9: 5185034.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zieliński M. Transcervical extended mediastinal lymphadenectomy. Thorac Surg Clin 2010; 20: 215–223. [DOI] [PubMed] [Google Scholar]
- 33.Osińska I, Domagała-Kulawik J. Plukanie oskrzelowo-pecherzykowe w raku pluca – znaczenie w diagnostyce i ocenie odpowiedzi ukladu odpornosciowego [Bronchoalveolar lavage in lung cancer – diagnostic value and assessment of the anti-cancer immune response]. Postepy Hig Med Dosw 2013; 67: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 34.Kwiecien I, Stelmaszczyk-Emmel A, Polubiec-Kownacka M, et al. Elevated regulatory T cells, surface and intracellular CTLA-4 expression and interleukin-17 in the lung cancer microenvironment in humans. Cancer Immunol Immunother 2017; 66: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domagała-Kulawik J, Guzman J, Costabel U. Immune cells in bronchoalveolar lavage in peripheral lung cancer – analysis of 140 cases. Respiration 2003; 70: 43–48. [DOI] [PubMed] [Google Scholar]
- 36.Domagala-Kulawik J, Hoser G, Safianowska A, et al. Elevated TGF-β1 concentration in bronchoalveolar lavage fluid from patients with primary lung cancer. Arch Immunol Ther Exp 2006; 54: 143–147. [DOI] [PubMed] [Google Scholar]
- 37.Osińska I, Stelmaszczyk-Emmel A, Polubiec-Kownacka M, et al. CD4+/CD25high/FoxP3+/CD127− regulatory T cells in bronchoalveolar lavage fluid of lung cancer patients. Hum Immunol 2016; 77: 912–915. [DOI] [PubMed] [Google Scholar]
- 38.Osińska I, Wołosz D, Domagała-Kulawik J. Association between M1 and M2 macrophages in bronchoalveolar lavage fluid and tobacco smoking in patients with sarcoidosis. Pol Arch Med Wewn 2014; 124: 359–364. [DOI] [PubMed] [Google Scholar]
- 39.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33: 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerr KM, Tsao MS, Nicholson AG, et al. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol 2015; 10: 985–989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
J. Domagala-Kulawik EDU-0019-2017_Domagala-Kulawik (1.2MB, pdf)