Key Clinical Message
Although tumor lysis syndrome is well described, it is rarely seen or suspected in solid malignancies. Early recognition of this entity is paramount in reducing morbidity and mortality. Treating physicians should be aware of this possibility in solid tumor patients with either bulky disease or extensive liver involvement.
Keywords: Colon cancer tumor lysis syndrome, solid tumors and spontaneous tumor lysis syndromes, spontaneous tumor lysis syndrome
Introduction
Tumor lysis syndrome (TLS) is a well‐described oncologic emergency. The syndrome is deemed spontaneous when it occurs before initiation of any cytotoxic or definite treatment. Here, we report a rare case of a 49‐year‐old woman diagnosed with metastatic colon cancer with extensive liver involvement manifesting with fatal spontaneous tumor lysis syndrome (STLS). Literature review revealed only one other case of spontaneous tumor lysis in colon cancer and 27 other cases of spontaneous tumor lysis in solid tumors, dating back to 1977 1, 2. We also compiled clinical data on patients with solid cancers presenting with STLS to better understand tumor characteristics, location, tumor burden, and eventual outcomes.
Case Presentation
A 49‐year‐old African American woman, who was in a normal state of health until two months prior to this current admission, presented with worsening abdominal pain associated with nausea, vomiting, loss of appetite, early satiety, and subjective weight loss. The patient also complained of postprandial abdominal pain in the right upper quadrant (RUQ), which was nonradiating. There were no associated hematemesis, hemoptysis, melena, bright red blood per rectum, dysuria, or frequency. Her past medical history included hypertension, gastroesophageal reflux, hyperlipidemia, and anemia. Her surgical history included a cholecystectomy done in 2011. Her family history was pertinent for a maternal grandmother who was diagnosed with breast cancer at the age of 62. Her social history was negative for tobacco use, ethanol use, or any illicit drug use.
Investigations and treatment
Pertinent physical examination findings demonstrated a morbidly obese (BMI > 45) patient with right upper quadrant tenderness on deep palpation without any signs of peritonitis, and bilateral pitting edema up to the knees. The cardiovascular, pulmonary, and neurological examinations were grossly normal. Computerized tomography (CT) scan of the abdomen and pelvis revealed hepatomegaly of 27.5 centimeters width by 14.5 centimeters anteroposterior diameter with multiple hypo‐attenuating lesions (Fig. 1D and F) along with mural thickening of the cecum. Pertinent laboratory findings at the time of presentation included: white blood cells: 12.14 × 103/μL, hemoglobin: 8.0 g/dL, platelets: 512 × 103/μL, potassium: 3.5 mmol/L, serum creatinine (Cr): 0.87 mg/dL, albumin: 2.7 g/dL, total bilirubin: 0.9 mg/dL, alanine aminotransferase: 57 U/L, aspartate aminotransferase: 212 U/L, alkaline phosphatase: 324 U/L, and a lactic acid: 2.3 mmol/L. CT‐guided liver biopsy demonstrated metastatic adenocarcinoma. Subsequent colonoscopy and biopsy of a near‐obstructing necrotic caecal mass (Fig. 1A and B) confirmed a moderately differentiated invasive adenocarcinoma. F‐18 Fluorodeoxyglucose positron emission tomography and computerized tomography (FDG PET/CT) scan revealed diffusely intense, FDG uptake in the liver (max standardized uptake value of 19.9) (Fig. 1C and E). The patient's health progressively started deteriorating with increasing edema of bilateral extremities associated with worsening dyspnea. Doppler ultrasound of bilateral extremities was negative for deep vein thrombosis, and the ventilation–perfusion scan was negative for pulmonary embolism. Transthoracic echocardiogram was also within normal limits. At this time, the patient was found to be in tumor lysis with lactate dehydrogenase levels: 10,853 U/L, uric acid: 20.3 mg/dL, and serum creatinine: 3.9 mg/dL (Table 1). Ultrasound of kidneys showed normal renal parenchyma and no evidence of obstructive uropathy. All the supportive measures including allopurinol, intravenous fluids, rasburicase, and dialysis were initiated. Despite aggressive resuscitative efforts, patient succumbed to death soon from multiorgan failure.
Figure 1.
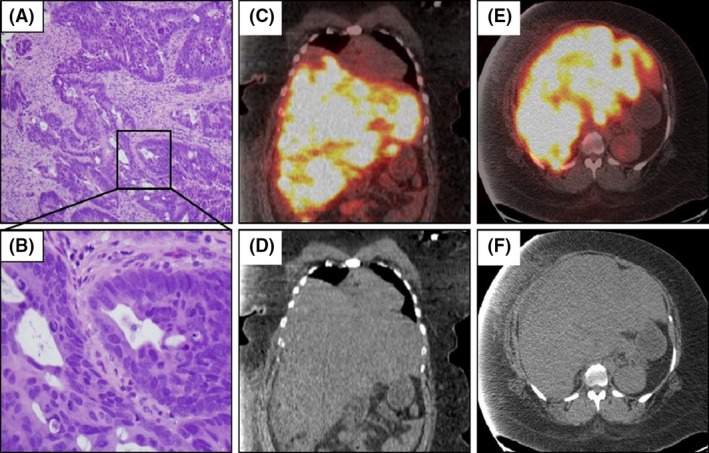
Biopsy of colonic mass and fused PET/CT images. H&E staining of colon mass at lower (A) and 40× (B) magnification. Fused PET‐CT sagittal (B,C) and coronal sections (D,E) revealing very avid uptake in the liver.
Table 1.
Laboratory trends in the patient presented
| Parameter | Day 1 | Day 13 | Day 21 | Day 23 |
|---|---|---|---|---|
| WBC (K/μL) | 12.14 | 13.29 | 16.95 | 22.05 |
| Hemoglobin (g/dL) | 8 | 7.7 | 7.1 | 7.5 |
| Platelets (K/μL) | 512 | 563 | 390 | 222 |
| Potassium (mmol/L) | 3.5 | 4.5 | 5.0 | 5.4 |
| BUN (mg/dL) | 6 | 24 | 73 | 72 |
| Creatinine (mg/dL) | 0.87 | 1.9 | 3.9 | 4.8 |
| Phosphorus | ‐ | 4.4 | 6.7 | |
| Calcium (mg/dL) | 8.5 | 8.9 | 8.2 | 8.1 |
| ALT (U/L) | 57 | 122 | 121 | 175 |
| AST (U/L) | 212 | 635 | 603 | 954 |
| Total Bilirubin (mg/dL) | 0.9 | 2.2 | 4.9 | 6.0 |
| Uric Acid (mg/dL) | – | – | 20.3 | 6.3 |
| LDH (U/L) | – | – | 10853 | >4000 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase.
Discussion
We present here a fatal case of spontaneous TLS (TLS) as a complication of metastatic colon adenocarcinoma. TLS is an oncological emergency precipitated by massive release of intracellular contents into the circulation, manifesting in distinct laboratory abnormalities including, but not limited to, hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. Along with kidney injury, each of these metabolic derangements has its own catastrophic consequences 3.
TLS is generally initiated by effective cytotoxic chemotherapy in a context of rapidly proliferating tumor or in patients with massive tumor burden. While TLS is most commonly seen in hematologic cancers, it can also be seen in solid malignancies with a large tumor burden 3. Cytotoxic chemotherapy, targeted antibody therapy, radiation, and even glucocorticoids are all well known to precipitate TLS 3. Spontaneous TLS can be defined as the TLS occurring in the absence of any definitive treatment 4. The initial step for diagnosis of TLS is based on the laboratory findings in an appropriate clinical context. Hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia are the primary indicators of TLS 5. Clinical tumor lysis syndrome is considered when the traditional laboratory findings of TLS lead to nausea, vomiting, diarrhea, lethargy, cardiac arrhythmias, seizures, or sudden death 4, 5. The Cairo‐Bishop definitions provide laboratory values for diagnosis as well as a grading system for TLS 5.
Care of the patient with established TLS should include astute monitoring of potassium, calcium, uric acid, phosphorous, lactate dehydrogenase and serum creatinine levels, along with cardiac monitoring and urine output. Hydration is a vital aspect of the treatment, and the remainder of the treatment is focused on specific electrolyte abnormalities, specifically management of hyperkalemia. 3. Hyperkalemia is one of the earliest and most ominous signs, a harbinger of life‐threatening arrhythmias, which warrants close telemetry monitoring 5. A parallel approach of correcting hypocalcemia and hyperphosphatemia, which often coexist, is important to avoid calcium phosphate precipitation 3, 5. Oral phosphorous binders not only decrease phosphorous levels, but also aid in correction of hypocalcemia 5. Uric acid crystal deposition in the distal renal tubules can cause obstructive nephropathy, and this along with hyperphosphatemia can lead to acute renal failure 5, 6, 7. Allopurinol can be used prophylactically in patients at risk of TLS to lower uric acid production. However, in patients with active TLS, allopurinol use may result in elevated levels of xanthine by inhibiting xanthine oxidase 6, 7, 8. Xanthine may also precipitate in the renal tubules which can contribute to obstructive uropathy in a similar manner as uric acid. 7, 8. For these reasons, rasburicase, which converts uric acid to soluble allantoin, is the recommended drug for hyperuricemia in tumor lysis syndrome 3, 4, 6, 7. In patients with low urine output, persistent hyperphosphatemia, or hypocalcemia, dialysis needs to be initiated 3.
The first reported case of spontaneous tumor lysis in a nonhematologic malignancy was reported in 1977 2. There have been 75–100 reported cases of tumor lysis in solid tumors, mostly after treatment, between 1977 and 2011 as described in the literature 6, 9. STLS is considered rare in all types of malignancy, but according to a study by Tsokos et al., three of 33 patients with non‐Hodgkin lymphoma already had hyperuricemia and kidney injury prior to treatment 10. Therefore, spontaneous TLS may be underdiagnosed at least in hematologic malignancies. Our literature review revealed only one other case of spontaneous tumor lysis in a patient with colon adenocarcinoma 1. This involved a 27‐year‐old man with a similar initial presentation as our patient.
Our literature review yielded 28 other cases of spontaneous tumor lysis in solid tumors (Table 2). There was a male predominance with 18 of 29 patients being male. The mean age at the time of diagnosis of STLS was 57.4 years. Although there was a variation in origin of primary tumors, extensive liver involvement is noted in 82.8% of total cases reported. When the liver is not involved, patients inevitably had a large tumor burden and necrosis. As noted by Gemici et al. 5, liver involvement seems to predispose patients to TLS. This may be due to a high purine pool in the liver which can be released during necrosis or impaired uric acid metabolism if hepatic function is impaired by a high tumor burden 5. The most common presenting symptom was abdominal pain or discomfort, seen in 48.3% of the patients. With our patient included, the mortality rate of these cases approaches 69%. TLS in solid tumors is unpredictable and carries a worse prognosis when compared to hematologic malignancies 5. There is a 20%–50% mortality in all cases of TLS in solid tumors if undiagnosed or if diagnosed too late 6, 11. United States Food and Drug Administration has approved rasburicase in 2009 for the initial management of plasma uric acid levels in adult patients with hematological and solid malignancies who are receiving anticancer therapy, expected to result in TLS. This approval was based on a phase III, randomized, multicenter, open‐label trial (EFC 4978) which demonstrated a significant improvement in uric acid response rates among patients treated with rasburicase compared to those receiving allopurinol 12. The mortality rate for our reviewed cases of spontaneous tumor lysis syndrome in which rasburicase was used is 87.5%. Whether rasburicase use, which is currently increasing, can contribute to increased survival is unknown secondary to very few cases of STLS in solid tumors reported in the literature.
Table 2.
Published cases from 1977 until 2016 of spontaneous tumor lysis syndromes in solid tumors
| Case author | Age/sex | Initial TLS symptom | Cancer type | Sites involved | Treatment | Initial outcome |
|---|---|---|---|---|---|---|
| Gbaguidi et al. 13 | 88/F | Vomiting | Renal Ca. | Large renal + bone + liver | Unknown | Died |
| Okamoto et al. 14 | 62/F | Abd distention | Uterine adeno Ca. | >20 cm pelvic mass + ascites | Chemo, surgery | Survived |
| Saleh et al. 15 | 56/F | Fatigue | Pancreatic adeno Ca. | Large pancreatic mass + liver | All, Rasb, K Binders | Died |
| Wang et al. 16 | 71/F | Skin nodules | GI or ovarian adeno Ca. | Skin + renal + adrenal + lung + liver | All, K Binders, Alk | Died |
| Frestad et al. 1 | 27/M | Abd Pain | Colon mucinous adeno Ca. | Liver + lung + pleura + LAD | Rasb, fibrinogen | Died |
| Norberg et al. 17 | 56/M | Back Pain | Renal Ca. | 10 cm Renal mass + liver + bone + lung | Fluids, all, HD | Died |
| Zakharia et al. 18 | 49/F | Abd Pain | Spindle cell sarcoma | 9 cm RP mass + liver + lung | Fluids, Rasb, HD | Died |
| Goyal et al. 19 | 51/M | Weakness | Gastric adeno Ca. | Liver + bone + adrenal + LAD | Fluids, All, HD | Survived |
| Ali et al. 20 | 66/M | Abd Pain | Cholangiocarcinoma | Extensive liver involvement | Fluids, All | Died |
| Mehrzad et al. 21 | 70/M | Unresponsive | Hepatocellular Ca. | 14 cm liver mass + LAD | Fluids, Alk | Died |
| Mouallem et al. 22 | 69/M | Rectal Bleed | Melanoma | Extensive liver involvement | Fluids | Died |
| Saini et al. 23 | 59/F | Vomiting | CUP: adeno Ca. | Large RP mass + liver + LAD | Fluids, Rasb, HD | Died |
| Kekre et al. 24 | 76/M | Vomiting | Hepatocellular Ca. | 19 cm liver mass | Fluids, All, HD | Died |
| Goyal et al. 25 | 51/F | Anuria | Ductal breast Ca. | 4 cm ulcerated breast mass | Fluids, All | Died |
| Murray et al. 26 | 13/F | Abd distention | GCT | 20 cm pelvic mass + peritoneum | Rasb, Chemo | Survived |
| D'Alessandro et al. 27 | 22/M | Abd distention | GCT: choriocarcinoma | 14 cm RP mass + lung + liver | Rasb, K Binders | Died |
| Abboud et al. 28 | 53/M | Abd Pain | Sq. cell Ca./maxillary sinus | Extensive liver involvement | All, Alk, Rasb | Died |
| Shenoy. 29 | 74/M | Anuria | Squamous cell Ca‐ lung | Bulky necrotic lung mass | All, HD, Chemo | Survived |
| Lin et al. 30 | 72/M | Anorexia | Prostate Ca. | Extensive liver + bone involvement | All, Furosemide, HD | Died |
| Vaisban et al. 31 | 82/F | Weakness | Colon Ca. | Extensive liver involvement | All, Alk | Survived |
| Vaisban et al. 31 | 80/M | Abd Pain | Pheochromocytoma | 20 cm Adrenal mass | All, Alk | Survived |
| Vaisban et al. 31 | 72/M | Weakness | Hepatocellular Ca. | Large liver lesion | All, Alk | Died |
| Pentheroudakis et al. 32 | 52/M | Abd pain | GCT: endodermal sinus | RP LAD + liver + lung | HD, Chemo | Survived |
| Pentheroudakis et al. 32 | 24/M | Abd pain | GCT: Seminoma | 25 cm RP mass + liver | HD, Chemo | Survived |
| Woo et al. 33 | 36/M | Abd distention | Gastric adeno Ca. | 7 cm gastric mass + liver + LADs | Alk, All, HD, Chemo | Died |
| Feld et al. 34 | 72/M | Abd distention | Lung adeno Ca. | Large lung mass + liver | Cal, K Binders, All | Died |
| Sklarin et al. 35 | 62/F | Bone pain | Inflammatory breast Ca. | Breast + liver + lung + bone marrow | All, Chemo | Survived |
| Crittenden et al. 2 | 50/M | Abd distention | CUP: adeno Ca. | Extensive liver + bone + LAD | All, Alk | Died |
Abd, abdominal; All, allopurinol; Alk, alkalinization; Ca, carcinoma; Cal, calcium supplementation; Chemo, chemotherapy; CUP, carcinoma of unknown primary; F, female; HD, hemodialysis; GCT, germ cell tumor; K Binders, potassium Binders; M, male; Mets, metastases; Rasb, rasburicase; RP, retroperitoneal; Sq Cell Ca, squamous cell carcinoma.
Conclusion
We believe that spontaneous TLS is an underdiagnosed and overlooked entity which is associated with poor outcomes. High index of suspicion for STLS should be exercised while managing patients with bulky disease, extensive liver involvement, or concurrent renal dysfunction. As laboratory testing for uric acid and LDH is universally available and can be easily performed, early recognition and aggressive management should be pursued to potentially reduce organ dysfunction and fatal outcomes.
Authorship
DS, PP, and AT: were responsible for manuscript preparation. RS: involved in pathology contribution and manuscript editing. All the authors: have approved the final manuscript.
Conflict of Interest
None declared.
Patient consent
Obtained.
Provenance and peer review
Not commissioned; externally peer reviewed.
Clinical Case Reports 2017; 5(12): 2121–2126
[Correction added on 22 November 2017 after first online publication: One of the author names was previously incorrect and has been corrected in this version.]
References
- 1. Frestad, D. , Perner A., and Pedersen U. G.. 2014. Acute onset and rapid progression of multiple organ failure in a young adult with undiagnosed disseminated colonic adenocarcinoma. BMJ Case Rep. 2014 Sep 24. https://doi.org/10.1136/bcr-2014-205002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crittenden, D. R. , and Ackerman G. L.. 1977. Hyperuricemic acute renal failure in disseminated carcinoma. Arch. Intern. Med. 137:97–99. [PubMed] [Google Scholar]
- 3. Coiffier, B. , Altman A., Pui C. H., Younes A., and Cairo M. S.. 2008. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence‐based review. J. Clin. Oncol. 26:2767–2778. [DOI] [PubMed] [Google Scholar]
- 4. Nagaiah, G. , Truong Q., and Monga M.. 2014. Oncologic emergencies and paraneoplastic syndromes in Abraham J., Gulley J. L. and Allegra C. J., eds. The Bethesda handbook of clinical oncology, 5th ed. Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 5. Gemici, C. 2006. Tumour lysis syndrome in solid tumours. Clin. Oncol. (R. Coll. Radiol.) 18:773–780. [DOI] [PubMed] [Google Scholar]
- 6. Vodopivec, D. M. , Rubio J. E., Fornoni A., and Lenz O.. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep. Med. 2012;2012:Article ID 468452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cairo, M. S. , and Bishop M.. 2004. Tumour lysis syndrome: new therapeutic strategies and classification. Br. J. Haematol. 127:3–11. [DOI] [PubMed] [Google Scholar]
- 8. LaRosa, C. , McMullen L., Bakdash S., Ellis D., Krishnamurti L., Wu H. Y., et al. 2007. Acute renal failure from xanthine nephropathy during management of acute leukemia. Pediatr. Nephrol Support. Care Cancer. 22:132–135. [DOI] [PubMed] [Google Scholar]
- 9. Kim, H. D. , Ha K. S., Woo I. S., Jung Y. H., Han C. W., and Kim T. J.. 2014. Tumor lysis syndrome in a patient with metastatic colon cancer after treatment with 5‐fluorouracil/leucovorin and oxaliplatin: case report and literature review. Cancer Res. Treat. 46:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsokos, G. C. , Balow J. E., Spiegel R. J., and Magrath I. T.. 1981. Renal and metabolic complications of undifferentiated and lymphoblastic lymphomas. Medicine 60:218–229. [DOI] [PubMed] [Google Scholar]
- 11. Coiffier, B. 2010. Acute tumor lysis syndrome – a rare complication in the treatment of solid tumors. Oncol. Res. Treat. 33:498–499. [DOI] [PubMed] [Google Scholar]
- 12. Pazdur, R. FDA Approval for Rasburicase [Internet]. National Cancer Institute; 2013. Available at https://www.cancer.gov/about-cancer/treatment/drugs/fda-rasburicase (accessed 23 September 2016). [Google Scholar]
- 13. Gbaguidi, X. , Goodrich L., Roca F., Suel P., and Chassagne P.. 2016. Bulky solid tumors in elderly adults: beware of spontaneous tumor lysis syndrome. J. Am. Geriatr. Soc. 64:235–237. [DOI] [PubMed] [Google Scholar]
- 14. Okamoto, K. , Kinoshita T., Shimizu M., Okura I., Kawada A., Mizobuchi K., et al. A case of spontaneous tumor lysis syndrome in a patient with ovarian cancer. Case Rep. Obstet. Gynecol. 2015. 2015:Article ID 461870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saleh, R. R. , Rodrigues J., and Lee T. C.. A tumour lysis syndrome in a chemotherapy naive patient with metastatic pancreatic adenocarcinoma. BMJ Case Rep. 2015. 29:bcr2014207748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang, Y. , Yuan C., and Liu X.. 2014. Cutaneous metastatic adenocarcinoma complicated by spontaneous tumor lysis syndrome: a case report. Oncol. Lett. 8:905–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norberg, S. M. , Oros M., Birkenbach M., and Bilusic M.. 2014. Spontaneous tumor lysis syndrome in renal cell carcinoma: a case report. Clin. Genitourin. Cancer 12:e225–e227. [DOI] [PubMed] [Google Scholar]
- 18. Zakharia, Y. , Mansour J., Vasireddi S., Zakharia K., Fatakhov E., Koch C., et al. 2014. Tumor lysis syndrome in a retroperitoneal sarcoma. J. Invest. Med. High Impact Case Rep. 2:2324709614542340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goyal, H. , Sawhney H., Bekara S., and Singla U.. 2014. Spontaneous acute tumour lysis syndrome in gastric adenocarcinoma: a case report and literature review. J. Gastrointest. Cancer 45:208–211. [DOI] [PubMed] [Google Scholar]
- 20. Ali, A. M. , Barbaryan A., Zdunek T., Khan M., Voore P., and Mirrakhimov A. E.. 2014. Spontaneous tumor lysis syndrome in a patient with cholangiocarcinoma. J. Gastrointest. Oncol. 5:E46–E49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehrzad, R. , Saito H., Krahn Z., and Feinstein A.. 2014. Spontaneous tumor lysis syndrome in a patient with metastatic hepatocellular carcinoma. Med. Princ. Pract. 23:574–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mouallem, M. , Zemer‐Wassercug N., Kugler E., Sahar N., Shapira‐Frommer R., and Schiby G.. 2013. Tumor lysis syndrome and malignant melanoma. Med. Oncol. 30:1–4. [DOI] [PubMed] [Google Scholar]
- 23. Saini, N. , Pyo Lee K., Jha S., Patel S., Bonthu N., Kansagra A., et al. 2012. Hyperuricemic renal failure in nonhematologic solid tumors: a case report and review of the literature. Case Rep. Med. 2012:Article ID 314056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kekre, N. , Djordjevic B., and Touchie C.. 2012. Spontaneous tumour lysis syndrome. Can. Med. Assoc. J. 184:913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal, H. , Sawhney H., and Singh J.. 2012. Spontaneous fatal recurrent tumor lysis syndrome in ductal breast carcinoma. Commun. Oncol. 9:136–137. [Google Scholar]
- 26. Murray, M. J. , Metayer L. E., Mallucci C. L., Hale J. P., Nicholson J. C., Kirollos R. W., et al. 2011. Intra‐abdominal metastasis of an intracranial germinoma via ventriculo‐peritoneal shunt in a 13‐year‐old female. Br. J. Neurosurg. 25:747–749. [DOI] [PubMed] [Google Scholar]
- 27. D'Alessandro, V. , Greco A., Clemente C., Sperandeo M., De Cata A., Di Micco C., et al. 2010. Severe spontaneous acute tumor lysis syndrome and hypoglycemia in patient with germ cell tumor. Tumori 96:1040. [PubMed] [Google Scholar]
- 28. Abboud, M. , and Shamseddine A.. 2009. Maxillary sinus squamous cell carcinoma presenting with fatal tumor lysis syndrome: a case report and review of the literature. Case Rep. Oncol. 2:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shenoy, C. 2009. Acute spontaneous tumor lysis syndrome in a patient with squamous cell carcinoma of the lung. QJM 102:71–73. [DOI] [PubMed] [Google Scholar]
- 30. Lin, C. J. , Hsieh R. K., Lim K. H., Chen H. H., Cheng Y. C., and Wu C. J.. 2007. Fatal spontaneous tumor lysis syndrome in a patient with metastatic, androgen‐independent prostate cancer. South. Med. J. 100:916–917. [DOI] [PubMed] [Google Scholar]
- 31. Vaisban, E. , Braester A., Mosenzon O., Kolin M., and Horn Y.. 2003. Spontaneous tumor lysis syndrome in solid tumors: really a rare condition? Am. J. Med. Sci. 325:38–40. [DOI] [PubMed] [Google Scholar]
- 32. Pentheroudakis, G. , O'Neill V., Vasey P., and Kaye S.. 2001. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Support. Care Cancer 9:554–557. [DOI] [PubMed] [Google Scholar]
- 33. Woo, I. S. , Kim J. S., Park M. J., Lee M. S., Cheon R. W., Chang H. M., et al. 2001. Spontaneous acute tumor lysis syndrome with advanced gastric cancer. J. Korean Med. Sci. 16:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feld, J. , Mehta H., and Burkes R. L.. 2000. Acute spontaneous tumor lysis syndrome in adenocarcinoma of the lung: a case report. Am. J. Clin. Oncol. 23:491–493. [DOI] [PubMed] [Google Scholar]
- 35. Sklarin, N. T. , and Markham M.. 1995. Spontaneous recurrent tumor lysis syndrome in breast cancer. Am. J. Clin. Oncol. 18:71–73. [DOI] [PubMed] [Google Scholar]


