Key Clinical Message
Temsirolimus did not demonstrate an efficacy advantage compared with sorafenib as second‐line therapy in patients with metastatic renal cell carcinoma (mRCC). Only a few patients achieved complete responses, and the median progression‐free survival rate remains short. We report one patient with mRCC who had a continuing response to temsirolimus.
Keywords: Mammalian target of rapamycin, metastatic renal cell carcinoma, temsirolimus
Introduction
The treatment of metastatic renal cell carcinoma (mRCC) has significantly improved due to the addition of targeted agents that inhibit elements of the vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) pathway. The treatment with temsirolimus for advanced renal cell carcinoma (RCC) has shown efficacy and safety in a phase II study on cytokine refractory patients 1. Temsirolimus is also considered to be the standard of care in first‐line therapy of mRCC patients with a poor‐risk prognosis. However, in a randomized phase III trial, temsirolimus did not demonstrate an efficacy advantage compared with sorafenib as second‐line therapy after disease progression in patients treated with sunitinib for mRCC 2. In addition, only a few patients achieved complete responses (CR) 3 and the median progression‐free survival (PFS) rate remains relatively low in patients with mRCC who received temsirolimus 1, 2, 4. Herein, we report one patient with mRCC who had a continuing response to temsirolimus for more than 3 years after failure of treatment with tyrosine kinase inhibitors (TKIs).
Case Report
A 51‐year‐old Japanese man presented at a community hospital with a chief complaint of continuous low‐grade fever. The patient had an abnormal chest X‐ray and visited our hospital in October 2012. Laboratory evaluations revealed the following findings: hemoglobin, 7.2 g/dL (normal range: 13.5–17.5 g/dL), corrected calcium level, 10.6 mg/dL (normal range: 8.3–10.3 mg/dL), and C‐reactive protein, 17.3 mg/dL (normal range: <0.3 mg/dL). Computed tomography (CT) revealed a hypervascular tumor (size, 9 × 8.5 cm) in the upper pole of the left kidney with multiple lung metastases (Fig. 1, 2A and B). The tumor was clinically diagnosed as a left renal cell carcinoma (RCC) and was classified as clinical T2bN2M1 according to the tumor–node–metastasis system 5.
Figure 1.
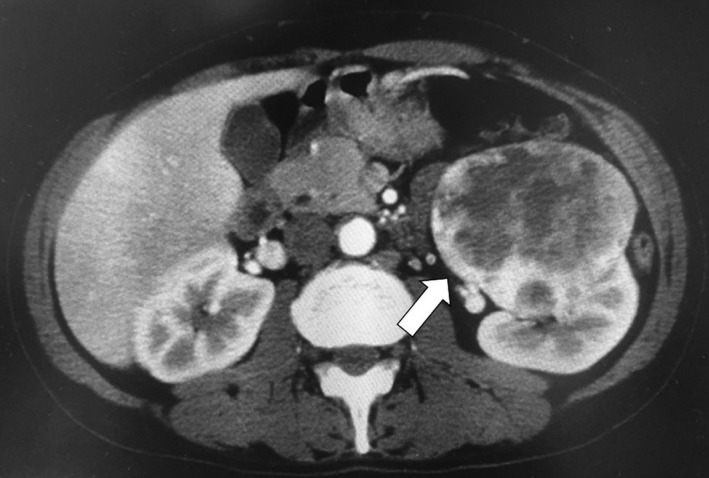
Abdominal computed tomography revealed a hypervascular tumor, measuring 9 × 8.5 cm, in the upper pole of the left kidney (arrow).
Figure 2.
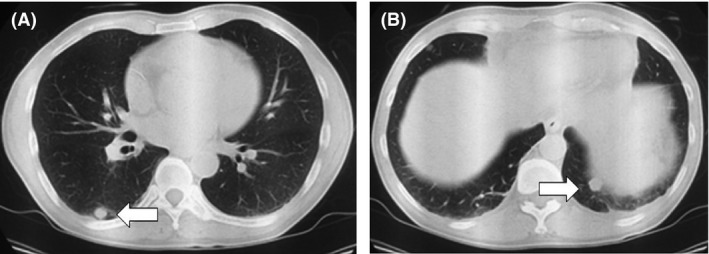
(A and B) Thoracic computed tomography revealed multiple lung metastases (arrow).
The patient underwent open radical nephrectomy in December 2012. Pathological analysis identified a pT3a clear cell RCC of Fuhrman grade 3 with hemorrhage and necrotic tissue. In January 2013, the patient received sunitinib at 50 mg/day for 2 weeks of every 3‐week cycle for the treatment of multiple lung metastases. After two cycles, a lung CT showed disease progression with lung metastases, which increased in size (Fig. 3A and B). The patient received axitinib at 10 mg/day starting in March 2013. After 5 months of axitinib treatment, the patient achieved a complete response (CR) with lung metastases 5. In February 2014, CT showed a mediastinal lymph node metastasis after a year of axitinib treatment (Fig. 4). The patient received temsirolimus at 25 mg/week beginning in March 2014. After 3 months of temsirolimus treatment, the patient achieved a CR with a mediastinal lymph node metastasis (Fig. 5). In November 2016, the treatment schedule of temsirolimus was changed from weekly to biweekly. To date, CT has shown no evidence of disease and treatment with temsirolimus is still ongoing. The patient did not experience any adverse events.
Figure 3.
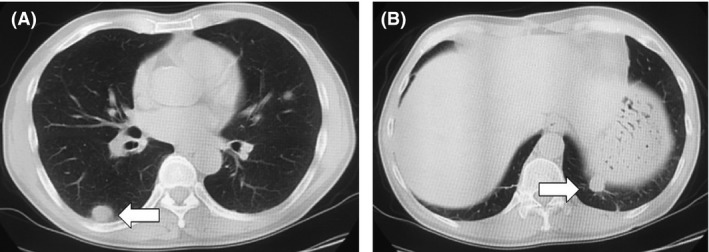
(A and B) Thoracic computed tomography revealed a disease progression with multiple lung metastases, which increased in size (arrow).
Figure 4.
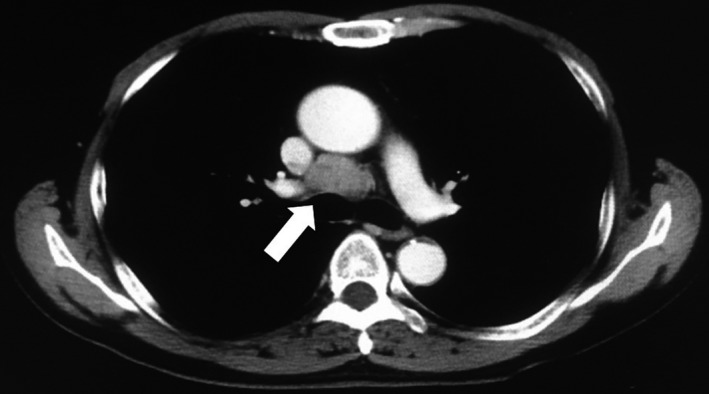
Thoracic computed tomography revealed a lymph node metastasis in the mediastinum (arrow).
Figure 5.
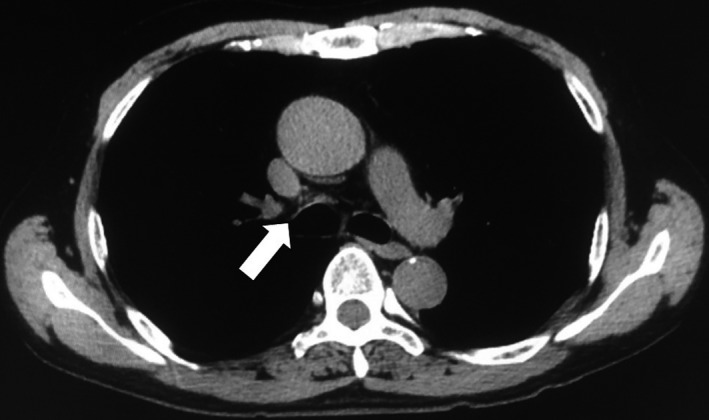
Thoracic computed tomography showed a complete response by RECIST criteria (arrow) 3.
Discussion
mTOR is a highly conserved serine–threonine kinase and is activated through the phosphoinositide 3‐kinase (PIK3)–Akt pathway after binding of the ligand to growth factor receptors 6. Activation of the PIK3/Akt/mTOR pathway plays a critical role in the proliferation and survival of malignant cells and has been recognized as a valuable therapeutic target 6. Temsirolimus is a prodrug and needs to be converted to its active metabolite, sirolimus, by CYP3A4 in the liver and has a half‐life of approximately 13 h 1.
Based on a multicenter, randomized phase III trial 7, the European Association of Urology guidelines recommended temsirolimus as a first‐line treatment in patients in the poor prognosis group according to the Memorial Sloan‐Kettering Cancer Center (MSKCC) criteria 8. Median PFS and overall survival were 3.8 and 10.8 months, respectively 7. Although TKIs or mTOR inhibitors are the standard therapy for mRCC, it is still difficult to achieve a CR in advanced RCC (aRCC) or mRCC. In fact, an objective response rate of only 8.6% has been reported among patients with poor prognosis mRCC receiving temsirolimus 7.
In a randomized phase III trial from investigating temsirolimus as second‐line therapy, temsirolimus did not demonstrate an efficacy advantage compared with sorafenib as second‐line therapy after disease progression in patients with mRCC who were receiving sunitinib 2. Several studies showed that everolimus offers superior OS compared with temsirolimus after disease progression during TKI therapy for patients with mRCC, although both agents were associated with similar response rates and PFS 9, 10. Iacovelli et al. reported that everolimus decreased the risk of death by 26% over temsirolimus 10. Recent studies have demonstrated that cabozantinib and nivolumab are superior to everolimus after progression during initial TKI therapy. Therefore, everolimus may be considered a treatment option as third‐ or fourth‐line therapy 11, 12.
To date, the optimal third‐line treatment has not been established. Jonasch et al. investigated current practice patterns, including the selection of first‐ and second‐line therapy and treatment sequences, for patients with mRCC 13. A total of 433 patients who received second‐line therapy were enrolled in this study. Only 21% of the patients received third‐line therapy 13. Of these, sunitinib–everolimus–bevacizumab was the most commonly used treatment sequence 13. Wells et al. reported the use of targeted third‐line therapy 14. OS after cessation of second‐line therapy was 14 months in patients who received third‐line therapy, and 2.1 months for those not receiving third‐line therapy (P < 0.001) 14. However, only 3.2% of the patients were administered temsirolimus as a third‐line therapy 14. Thus, the frequency of use of temsirolimus for patients with aRCC or mRCC may decrease in the future. However, the toxicity profile of patients who received temsirolimus was remarkably favorable with no grade 3/4 adverse events other than those who received TKI 15. In the present case report, although the patient who was administered axitinib as a second‐line TKI treatment achieved complete response for lung metastases, CT showed mediastinal lymph node metastasis. Therefore, we selected temsirolimus as a third‐line treatment.
Although many molecular‐targeted drugs are available, the choice of the best drug depends on the clinical situation. Temsirolimus may be a useful and valuable treatment in patients with aRCC or mRCC who failed to respond to TKIs.
Authorship
TS: evaluated the patient's medical records, wrote the article draft; TK: performed critical revisions of the text and figures, and is the corresponding author; HH: evaluated the patient's medical record; NT: performed the clinical evaluation of the patient and contributed to the writing process; SN: operated the patient, obtained the patient's consent, and contributed to the writing process; CO: coordinated and supervised the writing process. All authors approved the final version of the manuscript.
Consent for Publication
Informed written consent was obtained from the patient.
Conflict of Interest
No conflict of interests to report.
Acknowledgments
This study was supported by the following grants in aid for Scientific Research from the Japan Society for the Promotion of Science: 15H02563 (to CO), 15K15579 (to CO), and 17K11118 (to TK).
Clinical Case Reports 2017; 5(12): 1950–1953
References
- 1. Atkins, M. B. , Hidalgo M., Stadler W. M., Logan T. F., Dutcher J. P., Hudes G. R., et al. 2004. Randomized phase II study of multiple dose levels of CCI‐779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J. Clin. Oncol. 22:909–918. [DOI] [PubMed] [Google Scholar]
- 2. Hutson, T. E. , Escudier B., Esteban E., Bjarnason G. A., Lim H. Y., Pittman K. B., et al. 2014. Randomized phase III trial of temsirolimus versus sorafenib as second‐line therapy after sunitinib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 32:760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eisenhauer, E. A. , Therasse P., Bogaerts J., Schwartz L. H., Sargent D., Ford R., et al. 2009. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45:228–247. [DOI] [PubMed] [Google Scholar]
- 4. Park, K. , Lee J. L., Park I., Park S., Ahn Y., Ahn J. H., et al. 2012. Comparative efficacy of vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI) and mammalian target of rapamycin (mTOR) inhibitor as second‐line therapy in patients with metastatic renal cell carcinoma after the failure of first‐line VEGF TKI. Med. Oncol. 29:3291–3297. [DOI] [PubMed] [Google Scholar]
- 5. American Joint Committee on Cancer (AJC) . 2010. Kidney Pp. 479–489 in Edge S. B., Byrd D. R., Compton C. C., et al., eds. AJCC cancer staging manual, 7th ed. Springer, New York. [DOI] [PubMed] [Google Scholar]
- 6. Patel, S. B. , Stenehjem D. D., Gill D. M., et al. 2016. Everolimus versus temsirolimus in metastatic renal cell carcinoma after progression with previous systemic therapies. Clin. Genitourin Cancer 14:153–159. [DOI] [PubMed] [Google Scholar]
- 7. Hudes, G. , Carducci M., Tomczak P., et al. 2007. Temsirolimus, interferon alfa, or both for advanced renal‐cell carcinoma. N. Eng. J. Med. 356:2271–2281. [DOI] [PubMed] [Google Scholar]
- 8. Mekhail, T. M. , Abou‐Jawde R. M., Boumerhi G., et al. 2005. Validation and extension of the Memorial Sloan‐Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J. Clin. Oncol. 23:832–841. [DOI] [PubMed] [Google Scholar]
- 9. Wong, M. K. , Yang H., Signorovitch J. E., et al. 2014. Comparative outcomes of everolimus, temsirolimus and sorafenib as second targeted therapies for metastatic renal cell carcinoma: a US medical record review. Curr. Med. Res. Opin. 30:537–545. [DOI] [PubMed] [Google Scholar]
- 10. Iacovelli, R. , Santoni M., Verzoni E., et al. 2015. Everolimus and temsirolimus are not the same second‐line in metastatic renal cell carcinoma. A systematic review and meta‐analysis of literature data. Clin. Genitourin Cancer 13:137–141. [DOI] [PubMed] [Google Scholar]
- 11. Choueiri, T. K. , Escudier B., Powels T., et al. 2015. Cabozantinib versus everolimus in advanced renal‐cell carcinoma. N. Eng. J. Med. 373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Motzer, R. J. , Escudier B., McDermott D. F., et al. 2015. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N. Eng. J. Med. 373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jonasch, E. , Signorovitch J. E., Lin P. L., et al. 2014. Treatment patterns in metastatic renal cell carcinoma: a retrospective review of medical records from US community oncology practices. Curr. Med. Res. Opin. 30:2041–2050. [DOI] [PubMed] [Google Scholar]
- 14. Wells, J. C. , Stukalin I., Norton C., et al. 2017. Third‐line targeted therapy in metastatic renal cell carcinoma: results from the international metastatic renal cell carcinoma database consortium. Eur. Urol. 71:204–209. [DOI] [PubMed] [Google Scholar]
- 15. Gerullis, H. , Bergmann L., Maute L., et al. 2010. Feasibility of sequential use of sunitinib and temsirolimus in advanced renal cell carcinoma. Med. Oncol. 27:373–378. [DOI] [PubMed] [Google Scholar]


