Key Clinical Message
Esophageal self‐expandable metal stents and radiotherapy are valuable in combination for palliation and definitive treatment of esophageal cancer. However, risk of aortoesophageal fistula is significant in patients with evidence of malignant aortic invasion. Use of thoracic endovascular repair may represent an approach to early intervention in high‐risk patients.
Keywords: Aortoesophageal fistula, chemoradiation, esophageal cancer, self‐expandable metal stent, thoracic endovascular aortic repair
Introduction
Many patients with esophageal cancer present at an advanced stage, and the majority of these suffer from some degree of dysphagia. Palliative dysphagia interventions allow patients to maintain oral caloric intake, manage oropharyngeal excretions, and reduce aspiration risk, often in advance of definitive intervention such as surgical resection or chemoradiation (CRT). Self‐expandable metal stents (SEMS) provide instant relief for malignant dysphagia, however, have a high rate of dysphagia reoccurrence and need for reintervention. Radiation therapy (RT), of either palliative or definitive intent, provides durable though delayed symptomatic improvement in malignant dysphagia, often taking several weeks to achieve maximum benefit.
As such, there has been interest in combining the therapeutic benefits of palliative‐ or definitive‐intent RT with SEMS to provide both immediate and longlasting relief from dysphagia. Both nonrandomized prospective and randomized trials have demonstrated improvement in both survival and symptomatic relief outcomes with poststenting RT 1, 2, 3, 4. In the majority of cases, this combined regimen is effective and well tolerated; however, some studies have reported a higher incidence of severe complications 5, 6, 7, 8. In particular, massive hematemesis or other GI bleeding has been identified in nearly a quarter of patients across all stages of disease 7. Sumiyoshi and colleagues have previously reported on their experience of 22 patients undergoing SEMS placement after CRT, with 6 of 8 patients with clinical T4 disease and evidence of invasion into the aorta dying of massive hemorrhage 8. Additional toxicity may be attributable to alterations of dose distribution resulting from SEMS presence in field 9, 10, while some reports suggest the causative role of radiotherapy itself 11, 12.
To further characterize the potential risk of aortoesophageal fistula (AEF) in esophageal cancer patients undergoing RT after placement of SEMS, we describe a case of a patient who underwent definitive CRT after palliative esophageal SEMS placement. With this report, we describe the pathological changes seen after post‐SEMS placement EBRT, including the development of AEF.
Case Presentation
A 59‐year‐old otherwise healthy woman presented with dysphagia to solids and was diagnosed with stage III T4N0M0 invasive squamous cell carcinoma of the mid‐esophagus with concern for involvement of the aorta (Fig. 1).
Figure 1.
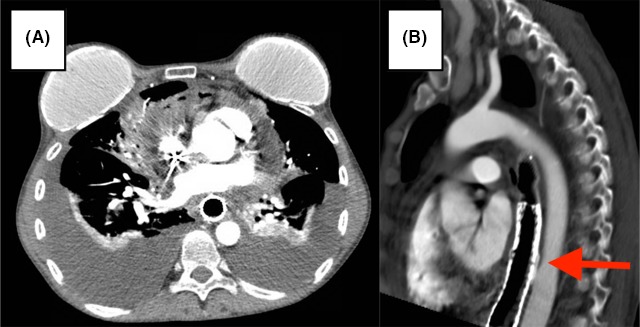
Pre‐treatment CT shows advanced esophageal carcinoma with infiltration of paraesophageal tissues around the aorta with intact fat plane.
A dedicated multidisciplinary foregut tumor board initially recommended neoadjuvant chemotherapy followed by definitive resection without radiation. Radiation was excluded from the original treatment plan due to concerns for a more challenging resection in the setting of radiation‐induced fibrosis due to the proximity of the mass to both the aorta and the membranous airways. The patient was given a jejunostomy feeding tube and underwent six cycles of doublet chemotherapy with carboplatin/paclitaxel. Upon restaging, the patient had evidence of local progression, was no longer considered a good candidate to achieve a R0 resection, and was referred for definitive chemoradiation.
Prior to chemoradiation, the patient underwent EGD with placement of a silicone‐coated nitinol esophageal stent (Wilson Cook Evolution fully covered, 18 mm × 10 cm) deployed across the stricture under endoscopic and fluoroscopic guidance (Fig. 2A–D) to palliate dysphagia. The stent spanned from 24 to 36cm from the incisors, completely crossing the distal edge of the tumor.
Figure 2.
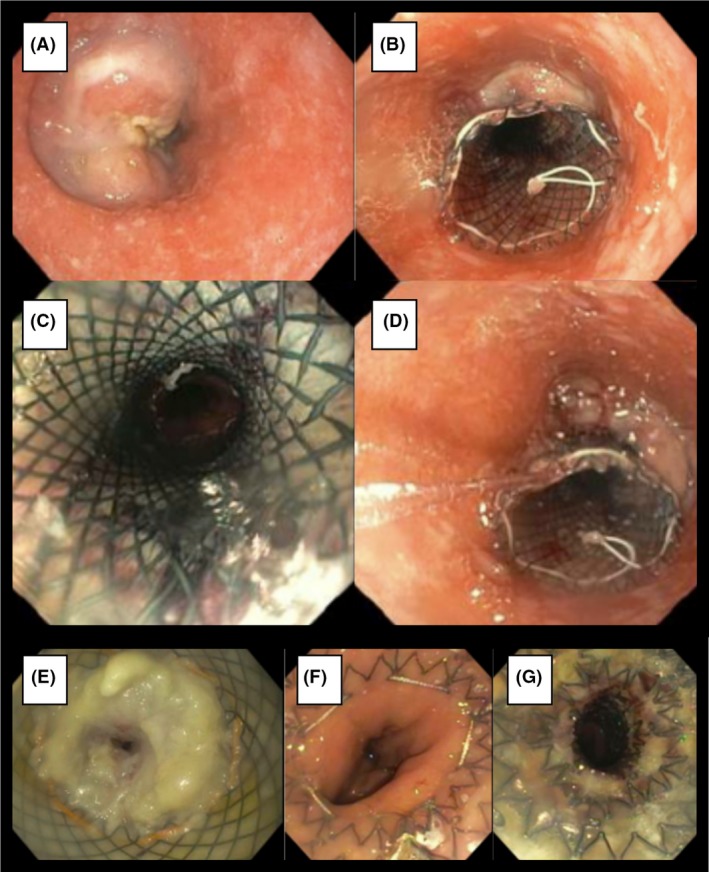
(A–D) Placement of first stent spanning from 25 to 35 cm from incisors. (E) Obstruction of initial stent. (F–G) Placement of second stent from 29–39 cm from incisors.
The patient subsequently received chemoradiation to 50Gy in 25 fractions over 35 elapsed days with a volumetric modulated arc therapy (VMAT) plan to the mid‐esophageal mass (Fig. 3A–C), by use of a single full arc with two coplanar sweeps using 6‐MV photon energy and weekly cisplatin/5‐fluorouracil. The dose–volume histogram revealed a maximum dose of 54.8 Gy to 0.035 cc of the aorta (Fig. 3D).
Figure 3.
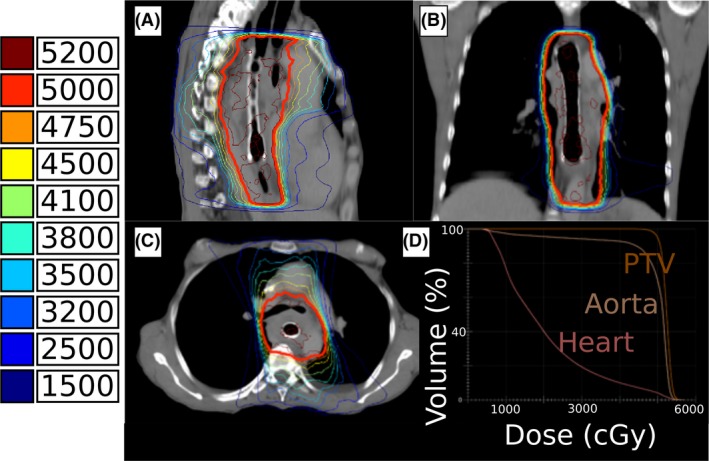
Sagittal (A), coronal (B), and axial (C) treatment planning isodose lines with color‐coded isodose levels shown in cGy. Dose–volume histogram (D) shown for the planning treatment volume (orange), descending aorta (tan), and heart (pink).
Two months later, the patient developed suspected stent obstruction. Chest CT and PET/CT at this time demonstrated persistent extramural invasion of tumor partially encasing the aorta, although less enhancement and slight interval decrease in size. There was no evidence of other disease or abnormality. On EGD, the distal end of the stent at 35 cm was obstructed by inflamed and malignant tissue. The stenosis was successfully traversed with an ultra‐thin scope, and a silicone‐coated nitinol esophageal stent (ENDOMAXX, 19 mm × 10 cm) was deployed under direct visualization over a wire guide from 29–39 cm (Fig. 2E–G).
Several weeks later, the patient was admitted with dyspnea and increasing lower extremity edema. She was found to have bilateral pleural effusions and pneumopericardium (Fig. 4). After admission, the patient developed mid‐thoracic pain, a single episode of hematemesis, and hemodynamic instability. At this time, she was considered not a candidate for surgical repair given instability and presumed malignant erosion into the pericardium and mediastinum. Hours later, during intubation for esophagogastroduodenoscopy to confirm source of bleeding, she had large volume hematemesis followed by pulseless arrest. At bedside, the patient's spouse confirmed her status as Do Not Resuscitate. She rapidly expired.
Figure 4.
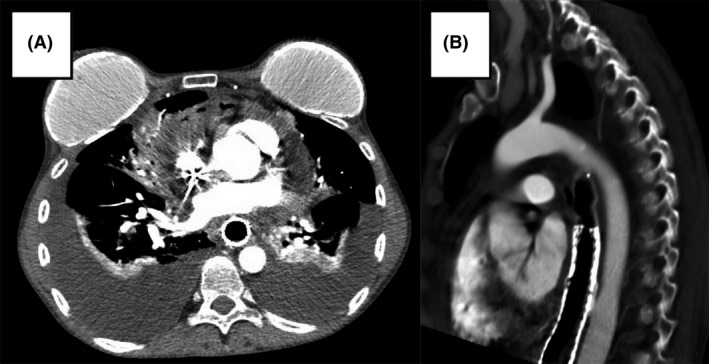
CT angiogram chest obtained during final hospitalization, prior to development of hematemesis, demonstrating (A) pericardial effusion containing locules of gas within pericardial thickening representing fistulization of the esophagus into the pericardium with significant bilateral layering pleural effusions and (B) potential site of aortoesophageal fistula.
An autopsy confirmed the diagnosis of locally advanced esophageal carcinoma without spread to the lymph nodes. The stent was without defect and did not appear to perforate the esophagus. Once the stent was removed, an AEF at the midway point of the stent was identified as the source of the bleeding (Fig. 5A). An esophagopericardial fistula was also identified as the source of the pneumopericardium (Fig. 5B), with approximately 70 mL of material consistent with refluxed gastric contents found in the pericardial sac (Fig. 5C). While microscopic inflammation and necrosis were noted at the sites of the fistulae, no active carcinoma was identified at either site.
Figure 5.
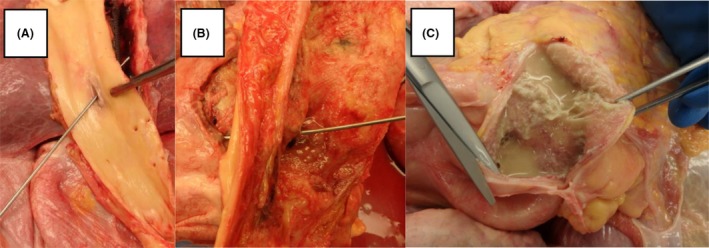
(A) A probe is passed through the wall of the thoracic aorta and an aortoesophageal fistula into the esophagus. (B) After opening the esophagus and removal of the stent, a probe is passed through the anterior wall of the esophagus and an esophagopericardial fistula into the pericardial sac. (C) The pericardial sac is opened anteriorly to reveal adherent, copious, yellow stringy, soft material, and thick yellow fluid.
Discussion
SEMS and EBRT are extremely effective in relieving dysphagia, a major concern in palliation of advanced esophageal cancer 13, 14. The potential for AEF resulting in massive hematemesis and exsanguination has been previously identified in the setting of SEMS and EBRT. This case is an example of both recurrent dysphagia following SEMS placement as well as pathologically confirmed AEF without evidence of direct tumor invasion on autopsy following palliative SEMS placement and EBRT.
AEF should be suspected in patients like this one with history of significant thoracic intervention presenting with elements of “Chiari's triad”—mid‐thoracic pain, sentinel arterial hemorrhage, and final exsanguination following symptom‐free interval 15. The three most common etiologies of AEF include thoracic aortic aneurysm (51.2%), foreign body ingestion (18.6%), and esophageal malignancy (17.0%) 16. Alarmingly, a growing number of cases have been published demonstrating formation of AEF independent of direct tumor involvement, mostly indicating external‐beam radiation therapy as the causative agent, perhaps resulting from radiation‐related damage to the vasa vasorum of the aorta 11, 12, 17, 18.
Although surgery remains the definitive management of AEF 19, many patients, like this one, are not surgical candidates at presentation. Thoracic endovascular aortic repair (TEVAR) is a rapid, less invasive, and effective alternative to surgical intervention in the urgent and emergent management of patients with AEF 20, 21 and can be used as a bridge to definitive surgical management 22. TEVAR involves advancing a folded endovascular stent graft through the lumen of an access vessel, usually the femoral artery, inside a delivery sheath before ultimate expansion and deployment in the thoracic or thoracoabdominal aorta. TEVAR provides rapid hemodynamic stabilization by controlling bleeding from the fistula site. However, outcomes following emergent intervention remain poor, suggesting a need for earlier intervention in high‐risk patients.
A gap exists in the early use of TEVAR in the context of malignancy‐, radiation‐ and SEMS‐associated AEF. Dual endoluminal intervention (i.e., concurrent SEMS placement and TEVAR) in the setting of esophageal–tracheobronchial strictures has been shown to be safe and effective at reducing dysphagia and dyspnea 23. In the urgent setting, three case reports describe successful use of TEVAR in combination with SEMS placement in management of AEF. Ghosh et al. describe development of AEF proximal to SEMS placed in a patient with esophageal cancer to palliate dysphagia. To manage acute hemodynamic instability, an additional SEMS was placed to cover the source of acute bleeding, without improvement in the patient's hemodynamic instability. The patient then underwent TEVAR and survived for 2 months before dying of disseminated malignancy 24. Civilini et al. describe a patient with a history of multiple thoracic surgeries who subsequently developed AEF, managed initially with TEVAR followed days later by SEMS after demonstration of persistent leak, who had recovered well at 5‐month follow‐up 25. Uchida et al. describe development of AEF in a patient with lung cancer following implantation of SEMS treated successfully with TEVAR 26.
In summary, special consideration should be applied when treating esophageal carcinoma with evidence of invasion into the aorta using SEMS combined with EBRT, as AEF formation is an under‐recognized yet fatal complication. Although further studies are needed to determine the role of prophylactic TEVAR in patients at high risk of developing AEF in the setting of esophageal cancer, our tumor board highly considers this approach when SEMS and EBRT are combined in this patient population.
Conflict of Interest
There is no conflict of interest to declare.
Authorship
GJM: wrote initial drafts and performed literature review. JH: provided content expertise and subsequent edits. CRT: provided content expertise and subsequent edits. NN: provided the concept and insights for this case, subsequent edits, and final approval.
Clinical Case Reports 2017; 5(12): 2074–2079
References
- 1. Zaidi, A. , Jelveh S., Mahmood J., and Hill R. P.. 2012. Effects of lipopolysaccharide on the response of C57BL/6J mice to whole thorax irradiation. Radiother. Oncol. 105:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu, Y.‐T. , Yang G., Liu Y., and Shen B.‐Z.. 2004. Clinical evaluation of radiotherapy for advanced esophageal cancer after metallic stent placement. World J. Gastroenterol. 10:2145–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ludwig, D. , Dehne A., Burmester E., Wiedemann G. J., and Stange E. F.. 1998. Treatment of unresectable carcinoma of the esophagus or the gastroesophageal junction by mesh stents with or without radiochemotherapy. Int. J. Oncol. 13:583–588. [DOI] [PubMed] [Google Scholar]
- 4. Zhong, J. , Wu Y., Xu Z., Liu X., Xu B., and Zhai Z.. 2003. Treatment of medium and late stage esophageal carcinoma with combined endoscopic metal stenting and radiotherapy. Chin. Med. J. (Engl.) 116:24–28. [PubMed] [Google Scholar]
- 5. Song, H.‐Y. , Lee D. H., Seo T.‐S., Kim S. B., Jung H. Y., Kim J. H., et al. 2002. Retrievable covered nitinol stents: experiences in 108 patients with malignant esophageal strictures. J. Vasc. Interv. Radiol. 13:285–293. [DOI] [PubMed] [Google Scholar]
- 6. Alberts, A. S. , Burger W., Greeff F., Schoeman L., Friediger D., Nel J., et al. 1992. Severe complications of 5‐fluorouracil and cisplatin with concomitant radiotherapy in inoperable non‐metastatic squamous cell oesophageal cancer after intubation–early termination of a prospective randomised trial. Eur. J. Cancer 28A:1005–1006. [DOI] [PubMed] [Google Scholar]
- 7. Nishimura, Y. , Nagata K., Katano S., Hirota S., Nakamura K., Higuchi F., et al. 2003. Severe complications in advanced esophageal cancer treated with radiotherapy after intubation of esophageal stents: a questionnaire survey of the Japanese Society for Esophageal Diseases. Int. J. Radiat. Oncol. Biol. Phys. 56:1327–1332. [DOI] [PubMed] [Google Scholar]
- 8. Sumiyoshi, T. , Gotoda T., Muro K., Rembacken B., Goto M., Sumiyoshi Y., et al. 2003. Morbidity and mortality after self‐expandable metallic stent placement in patients with progressive or recurrent esophageal cancer after chemoradiotherapy. Gastrointest. Endosc. 57:882–885. [DOI] [PubMed] [Google Scholar]
- 9. Francis, S. R. , Anker C. J., Wang B., Williams G. V., Cox K., Adler D. G., et al. 2013. Self‐expanding stent effects on radiation dosimetry in esophageal cancer. J. Appl. Clin. Med. Phys. 14:4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen, Y. K. , Schefter T. E., and Newman F.. 2011. Esophageal cancer patients undergoing external beam radiation after placement of self‐expandable metal stents: is there a risk of radiation dose enhancement? Gastrointest. Endosc. 73:1109–1114. [DOI] [PubMed] [Google Scholar]
- 11. Sivaraman, S. K. , and Drummond R.. 2002. Radiation‐induced aortoesophageal fistula: an unusual case of massive upper gastrointestinal bleeding. J. Emerg. Med. 23:175–178. [DOI] [PubMed] [Google Scholar]
- 12. Parikh, M. P. , Sherid M., Panginikkod S., Rawal H. A., and Gopalakrishnan V.. 2016. Radiation therapy‐induced aortoesophageal fistula: a case report and review of literature. Gastroenterol. Rep. 4:165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dua, K. S. 2007. Stents for palliating malignant dysphagia and fistula: is the paradigm shifting? Gastrointest. Endosc. 65:77–81. [DOI] [PubMed] [Google Scholar]
- 14. Javed, A. , Pal S., Dash N. R., Ahuja V., Mohanti B. K., Vishnubhatla S., et al. 2012. Palliative stenting with or without radiotherapy for inoperable esophageal carcinoma: a randomized trial. J. Gastrointest. Cancer 43:63–69. [DOI] [PubMed] [Google Scholar]
- 15. Carter, R. , Mulder G. A., Snyder E. N., and Brewer L. A.. 1978. Aortoesophageal fistula. Am. J. Surg. 136:26–30. [DOI] [PubMed] [Google Scholar]
- 16. Hollander, J. E. , and Quick G.. 1991. Aortoesophageal fistula: a comprehensive review of the literature. Am. J. Med. 91:279–287. [DOI] [PubMed] [Google Scholar]
- 17. Um, S.‐J. , Park B. H., and Son C.. 2009. An aortoesophageal fistula in patient with lung cancer after chemo‐irradiation and subsequent esophageal stent implantation. J. Thorac. Oncol. 4:263–265. [DOI] [PubMed] [Google Scholar]
- 18. Gabrail, N. Y. , Harrison B. R., and Sunwoo Y. C.. 1991. Chemo‐irradiation induced aortoesophageal fistula. J. Surg. Oncol. 48:213–215. [DOI] [PubMed] [Google Scholar]
- 19. Akashi, H. , Kawamoto S., Saiki Y., Sakamoto T., Sawa Y., Tsukube T., et al. 2014. Therapeutic strategy for treating aortoesophageal fistulas. Gen. Thorac. Cardiovasc. Surg. 62:573–580. [DOI] [PubMed] [Google Scholar]
- 20. Canaud, L. , Ozdemir B. A., Bee W. W., Bahia S., Holt P., and Thompson M.. 2014. Thoracic endovascular aortic repair in management of aortoesophageal fistulas. J. Vasc. Surg. 59:248–254. [DOI] [PubMed] [Google Scholar]
- 21. Ishikawa, N. , Maruta K., Oi M., Iizuka H., Kawaura H., and Omoto T.. 2013. Thoracic endovascular repair for aorto‐esophageal fistula in patients with esophageal carcinoma: report of 3 cases. Vasc. Endovasc. Surg. 47:65–69. [DOI] [PubMed] [Google Scholar]
- 22. Georvasili, V. K. , Bali C., Peroulis M., Kouvelos G., Avgos S., Godevenos D., et al. 2016. Management of an aorto‐esophageal fistula, complicating a descending thoracic aortic aneurysm endovascularly repaired. Gen. Thorac. Cardiovasc. Surg. 64:216–219. [DOI] [PubMed] [Google Scholar]
- 23. Nam, D. H. , Shin J. H., Song H. Y., Jung G. S., and Han Y. M.. 2006. Malignant esophageal‐tracheobronchial strictures: parallel placement of covered retrievable expandable nitinol stents. Acta Radiol. 47:3–9. [DOI] [PubMed] [Google Scholar]
- 24. Ghosh, S. K. , Rahman F. Z., Bown S., Harris P., Fong K., and Langmead L.. 2011. Survival following treatment of aortoesophageal fistula with dual esophageal and aortic intervention. Case Rep. Gastroenterol. 5:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Civilini, E. , Bertoglio L., Melissano G., and Chiesa R.. 2008. Aortic and esophageal endografting for secondary aortoenteric fistula. Eur. J. Vasc. Endovasc. Surg. 36:297–299. [DOI] [PubMed] [Google Scholar]
- 26. Uchida, N. , Katayama K., and Sueda T.. 2014. Endovascular stent graft for aortoesophageal fistula caused by esophageal stent. Asian Cardiovasc. Thorac. Ann. 22:368. [DOI] [PubMed] [Google Scholar]


