Abstract
Osteosarcoma is the most common bone cancer among those with non-hematological origin and affects mainly pediatric patients. In the last 50 years, refinements in surgical procedures, as well as the introduction of aggressive neoadjuvant and adjuvant chemotherapeutic cocktails, have increased to nearly 70% the survival rate of these patients. Despite the initial therapeutic progress the fight against osteosarcoma has not substantially improved during the last three decades, and almost 30% of the patients do not respond or recur after the standard treatment. For this group there is an urgent need to implement new therapeutic approaches. Oncolytic adenoviruses are conditionally replicative viruses engineered to selectively replicate in and kill tumor cells, while remaining quiescent in healthy cells. In the last years there have been multiple preclinical and clinical studies using these viruses as therapeutic agents in the treatment of a broad range of cancers, including osteosarcoma. In this review, we summarize some of the most relevant published literature about the use of oncolytic adenoviruses to treat human osteosarcoma tumors in subcutaneous, orthotopic and metastatic mouse models. In conclusion, up to date the preclinical studies with oncolytic adenoviruses have demonstrated that are safe and efficacious against local and metastatic osteosarcoma. Knowledge arising from phase I/II clinical trials with oncolytic adenoviruses in other tumors have shown the potential of viruses to awake the patient´s own immune system generating a response against the tumor. Generating osteosarcoma immune-competent adenoviruses friendly models will allow to better understand this potential. Future clinical trials with oncolytic adenoviruses for osteosarcoma tumors are warranted.
Keywords: Oncolytic adenovirus, Virotherapy, Osteosarcoma, Bones, Cancer, Tumor
1. Osteosarcoma: the disease
Compared to other tumors, bone cancers are relatively rare cancers with an average incidence less than 1 per 100,000 person-year [1], [2]. Bone cancers encompass different types of tumors, such as Ewing sarcoma and chondrosarcoma, but the most frequent among them is the osteogenic sarcoma, also known as osteosarcoma (OS), which comprises a 20–40% of total new diagnosed bone cancers [2], [3]. OS tumors are characterized by the overproduction of an aberrant osteoid matrix surrounding malignant spindle cells that leads to a high risk of fracture within the affected bone [3], [4]. Most of OS primary tumors are located in the metaphysis of the long bones in both upper and lower limbs, where there are developed about a 75–85% of the tumors [5], [6]. However, the main issue of OS tumors is their high spread of malignant cells along the organism and as a consequence most of the patients present metastasis at the diagnosis, thus worsening the prognosis of the disease.
The incidence of OS shows a bimodal age distribution with a major peak observed during childhood and puberty, and a second minor peak that appears in the elderly [2], [5], [7]. There are also gender related differences in the epidemiology of OS, with an overall higher incidence in males [1].
Although there is still a little knowledge about the etiology of OS, the incidence characteristics described above, as well as, the predominant location of these tumors in limbs suggest a relationship between bone growth and the development of the disease in young patients [8], [9], [10]. On the other hand, OS in adults often appears as a secondary malignancy [11] and the occurrence of these tumors has been linked to some predisposing syndromes like Li-Fraumeni syndrome [12].
2. Osteosarcoma: treatment, survival and further needs
Before the arising of chemotherapy, the only treatment available for OS was the amputation of the affected limb, even though the result was a poor survival rate below 20% of the patients [13]. Nowadays, the standard protocol for high-grade OS includes the surgical resection of the primary tumor and its metastases in combination with both neoadjuvant and adjuvant therapies using different cocktails of high-dose methotrexate with leucovorin rescue, adriamycin, cisplatin and cyclophosphamide or ifosfamide [13]. As a result, this regimen has increased the survival rate in the last 50 years up to a 60–70% of the patients, as well as the evolution of imaging and surgery techniques has improved the limb-salvage interventions over the 90% [13], [14]. However, with the current strategies the success of OS treatment has reached a plateau and the survival rate has not been increased in the last 30 years. Moreover, besides the primary location within long bones, OS is a highly metastatic tumor releasing invasive malignant cells that migrates through the organism, thus leading to the development of metastases in lungs [15], [16], [17] and, unfortunately, in those patients with metastatic OS the survival rate drops to a poor 30% [18]. The lack of progress in the fight against OS is associated with the presence of drug-resistant tumor cells [19]. In addition, some OS tumors cannot be completely removed due to their location, especially those affecting the axial skeleton.
On the other hand, the aggressiveness of the chemotherapeutic treatment is associated with severe side effects including oocyte destruction and infertility, hearing impairment, heart failure, hepatotoxicity and nephrotoxicity [20], [21], [22], [23], [24], [25], [26].
For all these reasons, it is urgent to implement new therapeutic approaches against osteosarcoma which allow improving the survival rate as well as reducing the side effects. In the last years virotherapy has emerged as a true potential strategy in cancer medicine. Currently several viruses are being tested as anticancer agents, and among them we will focus on oncolytic adenoviruses.
3. Adenoviruses: structure and viral cycle
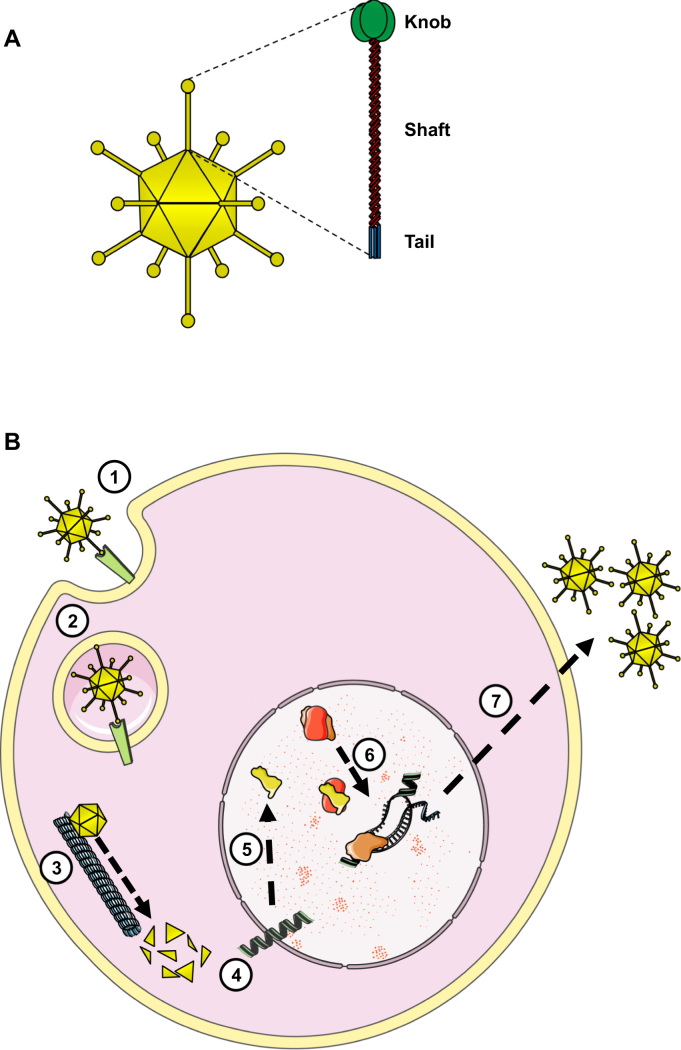
Although there are dozens of different adenovirus described, all of them share a common architecture. Adenoviruses are non-enveloped viruses with an icosahedral capsid composed by up to 7 different structural proteins. However, considering the scope of this review the most relevant viral protein is the fiber due to its role in adenovirus tropism. The fiber is a trimeric protein which is located on each of the 12 vertices of the virion and protrudes from the capsid as an elongated antenna [27]. The fiber protein is composed of three domains (Fig. 1A): 1) a proximal tail domain that anchors the protein to the capsid; 2) a distal globular knob domain that recognizes and binds to the cellular receptor, and 3) a fibrous shaft domain which keeps the knob away from the capsid, thus avoiding steric repulsion during the virus-cell interaction.
Fig. 1.
A) Illustration showing the location of the fiber protein on the adenovirus capsid, as well as the three structural domains of the fiber. B) Schematic adenovirus viral cycle. The adenovirus recognizes its specific receptor (1) triggering its internalization inside the cell (2). Then the virus migrates through the microtubules (3) and introduces the viral genome inside the nucleus (4). The E1A gene is expressed immediately (5) the E1A protein binds pRB releasing the transcription factor E2F and thus the cell cycle arrest (6), which in turn will promote the expression of viral proteins and the genome replication, obtaining the viral progeny (7).
On the other hand, the adenovirus genome is composed by a single linear dsDNA molecule of about 36 kb long and encodes for more than 40 gene products clustered into different transcription units, which in turn are named as early, intermediate or late depending on if they are transcribed before, during or after the DNA replication, respectively [28].
Regarding the viral cycle, first the adenovirus recognizes its specific receptor on the cell surface triggering its internalization. Once inside the cell the virus migrates through the microtubules and introduces the viral genome inside the nucleus. There the E1A gene is expressed immediately from the adenovirus genome. The E1A protein is able to bind pRb [29], [30], [31], releasing the transcription factor E2F and thus the arrest in the cell cycle. The release of E2F also triggers an orchestrated activation of the viral genes that eventually will lead to the generation of new virions, the lysis of the infected cell and the spread of the viral progeny (Fig. 1B).
Additionally, the E1B-55k viral protein is also transcribed during the initial stages of the infection and inhibits the tumor suppressor p53 to avoid its counterbalance effect in cell cycle progression as well as the E1A-induced apoptosis mediated by p53 [32], [33].
Among the different species and serotypes described, the human adenovirus serotype 5 (Ad5) is, by far, the most studied adenovirus. The Ad5 is a very common virus that has tropism for the upper respiratory tract and usually causes just flu-like mild infections. Nevertheless, besides its role as a natural human pathogen, in the last three decades the interest of this virus has been boosted because of its potential utility in different biomedical fields such as gene therapy, vaccination and virotherapy [34], [35], [36], [37]. Although there are some studies using other adenovirus serotypes, the vast knowledge about the biology of the Ad5, as well as its relatively harmless behavior are the main reasons that places this serotype as the reference adenovirus in biomedicine.
In this article we will review briefly the state-of-the art of Ad5 derivatives as therapeutic agents in the field of virotherapy and how are they being tested to treat osteosarcoma.
4. Oncolytic adenoviruses as therapeutic tools: tumor specificity
As described before, adenoviruses infect their target cells and subsequently replicates inside the nucleus and lyses the cells to spread the progeny to infect new cells. This viral lytic cycle could be exploited to destroy the cancer cells through several rounds of replication of the adenovirus within the tumor mass (Fig. 2). However, this strategy may confront some challenges to obtain a successful anti-cancer effect that can be summarized as a replication of the virus restricted to cancer cells and an enhanced tropism towards the tumor.
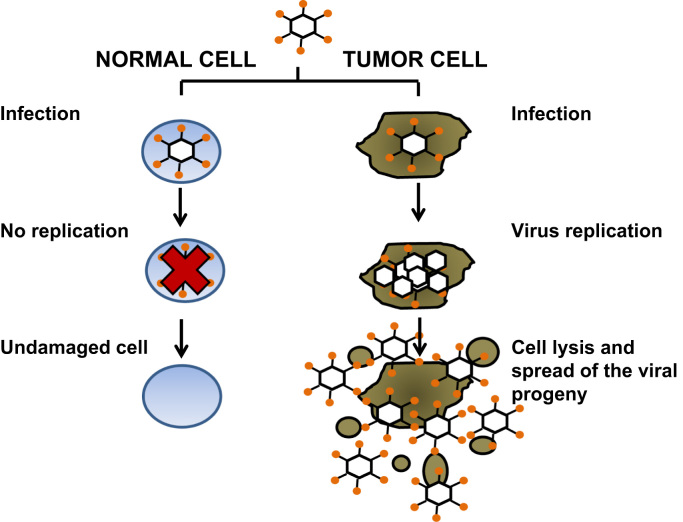
Fig. 2.
Schematic diagram representing the progression of the oncolytic adenovirus cycle following the infection of a tumor cell versus a healthy cell. Although the virus is able to infect normal healthy cells its replication is impaired, thus the cell remains undamaged. On the contrary, the virus replicates in a tumor cell and generates new viral particles, which in turn leads to the lysis of the cell and the spread of these particles to nearby cells.
In regard to the cancer selectivity, different strategies have been tested to generate conditionally replicative adenoviruses (CRAds) whose replication is permissive only in cancer cells while remaining innocuous to normal healthy cells (Fig. 3A). The prototypical CRAd was the dl1520 virus, named with the trademark ONYX-015, which is defective for E1B-55k protein [38]. Since E1B-55k is required for p53 shutdown, this virus was proposed as replicative only in p53 deficient tumor cells, while in normal cells the normal p53 function would block the viral replication [39]. Nevertheless, it was demonstrated that dl1520 replication is independent from the p53 status of the tumor [40], [41]. Moreover, the dl1520 selectivity replication seems to be related to other functions of E1B-55k like the late viral mRNA nuclear export [42] and therefore, dl1520 oncolysis would be limited to those tumors that complement the viral mRNA export.
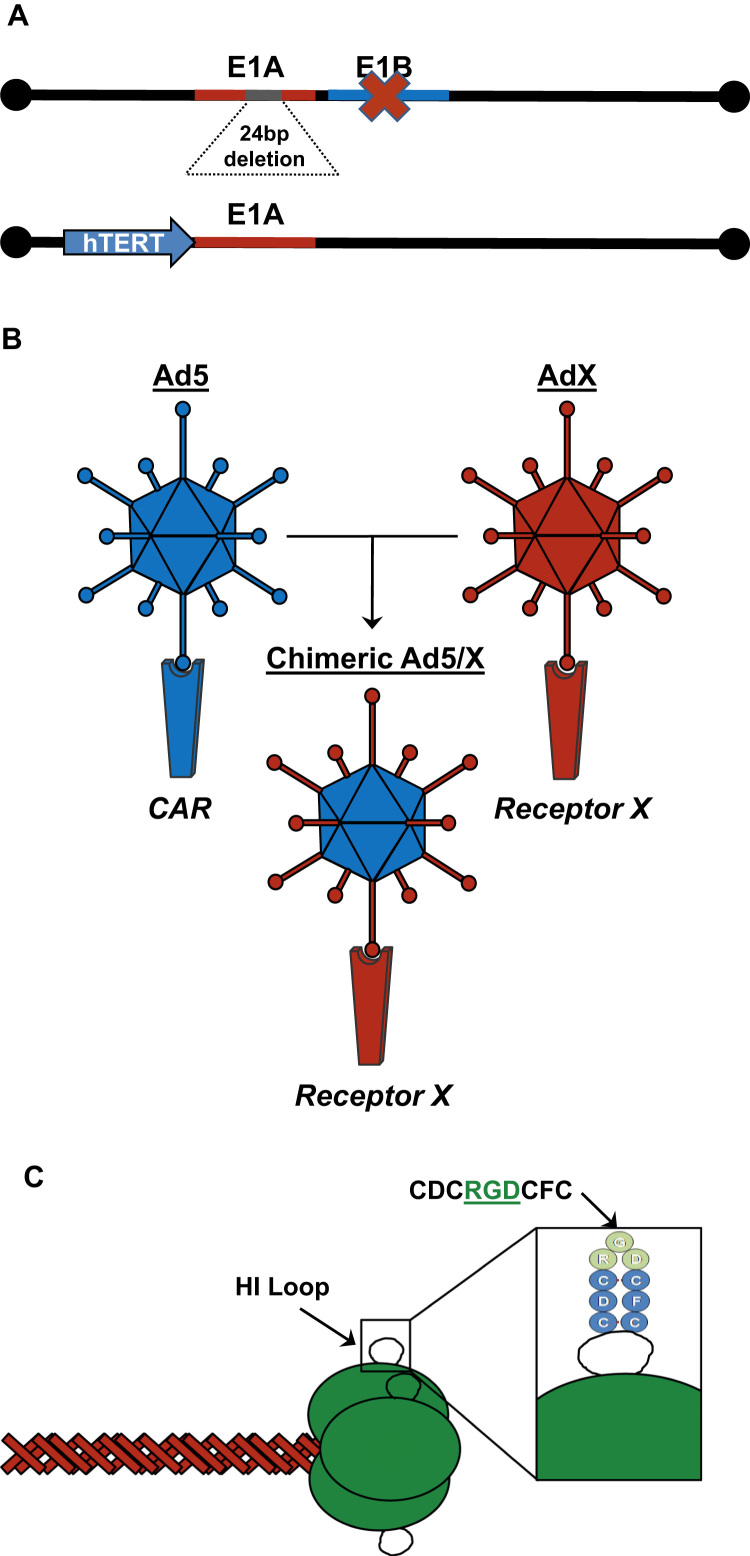
Fig. 3.
Examples of strategies to obtain oncolytic adenoviruses with tumor selective replication (A) or an enhanced tumor tropism (B and C). A) By mutating the E1A or E1B genes the replication is restricted to pRB or p53 deficient cells, respectively. The expression of E1A under tumor specific promoters, like human telomerase promoter (hTERT), allows viral replication only in tumor cells. B) Generation of chimeric Ad5 viruses by fiber replacement from another serotype (AdX), thus obtaining an Ad5 with the tropism of the AdX. C) Addition of the integrin binding motif CDCRGDCDC within the knob domain of the fiber.
Another strategy to achieve a cancer selective replication lies on the mutation of the E1A transcription unit. In 2000 it was published by Fueyo et al. [43] the generation of a CRAd named Delta24 carrying a 24-bp deletion in the second conserved region (CR2) of the E1A protein (Fig. 3A). Considering that this region is involved in the binding of E1A to pRb, the propagation of Delta24 is hindered in non-tumor cells because of its inability to release the E2F required to promote the viral replication. By contrast, the lack of a fully functional E1A protein does not affects the virus replication in tumor cells since in most of the tumors the pRb function is already impaired. Consequently, the Delta24 adenovirus replicates in and kills cancer cells while remains attenuated in normal cells.
A different approach to obtain CRAds is based on the replacement of the E1A transcription unit promoter by tumor specific promoters [44] (Fig. 3A), such as the promoters for the human telomerase (hTERT) [45], [46], [47], the prostatic specific antigen (PSA) [48] or the α-fetoprotein (AFP) [49], and so the viral gene expression and replication can only be achieved in those tumors which activate these transgenic promoters.
5. Oncolytic adenoviruses as therapeutic tools: widening adenoviruses tropism
Besides the ability of the virus to replicate selectively in cancer cells, it must be also considered its tropism towards the tumors. As described previously, the virus recognizes its target cells trough the interaction of the fiber protein with a specific viral receptor on the cell membrane. The most common Ad5 receptor is the CAR protein (Coxsackie and Adenovirus Receptor) [50], [51], which participates in intercellular unions within the tight junctions [52]. Nevertheless, the CAR protein expression is down-regulated in many aggressive tumors [53], [54], [55], [56], thus hampering the adenovirus infection and so its oncolytic effect. To overcome this barrier there have been engineered modifications of the adenovirus fiber protein in order to retarget the virus to different receptors that could mediated the virus internalization in the cancer cells (Figs. 3B and 3C).
One of the most preeminent examples of these modifications is the insertion of an RGD peptide in the HI loop of the fiber knob domain [57]. Since RGD is an αv-integrin binding motif, the addition of this peptide allows the adenovirus attachment to different integrins, like αvβ3 orαvβ5, and subsequently the virus infects the cell in a CAR-independent manner [58]. The ability of this modified fiber to mediate adenovirus infection in CAR-negative cells has been exploited in multiple virotherapy studies for a broad range of tumors as for example in glioma [59], [60], [61], osteosarcoma [62], [63], ovarian cancer [64], prostate cancer [65] or bladder cancer [66].
6. Clinical experience with oncolytic adenoviruses
Virotherapy has emerged as a novel innovative approach in the fight against cancer. Nevertheless, despite the booming starting of virotherapy its road to the clinic has been a long and windy one not exempt of difficulties and drawback. In the early 2000s, multiple phase I and II clinical trials were completed using the oncolytic adenovirus ONYX-015 obtaining as a result a safety profile and moderate but promising antitumor activity of the virus [67], [68], [69], [70]. However due to a switch in corporative strategies, the rights of ONYX-015 were sold to the Chinese company Shanghai Sunway Biotech [71], thus leading to the impression that ONYX-015 was an ineffective therapeutic agent. Therefore, the interest in oncolytic adenoviruses dropped during a few years. Later on, Shanghai Sunway Biotech finished its phase III clinical trial using the oncolytic adenovirus H101 (a modified ONYX-015), and its use has been approved by the Chinese authorities for the treatment of squamous cell cancer of head and neck (HNC) [72]. This adenovirus (marketed under the brand name Oncorine) has demonstrated its efficacy and safety in HNC in the phase III trial obtaining an overall response rate nearly 80% in combination with cisplatin with only mild flu-like symptoms as side effects [73]. However, it is important to highlight that this treatment was approved by the Chinese Regulatory Agency without any data regarding patient´s survival, nor the company has reported information about overall survival.
Although adenovirus virotherapy has not been approved yet by any Western country regulator, the “resurrection” of ONYX-015/H101 has awaked the interest in oncolytic adenoviruses. As a result, at the present we can find in the literature a plethora of clinical trials using oncolytic adenoviruses for the treatment of different cancers, although most of them are focused on the evaluation of the safety rather than efficacy of the treatment.
As an example, two phase I clinical trials for the treatment of patients affected by recurrent gynecologic cancers have been conducted using the Delta24-RGD virus [74] or the Ad5/3-Δ24 virus [75], which is a fiber chimeric Delta24 virus carrying the knob domain of the Ad3. In both studies, the results shows a safety profile of the treatment since only moderate side effect such as nausea or fatigue were attributable to the viruses. Moreover, adenoviral replication was detected in ascites of most of the patients, being detectable even nearly one month after the treatment in those patients who received the highest dose. In regard to clinical efficacy one month after the treatment with the oncolytic adenoviruses nearly 60% of the patients show a stable disease under RECIST criteria in both trials. Nevertheless, it must be considered that RECIST criteria could be underestimating the potency of the treatment since tumor pseudoprogression has been reported as a common occurrence in virotherapy [76].
Recently the oncolytic virus Talimogene laherparepvec (T-VEC), an attenuated herpes simplex type 1 (HSV-1) encoding the GM-CSF, has been approved by the Western countries regulatory agencies for the treatment of advanced melanoma [77]. Although T-VEC is not an adenovirus, this is an important milestone in the field of virotherapy since it is the first oncolytic virus being accepted by the Western agencies, thus paving the way in the development of other oncolytic viruses, such as adenoviruses.
7. Oncolytic adenovirus and osteosarcoma
In regard to osteosarcoma, there is still little data regarding the use of oncolytic adenoviruses as therapeutic agents. Even though scarce, several preclinical studies utilizing oncolytic adenoviruses have already shown encouraging antisarcoma effect in human and in dogs [62], [63], [78], [79], [80], [81]. Despite the highly interesting model of canine OS, in this study we focus in the use of oncolytic adenoviruses for human OS.
Since most of OS tumors are defective in the Rb pathway [82], the CRAd Delta24 is an attractive platform to develop virotherapeutic strategies against OS. One caveat to this strategy is that OS primary-tumor cells as well as most OS cell lines express low levels of CAR receptor. This is a major limiting factor to adenoviral infection [83], [84]. For this reason, one of the first works on the use of oncolytic adenovirus in OS, if not the first, employed a Delta24 virus containing the RGD-4C insertion in the HI loop of the fiber knob (Delta24-RGD) [62]. In this study, Witlox and colleagues demonstrated the presence of αvβ3/5 integrins in OS primary cultures and cell lines and also their higher susceptibility to an RGD modified virus infection in comparison with an unmodified virus [62]. Also the oncolytic effect was assessed in vitro obtaining as a result a massive reduction in OS primary and cell line cultures viability after its incubation with low doses of Delta24-RGD virus (MOI of 0.1 or 1), whereas at these doses the Delta24 virus has no effect in cell viability except in the CAR-positive SaOS-2 cell line. Then, the Delta24-RGD was administered intratumorally at 5×107 pfu in nude mice carrying subcutaneous primary human OS xenografts, obtaining a delay in tumor growth of 11 days compared to untreated animals. Moreover, they demonstrated that the virus was able to replicate within the tumor. However, the virus was unable to fully eradicate the xenograft, and the authors speculated this could be due to an inefficient spread of the virus through the tumor matrix. It must also be considered that this experiment was carried out in a subcutaneous model, and the tumor environment may impact on the tumor progression hampering the virus efficacy.
Later on, in 2008 the same group showed results obtained in a lung metastatic nude mice model after the systemic administration of Delta24-RGD [79]. The virus was administered intravenously at 1×109 pfu weekly during three consecutive weeks (weeks 1–3), and also they performed a second experiment delaying the administration of the virus 4 weeks (weeks 5–7). In both cases the systemic administration of the adenovirus induced a reduction in the number of pulmonary metastatic nodules. Again, they observed adenovirus replication 3 weeks after the last administration of the virus, indicating that tumor matrix did not block the spread of the virus within the tumor. Surprisingly, the antitumor efficacy of Delta24-RGD was slightly higher when the virus was administered with a 4-weeks delay. The authors suggested a relationship between a higher vascularization of the tumors and the ability of the virus to target the tumor cells through an intravenous administration. Nevertheless, this affirmation was just a hypothesis and was not contrasted.
As described above, tumor location could be an important factor when it comes to its own progression and how the virus will exert its anticancer effect. Therefore, it is important to evaluate the viral antitumor efficacy in relevant orthotopic models. Recently, our group published two studies where we evaluated the use of oncolytic adenoviruses in sarcoma in local orthotopic, tibial, and lung metastatic models [63], [81]. In the first of these studies, 531MII human primary OS cells were engrafted in the tibia of nude mice, and then Delta24-RGD was administered intratumorally at a dose 3.8×107 pfu once a week during three consecutive weeks, obtaining a significant reduction in tumor burden [63]. We also evaluated a possible synergistic effect of the Delta24-RGD in combination with cisplatin, which is used in the current chemotherapeutic protocols for OS. We showed that unlike other drugs used for the treatment of OS, such as doxorubicin, cisplatin does not interfere with viral replication. Moreover, the adenovirus mediates an enhanced antitumoral effect of cisplatin since its IC50 in vitro decreased to 1 logarithm due to the induction of autophagic cell death. Regarding the in vivo studies, mice bearing lung metastatic OS were treated with cisplatin and Delta24-RGD (intravenously at 2.5×108 pfu) weekly during three weeks. This combined regimen led to a reduction of the tumor burden in the lungs, indicating that the combination of Delta24-RGD and cisplatin could be an effective treatment for metastatic lesions in osteosarcoma.
In a second study, our group evaluated the efficacy of the VCN-01 virus as a therapeutic agent in both orthotopic and metastatic models of OS [81]. The VCN-01 is an oncolytic adenovirus whose E1A gene also contains the ∆24 deletion in the pRb binding site, thus leading to a selective replication in Rb deficient tumor cells [85]. However, this virus contains two additional modifications to enhance its anticancer effect. First, an RGD integrin binding motif has been placed in the fiber shaft, replacing the putative heparan-sulfate proteoglycan binding motif KKTK. This modification improves the tumoral tropism of the virus as well as it abrogates the liver sequestration of the virus [86]. On the other hand, this virus also encodes the PH20 hyaluronidase to facilitate the spread of the virus within the extracellular matrix of the tumor, allowing the virus to reach new target cells [87] (Fig. 4). To evaluate the oncolytic activity of the VCN-01 in OS, the virus was administered intratumorally at 107 or 108 pfu in nude mice bearing orthotopic intratibial tumors. As a result, only a 30% of the tibias treated with 107 pfu developed visible tumors at 90 days after the engraftment, and none of the treated with 108 pfu did. Moreover, visible tumors where observed in all untreated animals. Interestingly, 70% of the control animals presented also lung metastases, while the development of metastases was completely abolished in the animals treated with the higher dose of virus. These data underscored the potent antiosteosarcoma effect of the VCN-01 in both the growth of the primary tumors and the prevention of lung metastases. The efficacy of VCN-01 was assessed also in nude mice with lung metastatic OS tumors, and to this end the virus was administered intravenously at the same doses as before. Once again, the tumor burden was reduced in the animals treated with the VCN-01 compared to the control animals, as they developed less metastatic nodules, which in turn where also smaller. All together, the results obtained with the VCN-01 adenovirus demonstrate its potential use as an effective therapeutic agent for the treatment of osteosarcoma.
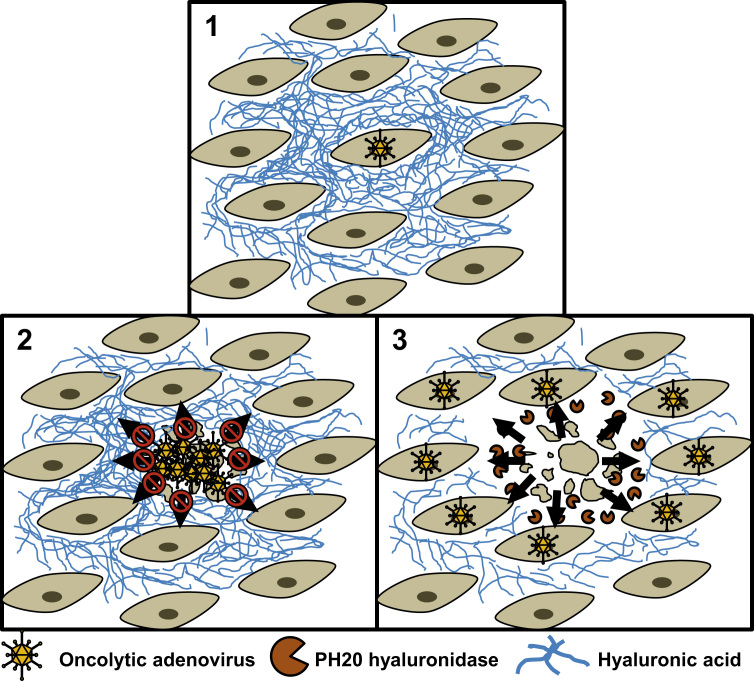
Fig. 4.
Schematic illustration showing an enhancement of the viral spread in the presence of a porous extracellular matrix (ECM). An infected tumor cell (1) will eventually produce new infectious viral particles. The spread of a normal oncolytic adenovirus is hindered by a dense hyaluronic acid ECM (2). The VCN-01 adenovirus encodes the human hyaluronidase PH20, which is secreted by the infected cell and, in turn, the hyaluronidase degrades the hyaluronic acid, facilitating the spread of the viral progeny through the ECM to new tumor cells (3).
8. Future perspectives: immuno-virotherapy?
In the different studies explained above, oncolytic adenoviruses showed promising results that validate these viruses as potential candidates in the fight against osteosarcoma. Nevertheless, all the studies published to date were performed in immunodeficient mice models. One important lesson that we have learned from recent oncolytic viruses-based clinical trials is that besides the lytic effect that they induce through replication they also trigger systemic antitumor immune responses. It is clear that a proper immune response against the tumor is a key element that helps to achieve a successful antitumor therapy, and the use of oncolytic adenoviruses may play an important role in stimulating such immune reaction against tumor cells by different mechanisms, such as an increase in the presentation of tumor associated antigens (TAAs). Eventually, the cell death mediated by the adenovirus would release tumor antigens which can be presented to T-cell lymphocytes by the antigen presenting cells (APC). The combination of the lytic activity of the virus and the enhancement of the immune reaction could lead to a synergistic antitumor effect. Therefore, in order to fully understand how preclinical studies will translate to the clinic it would be very important to have proper immunocompetent models and, consequently, lacking a complete immune system may alter the results thus hindering the prediction of these treatments in patients. Regarding immunocompetent models to evaluate the efficacy and immune mechanisms triggered by adenoviruses we come to a major hurdle which is the lack or low replication of adenoviruses in murine cells [88], [89], [90]. This limitation has led to a paucity of studies in immunocompetent models. Recently, several works have addressed the effect of oncolytic adenoviruses in the context of immunocompetent mice. Even though, one can argue these are not the best models, at least, these experimental approaches have provided clues regarding the possible effect of oncolytic viruses in humans. Thus, it would be imperative to develop new animal models which allow us to evaluate together the lytic activity of the virus and the enhancement of the immune reaction against the tumor. In the recent years, some groups have developed humanized mouse models which contain a human immune system so human tumor cells can be transferred to these mice, thus enabling to study the replication of the oncolytic virus in an immunocompetent environment.
In summary, it is clear that in preclinical studies oncolytic adenoviruses are effective against osteosarcoma. Further, clinical trials will uncover if these non-pathogenic viruses fulfill the promise seen in the lab. In addition, we are witnessing an exciting time for immunotherapy with the antibodies against the immune-checkpoints, CAR-T cells and other immune strategies achieving success in the clinic for some type of tumors. All these immune-therapies are amenable to combine with oncolytic viruses opening the door to possible synergistic combination. We hope that in the near future we can see the results in the form of successful clinical trial based oncolytic adenoviruses for those osteosarcomas that do not respond to the standard treatment.
Conflict of interest
No potential conflicts of interest to disclose.
Acknowledgments and grant support
This work was supported by the European Union (Marie Curie IRG270459 to MMA), the Instituto de Salud Carlos III y los Fondos Feder Europeos (PI13/125 to MMA), the Spanish Ministry of Economy and competitiveness (IEDI-2015-00638 to MMA), The L`OREAL-Unesco Foundation (to MMA), The Department of Health of the Government of Navarra 22/2015 (to MMA), The Basque Foundation for Health Research (BIOEF, BIO13/CI/005), The 9th GEIS grant Jose María Buesa for the Research in Sarcomas (to MMA) and Fundación Caja Navarra (Convocatoria de Ayudas 2015 to MMA).
References
- 1.Anfinsen K.P. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976–2005) Cancer Epidemiol. Biomark. Prev. 2011;20(8):1770–1777. doi: 10.1158/1055-9965.EPI-11-0136. [DOI] [PubMed] [Google Scholar]
- 2.Valery P.C., Laversanne M., Bray F. Bone cancer incidence by morphological subtype: a global assessment. Cancer Causes Control. 2015;26(8):1127–1139. doi: 10.1007/s10552-015-0607-3. [DOI] [PubMed] [Google Scholar]
- 3.Dorfman H.D., Czerniak B. Bone cancers. Cancer. 1995;75(1 Suppl):203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Gorlick R. Current concepts on the molecular biology of osteosarcoma. Cancer Treat. Res. 2009;152:467–478. doi: 10.1007/978-1-4419-0284-9_27. [DOI] [PubMed] [Google Scholar]
- 5.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and end results program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duong L.M., Richardson L.C. Descriptive epidemiology of malignant primary osteosarcoma using population-based registries, United States, 1999–2008. J. Regist. Manag. 2013;40(2):59–64. [PMC free article] [PubMed] [Google Scholar]
- 7.Mirabello L., Troisi R.J., Savage S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J. Cancer. 2009;125(1):229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraumeni J.F., Jr. Stature and malignant tumors of bone in childhood and adolescence. Cancer. 1967;20(6):967–973. doi: 10.1002/1097-0142(196706)20:6<967::aid-cncr2820200606>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Mirabello L. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control. 2011;22(6):899–908. doi: 10.1007/s10552-011-9763-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotterill S.J. Stature of young people with malignant bone tumors. Pedia. Blood Cancer. 2004;42(1):59–63. doi: 10.1002/pbc.10437. [DOI] [PubMed] [Google Scholar]
- 11.Hamre M.R. Osteosarcoma as a second malignant neoplasm. Radio. Oncol. 2002;65(3):153–157. doi: 10.1016/s0167-8140(02)00150-0. [DOI] [PubMed] [Google Scholar]
- 12.Mirabello L. Germline TP53 variants and susceptibility to osteosarcoma. J. Natl. Cancer Inst. 2015;107:7. doi: 10.1093/jnci/djv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamplot J.D. The Current and future therapies for human osteosarcoma. Curr. Cancer Ther. Rev. 2013;9(1):55–77. doi: 10.2174/1573394711309010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayerza M.A. Does increased rate of limb-sparing surgery affect survival in osteosarcoma? Clin. Orthop. Relat. Res. 2010;468(11):2854–2859. doi: 10.1007/s11999-010-1423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antunes M. Excision of pulmonary metastases of osteogenic sarcoma of the limbs. Eur. J. Cardiothorac. Surg. 1999;15(5):592–596. doi: 10.1016/s1010-7940(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 16.Jeffree G.M., Price C.H., Sissons H.A. The metastatic patterns of osteosarcoma. Br. J. Cancer. 1975;32(1):87–107. doi: 10.1038/bjc.1975.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price C.H., Jeffree G.M. Metastatic spread of osteosarcoma. Br. J. Cancer. 1973;28(6):515–524. doi: 10.1038/bjc.1973.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longhi A. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat. Rev. 2006;32(6):423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 19.He H., Ni J., Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review) Oncol. Lett. 2014;7(5):1352–1362. doi: 10.3892/ol.2014.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurisicova A. Molecular requirements for doxorubicin-mediated death in murine oocytes. Cell Death Differ. 2006;13(9):1466–1474. doi: 10.1038/sj.cdd.4401819. [DOI] [PubMed] [Google Scholar]
- 21.Skinner R. Ototoxicity of cisplatinum in children and adolescents. Br. J. Cancer. 1990;61(6):927–931. doi: 10.1038/bjc.1990.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz L. Auditory function in pediatric osteosarcoma patients treated with multiple doses of cis-diamminedichloroplatinum(II) Cancer Res. 1989;49(3):742–744. [PubMed] [Google Scholar]
- 23.Zhang S. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012;18(11):1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 24.Hegyi M. Clinical relations of methotrexate pharmacokinetics in the treatment for pediatric osteosarcoma. J. Cancer Res. Clin. Oncol. 2012;138(10):1697–1702. doi: 10.1007/s00432-012-1214-2. [DOI] [PubMed] [Google Scholar]
- 25.Holmboe L. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br. J. Clin. Pharm. 2012;73(1):106–114. doi: 10.1111/j.1365-2125.2011.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaffe N. Renal toxicity with cumulative doses of cis-diamminedichloroplatinum-II in pediatric patients with osteosarcoma. Effect on creatinine clearance and methotrexate excretion. Cancer. 1987;59(9):1577–1581. doi: 10.1002/1097-0142(19870501)59:9<1577::aid-cncr2820590908>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Russell W.C. Adenoviruses: update on structure and function. J. Gen. Virol. 2009;90(Pt 1):1–20. doi: 10.1099/vir.0.003087-0. [DOI] [PubMed] [Google Scholar]
- 28.McConnell M.J., Imperiale M.J. Biology of adenovirus and its use as a vector for gene therapy. Hum. Gene Ther. 2004;15(11):1022–1033. doi: 10.1089/hum.2004.15.1022. [DOI] [PubMed] [Google Scholar]
- 29.Bandara L.R., La Thangue N.B. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991;351(6326):494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- 30.Arroyo M., Raychaudhuri P. Retinoblastoma-repression of E2F-dependent transcription depends on the ability of the retinoblastoma protein to interact with E2F and is abrogated by the adenovirus E1A oncoprotein. Nucleic Acids Res. 1992;20(22):5947–5954. doi: 10.1093/nar/20.22.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyson N., Harlow E. Adenovirus E1A targets key regulators of cell proliferation. Cancer Surv. 1992;12:161–195. [PubMed] [Google Scholar]
- 32.Lowe S.W., Ruley H.E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7(4):535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 33.Debbas M., White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7(4):546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 34.Tatsis N., Ertl H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004;10(4):616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wold W.S., Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013;13(6):421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majhen D. Adenovirus-based vaccines for fighting infectious diseases and cancer: progress in the field. Hum. Gene Ther. 2014;25(4):301–317. doi: 10.1089/hum.2013.235. [DOI] [PubMed] [Google Scholar]
- 37.Rosewell Shaw A., Suzuki M. Recent advances in oncolytic adenovirus therapies for cancer. Curr. Opin. Virol. 2016;21:9–15. doi: 10.1016/j.coviro.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker D.D., Berk A.J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156(1):107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 39.Bischoff J.R. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 40.Rothmann T. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J. Virol. 1998;72(12):9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harada J.N., Berk A.J. P53-Independent and -dependent requirements for E1B–55K in adenovirus type 5 replication. J. Virol. 1999;73(7):5333–5344. doi: 10.1128/jvi.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Shea C.C. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6(6):611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Fueyo J. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19(1):2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 44.Hardcastle J. Oncolytic viruses driven by tumor-specific promoters. Curr. Cancer Drug Targets. 2007;7(2):181–189. doi: 10.2174/156800907780058880. [DOI] [PubMed] [Google Scholar]
- 45.Wirth T. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63(12):3181–3188. [PubMed] [Google Scholar]
- 46.Irving J. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Ther. 2004;11(3):174–185. doi: 10.1038/sj.cgt.7700666. [DOI] [PubMed] [Google Scholar]
- 47.Kawashima T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004;10(1 Pt 1):285–292. doi: 10.1158/1078-0432.ccr-1075-3. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez R. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57(13):2559–2563. [PubMed] [Google Scholar]
- 49.Zhang K.J. Complete eradication of hepatomas using an oncolytic adenovirus containing AFP promoter controlling E1A and an E1B deletion to drive IL-24 expression. Cancer Gene Ther. 2012;19(9):619–629. doi: 10.1038/cgt.2012.40. [DOI] [PubMed] [Google Scholar]
- 50.Bergelson J.M. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 51.Law L.K., Davidson B.L. What does it take to bind CAR? Mol. Ther. 2005;12(4):599–609. doi: 10.1016/j.ymthe.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 52.Coyne C.B., Bergelson J.M. CAR: a virus receptor within the tight junction. Adv. Drug Deliv. Rev. 2005;57(6):869–882. doi: 10.1016/j.addr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Kim M. The coxsackievirus and adenovirus receptor acts as a tumour suppressor in malignant glioma cells. Br. J. Cancer. 2003;88(9):1411–1416. doi: 10.1038/sj.bjc.6600932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tango Y. Late resistance to adenoviral p53-mediated apoptosis caused by decreased expression of Coxsackie-adenovirus receptors in human lung cancer cells. Cancer Sci. 2004;95(5):459–463. doi: 10.1111/j.1349-7006.2004.tb03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumoto K. Loss of coxsackie and adenovirus receptor expression is associated with features of aggressive bladder cancer. Urology. 2005;66(2):441–446. doi: 10.1016/j.urology.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 56.Anders M. Loss of the coxsackie and adenovirus receptor contributes to gastric cancer progression. Br. J. Cancer. 2009;100(2):352–359. doi: 10.1038/sj.bjc.6604876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki K. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin. Cancer Res. 2001;7(1):120–126. [PubMed] [Google Scholar]
- 58.Dmitriev I. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 1998;72(12):9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kleijn A. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS One. 2014;9(5):e97495. doi: 10.1371/journal.pone.0097495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang H. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS One. 2014;9(5):e97407. doi: 10.1371/journal.pone.0097407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamfers M.L. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62(20):5736–5742. [PubMed] [Google Scholar]
- 62.Witlox A.M. Conditionally replicative adenovirus with tropism expanded towards integrins inhibits osteosarcoma tumor growth in vitro and in vivo. Clin. Cancer Res. 2004;10(1 Pt 1):61–67. doi: 10.1158/1078-0432.ccr-0609-03. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Velez N. The oncolytic adenovirus Delta24-RGD in combination with cisplatin exerts a potent anti-osteosarcoma activity. J. Bone Min. Res. 2014;29(10):2287–2296. doi: 10.1002/jbmr.2253. [DOI] [PubMed] [Google Scholar]
- 64.Zhang B. A novel CRAd in combination with cisplatin enhanced the antitumor efficacy in ovarian cancer. Int J. Gynecol. Cancer. 2011;21(9):1540–1546. doi: 10.1097/IGC.0b013e31823105ed. [DOI] [PubMed] [Google Scholar]
- 65.Shen Y.H. Arg-Gly-Asp (RGD)-modified E1A/E1B Double mutant adenovirus enhances Antitumor activity in prostate cancer cells In vitro and in mice. PLoS One. 2016;11(1):e0147173. doi: 10.1371/journal.pone.0147173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y. RGD-modifided oncolytic adenovirus exhibited potent cytotoxic effect on CAR-negative bladder cancer-initiating cells. Cell Death Dis. 2015:6. doi: 10.1038/cddis.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganly I. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000;6(3):798–806. [PubMed] [Google Scholar]
- 68.Nemunaitis J. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60(22):6359–6366. [PubMed] [Google Scholar]
- 69.Nemunaitis J. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J. Clin. Oncol. 2001;19(2):289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 70.Vasey P.A. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J. Clin. Oncol. 2002;20(6):1562–1569. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- 71.Jia H., Kling J. China offers alternative gateway for experimental drugs. Nat. Biotechnol. 2006;24(2):117–118. doi: 10.1038/nbt0206-117. [DOI] [PubMed] [Google Scholar]
- 72.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006;98(5):298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 73.Xia Z.J. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng. 2004;23(12):1666–1670. [PubMed] [Google Scholar]
- 74.Kimball K.J. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin. Cancer Res. 2010;16(21):5277–5287. doi: 10.1158/1078-0432.CCR-10-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim K.H. A phase I clinical trial of Ad5/3-Delta24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol. Oncol. 2013;130(3):518–524. doi: 10.1016/j.ygyno.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hemminki A. Oncolytic immunotherapy: where are we clinically? Sci. (Cairo) 2014;2014:862925. doi: 10.1155/2014/862925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rehman H. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hemminki A. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol. Ther. 2003;7(2):163–173. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 79.Graat H.C. Intravenous administration of the conditionally replicative adenovirus Ad5-Delta24RGD induces regression of osteosarcoma lung metastases. Mol. Cancer. 2008;7:9. doi: 10.1186/1476-4598-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laborda E. A pRb-responsive, RGD-modified, and hyaluronidase-armed canine oncolytic adenovirus for application in veterinary oncology. Mol. Ther. 2014;22(5):986–998. doi: 10.1038/mt.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez-Velez N. The oncolytic adenovirus VCN-01 as therapeutic approach against pediatric osteosarcoma. Clin. Cancer Res. 2016;22(9):2217–2225. doi: 10.1158/1078-0432.CCR-15-1899. [DOI] [PubMed] [Google Scholar]
- 82.Chen X. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014;7(1):104–112. doi: 10.1016/j.celrep.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Witlox M.A. Epidermal growth factor receptor targeting enhances adenoviral vector based suicide gene therapy of osteosarcoma. J. Gene Med. 2002;4(5):510–516. doi: 10.1002/jgm.308. [DOI] [PubMed] [Google Scholar]
- 84.Graat H.C. Coxsackievirus and adenovirus receptor expression on primary osteosarcoma specimens and implications for gene therapy with recombinant adenoviruses. Clin. Cancer Res. 2005;11(6):2445–2447. doi: 10.1158/1078-0432.CCR-04-2375. (author reply2447-8) [DOI] [PubMed] [Google Scholar]
- 85.Rodriguez-Garcia A. Safety and efficacy of VCN-01, an oncolytic adenovirus combining fiber HSG-binding domain replacement with RGD and hyaluronidase expression. Clin. Cancer Res. 2015;21(6):1406–1418. doi: 10.1158/1078-0432.CCR-14-2213. [DOI] [PubMed] [Google Scholar]
- 86.Bayo-Puxan N. Replacement of adenovirus type 5 fiber shaft heparan sulfate proteoglycan-binding domain with RGD for improved tumor infectivity and targeting. Hum. Gene Ther. 2009;20(10):1214–1221. doi: 10.1089/hum.2009.038. [DOI] [PubMed] [Google Scholar]
- 87.Guedan S. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol. Ther. 2010;18(7):1275–1283. doi: 10.1038/mt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duncan S.J. Infection of mouse liver by human adenovirus type 5. J. Gen. Virol. 1978;40(1):45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- 89.Jogler C. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J. Virol. 2006;80(7):3549–3558. doi: 10.1128/JVI.80.7.3549-3558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blair G.E. Restricted replication of human adenovirus type 5 in mouse cell lines. Virus Res. 1989;14(4):339–346. doi: 10.1016/0168-1702(89)90026-9. [DOI] [PubMed] [Google Scholar]